Introduction
Glomerulus nephritis (GN) is an autoimmune system
disease with complex pathogenesis, diverse pathological
classifications and long disease course (1). It can be induced by various causes and
can be divided into primary glomerulonephritis (PGN) and secondary
glomerulonephritis (SGN) (2). The
pathogenic process of GN is divided into several stages: first
edema, hematuria or proteinuria can be seen in the body; then,
renal function weakens; eventually, patients suffer renal failure
(3). At present, the drugs used to
treat GN in clinic are few, and the efficacy of some drugs is poor,
causing adverse reactions. Moreover, the treatment is expensive
(4). Comprehensive preventive
measures should be taken in the treatment of GN to control
deterioration of renal function, alleviate the symptoms of
patients, reduce the incidence of serious complications and improve
patients' life quality (5).
Serum cystatin C (C's C) is a non-glycosylated small
protein, with 120 amino acid residues, and is a member of cysteine
protease inhibitors (6). The
production rate of C's C is relatively constant in body fluids and
nucleated cells in tissues of the body. C's C metabolizes and
regulates peptides and proteins in cells (7). In the blood circulation of the body,
C's C is filtered by glomerular filtration membrane and is
reabsorbed by proximal convoluted tubules. The glomerular
filtration function directly determines the level of C's C in the
serum (8,9).
Vascular endothelial growth factor (VEGF), a dynamic
source of division and growth of endothelial cells, facilitates
angiogenesis. VEGF can combine with soluble vascular endothelial
growth factor receptor 1 (sVEGFR1) to prevent VEGF membrane
receptors from mediating and taking a biological effect. sVEGFR1
and VEGF membrane receptors are competitive, when they are combined
with VEGF, the biological activity of sVEGFR1 and VEGF will be
regulated negatively (10,11). Studies have shown that when sVEGFR1
combines with VEGF in the blood circulation, the function of VEGF
will be regulated. Increasing the level of sVEGFR1 prevents VEGF
from facilitating angiogenesis and injuring endothelial cells,
which is closely related to kidney diseases (12).
Studies on the expression of serum C's C and sVEGFR1
in patients with GN and predictive function and evaluation function
of serum C's C and sVEGFR1 in diseases are scarce. Therefore,
expression levels of serum C's C and sVEGFR1 in the treatment of
patients with GN were detected and the monitoring function and
significance of serum C's C and sVEGFR1 in GN were investigated in
this study, in order to provide specific study data and theoretical
basis for clinical treatment of GN.
Patients and methods
General data
The medical records of 88 patients with GN who were
diagnosed in Weifang People's Hospital (Weifang, China) from March
2014 to June 2017 were collected, and their medical records were
considered as study group. The study group was divided to SGN group
and PGN group. There were 52 patients in SGN group and 36 patients
in PGN group. Physical examination data of 50 healthy volunteers
who were examined in Weifang People's Hospital during the same
period were considered as the control group. Among those patients,
there were 66 males and 72 females, with an average age of
45.0±15.7 years and a BMI of 21.0±3.6. There was no significant
difference in some data of the patients in two groups, including
gender, age and BMI (P>0.05). There were significant differences
in hypertension, edema and some other aspects (P<0.05) (Table I).
 | Table IGeneral data of the patients in the
study group and the control group [n (%)]. |
Table I
General data of the patients in the
study group and the control group [n (%)].
| Factors | Study group
(n=88) | Control group
(n=50) | χ2/t | P-value |
|---|
| Sex | | | 0.105 | 0.746 |
|
Male | 43 (48.86) | 23 (46.00) | | |
|
Female | 45 (51.14) | 27 (54.00) | | |
| Age (years) | | | 0.001 | 0.975 |
|
≤45 | 46 (52.27) | 26 (52.00) | | |
|
>45 | 42 (47.73) | 24 (48.00) | | |
| BMI
(kg/m2) | | | 0.178 | 0.673 |
|
≤21 | 42 (47.73) | 22 (44.00) | | |
|
>21 | 46 (52.27) | 28 (56.00) | | |
| Hypertension | | | 48.391 | <0.001 |
|
Yes | 63 (71.59) | 5 (10.00) | | |
|
No | 25 (28.41) | 45 (90.00) | | |
| Edema | | | 63.971 | <0.001 |
|
Yes | 62 (70.45) | 0 | | |
|
No | 26 (29.55) | 50 (100.00) | | |
| Hematuresis | | | 60.311 | <0.001 |
|
Yes | 60 (68.18) | 0 | | |
|
No | 28 (31.82) | 50 (100.00) | | |
| Proteinuria | | | 76.171 | <0.001 |
|
Yes | 68 (77.27) | 0 | | |
|
No | 20 (22.73) | 50 (100.00) | | |
| Urea nitrogen
(mmol/l) | 14.34±1.23 | 5.65±0.83 | 44.500 | <0.001 |
| Creatinine
(µmol/l) | 293.43±32.32 | 81.34±11.25 | 44.820 | <0.001 |
Inclusion and exclusion criteria
Inclusion criteria: Patients who were diagnosed with
GN by histopathology were included; Serum creatinine (SCr) <350
mol/l, and blood urea nitrogen (BUN) <17 mmol/l; urine RBC was
increased and showed tubular shape (13).
Exclusion criteria: i) patients with malignant
tumors; ii) patients complicated with solid lesions of other
organs; iii) patients complicated with blood diseases and
infection; iv) patients with cognitive disorder or communication
disorder; v) patients with poor compliance.
All the patients and their family members agreed to
participate in the experiment, and an informed consent form was
signed. This experiment was approved by the Medical Ethics
Committee of Weifang People's Hospital.
Reagents and materials
The serum separation centrifuge was purchased from
Zhengzhou Zhishi Changyun Technology Co., Ltd. C's C was was
detected by automatic biochemical analyzer BS-600 (Shenzhen Mindray
Biomedical Electronics Co., Ltd.); C's C test kits (Shenzhen ziker
Biological Technology Co., Ltd., ZK-H068); sVEGFR1 kits were from
R&D Systems (ML-ELISA-0073).
Experiment methods
The patients were treated for more than 3 cycles.
The specific therapeutic regimen was as follows: the patients took
valsartan dispersible tablets once a day, and the dosage was 80 mg
per time. Alprostadil injection (10 µg) was mixed with sodium
chloride solution with a concentration of 0.9%, then the patients
were injected with the mixture once a day, the dosage was 100 ml
per time. Fasting venous blood (5 ml) of the patients and the
healthy volunteers was collected in the morning, then the serum was
separated from the blood by serum separation centrifuge at a speed
of 1,500 x g at 4˚C for 10 min, and the serum was reserved. Serum
C's C of the patients was detected by immunoturbidimetry. The
expression level of sVEGFR1 in the serum was detected by
enzyme-linked immunosorbent assay (ELISA). The procedures were
carried out strictly according to the instructions of the kits.
Observation indicators
i) The expression levels of C's C and sVEGFR1 in
serum of the patients in the study group and the control group were
compared before treatment. ii) The changes of expression levels of
serum C's C of the patients in PGN group and SGN group were
compared before they were treated, after one cycle of treatment,
after three cycles of treatment and after discharged from the
hospital. iii) The changes of expression levels of sVEGFR1 of the
patients in PGN group and SGN group were compared before they were
treated, after one cycle of treatment, after three cycles of
treatment and after discharged from the hospital. iv) The
correlation between the expression of C's C and the expression of
sVEGFR1 in GN was analyzed by correlation analysis.
Statistical analysis
In this study, SPSS 19.0 (Bizinsight (Beijing)
Information Technology Co., Ltd.) was used to statistically analyze
the experimental data. The count data were analyzed by
χ2 test. The measurement data were expressed in the form
of mean value ± standard deviation. t-test was used in comparison
of data between two groups. Bonferroni test was the post hoc test.
Repeated measures analysis of variance was used in comparison of
the data between two groups at different time points in the
treatment of patients. Pearson analysis was used to carry out
correlation analysis of the data. The illustrations of this
experiment were drawn by GraphPad Prism 8. P<0.05 indicates
statistically significant difference.
Results
Comparison of expression levels of C's
C and sVEGFR1 of the patients in the study and the control
groups
The expression levels of C's C and sVEGFR1 of the
patients in the study group and the subjects in the control group
were compared before treatment. The expression level of C's C of
the patients in the study group was significantly higher than that
in control group. The expression level of sVEGFR1 of the patients
in study group was significantly higher than that in the control
group. Differences in expression levels of C's C and sVEGFR1 of the
patients in the two groups were statistically significant
(P<0.05) (Table II).
 | Table IIComparison of expression levels of
C's C and sVEGFR1 in the two groups. |
Table II
Comparison of expression levels of
C's C and sVEGFR1 in the two groups.
| Groups | Study group
(n=88) | Control group
(n=50) | t | P-value |
|---|
| C'sC (mg/l) | 2.43±0.45 | 0.83±0.18 | 24.040 | <0.001 |
| sVEGFR1 (µg/l) | 30.42±7.42 | 7.34±1.54 | 21.700 | <0.001 |
Comparison of the changes of
expression level of C's C of the patients in PGN and SGN groups
before treatment, one cycle after treatment, three cycles after
treatment and after discharge
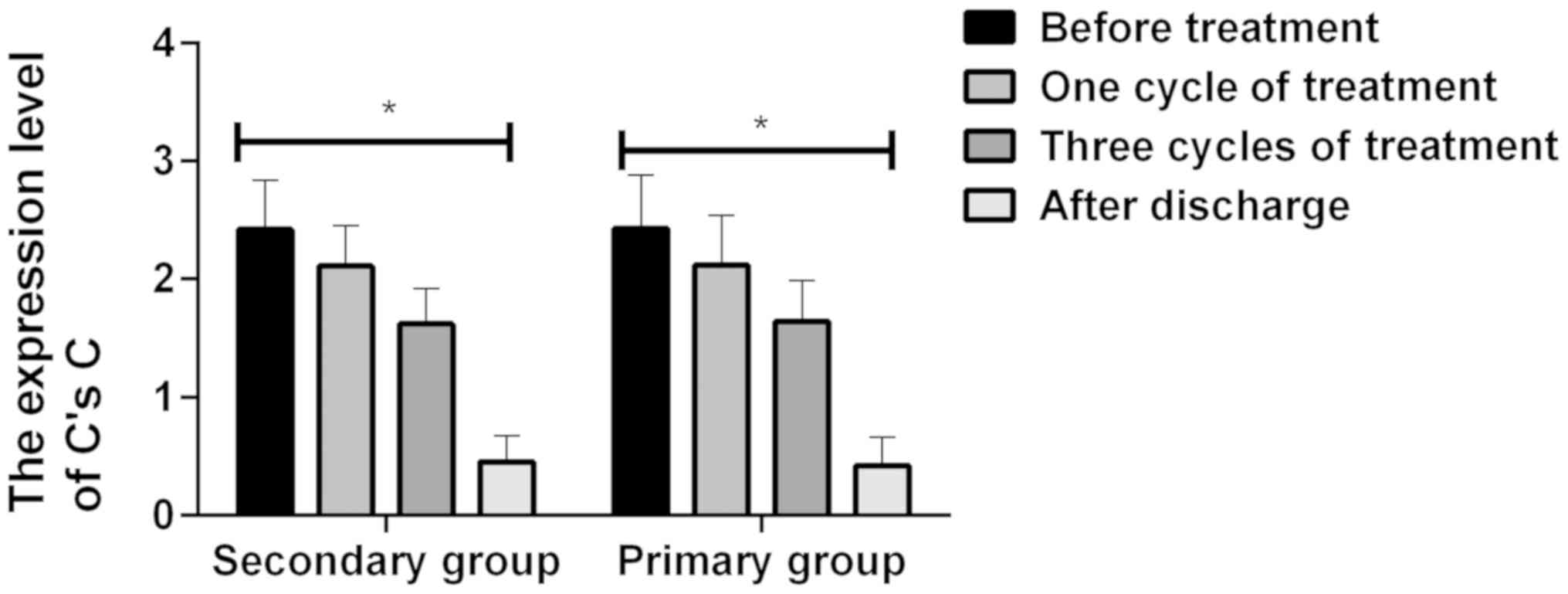
The expression level of C's C of the patients in PGN
group and SGN group was compared before treatment, after one cycle
of treatment, after three cycles of treatment, and after discharge
from hospital. It was found that the expression level of C's C of
the patients in PGN group and SGN group decreased gradually after
one cycle of treatment. The expression level of C's C of the
patients in PGN group and SGN group after one cycle of treatment
was lower than that before they were treated (P<0.05). The
expression level of C's C of the patients in PGN group and SGN
group decreased gradually after three cycles of treatment. The
expression level of C's C of the patients in PGN group and SGN
group after three cycles of treatment was lower than that after one
cycle of treatment (P<0.05). The expression level of C's C of
the patients in PGN group and SGN group decreased gradually after
discharge from hospital. The expression level of C's C of the
patients in PGN group and SGN group after discharged from the
hospital was lower than that after three cycles of treatment
(P<0.05). There was no significant difference between the
expression level of C's C of the patients in PGN group and that in
SGN group (P>0.05) (Table III
and Fig. 1).
 | Table IIIComparative analysis of the
expression level of C's C of the patients in PGN group and SGN
group before and after treatment. |
Table III
Comparative analysis of the
expression level of C's C of the patients in PGN group and SGN
group before and after treatment.
| Groups | Before patients
were treated | In one cycle after
patients were treated | In three cycles
after patients were treated | After patients were
discharged | F-value | P-value |
|---|
| SGN group
(n=52) | 2.42±0.42 |
2.11±0.34a |
1.62±0.30a,b |
0.45±0.22a-c | 0.842 | <0.001 |
| PGN group
(n=36) | 2.43±0.45 |
2.12±0.42a |
1.64±0.35a,b |
0.42±0.24a-c | 0.812 | <0.001 |
| t-test | 0.107 | 0.123 | 0.287 | 0.606 | | |
| P-value | 0.915 | 0.902 | 0.775 | 0.546 | | |
Comparison of the changes of
expression level of sVEGFR1 of the patients in PGN and SGN groups
before treatment, one cycle after treatment, in three cycles after
treatment and after discharge
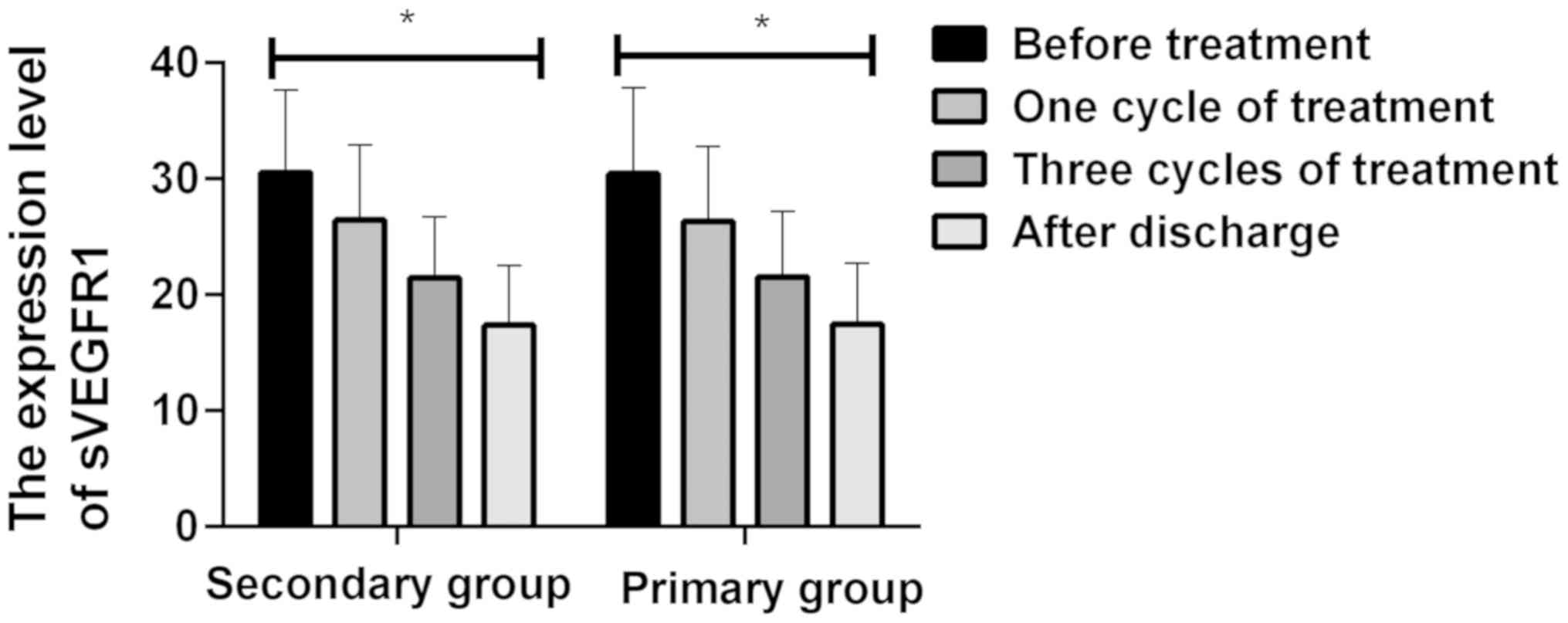
The expression level of sVEGFR1 of the patients in
PGN group and SGN group was compared before they were treated,
after a cycle of treatment, after three cycles of treatment, and
after they discharged from the hospital. It was found that the
expression level of sVEGFR1 of the patients in PGN group and SGN
group decreased gradually after a cycle of treatment. The
expression level of sVEGFR1 of the patients in PGN group and SGN
group after a cycle of treatment was lower than that before they
were treated (P<0.05). The expression level of sVEGFR1 of the
patients in PGN group and SGN group decreased gradually after three
cycles of treatment. The expression level of sVEGFR1 of the
patients in PGN group and SGN group after three cycles of treatment
was lower than that after one cycle of treatment (P<0.05). The
expression level of sVEGFR1 of the patients in PGN group and SGN
group decreased gradually after discharge from the hospital. The
expression level of sVEGFR1 of the patients in PGN group and SGN
group after discharge from hospital was lower than that after three
cycles of treatment (P<0.05). There was no significant
difference between the expression level of sVEGFR1 of the patients
in PGN group and that in SGN group (P>0.05) (Table IV and Fig. 2).
 | Table IVComparative analysis of the
expression level of sVEGFR1 of the patients in PGN group and SGN
group before and after treatment. |
Table IV
Comparative analysis of the
expression level of sVEGFR1 of the patients in PGN group and SGN
group before and after treatment.
| Groups | Before the patients
were treated | In one cycle after
the patients were treated | In three cycles
after the patients were treated | After the patients
were discharged | F-value | P-value |
|---|
| SGN group
(n=52) | 30.51±7.13 |
26.43±6.46a |
21.42±5.25a,b |
17.34±5.13a-c | 0.409 | <0.001 |
| PGN group
(n=36) | 30.42±7.42 |
26.32±6.42a |
21.53±5.63a,b |
17.45±5.26a-c | 0.387 | <0.001 |
| t-test | 0.057 | 0.079 | 0.094 | 0.098 | | |
| P-value | 0.955 | 0.937 | 0.926 | 0.922 | | |
Changes in renal function in different
periods of treatment
The results showed that with the increase of the
treatment cycle, the renal function indexes of patients in the
study group showed a downward trend (P<0.05) (Table V).
 | Table VChanges in renal function of the
study group in different periods of treatment. |
Table V
Changes in renal function of the
study group in different periods of treatment.
| Groups | Before patients
were treated | One cycle after
patients were treated | Three cycles after
patients were treated | After patients were
discharged | F-value | P-value |
|---|
| Urea nitrogen
(mmol/l) | 14.34±1.23 |
12.42±1.14a |
10.41±1.08a,b |
7.65±0.84a-c | 615.500 | <0.001 |
| Creatinine
(umol/l) | 293.43±32.32 |
241.52±31.02a |
174.41±30.73a,b |
161.41±28.26a-c | 440.900 | <0.001 |
Correlation analysis of expression of
C's C and sVEGFR1 in GN and the renal function indexes
According to Spearman's correlation analysis, the
expression of C's C and sVEGFR1 was positively correlated in GN
(r=0.740, P<0.001). Due to the positive correlation between the
expression of C's C and sVEGFR1, the association between the
expression of C's C and urea nitrogen, creatinine were analyzed.
Spearman's correlation results showed that C's C expression was
positively correlated with urea nitrogen (r=0.797, P<0.001), and
C's C expression was positively correlated with creatinine
(r=0.841, P<0.001) (Fig. 3).
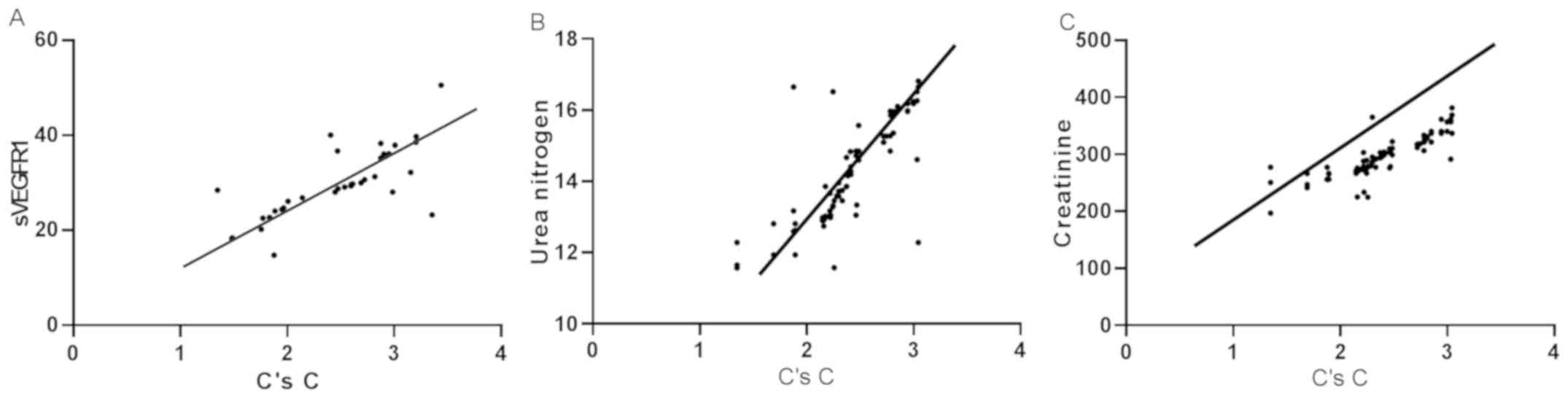 | Figure 3Correlation analysis of expression of
C's C and sVEGFR1 in GN, the renal function indexes. (A) According
to Spearman's correlation analysis, the expression of C's C and
sVEGFR1 was positively correlated in GN (r=0.740, P<0.001). (B)
C's C expression was positively correlated with urea nitrogen
(r=0.797, P<0.001). (C) C's C expression was positively
correlated with creatinine (r=0.841, P<0.001). C's C, serum
cystatin C; sVEGFR1, soluble vascular endothelial growth factor
receptor 1; GN, glomerulus nephritis; PGN, primary
glomerulonephritis; SGN, secondary glomerulonephritis. |
Discussion
At present, it is believed that GN can lead to
end-stage renal diseases and its pathological changes are complex.
For many GN diseases, cellular immunity plays a decisive role, and
humoral immunity is also clearly expressed during the pathogenic
process of GN diseases (14). In
addition, the disorder of blood coagulation mechanism and the
change of blood rheology have an effect on the occurrence and
progression of glomerular diseases (15). In clinic, the pathogenesis and
treatment of GN are known, the drugs used to treat GN are
immunosuppressive agents and glucocorticoid, but the efficacy of
many glomerular diseases is still unsatisfactory (16,17). In
order to evaluate the changes of patients' condition in the
occurrence and progression of GN disease and improve clinical
symptoms of the patients, and the cure rate, the expression of C's
C and sVEGFR1 in GN was investigated. C's C is an independent
endogenous marker, only filtered and metabolized by kidneys. C's C
is important in the early diagnosis of renal injury and is highly
recognized for evaluating glomerular function in clinic (18,19).
sVEGFR1 is produced in endothelial cells, monocytes and placenta.
As a soluble receptor of VEGF, it negatively regulates the activity
of VEGF (20). When sVEGFR1 is
combined with VEGF, the signal transduction acts on angiogenesis,
when this process is disrupted, a series of pathological conditions
will occur, such as the deterioration of renal function, which is
caused by injury of vascular endothelial cell function (21).
In this study, the expression levels of C's C and
sVEGFR1 of the patients in the study group and the control group
were compared. It was found that the expression levels of C's C and
sVEGFR1 of the patients in the study group were significantly
higher than those in the control group. The results indicated that
the expression levels of C's C and sVEGFR1 were diagnostic markers
in the occurrence and progression of glomerular nephropathy. Some
studies reported that the occurrence and progression of GN diseases
are related to cellular immunity, serum free radicals, and
coagulation mechanism, and that the occurrence and development of
GN diseases are closely related to cellular immunity (22). C's C is a cysteine protease inhibitor
that can be used to detect the filtration rate of glomeruli in
kidney injury. The practical method to detect C's C is immunoassay
(23). The signal transduction of
sVEGFR1 improves hematopoietic function, proliferative function,
differentiation function and immune function of monocytes and
macrophages of hematopoietic stem cells (HSC) (24). The changes of cellular immunity exist
in the expression of C's C and sVEGFR1, which indirectly confirms
the conclusion of this study. To investigate the specific changes
of the expression levels of C's C and sVEGFR1 in GN, the changes of
expression levels of C's C and sVEGFR1 were investigated before and
after treatment of patients. The results showed that expression
levels of serum C's C and sVEGFR1 of the patients in PGN group and
SGN group decreased gradually after a cycle of treatment.
Expression levels of serum C's C and sVEGFR1 of the patients in PGN
group and SGN group after a cycle of treatment were lower than
those before treatment (P<0.05). Expression levels of serum C's
C and sVEGFR1 of the patients in PGN group and SGN group decreased
gradually after three cycles of treatment. Expression levels of
serum C's C and sVEGFR1 of the patients in PGN group and SGN group
after three cycles of treatment were lower than those after a cycle
of treatment (P<0.05). Expression levels of serum C's C and
sVEGFR1 of the patients in PGN group and SGN group decreased
gradually after they discharged. Expression levels of serum C's C
and sVEGFR1 of the patients in PGN group and SGN group after
discharged from hospital were lower than those after three cycles
of treatment (P<0.05). Previous studies have shown that the
pathogenesis of secondary glomerular diseases and primary
glomerular diseases is completely different, while their clinical
manifestations and pathological changes are similar (25). Previous studies have demonstrated
that C's C can be expressed effectively in the occurrence and
progression of kidney diseases. With the deterioration of
pathological conditions, the filtration rate of glomeruli decreases
and the expression level of C's C increases (26). When the glomerular filtration barrier
is injured and the repair of endothelial cells is blocked, the
biological effects of VEGF will be inhibited and respond to the
expression of sVEGFR1 negatively (27). In this study, the changes of
expression of C's C and sVEGFR1 were investigated before and after
treatment. The result showed that the expression levels of C's C
and sVEGFR1 decreased with the continuous treatment of the
patients. This result indicates that the expression of C's C and
sVEGFR1 can be used as an indicator for monitoring the condition of
patients with GN when they are treated. The correlation between
expression of C's C and expression of sVEGFR1 was analyzed. The
result showed that the expression of C's C and sVEGFR1 was
positively correlated in GN. This result suggests that C's C and
sVEGFR1 are associated with the progression of GN. Some studies
(28) demonstrated that endogenous
creatinine clearance was an important indicator to evaluate the
filtration rate of glomeruli. Endogenous creatinine clearance and
expression of C's C are related to the pathogenic process of GN. At
present, studies on sVEGFR1 and the correlation between C's C and
sVEGFR1 are few, so the specific mechanism of C's C and sVEGFR1
needs further investigation.
In conclusion, C's C and sVEGFR1 are highly
expressed in serum of GN patients. As patients are treated, the
expression levels of C's C and sVEGFR1 decrease. C's C and sVEGFR1
play important roles in monitoring the condition of GN patients
during treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX analyzed and interpreted the patient general
data. LQ performed ELISA. LY was responsible for analysis of the
observation indicators. XX wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients and/or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao W, Ma Y, Wang M, Shi C, Sun J, Chu K
and Liu C: Expression of Foxp3 in renal tissue of patients with
HBV-associated glomerulonephritis and their clinical and
pathological characteristics. Exp Ther Med. 14:4928–4934.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Agrawal S, Zaritsky JJ, Fornoni A and
Smoyer WE: Dyslipidaemia in nephrotic syndrome: Mechanisms and
treatment. Nat Rev Nephrol. 14:57–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakagawa N, Hasebe N, Hattori M, Nagata M,
Yokoyama H, Sato H, Sugiyama H, Shimizu A, Isaka Y, Maruyama S, et
al: Clinical features and pathogenesis of membranoproliferative
glomerulonephritis: A nationwide analysis of the Japan renal biopsy
registry from 2007 to 2015. Clin Exp Nephrol. 22:797–807.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Komatsu H, Fujimoto S, Yoshikawa N,
Kitamura H, Sugiyama H and Yokoyama H: Clinical manifestations of
Henoch-Schönlein purpura nephritis and IgA nephropathy: Comparative
analysis of data from the Japan Renal Biopsy Registry (J-RBR). Clin
Exp Nephrol. 20:552–560. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gumber R, Cohen JB, Palmer MB, Kobrin SM,
Vogl DT, Wasserstein AG, Nasta SD, Bleicher MB, Bloom RD, Dember L,
et al: A clone-directed approach may improve diagnosis and
treatment of proliferative glomerulonephritis with monoclonal
immunoglobulin deposits. Kidney Int. 94:199–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pottel H, Delanaye P, Schaeffner E,
Dubourg L, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST,
Glassock RJ, et al: Estimating glomerular filtration rate for the
full age spectrum from serum creatinine and cystatin C. Nephrol
Dial Transplant. 32:497–507. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pottel H, Dubourg L, Schaeffner E, Eriksen
BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V,
et al: The diagnostic value of rescaled renal biomarkers serum
creatinine and serum cystatin C and their relation with measured
glomerular filtration rate. Clin Chim Acta. 471:164–170.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Serezlija E, Serdarevic N and Begic L: The
estimation of glomerular filtration rate based on the serum
cystatin C and creatinine values. Clin Lab. 63:1099–1106.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pottel H, Dubourg L, Schaeffner E, Eriksen
BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V,
et al: Data on the relation between renal biomarkers and measured
glomerular filtration rate. Data Brief. 14:763–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Trachtman H, Gipson DS, Somers M, Spino C,
Adler S, Holzman L, Kopp JB, Sedor J, Overfield S, Elegbe A, et al:
Randomized clinical trial design to assess abatacept in resistant
nephrotic syndrome. Kidney Int Rep. 3:115–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Abramowicz D, Oberbauer R, Heemann U,
Viklicky O, Peruzzi L, Mariat C, Crespo M, Budde K and Oniscu GC:
Recent advances in kidney transplantation: A viewpoint from the
Descartes advisory board. Nephrol Dial Transplant. 33:1699–1707.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bus P, Scharpfenecker M, Van Der Wilk P,
Wolterbeek R, Bruijn JA and Baelde HJ: The VEGF-A inhibitor sFLT-1
improves renal function by reducing endothelial activation and
inflammation in a mouse model of type 1 diabetes. Diabetologia.
60:1813–1821. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Atkinson JM, Pullen N, Da Silva-Lodge M,
Williams L and Johnson TS: Inhibition of thrombin-activated
fibrinolysis inhibitor increases survival in experimental kidney
fibrosis. J Am Soc Nephrol. 26:1925–1937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peng T, Xie T, Liu L, Zhen J and Yang X:
Analysis of clinical features and pathology of serum HBsAg positive
glomerulonephritis. J Med Virol. 90:612–615. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Güran T: Latest insights on the etiology
and management of primary adrenal insufficiency in children. J Clin
Res Pediatr Endocrinol. 9 (Suppl 2):9–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leon J, Pérez-Sáez MJ, Uffing A, Murakami
N, Watanabe A, Cureton P, Kenyon V, Keating L, Yee K, Fernandes
Satiro CA, et al: Effect of combined gluten-free, dairy-free diet
in children with steroid-resistant nephrotic syndrome: An open
pilot trial. Kidney Int Rep. 3:851–860. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krhač M and Lovrenčić MV: Update on
biomarkers of glycemic control. World J Diabetes. 10:1–15.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Leem AY, Park MS, Park BH, Jung WJ, Chung
KS, Kim SY, Kim EY, Jung JY, Kang YA, Kim YS, et al: Value of serum
cystatin C measurement in the diagnosis of sepsis-induced kidney
injury and prediction of renal function recovery. Yonsei Med J.
58:604–612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
De Vriese AS, Sethi S, Nath KA, Glassock
RJ and Fervenza FC: Differentiating primary, genetic, and secondary
FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol.
29:759–774. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matsui M, Takeda Y, Uemura S, Matsumoto T,
Seno A, Onoue K, Tsushima H, Morimoto K, Soeda T, Okayama S, et al:
Suppressed soluble Fms-like tyrosine kinase-1 production aggravates
atherosclerosis in chronic kidney disease. Kidney Int. 85:393–403.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li C, Liu B, Dai Z and Tao Y: Knockdown of
VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration,
angiogenesis and growth of clear cell renal cell carcinoma (CRCC).
Cancer Biol Ther. 12:872–880. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lewis EJ: The enigma of cellular immunity
in glomerulonephritis: Lupus nephritis is not necessarily a
prototype of immune complex-mediated glomerulonephritis. Nephron
Physiol. 112:6–10. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin H, Li L, Lei C, Xu X, Nie Z, Guo M,
Huang Y and Yao S: Immune-independent and label-free fluorescent
assay for cystatin C detection based on protein-stabilized Au
nanoclusters. Biosens Bioelectron. 41:256–261. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Murakami M, Iwai S, Hirasuka S, Buron F,
de Souza VC, Rimmelé T, Thaunat O, Badet L, Morelon E, et al:
VEGFR-1 (Flt-1) tyrosine kinase signaling enhances hematopoiesis,
proliferation/differentiation and immunity of monocyte/macrophage
from bone marrow hematopoietic stem cells, and promotes rheumatoid
arthritis. Transpl Int. 32:75–83. 2018.
|
|
25
|
Yang Y, Zhang Z, Zhuo L, Chen DP and Li
WG: The spectrum of biopsy-proven glomerular disease in China: A
systematic review. Chin Med J (Engl). 131:731–735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shen ZX, Liu Y, Liu M, Zheng H, Wu XJ and
Shen C: Evaluation and application of estimation of glomerular
filtration rate based on serum creatinine and cystatin C in renal
function staging]. Zhonghua Liu Xing Bing Xue Za Zhi. 38:1557–1562.
2017.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
27
|
Satchell SC, Anderson KL and Mathieson PW:
Angiopoietin 1 and vascular endothelial growth factor modulate
human glomerular endothelial cell barrier properties. J Am Soc
Nephrol. 15:566–574. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nix DE, Mayersohn M and Erstad BL: Should
estimates of glomerular filtration rate and creatinine clearance be
indexed to body surface area for drug dosing? Am J Health Syst
Pharm. 74:1814–1819. 2017.PubMed/NCBI View Article : Google Scholar
|