Introduction
Diabetes mellitus (DM) is recognized as a metabolic
disorder this is primarily caused by dysregulation in the
production, secretion or action of insulin, subsequently resulting
in a global life-threatening disease (1). Patient morbidity is closely associated
with DM-related complications, which can affect a number of vital
organs including the eyes, heart, kidneys and liver (2,3).
Statistically, 50-73% patients with end-stage liver disease are
found to be obese and diabetic, where liver damage is a major
complication of DM (3,4). Multiple mechanisms of DM-induced liver
damage have been identified, in which hyperglycemia is among the
predominant causative factors (4-6).
Due to sensitivity to glucose homeostasis and insulin, the liver is
highly susceptible to hyperglycemia-induced oxidative stress, which
activates the inflammatory cascade further (5).
A number of studies have previously highlighted that
under hyperglycemic conditions, a reduction in the levels of
antioxidants, including superoxide dismutase (SOD) and catalase
(CAT), coupled with increased levels of malondialdehyde (MDA),
causes an increase in reactive oxygen species (ROS), leading to
oxidation-mediated liver damage (4,5).
Oxidative stress has also been linked to the activation of NF-κB
during DM, which may subsequently upregulate the expression of
pro-inflammatory cytokines (7). In
hepatocytes, hyperglycemia-induced NF-κB activation is induced by
mediators released from Kupffer cells, such as interleukin (IL)-6
and tumor necrosis factor (TNF)-α. IL-6 and TNF-α form a positive
feedback loop to further stimulate activated NF-κB, thereby
increasing the infiltration of inflammatory cells to the site of
hepatic injury (8). In addition,
NF-κB forms the central link between hepatic inflammation and
fibrosis through the upregulation of its downstream inflammatory
effectors, namely transforming growth factor (TGF)-β1 and protein
kinase C (PKC) (9). A combination of
these deleterious events aggravates the diabetic liver. In some
cases, DM can also cause excessive adipocyte accumulation in the
liver, resulting in non-alcoholic fatty liver disease (NAFLD)
(5,10). Therefore, novel treatment strategies
that include the development of naturally occurring antioxidants
that resist oxidative stress and those that normalize inflammation,
may be of interest in the management of DM-induced liver
damage.
The potential of Pluchea indica (P.
indica) leaves as a traditional herbal medicine is a subject of
growing interest in the scientific community. Its uses have been
previously documented by various researchers, which include its
antioxidant (11,12), anti-inflammatory (13,14) and
anti-hyperglycemic (15-17)
properties. P. indica exerts its antioxidant properties by
scavenging free radicals involved in peroxidation, preventing the
expression of proteins associated with oxidative stress (11,12).
Recently, a mimic human-like type 1 DM model in BALB/C mice was
established using multiple low doses (MLD) of streptozotocin (STZ),
as previously described (17). This
mouse strain is advantageous for the little to no influence from
its genetic background, since most new cases of type 1 DM are
spasmodic and can develop in families with no previous history of
DM (18). Furthermore, STZ has a
short half-life, remaining biologically active in the serum for
only 15 min following intravenous injection (19) and its acute toxicity to the liver can
be neglected after hyperglycemia is induced (20). Consequently, after STZ is eliminated
out of the body, any further functional impairments in the liver
observed may be attributed to the effects of diabetic hyperglycemia
(19,20). Therefore, the MLD-STZ-induced model
of DM is a suitable model for studying both the pathology of DM and
complications related to the disease, as well as developing
possible interventions for DM.
Using the aforementioned MLD-STZ-induced model of
DM, it was previously found that P. indica leaf ethanol
extract (PILE) was able to reduce blood glucose levels, where the
associated underlying mechanism in the diabetic pancreas, including
suppression of cytokines and apoptotic markers, were elucidated
(17). Notably, enzymes associated
with total cholesterol, triglycerides, aspartate aminotransferase
(AST), alanine aminotransferase (ALT), and alkaline phosphatase
(ALP) were found to be significantly decreased in the serum of
PILE-treated animals, indicating an improvement in liver function
under diabetic conditions (17).
Therefore, the present study aimed to test the hypothesis that PILE
has the potential to ameliorate hepatocellular damage in
STZ-induced diabetic mice further, that its potentially beneficial
effects are associated with the attenuation of important molecular
targets associated with oxidative stress, inflammation and
apoptosis.
Materials and methods
Plant material. P. indica
leaves were verified and maintained at the
Herbarium, Department of Biology, Faculty of Science, Prince of
Songkla University (PSU Herbarium; Hat Yai, Thailand). The voucher
specimen number J.Nopparat-A.Nualla-ong 1 (PSU) was designated to
the plants. For the preparation of the ethanol extract, dried
leaves (10 g) of P. indica were extracted using 95% ethanol
at 37˚C for 3 days. The plant extract was concentrated and dried
under reduced pressure using a rotary vacuum evaporator at 112 mm
Hg and 40˚C and filtered using 0.45-µm filters. PILEs were then
stored at 4˚C until further use. Various desired concentrations of
PILE were prepared by dissolving with 5% (v/v) Tween-80 before use
in the present study. The phytochemical component analysis of PILE
by liquid chromatography-mass spectrometry and gas
chromatography-mass spectrometry was recently published in a
previous study (17).
Induction of diabetes in experimental
animals
The experimental design and induction of diabetes
was previously reported (17).
Therefore, the present study represents further evaluation of our
previous animal study. A total of 80 male BALB/C mice (5-6 weeks
old) were purchased from Nomura Siam International Co., Ltd. The
mice were maintained in the animal facility of PSU in a
well-ventilated humidified room (23˚C±2˚C; humidity, 50%±10%; with
alternating 12-h light/dark cycles). The animals received water
ad libitum and were fed standard chow. The experimental
protocols described in this study were approved and guided by the
Institutional Animal Care and Use Committee of Prince of Songkla
University (MOE 0521.11/124). The mice were randomly assigned into
one of the following four groups (n=10 per group): i) Group I
(control), where the mice were intraperitoneally injected with 0.1
M citrate buffer, pH 4.5 (diluent for STZ; Sigma-Aldrich; Merck
KGaA) and fed once daily with the diluent 5% (v/v) Tween-80; ii)
Group II (STZ), where the mice were intraperitoneally injected with
STZ (50 mg/kg) for 5 consecutive days and fed once daily with
diluent; iii) Group III [PILE (50 mg/kg) + STZ], where the mice
were pretreated with a dietary supplement of 50 mg/kg PILE (PILE
50) for 2 weeks before receiving STZ injection (50 mg/kg); and iv)
Group IV [PILE (100 mg/kg) + STZ], where the mice were pretreated
with a dietary supplement of 100 mg/kg PILE (PILE 100) for 2 weeks
before receiving STZ injection (50 mg/kg).
Following 2 weeks of pretreatment with the
aforementioned diet, diabetes was induced with 50 mg/kg STZ
dissolved in 0.1 M citrate buffer (pH 4.5) for 5 consecutive days
(21). The first injection day was
defined as day 0. To confirm diabetic status, fasting blood glucose
levels were measured in a drop of blood collected from the tail
vein using a blood glucose meter (Accu-Chek Active test strips;
Roche Diagnostics GmbH) 3 days following the final STZ injection.
All STZ-injected animals developed hyperglycemia (blood glucose
levels >200 mg/dl) and were retained for further experimentation
(17,22). Animals in group III and IV continued
to be fed with PILE 50 or PILE 100 by oral gavage once daily for 4
or 8 weeks. At the final 4- and 8-week time points, the mice were
anesthetized with thiopental (70 mg/kg), following which euthanasia
was immediately performed by cervical dislocation. The assessment
criteria for confirmation of animal death included lack of
heartbeat for >5 minutes, lack of movement and visible lack of
breathing. Subsequently, the right and left lateral lobes of the
liver were collected for further study.
Histological examination
Following sacrifice, the livers were immediately
removed and fixed in 10% neutral formalin for 24 h at room
temperature (RT). After fixation, the liver tissues were dehydrated
in ascending grades of ethanol, cleared in xylene and embedded in
paraffin blocks. Embedded liver tissues were cut into 5-µm-thick
sections and stained with hematoxylin and eosin for 1.5 h at RT
according to standard laboratory procedures. Histological
observations were performed under light microscopy (magnifications,
x200 and x400; Olympus D73 equipped with CellSens software v1.16;
Olympus Corporation).
Biochemical analysis of oxidative
stress markers
The expression levels of the antioxidant enzymes SOD
(cat. no. 19160; Sigma-Aldrich; Merck KGaA), CAT (cat. no. 707002;
Cayman Chemical Company) and MDA (a marker of lipid peroxidation;
cat. no. MAK085; Sigma-Aldrich; Merck KGaA) were measured using
corresponding assay kits. Briefly, liver tissues were isolated from
the euthanized mice, rinsed with PBS (pH 7.4), homogenized in lysis
buffer (150 mM Tris; pH 7.2) and centrifuged at 10,000 x g for 15
min at 4˚C. The supernatants were further processed according to
the manufacturer's protocols.
Immunohistochemical analysis
Immunohistochemistry (IHC) was conducted according
to the manufacturer's instructions (Vectastain Elite ABC-HRP kit;
cat. no. PK-6200; Vector Laboratories, Inc.; Maravai Life
Sciences). The following antibodies were purchased: rabbit
polyclonal anti-SOD (1:200; cat. no. ab13498; Abcam), rabbit
monoclonal anti-IL-6 (1:400; cat. no. 12912; Cell Signaling
Technology, Inc.), rabbit polyclonal anti-TNF-α (1:200; cat. no.
ab6671; Abcam) and mouse monoclonal anti-TGF-β1 (1:200; cat. no.
MAB240, R&D Systems, Inc.). Briefly, liver tissue sections were
deparaffinized with xylene and rehydrated in a graded ethanol
series. Antigen retrieval was performed by heating the tissues at
95˚C with citrate buffer (pH 6.0) for 20 min, followed by blocking
endogenous peroxidase activity in 0.3% H2O2
(in methanol) for 30 min at RT. Following three 5 min washes with
PBS with 0.1% Triton X-100 (PBST), normal horse serum (Vector
Laboratories, Inc.; Maravai Life Sciences) was applied for 1 h at
RT. Incubation with primary antibodies against SOD, CAT, IL-6,
TNF-α and TGF-β1 was performed overnight at 4˚C in a humidified
chamber. The tissues were then rinsed with PBST and incubated with
a biotinylated horse anti-mouse/rabbit immunoglobulin G universal
secondary antibody (Vectastain Elite ABC-HRP kit; cat. no. PK-6200,
Vector Laboratories, Inc.; Maravai Life Sciences) for 1 h at RT.
After washing with PBST, the tissues were stained with
diaminobenzidine (DAB), rinsed with distilled water and
counterstained with hematoxylin for 1 min at RT. The sections were
then dehydrated in a series of graded ethanol, cleared in xylene
and mounted for light microscopic examination. To confirm specific
labelling, negative control staining was performed by incubating
the tissue with PBS instead of primary antibody.
Morphometric analysis
Immunopositive staining was represented by brown
staining and images were photographed at x400 magnification. The
percentage of the immunopositive area (% IA) was calculated in the
captured representative fields (a standard area, 460,096
µm2) using ImageJ software version 1.52u (National
Institutes of Health). Digital images (a total of 36 fields per
experimental group: 3 fields per section, 3 sections per mouse with
a 20-µm interval between each section, 4 mice per group) were
randomly selected, followed by deconvolution using the color
deconvolution plug-in, resulting in separate DAB (brown),
hematoxylin (blue) and a complimentary image. On the composite
hematoxylin-DAB images, the photographs were set to 8-bit where the
threshold was then adjusted for DAB detection according to
intensity. The threshold parameters remained consistent for all
images. The % IA for each group was calculated by dividing the sum
of the IA by the sum of the area of each microscopic field
multiplied by 100(23).
Western blot analysis
The antibodies used for western blotting were
obtained from the following sources: Rabbit polyclonal anti-CAT
(1:1,000; cat. no. ab16731; Abcam); rabbit monoclonal anti-NF-κB
p65 (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.);
rabbit polyclonal anti-caspase-3 (1:1,000; cat. no. 9662; Cell
Signaling Technology, Inc.); mouse monoclonal anti-caspase-9
(1:1,000; cat. no. 9508; Cell Signaling Technology, Inc.); rabbit
monoclonal anti-Bcl-2 (1:1,000; cat. no. 3498; Cell Signaling
Technology, Inc.); rabbit monoclonal anti-PKC (1:1,000; cat. no.
ab179521; Abcam) and rabbit polyclonal anti-β-actin (1:3,000; cat.
no. ab8227; Abcam). The liver tissues were lysed and homogenized in
ice-cold RIPA buffer (Sigma-Aldrich; Merck-KGaA) supplemented with
protease inhibitor cocktail (Merck KGaA). Following centrifugation
at 14,000 x g for 30 min at 4˚C, the supernatant was collected and
total protein was quantified using a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). The samples
(~50 µg each) were separated on 12% SDS/PAGE and transferred to
PVDF membranes (Merck KGaA). The membranes were blocked with 5%
skim milk for 1 h at RT and then probed with primary antibodies
against SOD, CAT, TGF-β1, NF-κBp65, PKC, caspase-3, caspase-9,
Bcl-2 and β-actin overnight at 4˚C. Subsequently, the membranes
were incubated at RT for 1 h with appropriate horseradish
peroxidase (HRP)-linked secondary antibodies (1:5,000; goat
anti-rabbit IgG; cat. no. A3687, rabbit anti-mouse IgG; cat. no.
A9917, Sigma-Aldrich; Merck KGaA). Protein signal was visualized
using Luminata Crescendo Western HRP substrate (Merck KGaA)
according to the manufacturer's instructions. The density of each
of the immunoreactive bands was analyzed using the ImageJ software
version 1.52u (National Institutes of Health). The data were
normalized to the density of each β-actin band, calculated as a
fold change compared with the normal control group, and then
analyzed.
Statistical analysis
All results are expressed as the mean ± SEM unless
otherwise stated. Statistical analysis was performed using GraphPad
Prism 7.0 (GraphPad Software, Inc.). The data from multiple groups
were compared using one-way ANOVA followed by Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PILE treatment alleviates liver
hypertrophy and hepato-histological changes in STZ-induced diabetic
mice
The liver is highly susceptible to damage from
STZ-induced diabetes (7). To explore
the STZ-associated diabetic phenotype in the present study, the
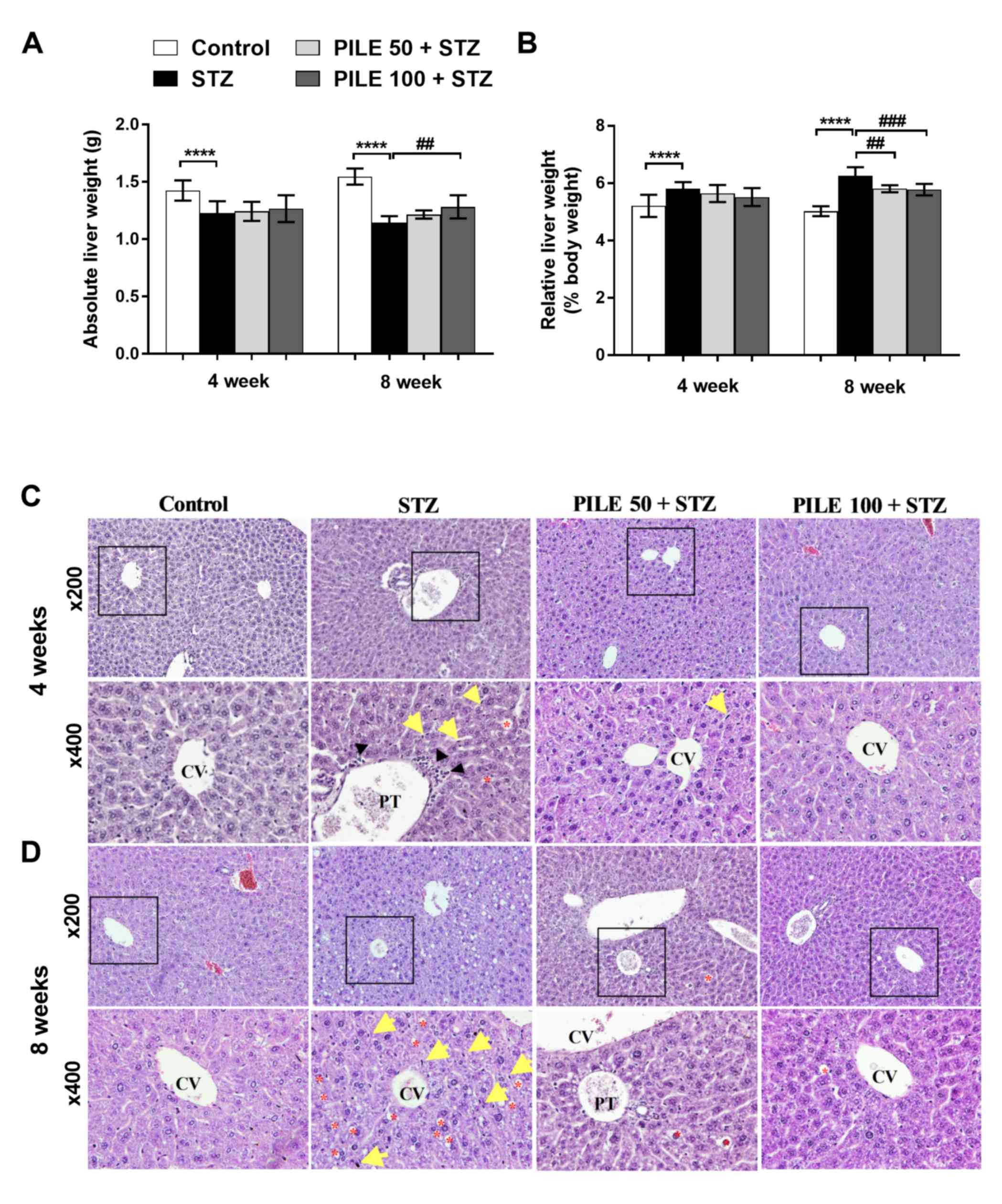
incidence of liver hypertrophy was investigated (Fig. 1A and B). A significant (P<0.0001) decrease in
the absolute liver weight was identified in the STZ mice compared
with the controls at both time points (Fig. 1A). However, a significant (P<0.01)
increase in the absolute liver weight was noted in the mice treated
with PILE 100 for 8 weeks compared with the STZ animals (Fig. 1A). The liver hypertrophy was revealed
when the ratio of liver weight with respect to body weight was
calculated (Fig. 1B). The ratio was
significantly higher (P<0.0001) in STZ groups compared with
control groups at both time points. This phenomenon was ameliorated
following PILE 50 (P<0.01) and PILE 100 (P<0.001) treatments
for 8 weeks (Fig. 1B). These results
suggested that the treatment with PILE has reversed the progressive
changes of liver weight in diabetic mice.
The presence of liver hypertrophy was reflected in
the histological alterations observed in the STZ mice compared with
control animals (Fig. 1C and
D). Liver sections from the control
mice exhibited normal liver sinusoids (not dilated) where the
hepatocyte architecture was arranged into sheets radiating from the
central vein (Fig. 1C and D). By contrast, the livers of the STZ group
exhibited marked hepatocyte degeneration (Fig. 1C and D), particularly at the central areas; the
disappearance of cell borders, disorganized hepatic cords, dilated
sinusoids and the presence of Kupffer cells and monocytes were also
observed (Fig. 1C). Following
progression to the 8-week time point, the hepatocytes of the STZ
mice developed a vacuolated cytoplasm with a ground-glass
appearance and markedly increased accumulation of lipid droplets
(Fig. 1D). The livers of the PILE 50
and PILE 100 groups exhibited less vacuolar degeneration,
preservation of hepatocyte structure, where the presence of Kupffer
cells was less apparent compared with the livers of STZ-only mice
(Fig. 1C and D). These observations provided evidence of
diabetes-associated histopathological alterations, where PILE
treatment exerted positive counteractive effects on STZ-induced
liver injury.
PILE treatment reduces the expression
of markers associated with oxidative stress in the liver
Oxidative stress is a common consequence of
prolonged hyperglycemia, as previously observed in STZ-induced
diabetic animals (7). Therefore, the
present study investigated the effects of PILE treatment on
STZ-induced oxidative damage by measuring levels of antioxidant
enzymes SOD, CAT, in addition to MDA, a surrogate marker for
oxidative stress, in liver homogenates. Biochemical analyses
revealed a significant reduction in the levels of SOD (P<0.0001;
Fig. 2A) and CAT (P<0.01 and
P<0.0001, 4 and 8 weeks, respectively; Fig. 2B), whilst those of MDA were
significantly increased (P<0.05 and P<0.001, 4 and 8 weeks,
respectively; Fig. 2C) in STZ
animals compared with those in control animals. However, there were
significant reversals on the STZ-induced effects on the levels of
SOD, CAT (both P<0.01, 8 weeks) and MDA (P<0.05 and
P<0.001, 4 and 8 weeks, respectively) following treatment with
PILE 100 (Fig. 2A-C). Western blot
analysis revealed a significant increase in the levels of SOD
(P<0.0001) and CAT (P<0.0001) in the livers of the STZ mice,
compared with control mice, at both time points (Fig. 2D-F). Treatment with PILE 100 resulted
in a significant reduction in SOD (P<0.05 and P<0.01, 4 and 8
weeks, respectively; Fig. 2E) and
CAT (P<0.05, both time points; Fig.
2F) compared with STZ mice. PILE 50 exerted a less pronounced
effect on CAT compared with PILE 100, especially at the 4-week time
point (Fig. 2E and F).
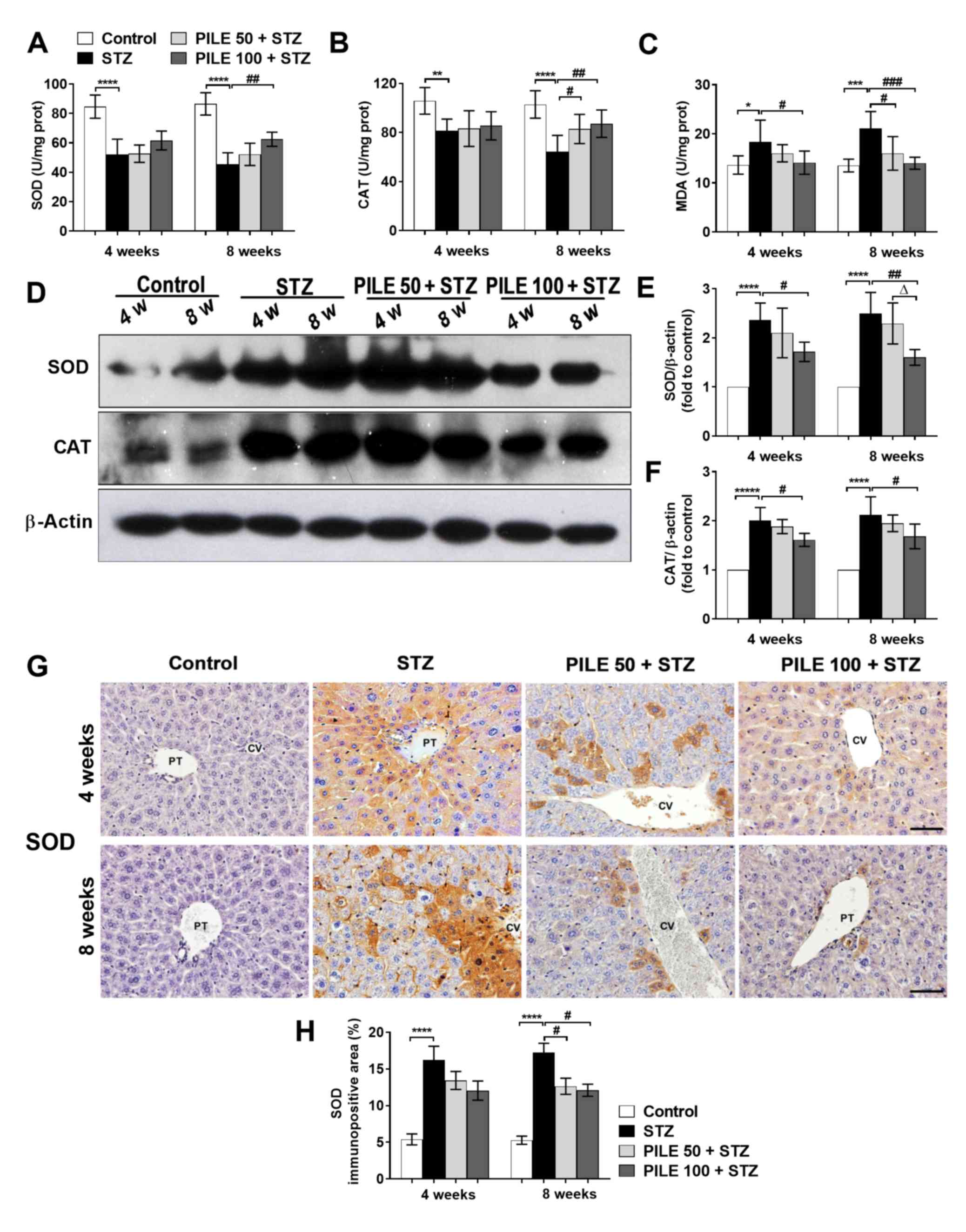 | Figure 2Effects of PILE treatment on the
hepatic oxidative status. Liver tissues from the different
treatment groups were isolated at 4 and 8 weeks, following which
the antioxidant properties of PILE were assessed. Biochemical
analysis of (A) SOD, (B) CAT and (C) MDA levels in liver
homogenates. n=6. (D) Representative western blot images of hepatic
SOD and CAT expression. Densitometric quantification of (E) SOD and
(F) CAT expression normalized to that of β-actin. n=4. (G)
Representative immunohistochemical images of hepatic SOD staining
at the 4- and 8-week time points. (H) Quantification of the
percentage of immunopositive areas of SOD staining. n=4.
Magnification, x400. Data are presented as the mean ± SD for
biochemical analysis and mean ± SEM for immunohistochemistry and
western blot assays. *P<0.05; **P<0.01;
***P<0.001 and ****P<0.0001;
#P<0.05; ##P<0.01 and
###P<0.001; ΔP<0.05. PILE, Pluchea
indica leaf ethanol extract; SOD, superoxide dismutase; CAT,
catalase; MDA, malondialdehyde; PT, portal triad; CV, central vein;
STZ, streptozotocin; PILE 50, mice treated with 50 mg/kg PILE; PILE
100; mice treated with 100 mg/kg PILE; w, weeks; prot, protein. |
Hepatic expression of SOD was subsequently validated
using IHC. The liver tissues of untreated STZ mice exhibited
significantly stronger SOD staining compared with that in control
mice (P<0.0001), which were predominantly distributed in the
hepatocytes around the central and portal triad areas (Fig. 2G). Conversely, at the 8-week time
point, the PILE 50 (P<0.05) and PILE 100 (P <0.05) groups
showed significantly reduced percentages of SOD staining compared
with those in the untreated STZ mice (Fig. 2H). These findings suggested that PILE
exerted protective effects against oxidative stress in STZ-induced
diabetic mice.
PILE treatment modulates the
upregulation of markers associated with inflammation in the
liver
Imbalance between the oxidative stress and
antioxidant defense system is closely associated with the
inflammatory response (24). This is
evident from the release of multiple inflammatory factors,
resulting in local inflammation and tissue injury (4,5,24). The expression of proinflammatory
cytokines IL-6 and TNF-α in the diabetic liver was therefore
investigated by western blotting (Fig.
3A-C) and immunohistochemical staining (Fig. 3D-G). STZ induced a significant
increase in hepatic IL-6 levels at both time points compared with
those in control mice (P<0.0001; Fig.
3A and B). By contrast, levels
of IL-6 were found to be significantly reduced by PILE 50
(P<0.05) at 4-week time point and PILE 100 at the 4-(P<0.01)
and 8-week (P<0.001) time points compared with those in the
untreated STZ mice (Fig. 3A and
B). The immunoreactivity of hepatic
IL-6 was also revealed to be significantly increased (P<0.0001)
in STZ animals compared with control mice at both time points,
which was significantly reversed by both PILE 50 (P<0.05) and
PILE 100 (P<0.01) at the 8-week time point compared with that in
the STZ mice (Fig. 3D and F).
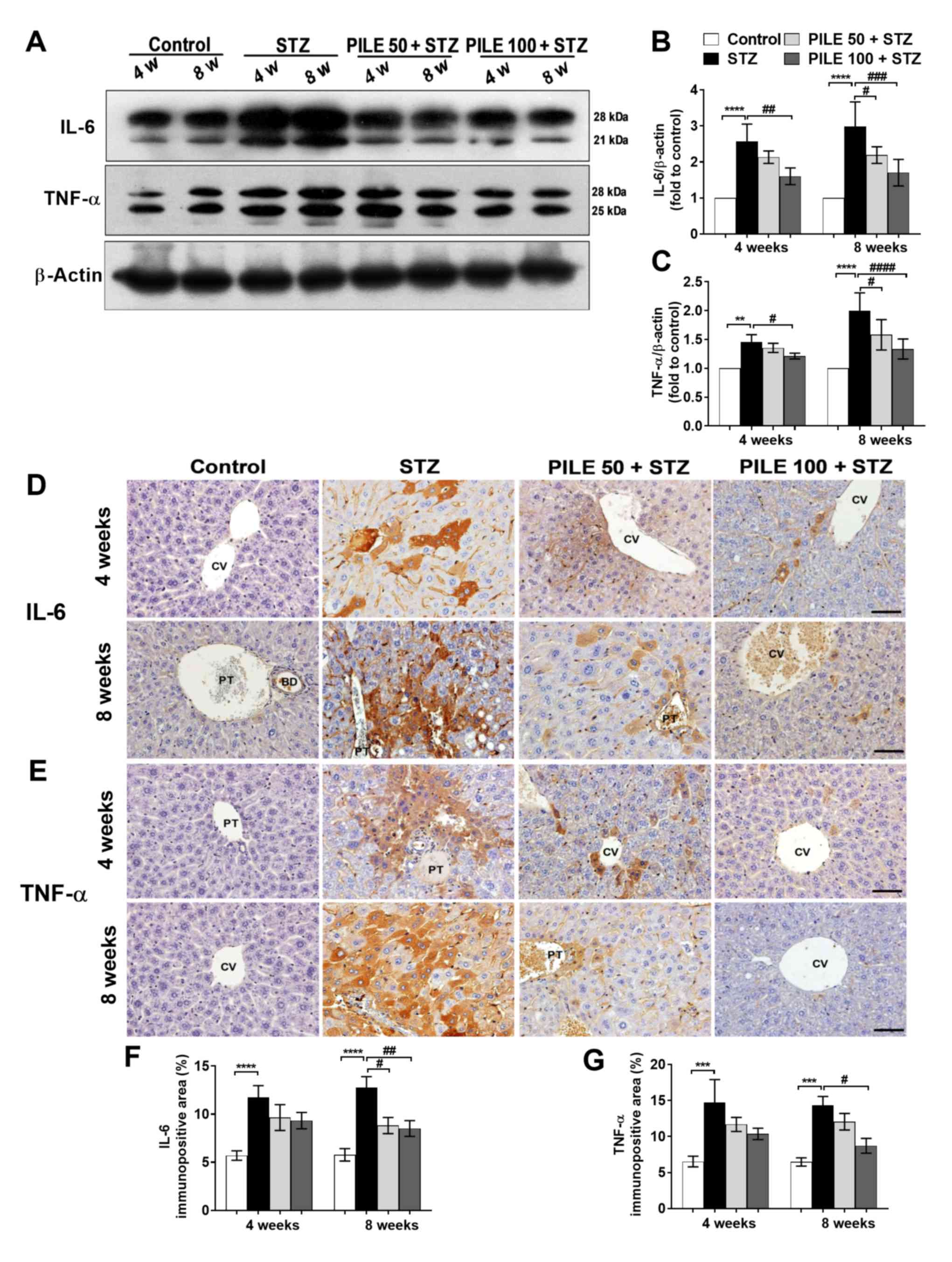 | Figure 3Effects of PILE treatment on hepatic
IL-6 and TNF-α levels in STZ mice. (A) Representative images of
western blot analysis of hepatic IL-6 and TNF-α expression.
Densitometric quantification of (B) IL-6 and (C) TNF-α expression
normalized to β-actin. Representative images of (D) hepatic IL-6
and (E) TNF-α immunoreactivity at the 4- and 8-week time points.
Quantification of the percentage immunopositive areas of (F) IL-6
and (G) TNF-α staining. Magnification, x400. Data represent the
mean ± SEM. n=4. **P<0.01, ***P<0.001
and ****P<0.0001; #P<0.05,
##P<0.01, ###P<0.001 and
####P<0.0001. PILE, Pluchea indica leaf
ethanol extract; IL-6, interleukin 6; TNF-α, tumor necrosis
factor-α; STZ, streptozotocin; PT, portal triad; CV, central vein;
BD, bile duct; PILE 50, mice treated with 50 mg/kg PILE; PILE 100;
mice treated with 100 mg/kg PILE; w, weeks. |
Similarly, levels of hepatic TNF-α were also found
to be significantly increased (P<0.01 and P<0.0001, 4 and 8
weeks, respectively) in the diabetic liver compared with those in
the control mice (Fig. 3A and
C), which were significantly reduced
by PILE 50 (P<0.05, 8 weeks) and PILE 100 (P<0.05 and
P<0.0001, 4 and 8 weeks, respectively). TNF-α immunoreactivity
manifested as a large area of accumulation at the center of the
cells and around the portal vein, particularly at the innermost
cell layer surrounding the vein (STZ group; Fig. 3E), which was not observed in the
control group (Fig. 3E). Although
the reduction in TNF-α staining intensity was not significant in
the PILE 50 or 100 groups at the 4-week time point, a significant
reduction was noted following treatment with PILE 100 at the 8-week
time point compared with that in the STZ group (P<0.05; Fig. 3E and G). These results indicated that PILE
treatment alleviated the hepatic inflammatory response.
PILE treatment attenuates the
STZ-induced upregulation of TGF-β1, NF-κB p65 and PKC expression in
the liver
The phosphorylated p65 subunit of NF-κB (NF-κB p65)
is a sensor of the immune response and serves a pivotal role in
oxidative stress (25). Therefore,
the potential association between STZ-induced oxidative damage and
the upregulation of hepatic NF-κB p65 was investigated. Western
blotting confirmed that hepatic NF-κB p65 protein expression was
significantly increased in untreated STZ mice compared with that in
control mice (P<0.01 and P<0.0001, 4 and 8 weeks,
respectively; Fig. 4A and B). By contrast, treatment with PILE 100
significantly counteracted this phenomenon at both time points
(P<0.05 and P<0.01, 4 and 8 weeks, respectively; Fig. 4A and B). Although PILE 50 also resulted in NF-κB
p65 downregulation at 4 and 8 weeks, the effect was not found to be
statistically significant compared with the STZ group (Fig. 4A and B). In addition, NF-κB p65 activation
involves alterations to its downstream targets, TGF-β1 and PKC
(9,26). In the present study, TGF-β1 and PKC
protein expression were significantly upregulated in the STZ group
compared with those in the control mice at both time points
(P<0.0001; Fig. 4A, C and D),
coinciding with the increase in NF-κB p65 aforementioned. However,
the upregulation of TGF-β1 (P<0.01 and P<0.05, 4 and 8 weeks,
respectively) and PKC (P<0.05 and P<0.0001, 4 and 8 weeks,
respectively) was reversed by PILE 100 (Fig. 4C and D). Furthermore, at the 8-week time point, a
significant reduction in PKC expression was observed in the PILE
100 group compared with that in the PILE 50 group (P<0.0001;
Fig. 4A and D).
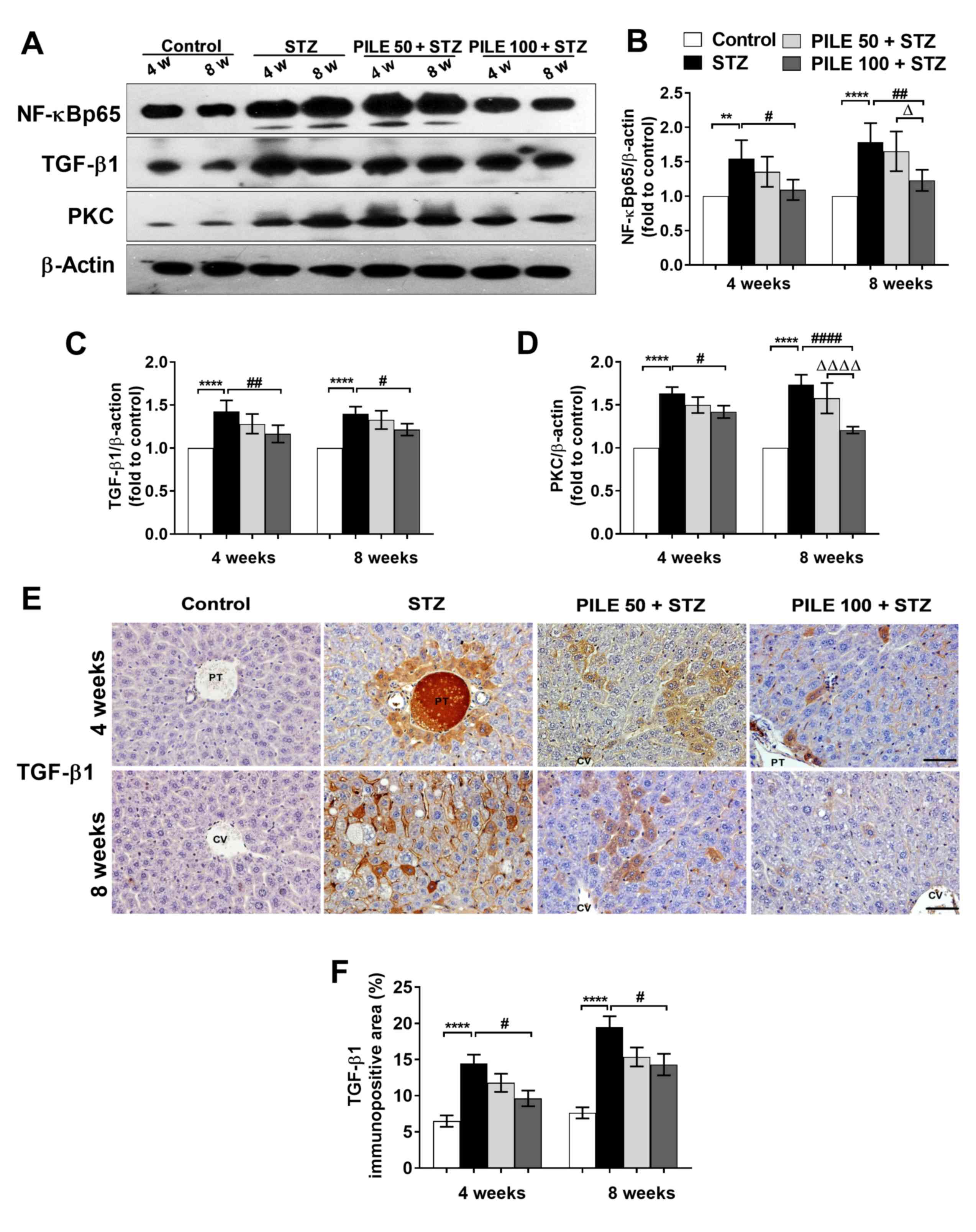 | Figure 4Effects of PILE treatment on hepatic
TGF-β1, NF-κB p65 and PKC expression in STZ mice. (A)
Representative western blot images of hepatic NF-κB p65, TGF-β1 and
PKC expression. Densitometric quantification of (B) NF-κB p65, (C)
TGF-β1 and (D) PKC expression levels normalized to β-actin. (E)
Representative TGF-β1 immunohistochemical staining images of the
liver tissues from different groups at 4- and 8-week time points.
(F) Quantification of the percentage immunopositive areas of TGF-β1
staining among the four experimental groups. Magnification, x400.
Data were presented as the mean ± SEM. n=4. **P<0.01
and ****P<0.0001; #P<0.05,
##P<0.01 and ####P<0.0001;
ΔP<0.05 and ΔΔΔΔP<0.0001. PILE,
Pluchea indica leaf ethanol extract; TGF-β1, transforming
growth factor-β1; PKC, protein kinase C; STZ, streptozotocin; PT,
portal triad; CV, central vein; BD, bile duct; PILE 50, mice
treated with 50 mg/kg PILE; PILE 100; mice treated with 100 mg/kg
PILE; w, weeks. |
According to immunohistochemical staining, TGF-β1
expression was predominantly localized to hepatocytes close to the
central and portal veins (Fig. 4E).
The intensity of TGF-β1 staining significantly increased in the
livers of untreated STZ mice compared with that in control mice
(P<0.0001), which was significantly reversed by PILE 100
treatment (P<0.05; Fig. 4F). In
summary, these observations suggest that PILE treatment attenuated
the inflammatory response in STZ-induced diabetic mice by
downregulating NF-κB p65 and its downstream effectors TGF-β1 and
PKC.
PILE treatment reduces levels of
caspase-associated apoptotic proteins in the liver
Apoptosis is a major pathway that is commonly
involved in liver dysfunction (6).
Therefore, western blot analysis was performed to elucidate the
protective effects of PILE on hepatocyte apoptosis, in addition to
the extent of inflammation-mediated hepatic injury (Fig. 5A-D). Compared with the control group,
mice in the STZ group exhibited significantly increased levels of
cleaved caspase-9 and -3 at both time points (P<0.0001; Fig. 5A-C). PILE 100 significantly
suppressed both cleaved caspase-9 (P<0.001, 8 weeks) and -3
(P<0.01 and P<0.0001, 4 and 8 weeks, respectively). Although,
PILE 50 (P<0.01) and PILE 100 (P<0.001) exerted a moderate
effect on caspase-9 at the 8-week time point (Fig. 5B), theirs effect on caspase-3 was
significantly more pronounced (both P<0.0001 at 8 weeks;
Fig. 5C).
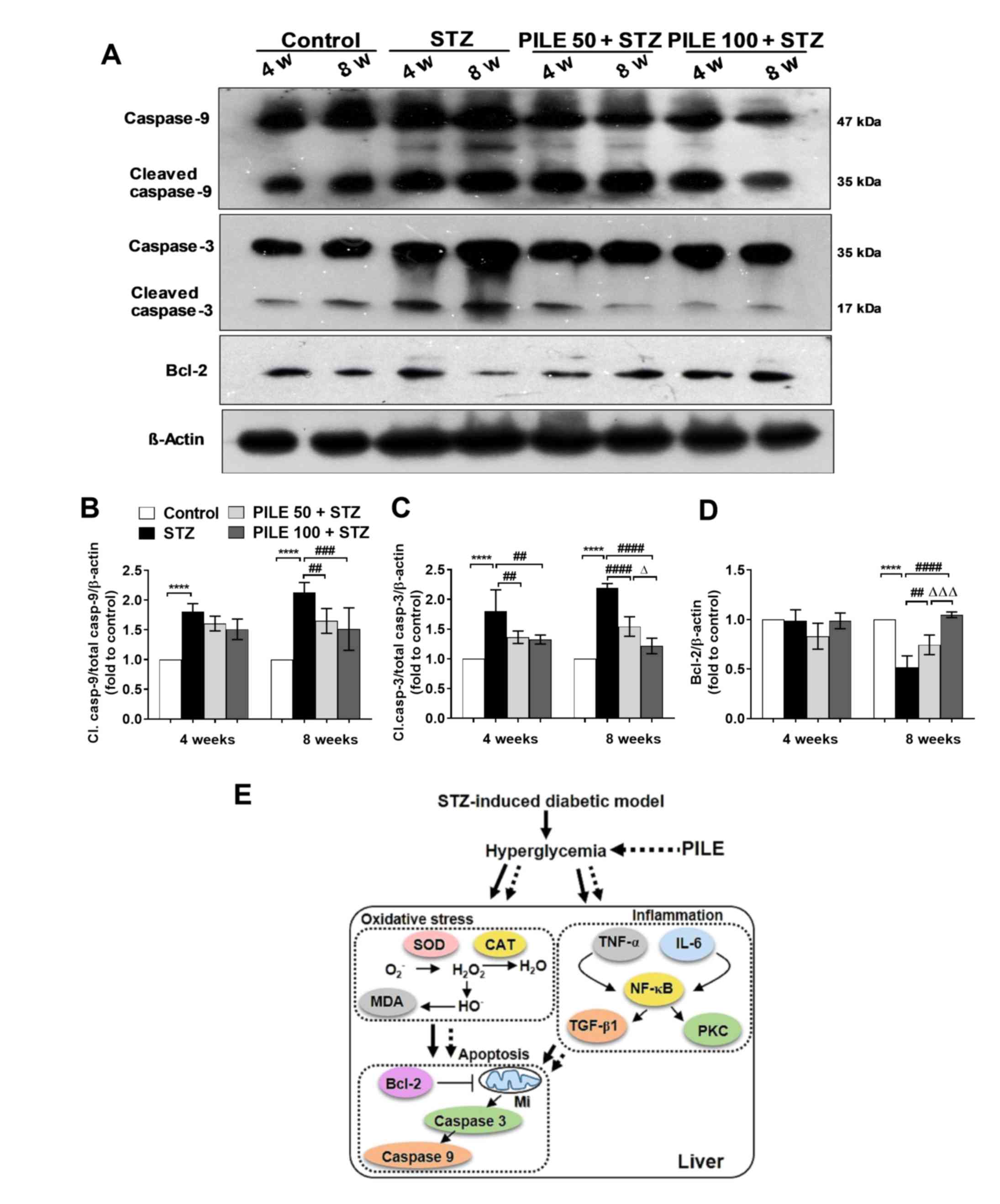 | Figure 5Effects of PILE on apoptosis in the
STZ mouse liver. (A) Representative western blot images of hepatic
protein expression of total and cleaved caspase-9, caspase-3 and
Bcl-2. Densitometric quantification of (B) cleaved and total
caspase-9, (C) cleaved and total caspase-3 and (D) Bcl-2 expression
normalized to β-actin. (E) Summary schematic diagram and proposed
roles of PILE against oxidative stress markers, inflammation
regulators and apoptosis in the hepatic tissues of STZ-induced
diabetic mice. Solid lines indicate increase in action or
synthesis; dashed lines indicate decrease or inhibition of action
or synthesis. Values represent the mean ± SEM. n=4.
****P<0.0001; ##P<0.01,
###P<0.001 and ####P<0.0001;
ΔP<0.05 and ΔΔΔP<0.001. PILE.
Pluchea indica leaf ethanol extract; STZ, streptozotocin;
Mi, mitochondria; Cl, cleaved; PILE 50, mice treated with 50 mg/kg
PILE; PILE 100; mice treated with 100 mg/kg PILE. SOD, superoxide
dismutase; CAT, catalase; MDA, malondialdehyde; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; TGF-β1, transforming growth
factor-β1; PKC, protein kinase C. |
To investigate the protective effects of PILE
further, the expression of hepatic Bcl-2, an anti-apoptotic
molecule, was also investigated (Fig.
5A and D). No significant
changes were observed in the levels of Bcl-2 at the 4-week time
point regardless of treatment. However, a significant reduction in
hepatic Bcl-2 expression was apparent in the STZ group at the
8-week time point compared with that in control mice (P<0.0001;
Fig. 5D). PILE 50 (P<0.01) and
PILE 100 (P<0.0001) significantly reversed the effects of STZ on
Bcl-2 expression to nearly that of control levels (Fig. 5D). Significantly higher levels of
Bcl-2 expression were also observed in the PILE 100 group compared
with those in the PILE 50 mice (P<0.001; Fig. 5D). These results implicate a role of
PILE in protecting against hepatic apoptosis in vivo.
Discussion
The liver is particularly susceptible to the effects
of hyperglycemia-induced oxidative stress (5). The mechanisms promoting hepatocyte
injury in the context of these risk factors are mainly linked to
the balance of oxidative stress and inflammation (5). Previous experimental evidence showed
that PILE can alleviate type 1 DM-related pathologies via a number
of mechanisms, including the promotion of antioxidant (11), anti-inflammatory (13,14) and
anti-hyperglycemic (15-17)
effects. Considering the phytochemical components of PILE, it was
previously reported, consistent with other studies, that it is a
rich source of phenolic acids and flavonoids, particularly
quercetin and resveratrol, known for their antioxidant and
anti-inflammatory properties (11,17,27-29).
Mukhopadhyay and Prajapati (29)
previously documented that quercetin has higher antioxidant
activity compared with other well-known antioxidant molecules such
as ascorbly and trolox, due to the number and positions of the free
hydroxyl groups in its structure. In addition, our earlier findings
indicated that PILE possesses anti-hyperglycemic activity (17). PILE mainly exerts this activity by
four mechanisms: i) Acting as free radical scavengers in
peroxidation and preventing the expression of oxidative
stress-related proteins (12,18,30);
ii) improving liver carbohydrate metabolism (31); iii) improving glucose uptake through
mediators of the insulin signaling pathway (23); and iv) possessing α-glucosidase
inhibitory effects, which can delay the breakdown of starch into
glucose, increasing glucose uptake from the circulation, lowering
blood glucose levels (32). Since a
previous study reported that treatment with PILE preserved liver
function by restoring AST, ALT and ALP levels in the mouse sera
(17), the role of PILE treatment on
hyperglycemia-induced liver damage was investigated in the present
study. Therefore, the present study aimed to determine the dose-
and time-dependent effects of PILE treatment to investigate its
protective effects against hepatic damage in STZ-induced diabetic
mice.
Firstly, hepatic abnormalities were demonstrated by
measuring the relative liver weights of STZ animals compared with
those of the normal control group, where STZ livers showed signs of
hypertrophy. Histological examination revealed that liver sections
obtained from untreated STZ mice exhibited severe deleterious
changes to the hepatic architecture, including degeneration of
hepatocytes, disorganization of hepatic cords, dilated sinusoids,
the appearance of Kupffer cells and monocyte infiltration.
Cytoplasm vacuolization was also observed in the STZ mice at the
8-week point, in addition to lipid droplets in the cytoplasm of
hepatocytes (classified as NAFLD) (4,10). Since
the present mouse model is not considered as an obesity model, this
phenomenon may be due to hypoinsulinemia increasing the influx of
fatty acids into the liver, accompanied by the low lipoprotein
excretion capacity of the liver (33). Hyperlipidemia may have also
influenced fatty liver formation (34). However, fewer pathological changes
and improved liver architecture were observed in PILE-pretreated
diabetic mice, with reduced liver fatty deposits and Kupffer cell
infiltration, indicating the protective effects of PILE treatment
against the hepatic damage associated with diabetes.
Hyperglycemia is a primary cause of increased ROS
generation, leading to oxidative stress (4,7). In the
present study, the occurrence of oxidative stress was assessed by
the detection of key antioxidant enzymes in the liver tissue.
Decreases in SOD and CAT expression, in addition to increases in
those of MDA, were observed in the liver tissues of STZ-induced
mice. Following treatment with PILE 100, the effects of STZ on the
levels of these oxidative markers were found to be significantly
reversed. Similar to the present results, Yang and Kang (35) previously found that quercetin and
resveratrol treatment restored the levels of hepatic glucose
metabolic enzymes, improved the antioxidant capacities and serum
lipid profiles of STZ-induced diabetic rats. Co-treatment with
quercetin and resveratrol exerted more pronounced effects against
all deleterious symptoms of diabetic conditions in rats (35). Therefore, findings of the present
study are consistent with previous observations that PILE possesses
strong antioxidant activity, which may contribute to its
prophylactic effect against organ dysfunction caused by prolonged
chronic hyperglycemia.
Accumulating evidence suggested that the
pathogenesis and progression of diabetic liver damage is due to the
feedback mechanism between oxidative stress and inflammation
(7,24,36).
Immune cells, including T cells, natural killer T cells and
macrophages, are the first line of defense in the innate immune
response, that impact the initial stages of diabetes-induced liver
damage (36,37). In particular, Kupffer cells, which
are liver-resident macrophages, are critical in the regulation of
the progression and resolution of tissue injury (37). Once oxidative stress occurs, Kupffer
cells become activated, leading to tissue injury by stimulating the
release of inflammatory cytokines, including IL-6 and TNF-α
(8). The role of IL-6 in metabolic
regulation is rather complex. Previous observations suggested that
IL-6-deficient mice (IL-6−/−)
developed mature-onset diabetes with liver inflammation and
hepatosteatosis (38), indicating a
protective role for IL-6 on hepatocytes. However, under
inflammatory conditions, IL-6 can be excessively excreted by
Kupffer cells, which can be regarded as a biomarker of acute liver
damage (39). Under the experimental
conditions considered in the present study, the expression of IL-6
and TNF-α were found to be significantly increased in the livers of
untreated STZ mice, whilst PILE 100 significantly attenuated the
levels of pro-inflammatory cytokines, which was reflected by a
reduction in liver inflammation. In the liver, IL-6 is not only
important for infection defense but also crucial for hepatocyte
homeostasis and regeneration (37,40).
Previous studies show that following hepatectomy or liver damage,
gut-derived factors such as lipopolysaccharides activate the
Kupffer cells, resulting in TNF-α-dependent secretion of IL-6,
consequently promoting liver regeneration (37,40).
Although liver regeneration was not investigated in the present
study, PILE-induced amelioration of IL-6 and TNF-α in STZ mice
could potentially provide favorable systemic conditions for
hepatocyte recovery from diabetes-associated cellular injury,
thereby restoring normal function.
The transcription factor NF-κB p65 is activated by
oxidative stress and the upregulation of IL-6 and TNF-α (25). Activated NFκB p65 then activates
downstream targets such as TGFβ1 and PKC, which further exacerbate
liver toxicity. TGF-β1 has long been regarded as a profibrogenic,
anti-inflammatory and immunosuppressive mediator (9,26,37). In
the present study, TGFβ1 and PKC levels were significantly
increased following STZ treatment under hyperglycemic conditions.
By contrast, strong inhibition of TGFβ1 and PKC was noted in PILE
100-treated mice compared with untreated STZ mice, which was more
pronounced when treatment was extended to 8 weeks. These findings
suggested that PILE attenuates the inflammatory response by
inhibiting the NFκB p65/TGFβ1/PKC pathway. In addition, TNF-α was
previously shown to be involved in apoptotic regulation through a
classical cascade pathway, resulting in hepatocellular death
(6). In the present study, the
administration of PILE downregulated the expression of cleaved
caspase-9 and caspase-3, whilst significantly upregulating Bcl-2.
These results suggested that PILE is able to counteract cell death
in the diabetic liver. Nevertheless, further studies are required
to clarify the detailed mechanism of PILE treatment and its
inhibitory effects on oxidant and inflammatory markers.
To the best of our knowledge, the results of the
present study demonstrated for the first time that PILE attenuated
diabetic liver damage by modulating oxidative stress and
inflammatory responses via the inhibition of TNF-α, IL-6, NF-κB
p65, TGF-β1 and PKC in STZ mice (Fig.
5E). This may lead to a reduction in apoptosis and
simultaneously improve cellular survival in the liver, thereby
preserving hepatocyte architecture. These findings validated the
ethanomedicinal applications of PILE for the management of diabetic
liver damage.
Acknowledgements
The authors also would like to thank Associate
Professor Dr Kitichate Sridith (Department of Biology, Faculty of
Science, Prince of Songkla University, Hat Yai, Thailand) for
kindly authenticating the plant material for this study.
Funding
This work was supported by research grants from
Prince of Songkla University (grant nos. SCI601252S and SCI600505N)
and the Thailand Research Fund (grant no. MRG6080028).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JN curated, performed the staining and western blot
analysis, analyzed the data, acquired the funding and drafted the
manuscript. AN and AP conceptualized the study and methodology,
prepared and analyzed the plant materials, acquired the funding and
reviewed and edited the manuscript. All authors read and approved
the final version of this manuscript.
Ethics approval and consent to
participate
The experimental protocols described in the present
study were approved and guided by the Institutional Animal Care and
Use Committee of Prince of Songkla University (Hat Yai, Thailand;
approval no. MOE 0521.11/124).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Danaei G, Finucane MM, Lin JK, Singh GM,
Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM,
et al: National, regional, and global trends in systolic blood
pressure since 1980: Systematic analysis of health examination
surveys and epidemiological studies with 786 country-years and 5·4
million participants. Lancet. 377:568–577. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee Yh, Cho Y, Lee BW, Park CY, Lee DH,
Cha BS and Rhee EJ: Nonalcoholic fatty liver disease in diabetes.
Part I: Epidemiology and diagnosis. Diabetes Metab J. 43:31–45.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adams LA, Harmsen S, St Sauver JL,
Charatcharoenwitthaya P, Enders FB, Therneau T and Angulo P:
Nonalcoholic fatty liver disease increases risk of death among
patients with diabetes: A community-based cohort study. Am J
Gastroenterol. 105:1567–1573. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hazlehurst JM, Woods C, Marjot T, Cobbold
JF and Tomlinson JW: Non-alcoholic fatty liver disease and
diabetes. Metabolism. 65:1096–1108. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garcia-Compean D, Jacquez-Quintana JO,
Gonzalez-Gonzalez JA and Maldonado-Garza H: Liver cirrhosis and
diabetes: Risk factors, pathophysiology, clinical implications and
management. World J of Gastroenterol. 15:280–288. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ingaramo PI, Ronco MT, Francés DE, Monti
JA, Pisani GB, Ceballos MP, Galleano M, Carrillo MC and Carnovale
CE: Tumor necrosis factor alpha pathways develops liver apoptosis
in type 1 diabetes mellitus. Mol Immunol. 48:1397–1407.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pan L, Weng H, Li H, Liu Z, Xu Y, Zhou C,
Lu X, Su X, Zhang Y and Chen D: Therapeutic effects of bupleurum
polysaccharides in streptozotocin induced diabetic mice. PLoS One.
10(e0133212)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liaskou E, Zimmermann HW, Li KK, Oo YH,
Suresh S, Stamataki Z, Qureshi O, Lalor PF, Shaw J, Syn WK, et al:
Monocyte subsets in human liver disease show distinct phenotypic
and functional characteristics. Hepatology. 57:385–398.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bilal HM, Riaz F, Munir K, Saqib A and
Sarwar MR: Histological changes in the liver of diabetic rats: A
review of pathogenesis of nonalcoholic fatty liver disease in type
1 diabetes mellitus. Cogent Med. 3(1)2016.
|
|
11
|
Vongsak B, Kongkiatpaiboon S, Jaisamut S
and Konsap K: Comparison of active constituents, antioxidant
capacity, and α-glucosidase inhibition in Pluchea indica
leaf extracts at different maturity stages. Food Biosci. 25:68–73.
2018.
|
|
12
|
Noridayu AR, Hii YF, Faridah A, Khozirah S
and Lajis N: Antioxidant and antiacetylcholinesterase activities of
Pluchea indica Less. Int Food Res J. 18:925–929. 2011.
|
|
13
|
Buapool D, Mongkol N, Chantimal J,
Roytrakul S, Srisook E and Srisook K: Molecular mechanism of
anti-inflammatory activity of Pluchea indica leaves in
macrophages RAW 264.7 and its action in animal models
ofinflammation. J Ethnopharmacol. 146:495–504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roslida AH, Erazuliana AK and Zuraini A:
Anti-inflammatory and antinociceptive activities of the ethanolic
extract of Pluchea indica (L) less leaf. Pharmacologyonline.
2:349–360. 2008.
|
|
15
|
Widyawati PS, Budianta TDW, Gunawan DI and
Wongso RS: Evaluation antidiabetic activity of various leaf
extracts of Pluchea indica less. Int J Pharmacogn Phytochem
Res. 7:597–603. 2015.
|
|
16
|
Pramanik KC, Bhattacharya P, Biswas R,
Bandyopadhyay D, Mishra M and Chatterjee TK: Hypoglycemic and
antihyperglycemic activity of leaf extract of Pluchea indica
Less. Orient Pharm Exp Med. 6:232–236. 2006.
|
|
17
|
Nopparat J, Nualla-Ong A and Phongdara A:
Ethanolic extracts of Pluchea indica (L.) leaf pretreatment
attenuates cytokine-induced β-cell apoptosis in multiple low-dose
streptozotocin-induced diabetic mice. PLoS One.
14(e0212133)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Landin-Olsson M: Latent autoimmune
diabetes in adults. Ann NY Acad Sci. 958:112–116. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eleazu CO, Eleazu KC, Chukwuma S and
Essien UN: Review of the mechanism of cell death resulting from
streptozotocin challenge in experimental animals, its practical use
and potential risk to humans. J Diabetes Metab Disord.
12(60)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tesch GH and Allen TJ: Rodent models of
streptozotocin-induced diabetic nephropathy. Nephrology.
12:261–266. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Furman BL: Streptozotocin-induced diabetic
models in mice and tats. Curr Protoc Pharmacol. 70:1–20. 2015.
|
|
22
|
Yu W, Zha W, Guo S, Cheng H, Wu J and Liu
C: Flos puerariae extract prevents myocardial apoptosis via
attenuation oxidative stress in streptozotocin-induced diabetic
mice. PLoS One. 9(e98044)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gallol LE and Mohamed FH:
Immunomorphometric variations of sustentacular cells of the male
viscacha adrenal medulla during the annual reproductive cycle.
Effects of androgens and melatonin. Acta Histochem. 120:363–372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Palsamy P, Sivakumar S and Subramanian S:
Resveratrol attenuates hyperglycemia-mediated oxidative stress,
proinflammatory cytokines and protects hepatocytes ultrastructure
in streptozotocin-nicotinamide-induced experimental diabetic rats.
Chem Biol Interact. 186:200–210. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Manna P, Das J, Ghosh J and Sil PC:
Contribution of type 1 diabetes to rat liver dysfunction and
cellular damage via activation of NOS, PARP, IkappaB
alpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways:
Prophylactic role of arjunolic acid. Free Radic Biol Med.
48:1465–1484. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dongiovanni P, Anstee QM and Valenti L:
Genetic predisposition in NAFLD and NASH: impact on severity of
liver disease and response to treatment. Curr Pharm Des.
19:5219–5238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirichaiwetchakoon K, Lowe GM, Thumanu K
and Eumkeb G: The Effect of Pluchea indica (L.) Less. Tea on
Adipogenesis in 3T3-L1 Adipocytes and Lipase Activity. Evid Based
Complement Alternat Med. 2018(4108787)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ruan J, Li Z, Yan J, Huang P, Yu H, Han L,
Zhang Y and Wang T: Bioactive constituents from the aerial parts of
Pluchea indica less. Molecules. 23(pii:
E2104)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mukhopadhyay P and Prajapati AK: Quercetin
in anti-diabetic research and strategies for improved quercetin
bioavailability using polymer-based carriers-a review. RSC
Advances. 5:97547–97562. 2015.
|
|
30
|
Jeong SM, Kang MJ, Choi HN, Kim JH and Kim
JI: Quercetin ameliorates hyperglycemia and dyslipidemia and
improves antioxidant status in type 2 diabetic db/db mice. Nutr Res
Pract. 6:201–207. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yazgan UC, Tasdemir E, Bilgin HM, Obay BD,
Sermet A and Elbey B: Comparison of the anti-diabetic effects of
resveratrol, gliclazide and losartan in streptozotocin-induced
experimental diabetes. Arch Physiol Biochem. 121:157–161.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arsiningtyas IS, Gunawan-Puteri MD, Kato E
and Kawabata J: Identification of α-glucosidase inhibitors from the
leaves of Pluchea indica (L.) Less., a traditional
Indonesian herb: Promotion of natural product use. Nat Prod Res.
28:1350–1353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ohno T, Horio F, Tanaka S, Terada M,
Namikawa T and Kitoh J: Fatty liver and hyperlipidemia in IDDM
(insulin-dependent diabetes mellitus) of streptozotocin-treated
shrews. Life Sci. 66:125–131. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chatrath H, Vuppalanchi R and Chalasani N:
Dyslipidemia in patients with nonalcoholic fatty liver disease.
Semin Liver Dis. 32:22–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang DK and Kang HS: Anti-diabetic effect
of cotreatment with quercetin and resveratrol in
streptozotocin-induced diabetic rats. Biomol Ther. 26:130–138.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Palsamy P and Subramanian S: Ameliorative
potential of resveratrol on proinflammatory cytokines,
hyperglycemia mediated oxidative stress, and pancreatic beta-cell
dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J
Cell Physiol. 224:423–432. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhan YT and An W: Roles of liver innate
immune cells in nonalcoholic fatty liver disease. World J
Gastroenterol. 16:4652–4660. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wallenius V, Wallenius K, Ahrén B, Rudling
M, Carlsten H, Dickson SL, Ohlsson C and Jansson JO:
Interleukin-6-deficient mice develop mature-onset obesity. Nat Med.
8:75–79. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matthews VB, Allen TL, Risis S, Chan MHS,
Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, et
al: Interleukin-6-deficient mice develop hepatic inflammation and
systemic insulin resistance. Diabetologia. 53:2431–2441.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kolios G, Valatas V and Kouroumalis E:
Role of Kupffer cells in the pathogenesis of liver disease. World J
Gastroenterol. 12:7413–7420. 2006.PubMed/NCBI View Article : Google Scholar
|