Introduction
Percutaneous coronary intervention (PCI) is one of
the most effective methods for the treatment of acute myocardial
infarction (AMI) (1). Cardiac
catheterization may be used to clear the narrowed or even occluded
coronary lumen, thereby improving myocardial perfusion. However,
certain studies have indicated that in the case of impaired
coronary microcirculation, even if cardiac revascularization is
successful, part of the blood flow to myocardial tissue may not
fully return to normal, which may lead to poor recovery of left
ventricular function (2).
Myocardial contrast echocardiography (MCE) is a
technique using microbubble distribution within the intravascular
space to reveal the status of microvascular perfusion (3). It enables direct and visual assessment
of microvascular perfusion. MCE has been used to predict the
prognosis for patients with myocardial infarction (4,5). MCE is
more objective to evaluate myocardial ischemia as compared with
normal echocardiography that visually observes the presence or
absence of segmental motor abnormalities. Dobutamine acts on the β1
and β2 receptors and has a different effect on the damaged
microcoronary and normal microcoronary vessels. Dobutamine is able
to activate viable myocardium and may induce more severe myocardial
ischemia. Through observing changes in the intramyocardial
development of the ultrasound enhancer under dobutamine stress,
surviving myocardium may be identified more objectively and
sensitively. Dobutamine is increasingly being used during MCE,
named as dobutamine stress MCE, and may significantly improve the
detection rate of myocardial ischemia and injury in patients with
coronary heart disease (CHD) (6,7).
However, there is currently a lack of research into the application
of stress MCE following PCI, its role in myocardial
microcirculation evaluation and the prediction of subsequent
cardiac function recovery. In the present study, the
microcirculation of 50 patients with AMI who underwent PCI using
MCE and low-dose stress MCE was assessed and the cardiac function
at 6 months following PCI using MCE was also evaluated.
Materials and methods
Patients
From June 2016 to August 2017, 50 patients (29 males
and 21 females) with AMI who underwent PCI were enrolled in the
present study. The characteristics of the patients are listed in
Table I. An appropriate stent length
and diameter were selected for all patients. The age of the
patients ranged from 45 to 76 years, with an average age of
61.3±12.2 years. The inclusion criteria were as follows: i)
Persistent chest pain and elevated troponin I; ii)
electrocardiogram with new myocardial ischemia changes, i.e. new ST
segment changes; iii) segmental wall motion abnormalities from
echocardiography; and iv) PCI performed within 24 h of the
occurrence of chest pain. Patients were excluded if they met the
following exclusion criteria: i) A congenital heart disease or
acute heart failure; ii) allergy to contrast agent; iii) malignant
arrhythmia and severe atrioventricular block; and iv) slow blood
flow or no reflow following PCI. The characteristics of all of the
patients are provided in Table
I.
 | Table IClinical characteristics of patients
(n=50). |
Table I
Clinical characteristics of patients
(n=50).
| Item | Value |
|---|
| Age (years) | 61.3±12.2 |
| Male sex | 29(58) |
| History of
smoking | 22(44) |
| Hyperlipidemia | 26(52) |
| Diabetes | 19(38) |
| Hypertension | 28(56) |
| Artery affected by
infarct | |
|
Front
descending branch | 38(76) |
|
Rotating
branch | 4(8) |
|
Right
crown | 8(16) |
Positron emission tomography
(PET)
PET was used as the gold standard for viable
myocardium detection. PET was performed within 3 days after stress
MCE. According to the PET results, the myocardial segments were
divided into the following groups: Normal myocardium group
(segments with normal perfusion and metabolism), viable myocardium
group (segments with a decrease in perfusion but with a normal
metabolism) and non-viable myocardium group (segments with a
defected perfusion and metabolism).
MCE
MCE was performed within 72 h following PCI. A
two-dimensional echocardiogram was performed prior to MCE and
SonoVue (Bracco Imaging S.p.A) was used as the contrast agent. A
total of 59 mg SonoVue was diluted in 20 ml normal saline and
shaken for 20 sec to obtain a white microbubble suspension.
Subsequently, the diluted SonoVue contrast agent was injected into
the upper limb anterior wall vein at a constant speed of 1.5
ml/min. After the contrast agent was stabilized in the myocardial
image for 2-3 min, high-mechanical index ultrasound beam emission
was used to destroy the contrast microbubbles in the myocardium,
and subsequently, the reperfusion process of the microbubbles was
observed in the apical view of the heart with a low mechanical
index (<0.2). Patients were prevented from using β-blockers and
drugs that may affect myocardial contractility 24 h prior to
MCE.
Low-dose dobutamine stress MCE
Low-dose dobutamine was injected at an initial dose
of 5 µg/kg/min and subsequently increased to 10 µg/kg/min and then
20 µg/kg/min every three minutes. Any changes in heart rate and
blood pressure were observed and MCE was performed a second time
using the same settings after reaching the loading dose.
Data analysis/image
interpretation
Semi-quantitative analysis of MCE was performed
using the following criteria: i) Uniform and sufficient contrast
agent display and good perfusion (1 point); ii) sparse contrast
agent display and weak perfusion or partial and flaky perfusion
(0.5 point); and iii) contrast agent filling defect or no perfusion
(0 point). The semi-quantitative index was statistically analyzed
using the contrast-enhanced index (CSI), which is calculated by
dividing the sum of the relevant segmental angiographic scores by
the number of segments.
Quantitative analysis of MCE was also performed. The
change in echo intensity (dB) of the contrast agent microbubble
signal in myocardial tissue over time was analyzed by placing the
region of interest (ROI) in the center of the wall of each segment
(the ROI size was set to a standard of 5 mm2). The
endometrium, epicardium and papillary muscles were avoided when the
area to be analyzed was selected. The QLAB quantification software
(Philips, version 10.5) automatically generates the time-perfusion
intensity curve and fits the function Y=A x (1-e-βt) +
C, where A is the peak intensity of the curve, reflecting the
myocardial blood volume, β, is the slope of the curve, reflecting
the myocardial blood flow (MBF) velocity and A x β reflects the MBF
(8,9).
Statistical analysis
Data were analyzed using SPSS v22.0 statistical
software (IBM, Corp.). The distribution of data was analyzed by
Shapiro-Wilk test, and normally distributed data were expressed as
the mean ± standard deviation. Categorical variables are expressed
as n (%). Differences between two independent groups were
determined using Student's t-test, while those among three groups
were assessed using one-way analysis of variance followed by a
Newman-Keuls post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Myocardial segment collection and
adverse effects
A total of 475 myocardial segments were collected
from the normal coronary blood supply area, used as normal
myocardium control. In the infarcted coronary blood supply area,
264 myocardial segments were collected, including 171 viable
myocardial segments and 93 non-viable myocardial segments. No
adverse effects, including abnormal heart rate, abnormal blood
pressure, chest tightness, belching or dizziness, were observed
during MCE.
Evaluation of microcirculation at 72 h
after PCI
Quantitative analysis revealed that A, β and A x β
of the normal coronary blood supply area were significantly larger
compared with those in the viable myocardium segment and the
non-viable myocardium segment (Table
II).
 | Table IIComparison of microcirculation between
normal myocardium, viable myocardium and non-viable myocardium at
72 h after surgery. |
Table II
Comparison of microcirculation between
normal myocardium, viable myocardium and non-viable myocardium at
72 h after surgery.
| Item | Normal myocardium
(n=475) | Viable myocardium
(n=171) | Non-viable myocardium
(n=93) | F-value | P-value |
|---|
| A (db) | 8.47±2.03 |
6.86±1.82a |
1.87±0.72a | 160.215 | <0.001 |
| β (1/sec) | 1.21±0.43 |
0.95±0.33a |
0.43±0.22a | 118.573 | <0.001 |
| A x β (db/sec) | 10.15±3.35 |
8.68±2.56a |
1.16±0.64a | 125.261 | <0.001 |
The microcirculation was also investigated following
low-dose dobutamine loading. Quantitative analysis revealed that
dobutamine loading significantly increased the values of A, β and A
x β of the normal coronary blood supply area. However, in the
viable myocardium, the segments A and A x β were markedly decreased
after dobutamine loading (Table
III); there was no significant change for segment β. As shown
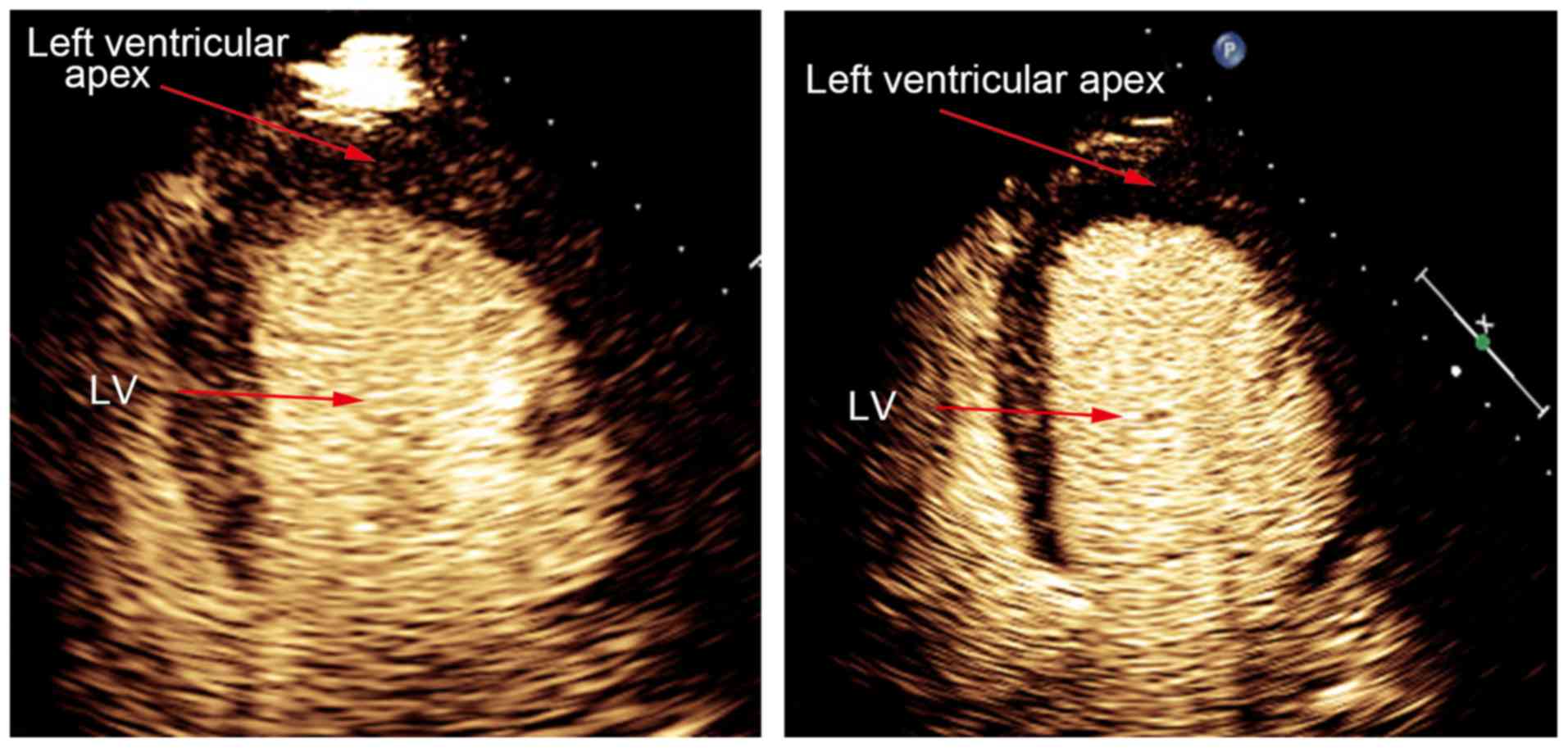
in Fig. 1, contrast agent filling
defect was enhanced after stress test in viable myocardial segment.
Furthermore, no significant changes were observed in the values of
A, β and A x β of the non-viable myocardium after loading (Table III).
 | Table IIIComparison of microcirculation prior
to and after dobutamine loading. |
Table III
Comparison of microcirculation prior
to and after dobutamine loading.
| | Normal myocardium
(n=475) | Viable myocardium
(n=171) | Non-viable myocardium
(n=93) |
|---|
| Item | Prior to loading | After loading | Prior to loading | After loading | Prior to loading | After loading |
|---|
| A (db) | 8.47±2.03 |
15.42±2.99a | 6.86±1.82 |
4.39±1.15a | 1.87±0.72 | 1.82±0.68 |
| β (1/sec) | 1.21±0.43 |
2.27±0.57a | 0.95±0.33 | 1.07±0.51 | 0.43±0.22 | 0.57±0.27 |
| A x β (db/sec) | 10.15±3.35 |
35.67±5.47a | 8.68±2.56 |
7.33±2.04a | 1.16±0.64 | 1.03±0.26 |
Recovery of left ventricular function
6 months after PCI
A total of 6 months following PCI, the values of A,
β and A x β of the viable myocardium group were significantly
increased compared with those at 72 h following surgery; while no
change was observed in the non-viable myocardium group (Table IV).
 | Table IVComparison of microcirculation between
6 months and 72 h after surgery. |
Table IV
Comparison of microcirculation between
6 months and 72 h after surgery.
| | Normal myocardium
(n=475) | Viable myocardium
(n=171) | Non-viable myocardium
(n=93) |
|---|
| Item | 72 h | 6 months | 72 h | 6 months | 72 h | 6 months |
|---|
| A (db) | 8.47±2.03 | 8.86±1.57 | 6.86±1.82 |
9.20±3.37a | 1.87±0.72 | 1.78±0.63 |
| β (1/sec) | 1.21±0.43 | 1.35±0.56 | 0.95±0.33 |
1.38±0.82a | 0.43±0.22 | 0.44±0.31 |
| A x β (db/sec) | 10.15±3.35 | 11.84±2.98 | 8.68±2.56 |
12.14±3.59a | 1.16±0.64 | 0.83±0.20 |
In order to evaluate the predictive value of
dobutamine stress MCE for cardiac function, patients were further
divided into two groups based on the change of CSI: i) Dobutamine
stress echocardiography (DSE)-positive group (increase or decrease
of CSI by >0.2 after dobutamine challenge) and ii) DSE-negative
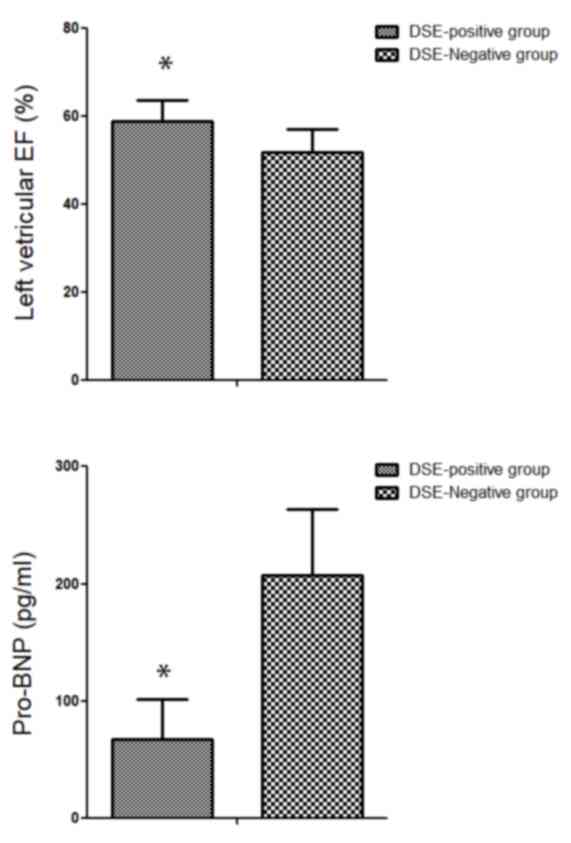
group (changes in CSI by <0.2). The results revealed that 6
months following intervention, the left ventricular ejection
fraction in the DSE-positive group (58.97±4.60%) was significantly
higher compared with that in the DSE-negative group (51.86±5.23%;
P<0.05; Fig. 2A). With regard to
the heart failure index, pro-B-type natriuretic peptide (pro-BNP),
a significant decrease of pro-BNP in the DSE-positive group
(67.73±33.79 pg/ml) was observed as compared with that in the
DSE-negative group (207.20±56.10 pg/ml; P<0.05; Fig. 2B). Taken together, CSI may
effectively predict the left ventricular function in patients with
AMI.
Discussion
Either DSE or MCE has been widely applied in the
evaluation of blood supply in CHD (10,11).
However, only few studies have been performed combining the two.
Dobutamine stress testing was used to detect viable myocardium
using a visual method, which was markedly affected by the
subjective effect of the ultrasound physician. MCE alone may only
reflect the myocardial microcirculation in the resting state and
false-positives may occur. Given their respective disadvantages,
the potential use of the combination of MCE with dobutamine stress
testing (low-dose dobutamine stress MCE) to evaluate myocardial
microcirculation perfusion and predict left ventricular function
was explored in the present study. The results suggested that
low-dose dobutamine stress MCE may be an effective method to
evaluate myocardial microcirculation perfusion in patients with AMI
after PCI. In addition, CSI as a simple semi-quantitative index is
able to predict left ventricular function in patients with AMI. By
combining wall motion and myocardial perfusion images, stress MCE
significantly improved the diagnostic value and diagnostic accuracy
for CHD. Furthermore, it is able to more accurately identify
surviving and dead myocardium and detect myocardial reserve
function (12-14).
Myocardial microcirculatory disorders caused by
ischemia-reperfusion injury following PCI include reversible and
irreversible damage. Galiuto et al (15) observed that in 50% of patients with
perfusion defects who underwent MCE 24 h following PCI,
self-healing was evident at follow-up, while the other 50% of
patients had persistent perfusion defects. Repeated MCE examination
prior to reperfusion therapy and early after reperfusion therapy
was able to simultaneously evaluate the effects of myocardial
microcirculation and interventional therapy (16).
Complete cardiomyocyte function is excitatory,
conductive and contractile, and good blood perfusion and myocardial
cell metabolic activity are necessary to maintain its normal
physiological activities (17,18). It
is generally thought that necrotic and scarred myocardium is a type
of myocardial damage, which cannot be reversed. An important
characteristic of viable myocardium is that myocardial dysfunction
may be reversed (19), that is, the
left ventricular function index may be significantly improved
following revascularization, and may benefit patients (20). It is difficult to confirm whether the
perfusion defect area in the MCE examination after AMI is a
complete structural injury or only a reversible injury. Therefore,
a load test is required to further determine whether
vasoconstriction in the perfusion defect area and abnormal
dilatation of blood vessels outside the perfusion defect area
exist.
In the present study, patients receiving PCI within
24 h of chest pain were included. It is generally thought that PCI
should be performed within 24 h of chest pain in order to achieve
timely rescue of ischemic myocardium. Furthermore, the difference
in the microcirculation status after the blood supply of large
vessels was restored. An increase in the values of A, β and A x β
was observed in normal coronary blood supply areas and a decrease
in viable myocardium areas was identified after low-dose dobutamine
loading.
The possible reason may be attributable to the
direct expansion of the coronary artery through stimulation of the
β2 receptor by dobutamine. On the other hand, dobutamine, as a
positive inotropic drug, may increase myocardial contractility,
thereby increasing the myocardial oxygen demand. The increase in
metabolites is able to dilate the inner diameter of the blood
vessels and reduce the resistance through acting on the tiny
vessels of the coronary arteries and thus increase the coronary
blood flow (21). The maximum
expansion of coronary vessels (coronary flow reserve) depends on
the expansion ability of tiny blood vessels and the damaged
coronary microvascular expansion ability is weakened, and coronary
steal is likely to occur after the load. Therefore, the status of
microvessels may be indirectly evaluated by detecting the coronary
flow reserve by using a load test (16). In the present study, a decrease of
the A, and A x β after dobutamine challenge was revealed as
compared with that in resting MCE. This is consistent with the
results of Kawamoto et al (22). However, in the study by Galiuto et
al (8), no significant change in
the filling of contrast agent in the perfusion defect area under
adenosine stress was observed. The reason may be the recovery of
reversible injury of coronary microcirculation, as the load test
was completed 7 days after PCI in the study by Galiuto et al
(8).
Research into stress MCE is currently limited
(8,23). The dose and timing of the drugs and
contrast agents used in the ultrasound stress test need further
standardization. Dobutamine has a wide range of clinical
applications. Due to its long-term use in clinical practice, its
availability and side effects can be predicted, especially since
low doses are considered safe. The safety of dobutamine stress MCE
has been proved by several studies (24,25). In
the present study, no adverse effects, including abnormal heart
rate, abnormal blood pressure, chest tightness, belching and
dizziness during MCE, were observed.
CSI, as a simple semi-quantitative index, has been
used to predict cardiac function 6 months following PCI. The
present study indicated that patients with a CSI change of >0.2
following dobutamine loading have higher left ventricular ejection
fraction and lower pro-BNP levels. For patients with
microcirculation dysfunction, cardiologists may consider the
addition of drugs to improve myocardial microcirculation, e.g.
nicorandil, or using auxiliary Traditional Chinese Medicine.
The small sample size is one limitation of the
present study. The major reason is that a high number of patients
refuse PET examination, as PET is not only a radioactive detection
method, but also a relatively high-cost non-medical insurance item
in China. However, this suggests, from another perspective, the
necessity to develop a more efficient and cost-effective method for
detecting surviving myocardium. Another limitation is that the
correlation between myocardial enzymes and MCE and DSE was not
analyzed. In the present study, the cardiac enzyme had been tested
only for the diagnosis of AMI when patients were admitted to the
hospital due to chest pain. Once patients were diagnosed with AMI,
PCI treatment must be performed as soon as possible. However, the
timing for subsequent consultation of each individual with chest
pain is not certain, so it is not guaranteed that myocardial enzyme
was at its peak level. Therefore, the correlation between
myocardial enzymes and MCE and DSE was not analyzed. Furthermore,
only patients with single coronary artery disease were included in
the present study. Further studies should involve a wide range
regarding the extent or size of myocardial infarction, which may
have an impact on the recovery of coronary microcirculation.
In conclusion, low-dose dobutamine stress MCE was
indicated to be a safe and effective method to evaluate myocardial
microcirculation perfusion in patients with acute myocardial
infarction after PCI. In addition, CSI, as a simple
semi-quantitative index, is able to predict left ventricular
systolic function in patients with AMI.
Acknowledgements
Not applicable.
Funding
The present study was funded by the 2018
Hospital-level Project of Tianjin Chest Hospital (Tianjin, China;
grant no. 2018XKC11).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YuL conceived the design of the study, analyzed and
interpreted patient data and drafted the manuscript. XG conceived
the design of the study, interpreted patient data, edited the
manuscript and approved the final version of manuscript. KR was
responsible for case follow-up and data analysis. YZ, YaL and YS
conducted clinical examinations and participated in data analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Chest Hospital (Tianjin, China) and informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uyarel H, Uzunlar B, Unal Dayi S, Tartan
Z, Samur H, Kasikcioglu H, Akgul O, Simsek D, Erdem I, Okmen E and
Cam N: Effect of tirofiban therapy on ST segment resolution and
clinical outcomes in patients with ST segment elevated acute
myocardial infarction undergoing primary angioplasty. Cardiology.
105:168–175. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ito H, Tomooka T, Sakai N, Yu H, Higashino
Y, Fujii K, Masuyama T, Kitabatake A and Minamino T: Lack of
myocardial perfusion immediately after successful thrombolysis. A
predictor of poor recovery of left ventricular function in anterior
myocardial infarction. Circulation. 85:1699–1705. 1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Danijela T, Jelena D, Olga P and Zorana
VP: Assessment of coronary microcirculation with myocardial
contrast echocardiography. Curr Pharm Des. 24:2943–2949.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Senior R: Role of myocardial contrast
echocardiography in the clinical evaluation of acute myocardial
infarction. Heart. 89:1398–1400. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hayat SA and Senior R: Myocardial contrast
echocardiography in ST elevation myocardial infarction: Ready for
prime time? Eur Heart J. 29:299–314. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aggeli C, Polytarchou K, Felekos I,
Zisimos K, Venieri E, Verveniotis A, Varvarousis D, Toutouzas K,
Tsiamis E and Tousoulis D: Effect of gender on the prognostic value
of dobutamine stress myocardial contrast echocardiography. Hellenic
J Cardiol. 58:419–424. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Agati L, Voci P, Autore C, Luongo R, Testa
G, Mallus MT, Di Roma A, Fedele F and Dagianti A: Combined use of
dobutamine echocardiography and myocardial contrast
echocardiography in predicting regional dysfunction recovery after
coronary revascularization in patients with recent myocardial
infarction. Eur Heart J. 18:771–779. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Galiuto L, Locorotondo G, Paraggio L, De
Caterina AR, Leone AM, Fedele E, Barchetta S, Porto I, Natale L,
Rebuzzi AG, et al: Characterization of microvascular and myocardial
damage within perfusion defect area at myocardial contrast
echocardiography in the subacute phase of myocardial infarction.
Eur Heart J Cardiovasc Imaging. 13:174–180. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abdelmoneim SS, Basu A, Bernier M, Dhoble
A, Abdel-Kader SS, Pellikka PA and Mulvagh SL: Detection of
myocardial microvascular disease using contrast echocardiography
during adenosine stress in type 2 diabetes mellitus: Prospective
comparison with single-photon emission computed tomography. Diab
Vasc Dis Res. 8:254–261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guiducci V, Fioroni S, Giacometti P,
Manari A and Gaddi O: Early evaluation of coronary microcirculation
by echocardiography with contrast medium in patients with acute
myocardial infarction treated with primary percutaneous
transluminal coronary angioplasty. Minerva Cardioangiol.
53:157–164. 2005.PubMed/NCBI
|
|
11
|
Marcovitz PA and Armstrong WF: Accuracy of
dobutamine stress echocardiography in detecting coronary artery
disease. Am J Cardiol. 69:1269–1273. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aggeli C, Bonou M and Stefanadis C:
Potential clinical applications of myocardial contrast
echocardiography in evaluating myocardial perfusion in coronary
artery disease. Int J Cardiol. 104:1–9. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vancraeynest D, Kefer J, Hanet C, Fillee
C, Beauloye C, Pasquet A, Gerber BL, Philippe M and Vanoverschelde
JL: Release of cardiac bio-markers during high mechanical index
contrast-enhanced echocardiography in humans. Eur Heart J.
28:1236–1241. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moir S, Haluska BA, Jenkins C, Fathi R and
Marwick TH: Incremental benefit of myocardial contrast to combined
dipyridamole-exercise stress echocardiography for the assessment of
coronary artery disease. Circulation. 110:1108–1113.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Galiuto L, Lombardo A, Maseri A, Santoro
L, Porto I, Cianflone D, Rebuzzi AG and Crea F: Temporal evolution
and functional outcome of no reflow: Sustained and spontaneously
reversible patterns following successful coronary recanalisation.
Heart. 89:731–737. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Galiuto L, Garramone B, Burzotta F,
Lombardo A, Barchetta S, Rebuzzi AG and Crea F: REMEDIA
Investigators. Thrombus aspiration reduces microvascular
obstruction after primary coronary intervention: A myocardial
contrast echocardiography substudy of the REMEDIA Trial. J Am Coll
Cardiol. 48:1355–1360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arrighi JA and Dilsizian V: Multimodality
imaging for assessment of myocardial viability: Nuclear,
echocardiography, MR, and CT. Curr Cardiol Rep. 14:234–243.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu M, Ma Z, Guo X, Zhu J and Su J:
Technetium-99m-labelled HL91 and technetium-99m-labelled MIBI SPECT
imaging for the detection of ischaemic viable myocardium: A
preliminary study. Clin Physiol Funct Imaging. 32:25–32.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fernandes H, Sousa A, Campos J, Patrício
J, Oliveira P, Vieira T, Oliveira A, Faria T, Perez B, Martins E
and Pereira J: Myocardial viability assessment. Acta Med Port. 24
(Suppl 4):S989:–S994. 2011.PubMed/NCBI(In Portuguese).
|
|
20
|
Kobylecka M, Maczewska J,
Fronczewska-Wieniawska K, Mazurek T, Płazińska MT and Królicki L:
Myocardial viability assessment in 18FDG PET/CT study (18FDG PET
myocardial viability assessment). Nucl Med Rev Cent East Eur.
15:52–60. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takehana K, Ruiz M, Petruzella FD, Watson
DD, Beller GA and Glover DK: Response to incremental doses of
dobutamine early after reperfusion is predictive of the degree of
myocardial salvage in dogs with experimental acute myocardial
infarction. J Am Coll Cardiol. 35:1960–1968. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kawamoto T, Yoshida K, Akasaka T, Hozumi
T, Takagi T, Kaji S and Ueda Y: Can coronary blood flow velocity
pattern after primary percutaneous transluminal coronary
angioplasty [correction of angiography] predict recovery of
regional left ventricular function in patients with acute
myocardial infarction? Circulation. 100:339–345. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taghizadeh Asl M, Mandegar MH, Roshanali F
and Assadi M: Comparison of stress dobutamine echocardiography and
stress dobutamine gated myocardial SPECT for the detection of
viable myocardium. Nucl Med Rev Cent East Eur. 17:18–25.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aggeli C, Giannopoulos G, Roussakis G,
Christoforatou E, Marinos G, Toli C, Pitsavos C and Stefanadis C:
Safety of myocardial flash-contrast echocardiography in combination
with dobutamine stress testing for the detection of ischaemia in
5250 studies. Heart. 94:1571–1577. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Timperley J, Mitchell AR, Thibault H,
Mirza IH and Becher H: Safety of contrast dobutamine stress
echocardiography: A single center experience. J Am Soc
Echocardiogr. 18:163–167. 2005.PubMed/NCBI View Article : Google Scholar
|