Introduction
Prostate cancer development is characterized
primarily by dependence on the androgen/androgen receptor (AR) axis
(1). Therefore, therapeutics
targeting this axis, such as androgen-deprivation therapy (ADT),
are the standard treatments for advanced prostate cancer (2). However, the major concern with
treatment of advanced prostate cancer is the development of
resistance to ADT. ADT-resistant prostate cancer is still dependent
on AR due to AR modifications, AR gene point mutations,
upregulation of AR splice variants, and ligand-independent AR
activation (3-5).
Although the underlying androgen resistance mechanisms have been
identified, these mechanisms only partially explain AR
insensitivity.
Glucocorticoids are currently used to treat prostate
cancer, as they suppress the secretion of adrenocorticotrophic
hormone, resulting in reduced expression of adrenal androgen
(6). Recently, elevated expression
of the glucocorticoid receptor (GR) has been linked to prostate
cancer resistance to ADT and disease progression (7,8). In
fact, a previous study observed GR upregulated expression in
pathological tissue specimens from patients with
androgen-independent prostate cancer (7). This is likely because AR can directly
repress GR expression via a negative androgen response element in
the GR promoter (9). GR can
functionally replace AR by blocking AR signaling (9). These findings suggest that AR and GR
potentially have significant overlap in their gene targets and
interacting proteins (7,10). Thus, novel and effective therapeutic
agents are needed to treat prostate cancer by targeting not only AR
but also GR.
The reduced expression in immortalized cells
(REIC) gene, which was initially discovered as a tumor
suppressor gene, is identical to Dickkopf-3 (Dkk-3), a
member of the Dickkopf gene family (11). REIC/Dkk-3 is ubiquitously expressed
in normal cells but significantly downregulated in various cancers,
including prostate cancer (12).
Many studies have shown that REIC/Dkk-3 downregulation is closely
associated with the pathological malignancy of various cancer types
(12,13). An adenovirus vector carrying
REIC/Dkk-3 selectively induced apoptosis in prostate cancer cells,
but not in normal cells (14,15). The
induction of apoptosis is dependent on activation of
c-jun-NH2 kinase and c-Jun by cellular endoplasmic
reticulum stress signaling (16).
We previously demonstrated that small glutamine-rich
tetratricopeptide repeat-containing protein α (SGTA) is a novel
interaction partner of REIC/Dkk-3(17). SGTA is ubiquitously expressed in
almost all tissues, and recently characterized as a heat shock
protein (Hsp)70- and Hsp90-associated co-chaperone that
specifically downregulates AR signaling (18-21).
Therefore, SGTA can influence the actions of androgens and be
consequently involved in hormone-mediated cancer progression. AR
signaling consists of AR maturation and transport to the nucleus
via the dynein motor axis. SGTA dimerization stabilizes the AR
complex and limits AR transport to the nucleus. REIC/Dkk-3
interferes with SGTA dimerization, promotes dynein-dependent AR
transport to the nucleus, and subsequently upregulates AR signaling
in human prostate cancer PC3 cells treated with dihydrotestosterone
(17).
Although REIC/Dkk-3 may have a role in
androgen-independent prostate cancer progression via regulation of
AR signaling, the ability of REIC/Dkk-3 to regulate other steroid
hormone receptors remains unclear. We herein investigated the
regulation of GR by REIC/Dkk-3 to further evaluate the mechanism by
which REIC/Dkk-3 regulates steroid receptor transport in human
prostate cancer cells.
Materials and methods
Cell culture and plasmid vectors
The human prostate cancer cell line PC3 and the
human embryonic kidney cell line 293T were provided by the American
Type Culture Collection. PC3 cells were maintained in Ham's F12
medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum, penicillin (50 IU/ml), and streptomycin (50 µg/ml)
under a humidified atmosphere with 5% CO2 at 37̊C. The
293T cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific) for 18-24 h until the cells
reached 60-80% confluence. The shRNA oligonucleotide (TG309472B;
Origene) and matched control shRNA were transfected into PC3 and
293T cells. Cell lysates were obtained at the indicated time points
for western blot analysis. As for construction of the plasmid
vectors, the full length cDNA of human SGTA and human REIC/Dkk-3
were cloned into the pMACS Kk.HA-C vector (Miltenyi Biotec).
Western blot analysis
Both PC3 and 293T cells were transfected using
Lipofectamine 3000™ transfection reagent (Thermo Fisher
Scientific, Inc.) with 250 ng of one of the following vectors:
pMACS Kk.HA-C-SGTA, pMACS Kk.HA-C-REIC/Dkk-3, pGFP-V-RS containing
SGTA-specific shRNA, or pMACS Kk.HA-C empty vector as the control.
The cells were harvested at 48 h after transfection with the
vectors. Approximately 5 µg extracted proteins were subjected to
Western blot analysis using the following specific primary
antibodies: Monoclonal mouse anti-human REIC/Dkk-3 antibody (raised
in our laboratory) (17), rabbit
polyclonal anti-SGTA (cat. no. 11019-2-AP; Protein Tech), and
anti-β-actin (cat. no. 4967S; Cell Signaling Technology). The
secondary antibodies used were horseradish peroxidase-conjugated
anti-mouse IgG or anti-rabbit IgG (GE Healthcare). Western blot
analysis was performed as described previously (13).
Analyses of GR translocation to the
nucleus
Approximately 1.25x105 PC3 cells and
5.0x105 293T cells on 24-well plates were transfected
with 100 ng pMACS Kk.HA-C-SGTA, pMACS Kk.HA-C-REIC/Dkk-3, pGFP-V-RS
containing SGTA-specific shRNA, pBIND-GR containing the GR
ligand-biding domain and renilla luciferase reporter gene (cat. no.
E158A; Promega), or pGL4.35(luc2P/9XGAL4 UAS/Hygro) containing the
firefly luciferase reporter gene (cat. no. E137A; Promega). At 24 h
post-transfection, the cells were treated for 24 h with 1 µM
dexamethasone (cat. no. D4902; Sigma) or the control (ethanol+DMEM)
and then subjected to luciferase assays using the
Dual-Luciferase® Reporter Assay System (Promega)
(22). Luciferase was added to the
culture media for 15 min, after which firefly luminescence was
measured using the Multi-detection Reader Flex Station 3 (Molecular
Device). Subsequently, Stop and Glo Reagent was added to the
culture medium, and renilla luminescence was measured immediately.
Relative promoter activity was calculated as the ratio of firefly
luciferase to renilla luciferase luminescence. All transfection
mixes were balanced with the appropriate empty vectors in terms of
the ratio of the expression vectors and total plasmids. The
firefly/renilla dual luciferase vector encoding the Gal4
DNA-binding domain fused to the glucocorticoid receptor
ligand-binding domain allows measurement of both firefly and
renilla luciferase activity in the same sample with high
sensitivity and linearity. The dual luciferase reporter assay is a
screening tool to detect the simultaneous expression and
measurement of each reporter enzyme within a single system.
Normalizing the activity of the experimental reporter to the
activity of the internal control minimizes experimental variability
caused by differences in cell viability or transfection efficiency.
Furthermore, other variabilities such as differences in pipetting
volumes, cell lysis efficiency and assay efficiency can be
effectively eliminated. Thus, dual-reporter assays often allow more
reliable interpretation of the experimental data by reducing
extraneous influences (23).
Statistical analyses
The data are presented as means ± standard error.
The unpaired Student's t-test was performed for comparisons between
two groups. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
StatView version 4.5 software (Abacus Concept).
Results
Expression of SGTA and REIC/Dkk-3 in
prostate cancer PC3 and 293T cell lines
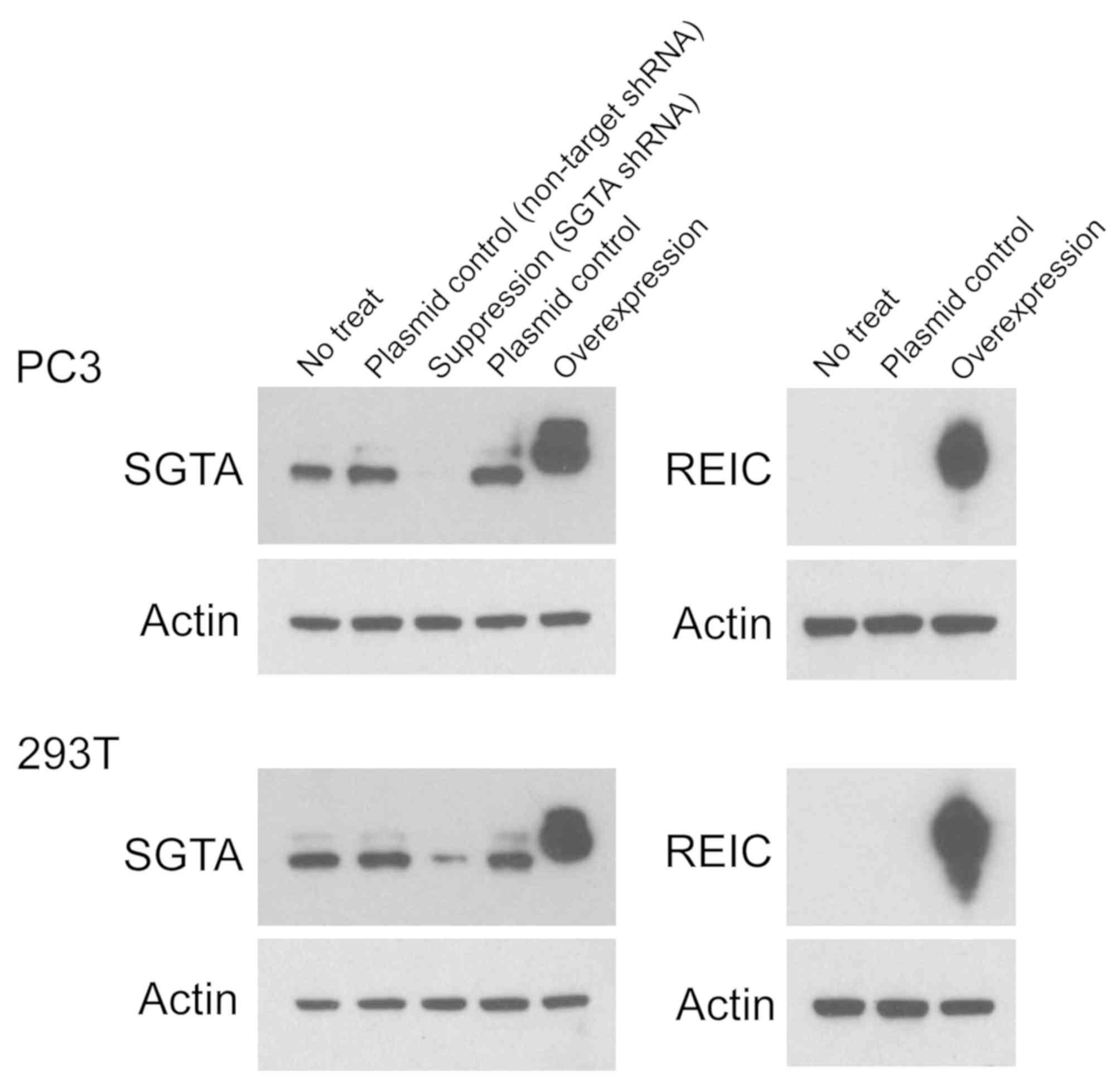
To assess the expression of SGTA and REIC/Dkk-3
proteins, we performed Western blot analyses in PC3 and 293T cells.
SGTA and REIC/Dkk-3 proteins were overexpressed in PC3 and 293T
cells by transfection of the vectors containing the human SGTA and
human REIC genes, respectively (Fig.
1). SGTA expression was suppressed in PC3 and 293T cells by
transfection of the pGFP-V-RS vector containing SGTA-specific shRNA
(Fig. 1).
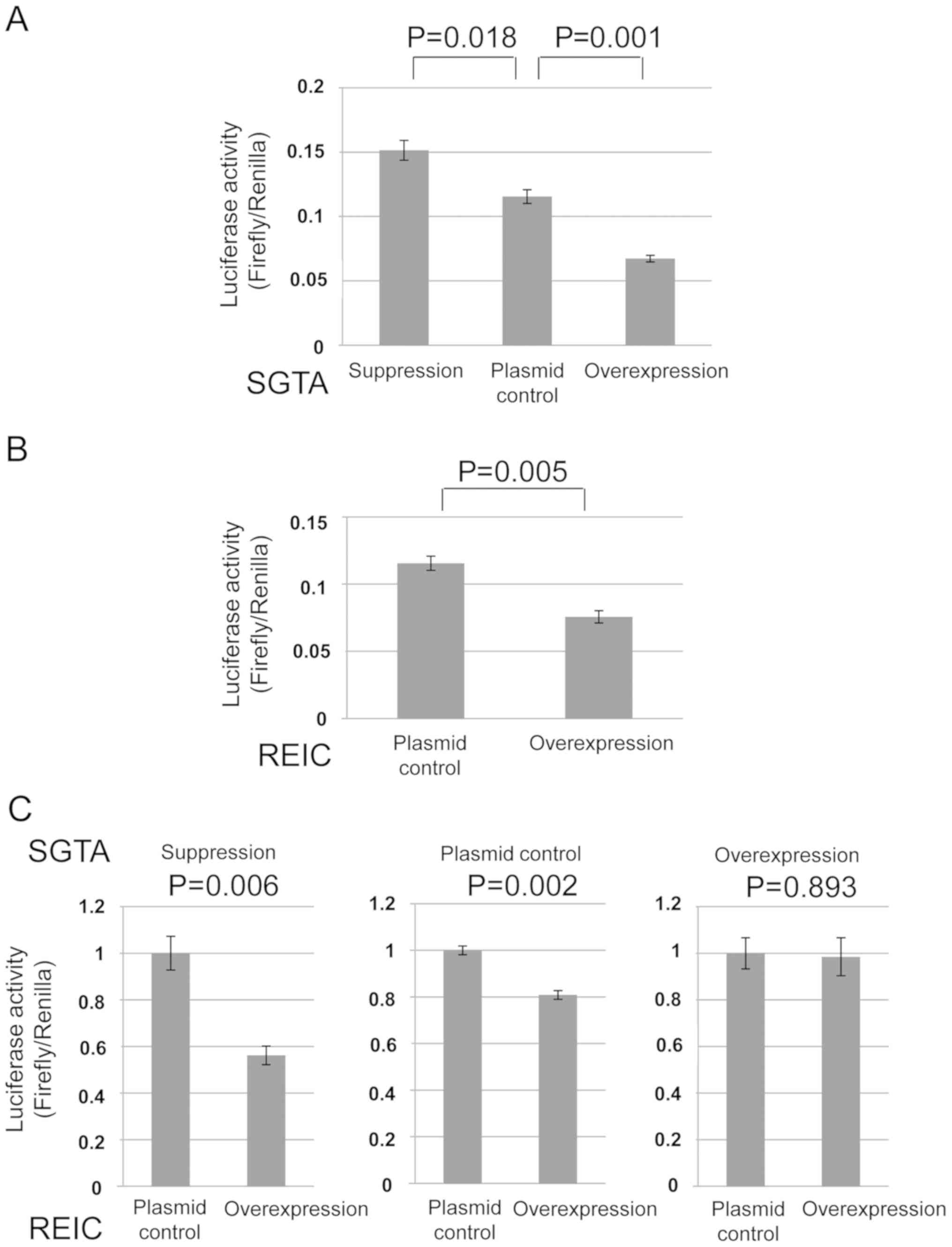
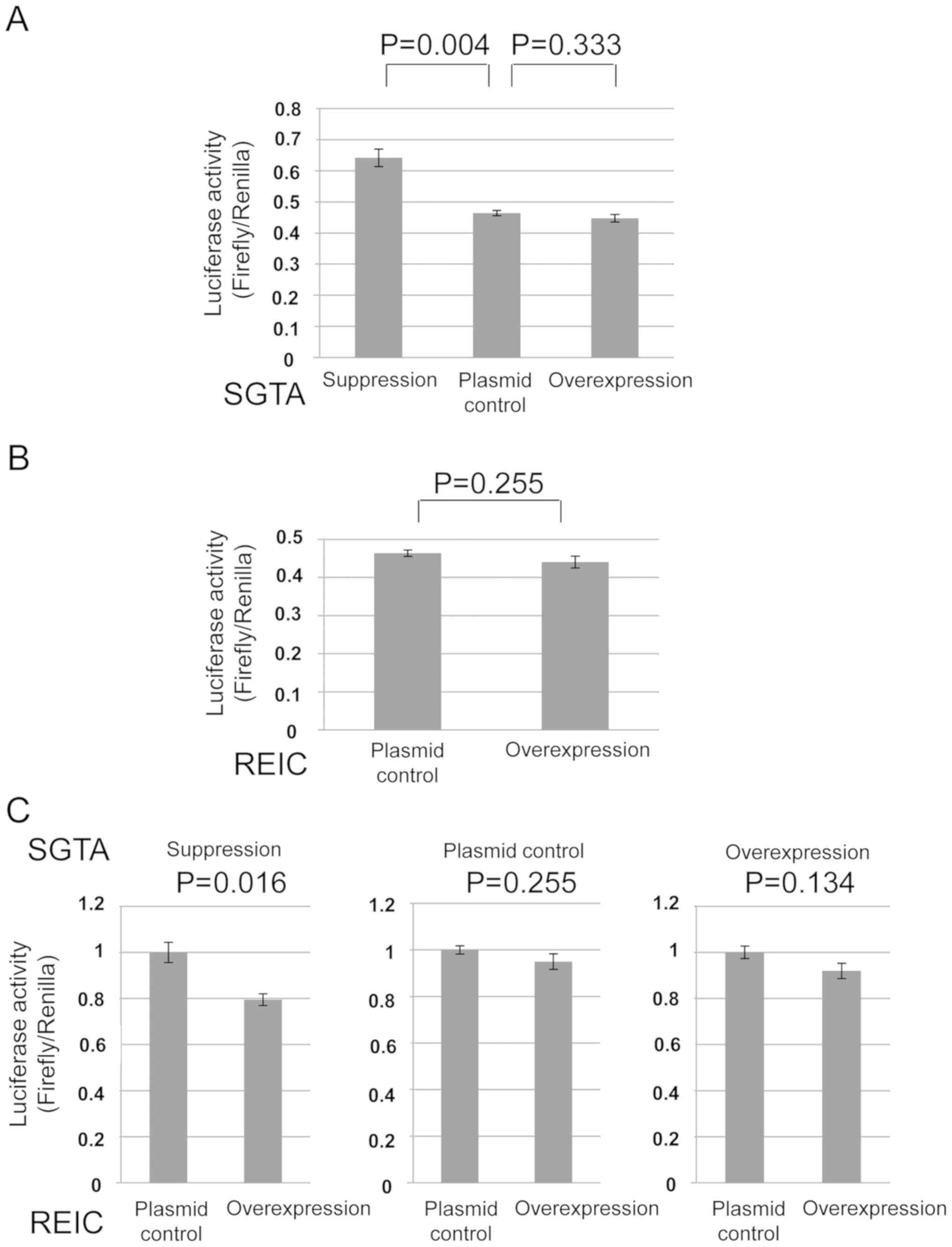
Inhibitory effects of SGTA and
REIC/Dkk-3 in GR signaling
To investigate the roles of SGTA and REIC/Dkk-3 in
GR transport to nucleus, we performed in vitro luciferase
reporter assays for the cytoplasmic GR transport in human prostate
cancer PC3 cells and 293T cells. As for the SGTA protein, the
amount of GR transport to nucleus was oppositely affected in
comparison to the levels of SGTA expression in the both cells
(Figs. 2A and 3A). As for the REIC/Dkk-3 protein, the GR
transport was inhibited by REIC/Dkk-3 overexpression only in the
PC3 cells (Figs. 2B and 3B). These results indicate that both of the
SGTA and REIC/Dkk-3 inhibit the cytoplasmic transport of GR to
nucleus in human prostate cancer PC3 cells.
Inhibitory effect of REIC/Dkk-3 on the
GR transport is augmented under the SGTA depleted condition
We previously disclosed that intracellular
REIC/Dkk-3 interacts with SGTA and the interaction modify the
cytoplasmic androgen receptor (AR) transport in the PC3 cells
treated with dihydrotestosterone (17). Since we herein demonstrated that both
SGTA and REIC/Dkk-3 inhibit the GR transport to nucleus in human
prostate cancer PC3 cells, it is conceivable that the expressional
state of REIC/Dkk-3 and SGTA protein may modify their inhibitory
effects on GR transport to nucleus in the cells. To examine the
mutual effects of SGTA and REIC/Dkk-3 on each other in terms of GR
transport, we simultaneously manipulated the expression levels of
these proteins in PC3 and 293T cells. Under the depleted condition
of SGTA by shRNA, the downregulation of GR transport by REIC/Dkk-3
was significantly augmented in comparison to the non-depleted
condition, indicating a compensatory association of REIC/Dkk-3 in
the SGTA mediated inhibition of GR transport (Figs. 2C and 3C). On the other hands, under the
overexpressed condition of SGTA, the inhibitory effect of
REIC/Dkk-3 on the GR transport was disappeared in PC3 cells
(Fig. 2C).
Discussion
The present study demonstrated that REIC/Dkk-3
expression significantly inhibited GR transport to the nucleus in
human prostate cancer PC3 cells. After shRNA-mediated depletion of
SGTA expression, downregulation of GR transport by REIC/Dkk-3 was
significantly enhanced compared to the control cells with normal
SGTA expression, suggesting a compensatory role of REIC/Dkk-3 for
the inhibitory function of SGTA in GR transport. We are the first
to demonstrate that both REIC/Dkk-3 and SGTA inhibit cytoplasmic
transport of GR to the nucleus in human prostate cancer cells.
Steroid hormone receptors are ligand-dependent
transcription factors that require dynamic, ordered assembly of the
chaperone and co-chaperone machinery to obtain a functional
conformation (19). Hsp70 and Hsp90
are key factors in this process and several Hsp70- and
Hsp90-associated co-chaperones interact with receptor-chaperone
complexes to functionally affect a wide variety of steps within the
receptor-folding process (24,25).
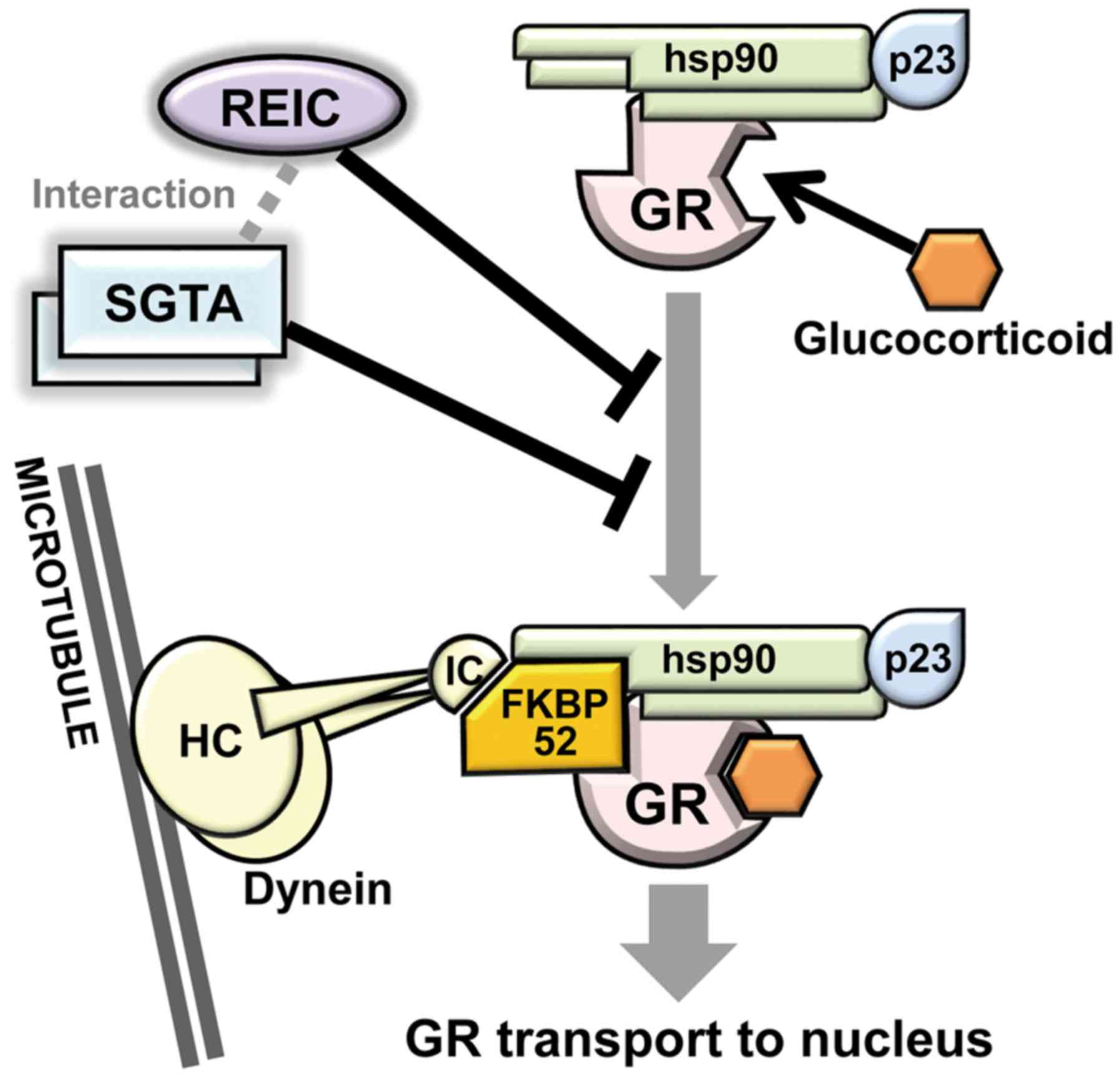
Referring the published reports, we herein proposed the role of
SGTA and REIC/Dkk-3 in cytoplasmic GR transport to nucleus
(Fig. 4) (17,20,21,26-29).
GR transport consists of the GR maturation and GR complex transport
to nucleus via the dynein motor axis. In this study, we
demonstrated that both SGTA and REIC/Dkk-3 inhibit the GR transport
to nucleus in human prostate cancer PC3 cells. Although the PC3
cell line was reported to be AR-negative, Akimirah et al
demonstrated that treatment of the PC3 cell line with
dihydrotestosterone resulted in measurable increases in the AR
protein levels and considerable nuclear accumulation (30). We previously indicated that
cytoplasmic REIC/Dkk-3 upregulates dynein motor-dependent AR
transport via its interaction with the co-chaperone SGTA. In
addition, the interaction between REIC/Dkk-3 and SGTA modify the
degree of cytoplasmic AR transport in the PC3 cells treated with
dihydrotestosterone (17). Based on
these findings, it is likely that the interaction between
REIC/Dkk-3 and SGTA protein modifies their own inhibitory roles of
GR transport in the cells.
The precise molecular mechanism by which REIC/Dkk-3
suppresses GR transport remains to be determined. REIC/Dkk-3 and
SGTA were shown cytoplasmic localization with a similar
distribution pattern. We previously reported that AR was
predominantly located in the cytoplasm when SGTA was expressed and
the expression of REIC/DKK-3 facilitated AR transport to nucleus
(17). Furthermore, REIC/Dkk-3 binds
to SGTA, and their interaction involves the 78 N-terminal amino
acids of both proteins (17). Based
on the binding, REIC/Dkk-3 interferes with the dimerization of SGTA
and abolishes SGTA dependent negative regulation of AR transport,
resulting in enhanced AR signaling in the PC3 prostate cancer
cells. However, in the present study, REIC/Dkk-3 unexpectedly
inhibited the GR transport to the nucleus and enhanced the degree
of inhibition under the SGTA depleted condition. As for the GR
transport, it is likely that REIC/Dkk-3 protein possesses similar
molecular activity with SGTA and the proteins work as co-effectors
in the process of GR transport inhibition. Further experiments are
necessary to reveal whether these two proteins interact with each
other to cooperate in the inhibition of GR transport, and whether
the proteins cooperate in regulating their own functions.
A recent study suggested that GR expression is
associated with resistance to the ADT in androgen-independent
prostate cancer (7,8). GR is elevated in bone metastases of
prostate cancer patients following enzalutamide therapy, and high
GR expression is associated with poor prognosis (7). In addition, GR is upregulated in
LNCaP/AR xenografts and in the cell lines that are able to grow in
the presence of enzalutamide, suggesting that GR can drive the
expression of AR target genes (7).
These findings indicate a significant role of GR signaling in the
resistance to anti-androgen therapy. In this study, we demonstrated
that intracellular REIC/Dkk-3 is potentially an inhibitor of GR
transport. In our previous study, we showed that REIC/Dkk-3
activates AR transport and signaling by binding to and interfering
with SGTA, a negative regulator of cytoplasmic AR transport
(17). Therefore, intracellular
REIC/Dkk-3 has two aspects of a GR inhibitor and AR activator as a
modifier of the steroid signaling.
The present study has some limitations. We only used
the PC3 cell line which was reported androgen-independent prostate
cancer cells, and further study is needed to verify the effect of
SGTA and REIC/Dkk-3 proteins on the GR axis in the other cell lines
(e.g. LNCaP or DU145). We did not investigate the changes of AR and
GR cellular distributions depending on expression level of SGTA and
REIC/Dkk-3, and also not investigate the protein-protein
interactions involved in this process. Despite these limitations,
this study could be significant for developing novel therapeutic
approach for androgen-independent prostate cancer.
In conclusion, REIC/Dkk-3 and SGTA inhibit
cytoplasmic transport of GR to the nucleus in human prostate cancer
PC3 cells. The findings could be of significance for understanding
GR signaling in the androgen-independent prostate cancer cells. The
mechanism of interaction between REIC/Dkk-3 and SGTA associated
with the GR complex provides new insights for developing novel
medicine targeting GR signaling.
Acknowledgements
The authors thank Ms. Fukasa Oonari (Center for
Innovative Clinical Medicine, Okayama University Hospital, Okayama
700-8558, Japan) and Ms. Shun-Ai Li (Department of Neuroscience,
Okayama University Graduate School of Medicine, Dentistry and
Pharmaceutical Sciences, Okayama 700-8558, Japan) for their
valuable assistance with the acquisition and interpretation of the
data in the present study.
Funding
The current study was supported by scientific
research grants from the Ministry of Education, Culture, Sports,
Science and Technology of Japan, and by scientific research grants
from the Teraoka Scholarship Foundation (JSPS KAKENHI grant nos.
JP19H01064 and JP18K09167).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TI wrote the manuscript and performed the
experiments. TSad made substantial contributions to the conception
and design of the present study and the interpretation of data. HU
made substantial contributions to the acquisition of the data and
interpretation of data in the present study. PH, MA and TW made
substantial contributions to the interpretation of data in the
present study. KO, TSas, MW and YN made substantial contributions
to the conception and design of the study, critical point of
discussion and the completion of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shiota M, Fujimoto N, Kashiwagi E and Eto
M: The role of nuclear receptors in prostate cancer. Cells.
8(602)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shiota M and Eto M: Current status of
primary pharmacotherapy and future perspectives toward upfront
therapy for metastatic hormone-sensitive prostate cancer. Int J
Urol. 23:360–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim JY, Yu J, Abdulkadir SA and
Chakravarti D: KAT8 regulates androgen signaling in prostate cancer
cells. Mol Endocrinol. 30:925–936. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thadani-Mulero M, Portella L, Sun S, Sung
M, Matov A, Vessella RL, Corey E, Nanus DM, Plymate SR and
Giannakakou P: Androgen receptor splice variants determine taxane
sensitivity in prostate cancer. Cancer Res. 74:2270–2282.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy
DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, et al: An F876L
mutation in androgen receptor confers genetic and phenotypic
resistance to MDV3100 (enzalutamide). Cancer Discov. 3:1030–1043.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ndibe C, Wang CG and Sonpavde G:
Corticosteroids in the management of prostate cancer: A critical
review. Curr Treat Options Oncol. 16(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arora VK, Schenkein E, Murali R, Subudhi
SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis
C, et al: Glucocorticoid receptor confers resistance to
Antiandrogens by bypassing androgen receptor blockade. Cell.
155:1309–1322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Isikbay M, Otto K, Kregel S, Kach J, Cai
Y, Vander Griend DJ, Conzen SD and Szmulewiz RZ: Glucocorticoid
receptor activity contributes to resistance to androgen-targeted
therapy in prostate cancer. Horm Cancer. 5:72–89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie N, Cheng H, Lin D, Liu L, Yang O, Jia
L, Fazli L, Gleave ME, Wang Y, Rennie P and Dong X: The expression
of glucocorticoid receptor is negatively regulated by active
androgen receptor signaling in prostate tumors. Int J Cancer.
136:E27–E38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lempiäinen JK, Niskanen EA, Vuoti KM,
Lampinen RE, Göös H, Varjosalo M and Palvimo JJ: Agonist-specific
protein interactomes of glucocorticoid and androgen receptor as
revealed by proximity mapping. Mol Cell Proteomics. 16:1462–1474.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biopsy Res Commum. 268:20–24. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hirata T, Watanabe M, Kaku H, Kobayashi Y,
Yamada H, Sakaguchi M, Takei K, Huh NH, Nasu Y and Kumon H:
REIC/Dkk-3-encoding adenoviral vector as a potentially effective
therapeutic agent for bladder cancer. Int J Oncol. 41:559–564.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Watanabe M, Sakaguchi M, Kinoshita R, Kaku
H, Ariyoshi Y, Ueki H, Tanimoto R, Ebara S, Ochiai K, Futami J, et
al: A novel gene expression system strongly enhances the anticancer
effects of a REIC/Dkk-3-encoding adenoviral vector. Oncol Rep.
31:1089–1095. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abarzua F, Sakaguchi M, Tanimoto R,
Sonegawa H, Li DW, Edamura K, Kobayashi T, Watanabe M, Kashiwakura
T, Kaku H, et al: Heat shock proteins play a crucial role in
tumor-specific apoptosis by REIC/Dkk-3. Int J Mol Med. 20:37–43.
2007.PubMed/NCBI
|
|
15
|
Kumon H, Sasaki K, Ariyoshi Y, Sadahira T,
Ebara S, Hiraki T, Kanazawa S, Yanai H, Watanabe M and Nasu Y:
Ad-REIC gene therapy: Promising results in a patient with
metastatic CRPC following chemotherapy. Clin Med Insights Oncol.
9:31–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ochiai K, Morimatsu M, Kato Y,
Ishiguro-Oonuma T, Udagawa C, Rungsuriyawiboon O, Azakami D,
Michishita M, Ariyoshi Y, Ueki H, et al: Tumor suppressor
REIC/Dkk-3 and co-chaperone SGTA: Their interaction and roles in
the androgen sensitivity. Oncotaget. 7:3283–3296. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu FH, Wu SJ, Hu SM, Hsiao CD and Wang C:
Specific interaction of the 70-kDa heat shock cognate protein with
the tetratricopeptide repeats. J Bio Chem. 247:34425–34432.
1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Paul A, Garcia YA, Zierer B, Patwardhan C,
Gutierrez O, Hildenbrand Z, Harris DC, Balsiger HA, Sivils JC,
Johnson JL, et al: The cochaperone SGTA demonstrates regulatory
specificity for the androgen, glucocorticoid, and progesterone
receptors. J Biol Chem. 289:15297–15308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Buchanan G, Ricciardeli C, Harris JM,
Prescott J, Yu ZC, Jia L, Butler LM, Marshall VR, Scher HI, Gerald
WL, et al: Control of androgen receptor signaling in prostate
cancer by the cochaperone small glutamine rich tetraticopeptide
repeat containing protein alpha. Cancer Res. 67:10087–10096.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Philp LK, Butler MS, Hichey TE, Bulter LM,
Tilley WD and Day TK: SGTA: A new player in the molecular
co-chaperone game. Horm cancer. 4:343–357. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaczmarczyk SJ and Green JE: A single
vector containing modified cre recombinase and LOX recombination
sequences for inducible tissue-specific amplification of gene
expression. Nucleic Acids Res. 29(E56)2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dual-Luciferase® Reporter Assay
System Technical Manual. https://www.promega.com/-/media/files/resources/protocols/technical-manuals/0/dual-luciferase-reporter-assay-system-protocol.pdf.
|
|
24
|
Echeverria PC and Picard D: Molecular
chaperones, essential partners of steroid hormone receptors for
activity and mobility. Biochim Biophys Acta. 1803:641–649.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Smith DF and Toft DO: Minireview: The
intersection of steroid receptors with molecular chaperones:
Observations and questions. Mol Endocrinol. 22:2229–2240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pratt WB, Galigniana MD, Morishima Y and
Murphy PJ: Role of molecular chaperones in steroid receptor action.
Essays Biochem. 40:41–58. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Grad I and Picard D: The glucocorticoid
responses are shaped by molecular chaperones. Mol Cell Endocrinol.
275:2–12. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pérez MH, Cormack J, Mallinson D and
Mutungi G: A membrane glucocorticoid receptor mediates the
rapid/non-genomic actions of glucocorticoids in mammalian skeletal
muscle fibres. J Physiol. 591:5171–5185. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cato L, Neeb A, Brown M and Cato AC:
Control of steroid receptor dynamics and function by genomic
actions of the cochaperones p23 and Bag-1L. Nucl Recept Signal.
12(e005)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Akimirah F, Chen J, Basrawala Z, Xin H and
Choubey D: DU-145 and PC-3 human prostate cancer cell lines express
androgen receptor: Implications for the androgen receptor functions
and regulation. FEBS Lett. 2294–2300. 2006.PubMed/NCBI View Article : Google Scholar
|