Introduction
Preeclampsia (PE) is a disease during pregnancy, and
its major symptoms include hypertension, proteinuria, edema and
systemic organ damage after 20 weeks of pregnancy (1). According to the epidemiological
studies, the morbidity rate of PE is 9.4% in China and 7-12%
worldwide, which is the main cause of maternal death, fetal growth
restriction and high fetal mortality (2).
There are many causes of PE, with a complicated
pathogenesis, and its specific mechanism of action remains unclear
(3). At present, the theory of
trophoblastic ischemia and hypoxia is widely recognized, which
holds that during the placental formation of PE patients, excessive
trophoblast apoptosis weakens the invasion ability of extravillous
trophoblasts, leads to the disorders of vascular remolding of
uterine spiral arterioles, and makes the maternal body unable to
supply enough blood and oxygen to the placenta, ultimately
resulting in the systemic inflammatory response and systemic
vascular endothelial injury, and inducing various clinical symptoms
of PE (4).
Micro ribonucleic acids (miRNAs) are endogenous
non-coding single-stranded small-molecule RNAs with 19-22 nt in
length. The major mechanism of action of miRNAs includes the
complementary binding to the target gene mRNA 3'-untranslated
region (3'-UTR) to directly degrade mRNA or inhibit its
translation, thereby regulating the gene expression at the
transcriptional level. Currently, studies have found that miRNAs
play very important roles in such biological processes as cell
proliferation, differentiation and apoptosis (2-4). As
a widely-studied miRNA at present, miR-30 plays a vital role in the
occurrence and development of cardiovascular diseases, metabolic
diseases and tumors (5-7).
There are studies showing that miR-30 can inhibit the transforming
growth factor-β (TGF-β)-induced organ fibrosis, which is associated
with epithelial cell apoptosis involving TGF-β (8,9).
Currently, studies have demonstrated that the
occurrence and development of PE are closely related to the
proliferation of trophoblasts. The present study, therefore,
explored whether miR-30 can affect the proliferation ability of
trophoblasts in PE through the mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK) pathway.
Materials and methods
Cell culture
The human choriocarcinoma HTR8/SVNEO cell lines used
in this study were purchased from American Type Culture Collection
(ATCC), and cultured in Roswell Park Memorial Institute 1640 (RPMI
1640) medium (Invitrogen; Thermo Fisher Scientific, Inc.; batch no:
991015) containing 10% fetal bovine serum (FBS) (Invitrogen; Thermo
Fisher Scientific, Inc.; batch no: 423257) in an incubator with 5%
CO2 at 37˚C.
Cell experimental scheme
In the present study, all cells were divided into
hypoxia group, miR-30 Mimic group (hypoxia + transfection with
miR-30 mimic) and CTL group (normal HTR8/SVNEO cells). In hypoxia
group, HTR8/SVNEO cells were transfected with 50 nM of miRNA
negative control while in miR-30 Mimic group, HTR8/SVNEO cells were
transfected with 50 nM of miR-30 mimic (Shanghai Genechem Co.,
Ltd.) using the Lipofectamine 2000 kit (Invitrogen; Thermo Fisher
Scientific, Inc.; batch no: 11573019) in strict accordance with the
instructions. Cells were seeded in 24-well plates for 24 h before
transfection. miRNA negative control or miR-30 mimics were diluted
and then mixed with Lipofectamine® 2000. The mixture was
added to each well after maintained at room temperature for 20 min.
The cells were cultured in a humidified atmosphere containing 5%
CO2 at 37˚C for 6 h, then the medium was replaced. At 48
h after transfection, the cells were cultured in a tri-gas
incubator (1% O2 and 99% N2) for 24, 48 and
72 h, followed by subsequent experiments. The sequences are as
follows: miRNA negative control F: 5'-TCTGAGGCTA ACCACGGTCTGTA-3'
and R: 5'-CTGATTAAGTGTCAT ACTCATAC; miR-30 mimics F:
5'-TGTAAACATCCTACAC TCTCAGC-3' and R: 5'-CTCGCTTCGGCAGCACACCG
ACT-3'.
Detection of cell proliferation via
methyl thiazolyl tetrazolium (MTT) assay
First, the cells treated in each group were cultured
in vitro, paved onto the plate, digested and counted. Then
the cells were diluted to 5x104/ml, and 100 μl of
diluent was added to a well of a 96-well plate, with the number of
cells controlled at 5,000 cells/well. At different time points
during growth, 5 mg/ml MTT stock solution (Sigma-Aldrich; Merck
KGaA) was prepared, diluted at 1:10 and added into cells for
reaction in the incubator for 4 h. After MTT solution was removed,
the wells were dried, 150 μl of DMSO was added into each
well, and the plate was placed in the incubator for 15 min.
Finally, the optical density (OD) value of cells was measured at
490 nM using a microplate reader to evaluate the number of viable
cells. The higher OD value corresponds to more cells.
Detection of apoptosis via flow
cytometry
The cells were suspended, directly centrifuged at
500 x g at 4˚C for 5 min and collected. The adherent cells were
digested with trypsin containing EDTA (endothelial cell medium) for
an appropriate time, and the reaction was terminated with complete
medium. Then the cells were rinsed with phosphate buffered saline
(PBS), counted and centrifuged at 500 x g at 4˚C for 5 min. Cells
(1-5x105) were collected, resuspended with 500 μl
of binding buffer, and mixed evenly with 5 μl of Annexin
V-Light 650 and 10 μl of propidium iodide (PI), followed by
reaction at room temperature in the dark for 5-15 min. Flow
cytometry was performed within 1 h, and the Annexin V-Light 650
fluorescence signal and PI fluorescence signal were detected
through the FL4 channel, and the FL2 or FL3 channel, respectively.
Finally, the Annexin V-Light 650 single positive tube and PI single
positive tube were detected simultaneously to determine the
fluorescence compensation value and the position of cross quadrant
gate.
Animal feeding, treatment and
grouping
A total of 30 healthy wild-type pregnant
Sprague-Dawley (SD) rats aged 5-6 weeks and weighing 200-250 gr
purchased from Shanghai BRL Biotechnology Co., Ltd. were fed in the
specific pathogen-free animal room at 22˚C, relative humidity of
60% and 12/12 h light/dark cycle, and they had free access to food
and water. The day when sperms were found in the vagina of rats and
the vaginal plug fell was determined as the first day of pregnancy.
The SD rats were randomly divided into control group (CTL group,
n=10), PE rat group (PE group, n=10) and PE+miR-30 Mimic group
(PE+agomiR-30 group, n=10). AgomiR-30 control group was not
included in this study. In PE+agomiR-30 group, agomiR-30 was
injected via the caudal vein (10 nmol/day) once every 2 days for a
total of 7 days. This study was approved by the Animal Ethics
Committee of Qinghai Provincial People's Hospital (Qinghai,
China).
Animal modeling method
It is currently recognized that placental ischemia
and hypoxia are important causes of PE, so the chronic placental
hypoxia model was established in this study using L-nitro-arginine
methyl ester (L-NAME), a NOS inhibitor (10). L-NAME was subcutaneously injected
(100 mg/kg/d) in PE group and PE+agomiR-30 group for 7 days from
12th day after pregnancy, while the same volume of normal saline
was subcutaneously injected in CTL group. The rats were sacrificed
using 5% isoflurane followed by cervical dislocation after 7 days
of treatment.
Detection of protein expression using
western blot analysis
The placental tissues of SD rats were cut into
pieces, homogenized and added with lysis buffer, followed by
centrifugation in 4,000 x g and 4˚C for 30 min. The total protein
concentration was measured using the bicinchoninic acid (BCA)
protein assay kit (Pierce Protein Biology; Thermo Fisher
Scientific, Inc.). After sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE, 12% gel), the protein (10 μl) was
transferred onto a polyvinylidene fluoride (PVDF) membranes (Merck
Millipore; IPVH00010), and incubated with phosphorylated ERK
(p-ERK)1/2 (1:1,000; cat no 4370), ERK1/2 (1:1,000; cat no 4695),
PCNA (1:1,000; cat no 13110) and tubulin (1:1,000; cat no 2148)
primary antibodies (Cell Signaling Technology, Inc.) at 4˚C
overnight. After washing, the protein was incubated again with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:1,000; cat no 7075; Cell Signaling Technology, Inc.) for 1 h.
Finally, the electrochemiluminescence (ECL) mixture was added to
obtain images using the fluorescence development technique.
Quantity One (version 4.0; Bio-Rad Laboratories, Inc.) was used for
densitometric analysis. Protein expression levels were calculated
as the relative band density to tubulin.
Detection of mRNA expression levels of
ERK1/2 via reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
The mRNA was extracted from tissues in each group
using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., cat no. 10296028), and reversely transcribed into
complementary deoxyribose nucleic acid (cDNA) using Takara
PrimeScriptTM RT Master Mix kit (Takara Biotechnology
Co., Ltd.; cat no RR036A). Reverse transcription reaction was
performed at 50˚C for 60 min, then the reverse transcriptase was
inactivated at 85˚C for 5 min. A total of 2 μl of
5xPrimeScript RT Master Mix was added into 500 ng of RNA, and the
total reaction system was 10 μl. Then PCR amplification was
performed using SYBR® Green Master Mix (Takara Bio,
Inc.; cat no MB3353). cDNA (2 μl) of was added with 10
μl of SYBR Premix Ex Taq II (Tli RNaseH Plus) (2x), 0.8
μl of forward primers, 0.8 μl of reverse primers, and
0.4 μl of ROX Reference Dye II (50x), and deionized water
was added to total volume of 20 μl. PCR amplification
conditions: pre-denaturation at 94˚C for 5 min, followed by 94˚C
for 30 sec, 55˚C for 30 sec, and 72˚C for 1 min and 30 sec for 40
cycles. The mRNA expression level was calculated using the cycle
threshold, with β-actin as an internal reference. The
2-ΔΔCq method was used to determine the relative
expression levels (11). The primer
sequences are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | Forward (5'-3') | Reverse (5'-3') |
|---|
| ERK |
AGAGTTGAAGGATGATGACT |
CACTCATGCAGCACCTGCAG |
| β-actin |
GCAGAAGGAGATTACTGCCCT |
GCTGATCCACATCTGCTGGAA |
Immunohistochemistry (IHC)
First, the placenta tissue was obtained from the rat
and paraffin sections were routinely prepared, deparaffinized,
incubated with 3% H2O2 - 60% methanol at room
temperature for 30 min, and washed with PBS 3 times, followed by
membrane permeabilization with 0.1% Triton X 100+PBS for 20 min,
and incubation with 5% normal goat serum at room temperature for 20
min, rabbit anti-mouse PCNA monoclonal antibody (1:200; cat no
13110) in a refrigerator at 4˚C overnight, and biotinylated goat
anti-rabbit IgG secondary antibody at 37˚C for 1 h. After washing
with PBS 3 times, the sections were incubated with HRP-labeled
streptavidin antibody at 37˚C for 30 min, followed by
diaminobenzidine (DAB) staining (Beijing Solarbio Science &
Technology Co., Ltd.) in the dark at room temperature, hematoxylin
counterstaining for 30 min, dehydration with gradient ethanol,
transparentization with xylene and sealing with neutral balsam.
Finally, the sections were observed under an inverted fluorescence
microscope.
Positive expression as dark brown
particles in cells
The mean OD value of IHC-positive particles was
determined using ImageJ professional image analysis system, and the
PCNA protein expression was semi-quantitatively analyzed.
Statistical analysis
GraphPad Prism 6.0 (La Jolla) was used for the
statistical analysis of data. Three batches of the cell culture
were carried out and the data were expressed as mean ± SEM
(standard error of the mean). Comparisons between multiple groups
was performed using one-way analysis of variance test followed by
Least Significant Difference post hoc test. P<0.05 indicates
statistically significant difference.
Results
Effect of miR-30 on proliferation of
trophoblast HTR8/SVNEO cells
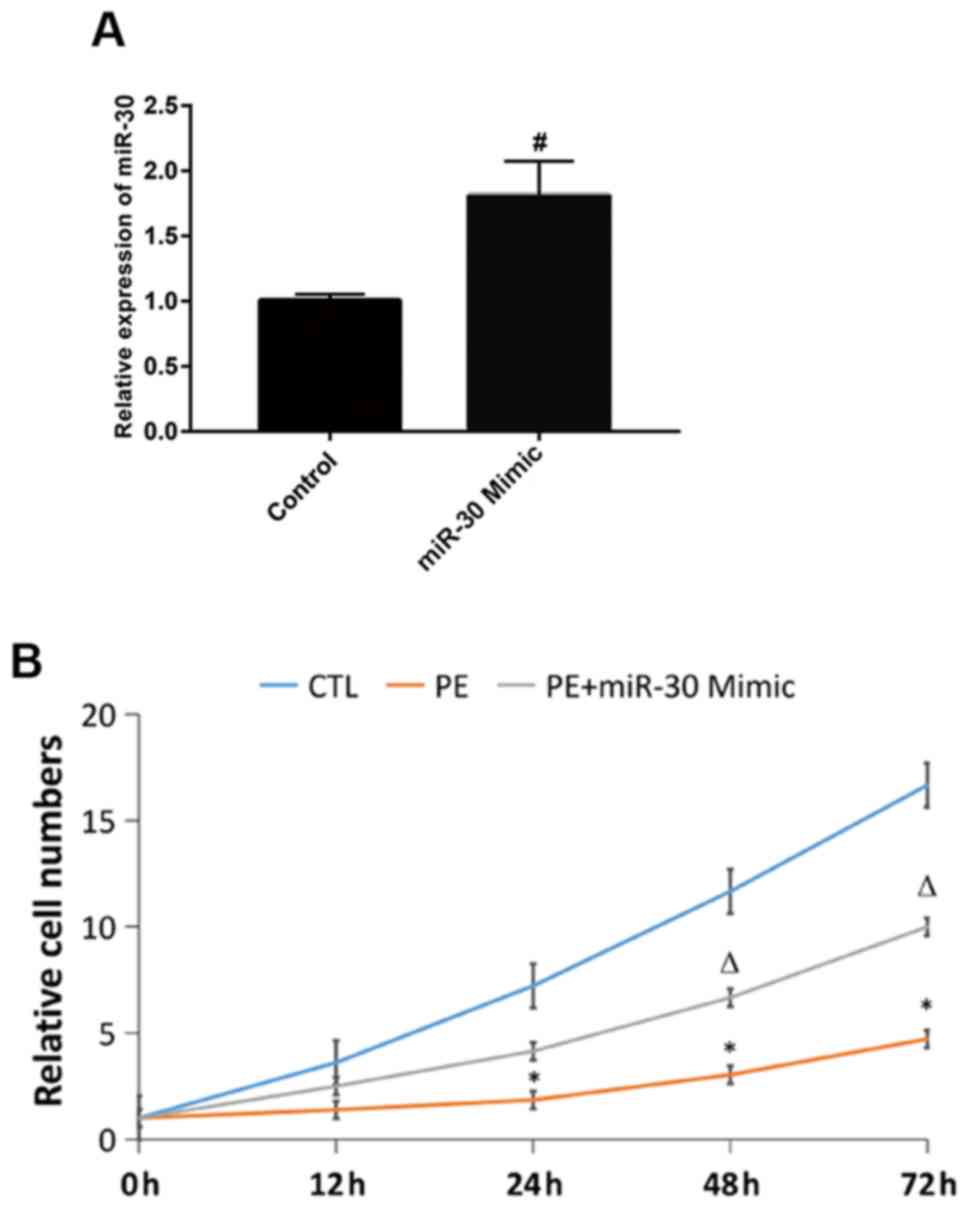
First, MTT assay was performed to detect the cell
proliferation in each group. It was found that the number of
proliferating cells in hypoxia group was significantly smaller than
that in CTL group at 24, 48 and 72 h, while the cell proliferation
ability was stronger in miR-30 Mimic group than that in hypoxia
group at 48 and 72 h (Fig. 1). The
above findings suggest that the proliferation of HTR8/SVNEO cells
significantly declines in hypoxic environment, while miR-30 can
promote the proliferation of HTR8/SVNEO cells and alleviate the
hypoxia-induced inhibition on cell proliferation.
Effect of miR-30 on hypoxia-induced
apoptosis of HTR8/SVNEO cells
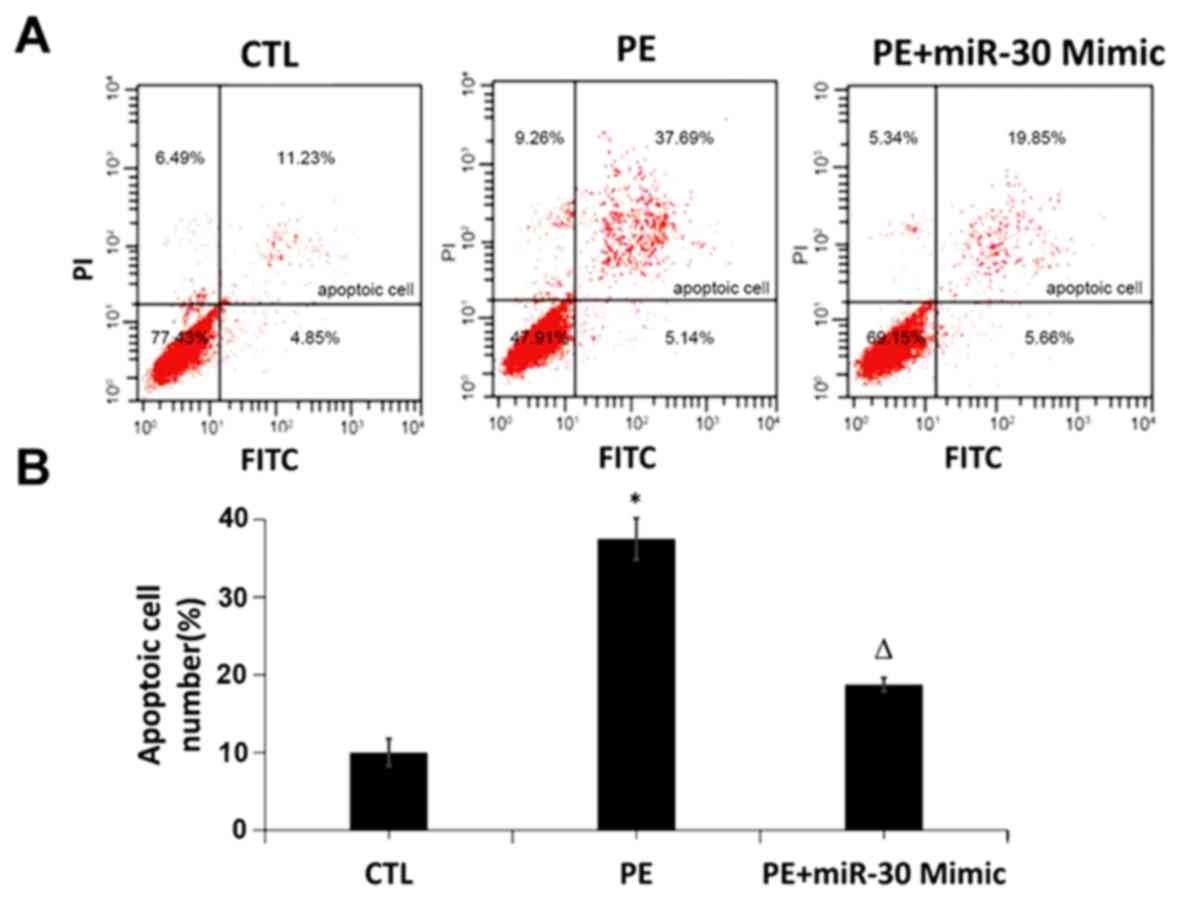
The effect of miR-30 on hypoxia-induced apoptosis of
HTR8/SVNEO cells was detected via Annexin V-FITC and PI
fluorescence labeling using a flow cytometer. The results showed
that the trophoblast apoptosis rate was increased in hypoxia group
compared with that in CTL group, while it was significantly
decreased in miR-30 Mimic group compared with that in hypoxia group
(Fig. 2), indicating that miR-30 can
inhibit hypoxia-induced trophoblast apoptosis.
Effect of miR-30 on expression level
of PCNA in placental tissues
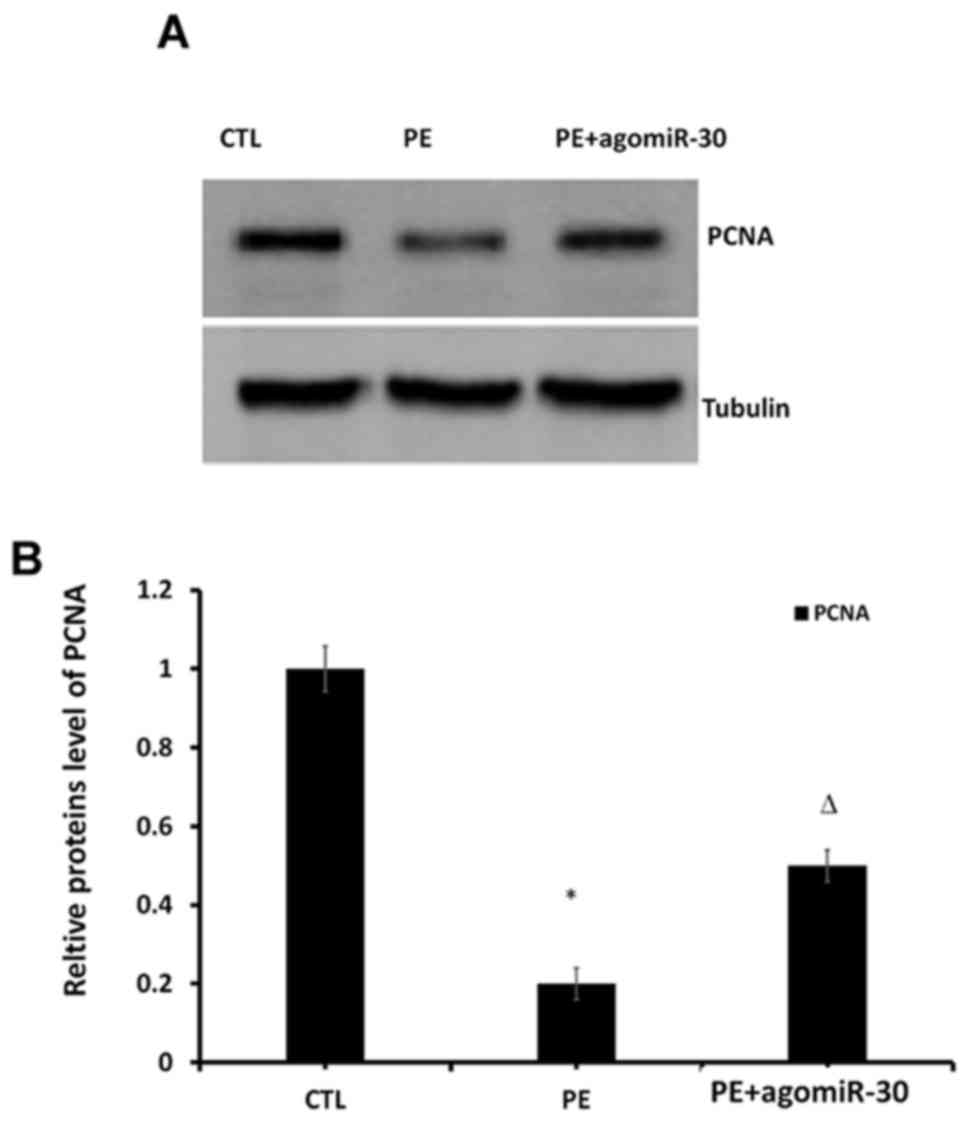
To explore the effect of miR-30 expression on the
proliferation of placental tissues, western blot analysis was
performed to detect the protein expression of PCNA in placental
tissues in the three groups. The results revealed that the PCNA
level evidently declined in PE group compared with that in CTL
group (P<0.01), while it was evidently increased in PE+agomiR-30
group compared with that in PE group (P<0.01) (Fig. 3), suggesting that the cell
proliferation level in placental tissues was significantly
decreased in PE group, and significantly increased in PE+agomiR-30
group.
Effect of miR-30 on PCNA level
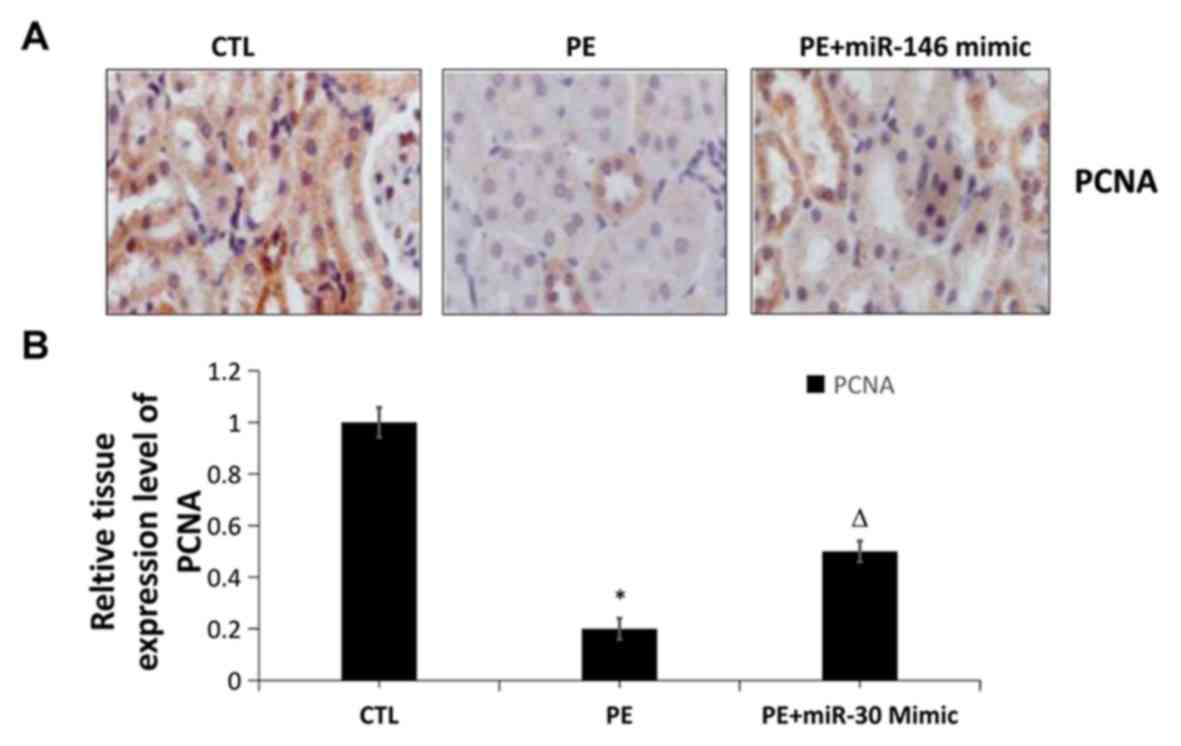
To obtain further evidence, the changes in the
protein expression of PCNA in placental tissues in the three groups
were determined using IHC. The PCNA level evidently declined in PE
group compared with that in CTL group (P<0.01), while it was
evidently increased in PE+agomiR-30 group compared with that in PE
group (P<0.01), consistent with the results of western blot
analysis (Fig. 4). The cell
proliferation level in placental tissues significantly declined in
PE group, and significantly increased in PE+agomiR-30 group.
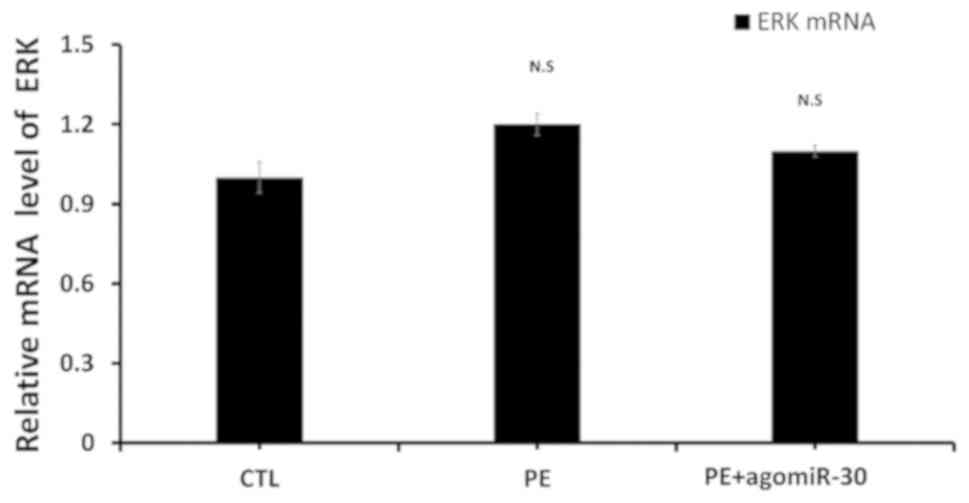
Effect of miR-30 expression on mRNA
level of ERK1/2
To explore the specific mechanism of miR-30 in
promoting the proliferation of placental tissues, RT-qPCR was
performed, and the results showed that there was no significant
difference in the mRNA level of ERK among CTL, PE and PE+agomiR-30
group (Fig. 5).
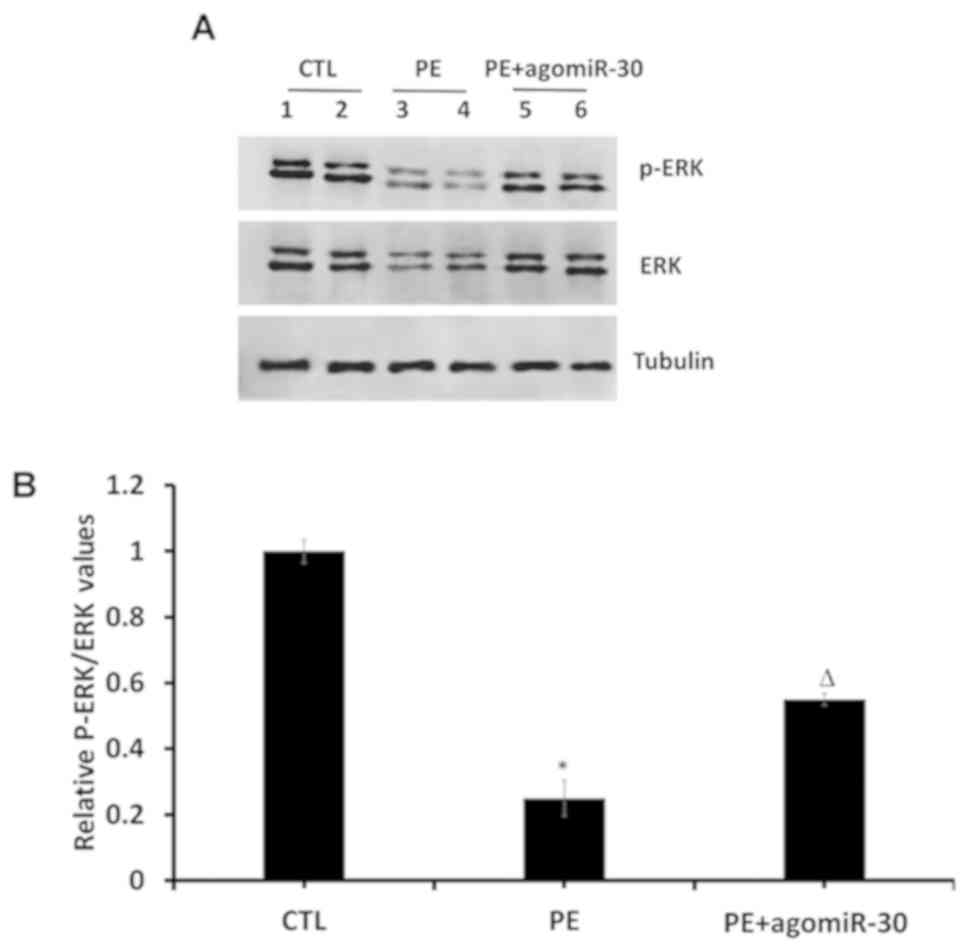
Effect of miR-30 on MAPK/ERK signaling
pathway in placenta tissues
Protein levels of P-ERK1/2, ERK1/2 and tubulin in
placenta tissues were detected using western blot analysis. The
results showed that PE group had extremely decreased p-ERK and
p-ERK/ERK ratio in placental tissues compared with CTL group
(P<0.01), while PE+agomiR-30 group had obviously increased p-ERK
as well as p-ERK/ERK ratio in placental tissues compared with PE
group (P<0.05) (Fig. 6),
demonstrating that the MAPK/ERK signaling pathway is suppressed in
PE rats, and it can be activated by miR-30.
Discussion
miRNAs are a class of endogenous non-coding
single-stranded small-molecule RNAs 19-22 nt in length, which
regulate and inhibit the target gene expression mainly at the
transcriptional level (12). The
maturation of miRNAs involves two processes. First, the pri-RNA
molecules in the nucleus are processed by Drosha protein and DGCR8
protein into pre-miRNA with 70 nt in length. Then under the action
of nuclear export protein Exportin5/Ran GTP, pre-miRNA is
transported from the nucleus to the cytoplasm, and further
processed by Dicer into mature miRNA about 21 nt in length. miRNA
molecules form the RNA-induced silencing complex through the target
gene mRNA, thus inhibiting or degrading the target gene expression
(13).
As a widely-studied miRNA at present, miR-30 plays
an important role in the occurrence and development of
cardiovascular diseases, metabolic diseases and tumors. MiR-30
includes 5 family members: miR-30a, miR-30b, miR-30c, miR-30d and
miR-30e (14). Studies have shown
that miR-30 can inhibit the TGF-β-induced organ fibrosis, which is
associated with epithelial cell apoptosis involving TGF-β.
Overexpression of TGF-β can increase Caspase-3 expression to
facilitate apoptosis, while miR-30 is able to inhibit epithelial
cell apoptosis through suppressing TGF-β expression (15).
MAPK is a kind of serine/threonine protein kinase in
cells (16). Multiple parallel MAPK
signaling pathways are found in lower prokaryotic cells and higher
mammalian cells, and they mediate different cellular biological
responses, in which MAPK/ERK signaling pathway is an important one
participating in and playing a vital role in cell proliferation,
differentiation and apoptosis (17-19).
PE is an idiopathic placenta-derived disease during pregnancy, as
well as a polygenic disease whose pathogenesis has not been fully
clarified yet (20,21). Currently, studies on signaling
pathways in PE mostly focus on the adhesion protein, PI3K and
TGF-Smad pathways. PI3K is related to the differentiation and
invasion of trophoblasts, and MAPK is an intracellular
serine/threonine protein kinase. MAPK signals are activated by
different molecules, thereby inducing such biological reactions as
cell proliferation, differentiation, transformation and apoptosis
(22). Moreover, the MAPK signaling
pathway is suppressed in PE patients, and it can be activated by
miR-30.
In the present study, the effect of miR-30 on the
trophoblast proliferation in PE through the MAPK/ERK signaling
pathway was explored. The effect of expression level of miR-30 on
the proliferation of HTR8/SVNEO cells under hypoxia was detected
via MTT assay. Results showed that the trophoblast proliferation
significantly declined in hypoxic environment, while miR-30
promoted cell proliferation and alleviate hypoxia-induced
inhibition on cell proliferation. It was found through flow
cytometry that the level of hypoxia-induced trophoblast apoptosis
obviously declined in miR-30 Mimic group compared with that in
hypoxia group. The results of the in vivo western blot
analysis and IHC manifested that the protein expression of PCNA in
placental tissues was evidently decreased in PE group, while it was
evidently raised by miR-30. In addition, the cell proliferation
ability in placental tissues was remarkably weakened in PE group,
whereas it was remarkably enhanced in PE+agomiR-30 group. According
to the results of RT-qPCR and western blot analysis, miR-30
phosphorylated ERK1/2 to activate the MAPK/ERK signaling pathway,
which may play an important role in the enhanced proliferation of
placental tissues. However, there are some limitations in this
study. AgomiR-30 control was not used in any of the groups to
exclude non-specific effects, which could impact on the conclusion.
MAPK signals contain EKR, JNK and p38, whether miR-30 affects JNK
and p38 phosphorylation is still unknown.
In conclusion, miR-30 activates the MAPK/ERK
signaling pathway and increases the expression level of PCNA
through raising the p-ERK level and p-ERK/ERK ratio, thereby
inhibiting cell apoptosis and promoting cell proliferation.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW, LJ and HGu designed the study and performed the
experiments. YW and HGo established the animal models. LJ and YuL
collected the data. AX and YaL analyzed the data. YW, LJ and HGu
prepared the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Qinghai Provincial People's Hospital (Qinghai,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Jiang J and Zhao ZM: LncRNA HOXD-AS1
promotes preeclampsia progression via MAPK pathway. Eur Rev Med
Pharmacol Sci. 22:8561–8568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boehme KA and Rolauffs B: Onset and
progression of human osteoarthritis - Can growth factors,
inflammatory cytokines, or differential miRNA expression
concomitantly induce proliferation, ECM degradation, and
inflammation in articular cartilage? Int J Mol Sci.
19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guo J, Zeng X, Miao J, Liu C, Wei F, Liu
D, Zheng Z, Ting K, Wang C and Liu Y: MiRNA-218 regulates
osteoclast differentiation and inflammation response in
periodontitis rats through Mmp9. Cell Microbiol.
21(e12979)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Galardi S, Mercatelli N, Giorda E,
Massalini S, Frajese GV, Ciafrè SA and Farace MG: miR-221 and
miR-222 expression affects the proliferation potential of human
prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem.
282:23716–23724. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang S, Miao D, Wang M, Lv J, Wang Y and
Tong J: MiR-30-5p suppresses cell chemoresistance and stemness in
colorectal cancer through USP22/Wnt/β-catenin signaling axis. J
Cell Mol Med. 23:630–640. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lang Y, Zhao Y, Zheng C, Lu Y, Wu J, Zhu
X, Zhang M, Yang F, Xu X, Shi S, et al: MiR-30 family prevents
uPAR-ITGB3 signaling activation through calcineurin-NFATC pathway
to protect podocytes. Cell Death Dis. 10(401)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yi J, Liu D and Xiao J: LncRNA MALAT1
sponges miR-30 to promote osteoblast differentiation of
adipose-derived mesenchymal stem cells by promotion of Runx2
expression. Cell Tissue Res. 376:113–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu
J, Chen J, Dong L and Zhang J: miR-30 inhibits TGF-β1-induced
epithelial-to-mesenchymal transition in hepatocyte by targeting
Snail1. Biochem Biophys Res Commun. 417:1100–1105. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi S, Yu L, Zhang T, Qi H, Xavier S, Ju W
and Bottinger E: Smad2-dependent downregulation of miR-30 is
required for TGF-β-induced apoptosis in podocytes. PLoS One.
8(e75572)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ijomone OK, Shallie PD and Naicker T:
L-nitro-l-arginine methyl model of pre-eclampsia elicits
differential IBA1 and EAAT1 expressions in brain. J Chem Neuroanat.
100(101660)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lan W, Wang J, Li M, Liu J, Wu FX and Pan
Y: Predicting MicroRNA-disease sssociations based on improved
MicroRNA and disease similarities. IEEE/ACM Trans Comput Biol
Bioinform 2018; 15: 1774-1782. https://doi.org/10.1109/TCBB.2016.2586190.
|
|
13
|
Han J, Lee Y, Yeom KH, Kim YK, Jin H and
Kim VN: The Drosha-DGCR8 complex in primary microRNA processing.
Genes Dev. 18:3016–3027. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mao L, Liu S, Hu L, Jia L, Wang H, Guo M,
Chen C, Liu Y and Xu L: miR-30 Family: A promising regulator in
development and disease. BioMed Res Int.
2018(9623412)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang Q, Sun M, Chen Y, Lu Y, Ye Y, Song H,
Xu X, Shi S and Wang J: Triptolide protects podocytes from
TGF-β-induced injury by preventing miR-30 downregulation. Am J
Transl Res. 9:5150–5159. 2017.PubMed/NCBI
|
|
16
|
You Z, Liu SP, Du J, Wu YH and Zhang SZ:
Advancements in MAPK signaling pathways and MAPK-targeted therapies
for ameloblastoma: A review. J Oral Pathol Med. 48:201–205.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y, Pizzute T and Pei M: A review of
crosstalk between MAPK and Wnt signals and its impact on cartilage
regeneration. Cell Tissue Res. 358:633–649. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hindley A and Kolch W: Extracellular
signal regulated kinase (ERK)/mitogen activated protein kinase
(MAPK)-independent functions of Raf kinases. J Cell Sci.
115:1575–1581. 2002.PubMed/NCBI
|
|
20
|
Ramos JGL, Sass N and Costa SHM:
Preeclampsia. Rev Bras Ginecol Obstet. 39:496–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Saito S and Nakashima A: A review of the
mechanism for poor placentation in early-onset preeclampsia: The
role of autophagy in trophoblast invasion and vascular remodeling.
J Reprod Immunol. 101-102:80–88. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martinez-Lopez N and Singh R: ATGs:
Scaffolds for MAPK/ERK signaling. Autophagy. 10:535–537.
2014.PubMed/NCBI View Article : Google Scholar
|