Introduction
Human rheumatoid arthritis (RA) is characterized by
chronic inflammation and destruction of multiple joints (1). Fibroblast-like synoviocytes (FLS) in
synovial tissues play critical roles in the pathogenesis of RA,
such as initiation of inflammatory responses and
inflammation-associated cartilage damage (2). In addition, FLS can promote various
processes in RA by secreting different types of inflammatory
cytokines, which has been demonstrated to be closely associated
with the activation of cellular mitogen-activated protein kinase
(MAPK) and nuclear factor (NF)-κB signaling pathways (3-5).
Transforming growth factor β-activated kinase-1 (TAK1) is a member
of the MAPK family. A recent study has reported that TAK1 is
involved in cartilage and bone destruction through its downstream
signaling molecules, which are involved in pro-inflammatory
cytokine signaling, including c-Jun N-terminal kinase (JNK), p38
and NF-κB (6). Choi et al
(7) demonstrated that TAK1, a
dominant MAP3K, is involved in JNK activation in RA-FLS. Knockdown
of TAK1 in RA-FLS effectively inhibits the activation of
interleukin (IL)-1β-induced activator protein 1 and matrix
metalloproteinase (MMP)3 expression, and IL-6 production. Notably,
in RA-FLS, signaling molecules are dependent on TAK1, such as tumor
necrosis factor, toll-like receptor (TLR)2 and IL-1, but not
TLR4(8). These findings demonstrated
TAK1 to be a central regulator of cytokine signaling networks in
RA, and a therapeutic strategy for inhibiting TAK1 may prove to be
beneficial for the treatment of RA.
Sex may affect susceptibility to certain autoimmune
diseases, such as RA, which has a 3:1 female:male ratio. Sex
hormones and their receptors are the basis of sex-related
differences in RA activity (9).
Estrogen treatment delays arthritis progression, which has been
reported in collagen-induced arthritis (CIA), a well-established
experimental model (10,11). However, it is difficult to describe
the association between estrogen and RA. Since the pathogenesis of
RA involves both estrogen and TAK1, it is important to determine
whether TAK1 is an estrogen-responsive gene.
The aim of the present study was to investigate the
levels of TAK1 in synoviocytes of patients with RA, and determine
whether the effect of 17β-estradiol (E2) on the level of TAK1 mRNA
in FLS is dependent on estrogen receptor (ER). The findings of the
present study may help improve our understanding of the association
between estrogen and TAK1 and determine whether TAK1 may serve as a
potential diagnostic and therapeutic target in the treatment of
RA.
Materials and methods
Materials and reagents
E2, ICI 182,780, bovine type II collagen and
Freund's Complete Adjuvant were purchased from Sigma-Aldrich; Merck
KGaA. Mouse monoclonal antibody against TAK1 (1:200, cat. no.
sc-7967), mouse anti-GAPDH monoclonal antibody (1:1,000, cat. no.
sc-365062) and mouse IgGκ light chain binding protein conjugated to
horseradish peroxidase (1:1,000, cat. no. sc-516102) were obtained
from Santa Cruz Biotechnology, Inc.
Human synovial tissues and FLS
Human synovial tissue specimens were obtained from 6
healthy controls (3 men and 3 women, with ages ranging between 40
and 72 years and a mean age of 55.5±2.6 years) who had undergone
knee arthroscopic inspection, 6 patients with osteoarthritis (OA; 3
men and 3 women, with ages ranging between 46 and 72 years and a
mean age of 53.0±4.5 years) and 6 patients with RA (3 men and 3
women, with ages ranging between 38 and 68 years and a mean age of
52.5±2.8 years) who had undergone joint replacement surgery or
synovectomy at the Department of Sports Medicine and Joint Surgery,
The First Affiliated Hospital of China Medical University between
January 2007 and December 2007. The present study was approved by
the Ethics Committee of China Medical University and all patients
provided written informed consent. The RA patients fulfilled the
1987 revised criteria or the 2010 criteria for the disease
(12,13). FLS were prepared from synovial tissue
as previously described (14). All
specimens were stored in liquid nitrogen (195.79˚C) prior to use.
For functional assays, FLS were cultured in 0.1% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RNA isolation was performed using the RNeasy Mini
Kit (Qiagen, Inc.). The QuantiTect Reverse Transcription kit
(Qiagen, Inc.) was used to synthesize complementary DNA under the
following conditions: 42˚C for 15 min and 95˚C for 3 min. qPCR was
performed using RT² SYBR® Green qPCR Master Mix (cat.
no. 330500; Qiagen, Inc.). Samples were normalized to internal
control GAPDH and compared to the control group. The primers used
in the present study were as follows: TAK1 forward,
5'-ATCGTCATATCAGGCAACG GAC-3' and reverse, 5'-TGAGGTTGGTCCTGA
GGTAGT-3'; GAPDH forward, 5'-GCACCGTCAAGGCTGA GAAC-3' and reverse,
5'-TGGTGAAGACGCCAGTGGA-3'. The qPCR reaction conditions were as
follows: 10 min at 95˚C, followed by 40 cycles of 15 sec at 95˚C
and 1 min at 60˚C. Gene expression values were calculated using the
2-ΔΔCq relative quantification method, wherein the
control group was designated as the calibrator (15).
Western blot analysis
All proteins were isolated using RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.) from
E2-treated and untreated FLS with a bicinchoninic acid assay to
measure protein concentration. The whole-cell extracts (20 mg
protein) were fractionated by 10% SDS-PAGE and blotted onto PVDF
membranes. Following blocking of non-specific protein binding in 5%
skimmed milk at room temperature for 1 h, the membranes were
incubated at 25˚C for 4 h with primary antibodies diluted in
primary antibody dilution buffer (Beyotime Institute of
Biotechnology). Then, the membranes were further incubated with
horseradish peroxidase-conjugated secondary antibody at 25˚C for 2
h. The membranes were then visualized using enhanced
chemiluminescence (Sigma-Aldrich; Merck KGaA).
CIA
CIA was induced as previously described (10,11).
Briefly, bovine type II collagen (5 mg) was dissolved in 25 ml
acetic acid with a concentration of 0.1 mol/l, which was prepared
as a solution with a concentration of 2 mg/ml, followed by
preservation overnight at 4˚C in a refrigerator. Subsequently, 2.5
ml Freund's Complete Adjuvant was added to prepare the collagen
emulsion, which was stored at 4˚C until use. A total of 30 female
rats (4 months old, weighing 80-100 g) were randomly divided into
the normal control (n=10), CIA model (n=10) and E2-CIA (n=10)
groups. Rats were purchased from Beijing Vital River Laboratory
Animal Technology, Co. All animals were housed at 25˚C with a 12-h
light/dark cycle, with a relative humidity of 40-70%, and had free
access to food and water. The experimental protocol conformed to
the Animal Welfare Act Guide for Use and Care of Laboratory Animals
and was approved by the Institutional Animal Care and Use Committee
of China Medical University. Rats in the normal control group were
injected via the tail vein with 0.1 ml normal saline, and rats in
the CIA model and E2-CIA groups received an injection of 0.1 ml
collagen emulsion via the tail vein. At 21 days after the model
establishment, drug administration was performed, in which 100 µg
E2 was injected into rats of the E2-CIA group for 3 days, and
normal saline was given to rats in the normal control and CIA model
groups. The injection dose of E2 was significantly higher compared
with endogenous levels, therefore, normal secretions may not be
taken into consideration (16).
Assessment of arthritis severity was performed using scores between
0 and 3 for each paw, and each rat was given a maximum of 12
points, determined as follows: 1, Swelling or erythema in one
joint; 2, swelling or erythema in two joints; and 3, severe
swelling or erythema of more than two joints, or ankylosis of the
entire paw (17). Weighing the uteri
at termination confirmed successful estrogen treatment. The rats
were euthanized with an overdose of pentobarbital (200 mg/kg) and
death was confirmed through observing respiratory and cardiac
arrest and the absence of any pain response to needle puncture of
the extremities.
Statistical analysis
Statistical analyses were performed using SPSS
version 16.0 (SPSS, Inc.). Data are presented as the mean and
standard deviation. Student's t test was used to compare two groups
and one way ANOVA with Dunnett's post hoc test was used to compare
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
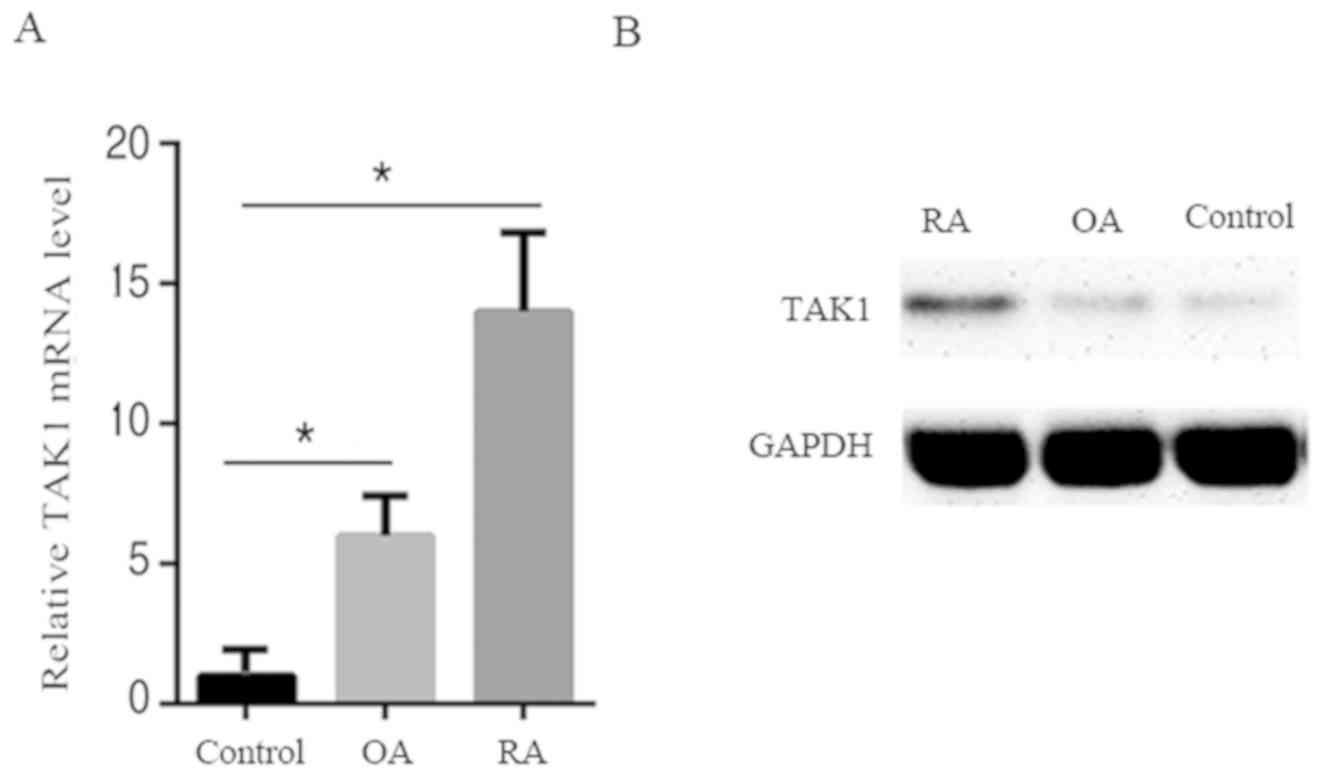
TAK1 level is upregulated in
synoviocytes of patients with RA
To investigate the role of TAK1 in patients with RA,
TAK1 expression levels were first determined in synoviocytes from
patients with RA, patients with OA and healthy controls. As shown
in Fig. 1A and B, compared with OA patients and healthy
controls, patients with RA exhibited an increase in TAK1 mRNA and
protein levels. This suggests that an increase of TAK1 expression
may be implicated in RA pathogenesis.
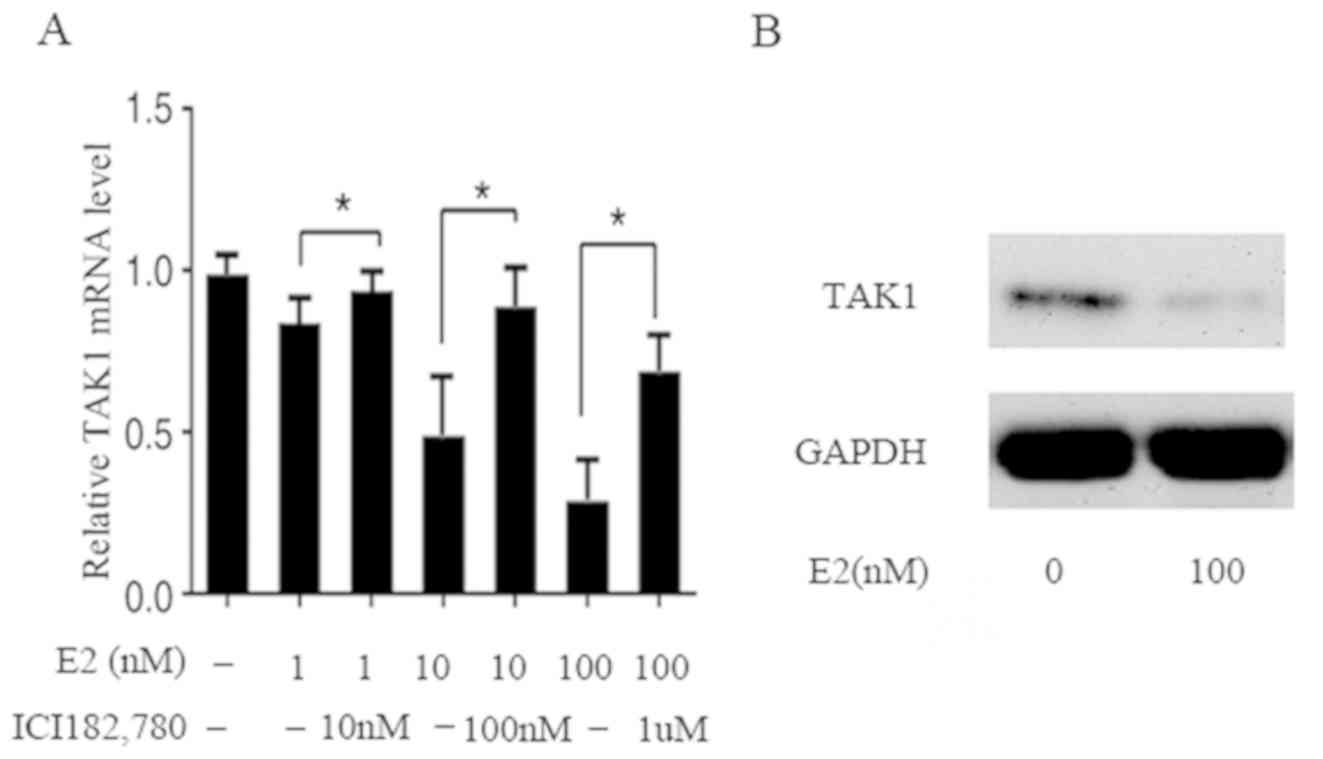
Downregulation of TAK1 expression by
E2 in FLS
To assess whether the expression of TAK1 is
regulated by E2, the TAK1 mRNA and protein levels were examined in
FLS. RT-qPCR analysis demonstrated that TAK1 mRNA expression was
evidently decreased by E2 in a dose-dependent manner; compared with
the control, mRNA levels decreased by 15, 50 and 70% at E2
concentrations of 1, 10 and 100 nM, respectively. In addition,
concomitant treatment with ICI 182,780, an ER antagonist, restored
the inhibition (Fig. 2A). The
results of western blot analysis demonstrated that after a 24
h-treatment of FLS with E2 (100 nM), the protein level was also
decreased, which is in agreement with the decrease in mRNA
(Fig. 2B). These results indicate
that E2 affects the mRNA and protein expression of TAK1 in the
genomic pathway involving ER.
Systemic administration of E2
attenuates the severity of CIA by regulating TAK1 gene expression
in vivo
The present data suggested that TAK1 and E2 may both
play important roles in the pathogenesis of osteoarthritis in human
RA. Therefore, it was next investigated whether E2 could alleviate
the inflammation and joint destruction observed in CIA by
regulating TAK1 in mice. Mice were randomly assigned to receive
treatment with E2 or saline and CIA was established; arthritis
development was then assessed. Measurement of swelling degree of
the toes at 21 days after model establishment revealed a
significant difference in joint swelling degree of the rats' left
feet between the CIA model group and the E2-CIA and control groups
(P<0.01), indicating that the models were successfully
established. E2 was administered at 21 days after model
establishment. The left foot joint swelling degrees of the rats in
the normal control, CIA model and E2-CIA groups were detected at 21
days after drug administration. The results demonstrated that there
was a significant difference between the E2-CIA and CIA model
groups (P<0.01; Table I).
 | Table IComparison of joint swelling degrees
of the left posterior foot among three groups of rats (n=10). |
Table I
Comparison of joint swelling degrees
of the left posterior foot among three groups of rats (n=10).
| | Swelling degree of
the left posterior foot after model establishment | Swelling degree of
the left posterior foot after drug administration |
|---|
| Groups | 21 days | 7 days | 14 days | 21 days |
|---|
| Control | 0 | 0 | 0 | 0 |
| CIA | 2.1±0.2a | 2.5±0.4a | 2.8±0.2a | 2.5±0.4a |
| E2-CIA | 2.0±0.1a | 1.6±0.2a,b | 1.2±0.1a,b | 1.1±0.3a,b |
By using X-ray imaging, the effect pre- and
post-administration of E2 on joint swelling and destruction in CIA
mice was also observed. The results demonstrated that the soft
tissue in the control group was not swollen and the joint remained
intact. In the CIA group, the soft tissue was swollen and the
tarsal bones were scattered; osteophytes were seen around the
tarsal bones, and free ‘articular bones’ were observed. However,
soft tissue swelling and joint damage were significantly relieved
in the E2-CIA group following administration of E2 (Fig. 3A).
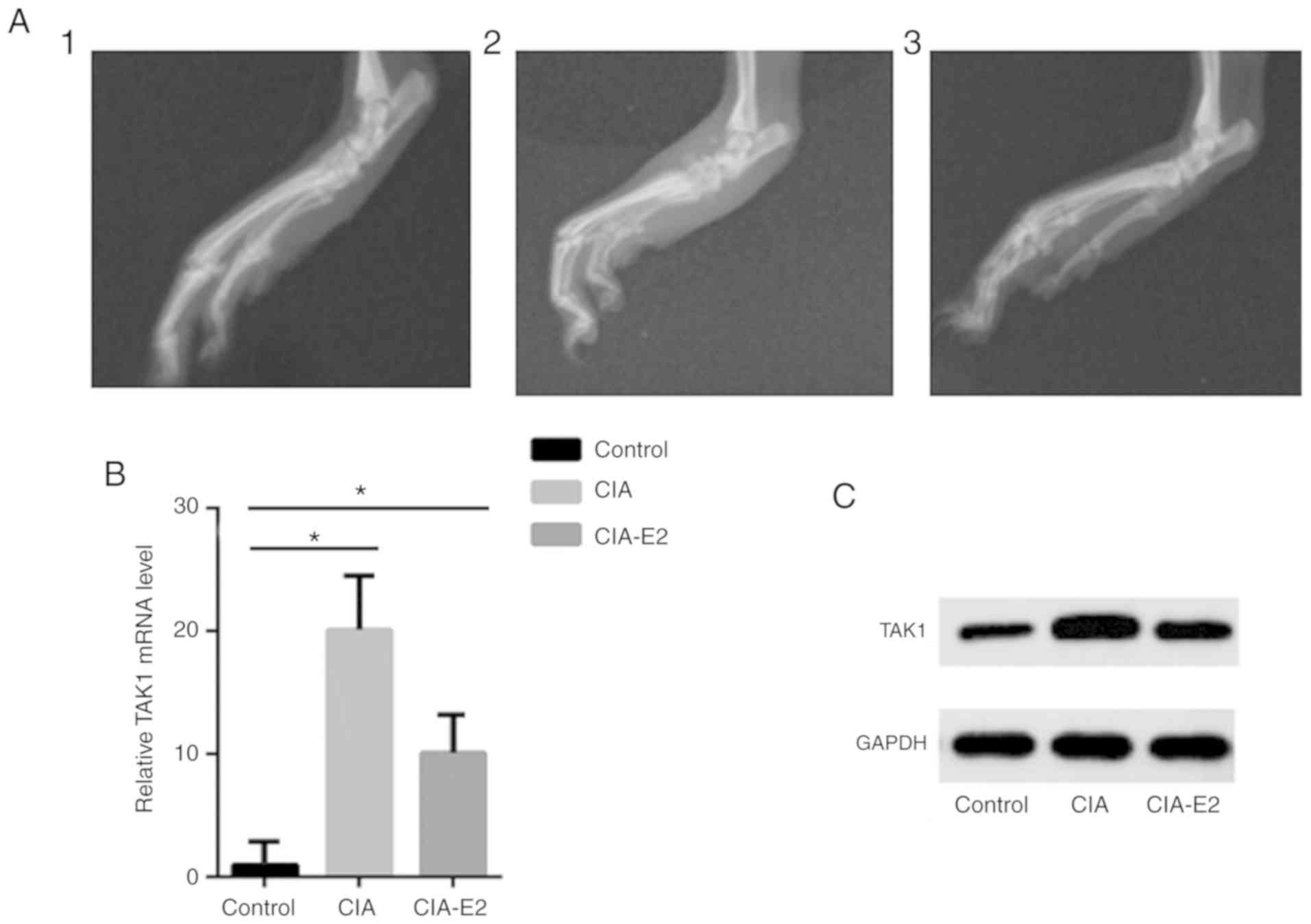 | Figure 3Systemic administration of E2
attenuates the severity of CIA by regulating TAK1 gene expression
in vivo. CIA was treated with E2 (100 µg/day) or saline for 3 days.
Arthritis severity was assessed by macroscopic scoring of arthritis
in the paws. (A) X-ray imaging demonstrates joint damage. 1,
control group; 2, CIA group; 3, E2-CIA group. Macroscopic evidence
of arthritis, such as swelling or scattered tarsal bones, was
markedly observed in the CIA group, while treatment with E2
significantly attenuated arthritis severity in CIA. (B) The mRNA
level of TAK1 was determined by reverse transcription-quantitative
PCR analysis. Data are presented as relative expression units,
normalized to GAPDH. Values are the mean ± standard error of the
mean from three independent experiments. *P<0.05. (C)
TAK1 protein expression was determined by western blotting. Data
are presented as the mean ± standard error of the mean. CIA,
collagen-induced arthritis; E2, 17β-estradiol; TAK1, transforming
growth factor β-activated kinase-1. |
TAK1 is a well-known key player in the pathogenesis
of RA. Therefore, the present study examined whether administration
of E2 was associated with the level of TAK1 in vivo. RT-qPCR
analysis and western blotting revealed a significant decrease in
TAK1 expression following treatment with E2. As shown in Fig. 3B and C, the expression of TAK1 in joint soft
tissue in the CIA model group was significantly increased compared
with that in the control group (P<0.05). TAK1 expression was
significantly lower in the E2-CIA group compared with that in the
CIA group (P<0.05). In summary, these results suggest that
systemic administration of E2 ameliorates the incidence and
severity of CIA through regulation of TAK1 expression in mice.
Discussion
To the best of our knowledge, the present study was
the first to report that TAK1 is present in the synoviocytes of
patients with RA. In addition, E2 decreased the expression of TAK1
in FLS, an effect that was dependent on ER. Furthermore, systemic
administration of E2 markedly reduced the incidence and symptoms of
arthritis, and decreased the expression of TAK1 in mice with
CIA.
In recent years, a number of studies have suggested
that TAK1 is implicated in the pathogenesis of RA. According to an
epistasis analysis in a study by Gottar-Guillier et al, as a
downstream mediator of TAK1 in the inflammatory signaling pathways,
IL-8 production in primary human endothelial cells was inhibited by
small interfering RNA knockdown of the tyrosine kinase bone marrow
kinase on chromosome X (BMX). Furthermore, BMX-deficient mice were
protected from developing K/BxN serum transfer-induced arthritis.
This indicates a potential role for TAK1 in RA pathogenesis through
BMX possibly working together with the TAK1-TAB1 complex (18).
The peak incidence of RA in women coincides with the
perimenopausal period, when estrogen levels rapidly decline
(19). During pregnancy, when the
levels of sex hormones increase, ≤75% patients with RA experience
symptom relief, which suggests an association between estrogen
deficiency and the development of RA (20). By contrast, men have fairly
continuous levels of estrogen throughout their adult lives
(21). In addition, estrogen levels
tend to be lower in postmenopausal women compared with those in men
of similar ages (22). A major
pathological RA manifestation is reorganization of the synovial
architecture, with immune cells infiltrating the synovium, FLS
proliferation, synovial inflammation and pannus formation (23). Estrogen exerts a direct effect on
monocytes and macrophages. It can increase the production of
proinflammatory cytokines, which promote cartilage reabsorption,
inhibit proteoglycan synthesis and trigger inflammation, which are
all characteristic of RA (24-26).
However, to date, there is limited evidence on the association
between TAK1 and E2. In the present study, E2, a terminal active
estrogen with the highest affinity for ER, was used to determine
the effect of estrogen on TAK1 expression in FLS. It was observed
that E2 decreased the mRNA level of TAK1 in a dose-dependent
manner, and this decrease was prevented by the selective ER
antagonist ICI 182,780. In agreement with previous findings, the
results of the present study indicated that the effects of estrogen
can be exerted directly through the ER in monocytes/macrophages
(27). This is also in agreement
with another report demonstrating that the expression and
phosphorylation level of TAK1 significantly decreased following
treatment with estradiol in PC12 cells (28).
A previous study reported that treatment with E2
significantly inhibited experimental autoimmune arthritis (29). However, the mechanism underlying
these effects has yet to be fully elucidated. The data of the
present study demonstrated that treatment with E2 decreased the
incidence and severity of CIA and also decreased the expression of
TAK1. However, the molecular mechanisms underlying the role of E2
in regulating the expression of TAK1 must be further investigated
in future studies.
In summary, the results of the present study
uncovered a previously unidentified role of E2 in regulating TAK1
expression and suppressing the pathological process of CIA. Thus,
TAK1 may serve as a potential diagnostic and therapeutic target for
the treatment of RA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Fund Project (grant no. 81401334), the Natural Science Fund
Projects of Liaoning Province (grant no. 20170540543) and the
Scientific Project from Liaoning Education Department (grant no.
LQNK201730).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XL collected clinical tissues and conducted the
experiments. ML designed the study and wrote the manuscript. Both
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of China Medical University (Shenyang, China). Written
informed consent was obtained from all participants.
Patient consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Viatte S, Plant D and Raychaudhuri S:
Genetics and epigenetics of rheumatoid arthritis. Nat Rev
Rheumatol. 9:141–153. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Juarez M, Filer A and Buckley CD:
Fibroblasts as therapeutic targets in rheumatoid arthritis and
cancer. Swiss Med Wkly. 142(w13529)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huh JE, Seo BK, Park YC, Kim JI, Lee JD,
Choi DY, Baek YH and Park DS: WIN-34B, a new herbal medicine,
inhibits the inflammatory response by inactivating IκB-α
phosphorylation and mitogen activated protein kinase pathways in
fibroblast-like synoviocytes. J Ethnopharmacol. 143:779–786.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu L, Feng X, Tan W, Gu W, Guo D, Zhang M
and Wang F: IL-29 enhances Toll-like receptor-mediated IL-6 and
IL-8 production by the synovial fibroblasts from rheumatoid
arthritis patients. Arthritis Res Ther. 15(R170)2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Yoshioka Y, Kozawa E, Urakawa H, Arai E,
Futamura N, Zhuo L, Kimata K, Ishiguro N and Nishida Y: Suppression
of hyaluronan synthesis alleviates inflammatory responses in murine
arthritis and in human rheumatoid synovial fibroblasts. Arthritis
Rheum. 65:1160–1170. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hammaker DR, Boyle DL, Inoue T and
Firestein GS: Regulation of the JNK pathway by TGF-beta activated
kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther.
9(R57)2007.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Choi YS, Park JK, Kang EH, Lee YK, Kim TK,
Chung JH, Zimmerer JM, Carson WE III, Song YW and Lee YJ: Cytokine
signaling-1 suppressor is inducible by IL-1beta and inhibits the
catabolic effects of IL-1beta in chondrocytes: Its implication in
the paradoxical joint-protective role of IL-1beta. Arthritis Res
Ther. 15(R191)2013.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Geurts J, van den Brand BT, Wolf A,
Abdollahi-Roodsaz S, Arntz OJ, Kracht M, van den Berg WB and van de
Loo FA: Toll-like receptor 4 signalling is specifically
TGF-beta-activated kinase 1 independent in synovial fibroblasts.
Rheumatology (Oxford). 50:1216–1225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alamanos Y and Drosos AA: Epidemiology of
adult rheumatoid arthritis. Autoimmun Rev. 4:130–136.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Engdahl C, Jochems C, Windahl SH,
Börjesson AE, Ohlsson C, Carlsten H and Lagerquist MK: Amelioration
of collagen-induced arthritis and immune-associated bone loss
through signaling via estrogen receptor alpha, and not estrogen
receptor beta or G protein-coupled receptor 30. Arthritis Rheum.
62:524–533. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Holmdahl R, Carlsten H, Jansson L and
Larsson P: Oestrogen is a potent immunomodulator of murine
experimental rheumatoid disease. Br J Rheumatol. 28 (Suppl
1):54–58; discussion 69-71. 1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Villeneuve E, Nam J and Emery P: 2010
ACR-EULAR classification criteria for rheumatoid arthritis. Rev
Bras Reumatol. 50:481–483. 2010.
|
|
14
|
Bartok B, Boyle DL, Liu Y, Ren P, Ball ST,
Bugbee WD, Rommel C and Firestein GS: PI3 kinase δ is a key
regulator of synoviocyte function in rheumatoid arthritis. Am J
Pathol. 180:1906–1916. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim JM, Shin SC, Park GC, Lee JC, Jeon YK,
Ahn SJ, Thibeault S and Lee BJ: Effect of sex hormones on
extracellular matrix of lamina propria in rat vocal fold.
Laryngoscope. 30:732–740. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andersson A, Grahnemo L, Engdahl C,
Stubelius A, Lagerquist MK, Carlsten H and Islander U:
IL-17-producing γδT cells are regulated by estrogen during
development of experimental arthritis. Clin Immunol. 161:324–332.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gottar-Guillier M, Dodeller F, Huesken D,
Iourgenko V, Mickanin C, Labow M, Gaveriaux S, Kinzel B, Mueller M,
Alitalo K, et al: The tyrosine kinase BMX is an essential mediator
of inflammatory arthritis in a kinase-independent manner. J
Immunol. 186:6014–6023. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tedeschi SK, Bermas B and Costenbader KH:
Sexual disparities in the incidence and course of SLE and RA. Clin
Immunol. 149:211–218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Islander U, Jochems C, Lagerquist MK,
Forsblad-d'Elia H and Carlsten H: Estrogens in rheumatoid
arthritis; the immune system and bone. Mol Cell Endocrinol.
335:14–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kvien TK, Uhlig T, Ødegård S and Heiberg
MS: Epidemiological aspects of rheumatoid arthritis: The sex ratio.
Ann N Y Acad Sci. 1069:212–222. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khosla S, Melton LJ III, Atkinson EJ and
O'Fallon WM: Relationship of serum sex steroid levels to
longitudinal changes in bone density in young versus elderly men. J
Clin Endocrinol Metab. 86:3555–3561. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jung SM, Kim KW, Yang CW, Park SH and Ju
JH: Cytokine-mediated bone destruction in rheumatoid arthritis. J
Immunol Res. 2014(263625)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cutolo M, Accardo S, Villaggio B, Clerico
P, Bagnasco M, Coviello DA, Carruba G, lo Casto M and Castagnetta
L: Presence of estrogen-binding sites on macrophage-like
synoviocytes and CD8+, CD29+,
CD45RO+ T lymphocytes in normal and rheumatoid synovium.
Arthritis Rheum. 36:1087–1097. 1993.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cutolo M, Capellino S, Montagna P, Sulli
A, Seriolo B and Villaggio B: Anti-inflammatory effects of
leflunomide in combination with methotrexate on co-culture of T
lymphocytes and synovial macrophages from rheumatoid arthritis
patients. Ann Rheum Dis. 65:728–735. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dubey RK, Tofovic SP and Jackson EK:
Cardiovascular pharmacology of estradiol metabolites. J Pharmacol
Exp Ther. 308:403–409. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cutolo M, Villaggio B, Bisso A, Sulli A,
Coviello D and Dayer JM: Presence of estrogen receptors in human
myeloid monocytic cells (THP-1 cell line). Eur Cytokine Netw.
12:368–372. 2001.PubMed/NCBI
|
|
28
|
Peng R, Dai W and Li Y: Neuroprotective
effect of a physiological ratio of testosterone and estradiol on
corticosterone induced apoptosis in PC12 cells via Traf6/TAK1
pathway. Toxicol In Vitro. 50:257–263. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Andersson A, Stubelius A, Karlsson MN,
Engdahl C, Erlandsson M, Grahnemo L, Lagerquist MK and Islander U:
Estrogen regulates T helper 17 phenotype and localization in
experimental autoimmune arthritis. Arthritis Res Ther.
17(32)2015.PubMed/NCBI View Article : Google Scholar
|