Introduction
Corticosteroids can effectively control intraocular
inflammation in patients with uveitis. Intraocular inflammation in
uveitis causes vitreous opacity, retinal exudates, retinal
haemorrhages, serous detachment, retinal neovascularization and
cystoid macular edema (1,2). Corticosteroids have been used since
1950 for the treatment of ocular inflammatory diseases (3). They have anti-inflammatory,
antiangiogenic, and anti-permeability properties that make them a
viable therapeutic option for a range of posterior segment
diseases. The reduction of exudation, the stabilization of the
blood-retinal barrier and the downregulation of inflammatory
stimuli are among the main effects of steroids, even though the
specific mechanisms remain unexplained. Steroids act by induction
of proteins called lipocortins, in particular phospholipase A2. The
proteins have multiple roles, such as the reduction of leukocyte
chemotaxis, the control of biosynthesis, and the inhibition of
arachidonic acid release from the phospholipid membrane. The
arachidonic acid represents the most important precursor of potent
inflammatory cell mediators, such as prostaglandins and
leukotrienes. This regulation influences the expression of vascular
endothelial growth factors (VEGF), inhibits pro-inflammatory genes,
such as tumor necrosis factor-alpha (TNF-α) and other inflammatory
chemokines, and induces the expression of anti-inflammatory
factors, such as pigment-derived growth factor (PEDF) (4,5).
Moreover, steroids seem to reduce the expression of matrix
metalloproteinases (MMPs) and to downregulate intercellular
adhesion molecule 1 (ICAM-1) on choroidal endothelial cells
(6).
Several routes of administration have been
considered for the treatment of various ocular diseases. Direct
injection through the pars plana leads the steroids to the vitreous
cavity. Many authors have suggested and reported that local
intravitreal delivery of steroids inhibits proliferation of cells,
intraocular inflammation and neovascularization (7). By using the intravitreal delivery
method, the adverse systemic side effects of steroids are avoided.
Intravitreal steroid path bypasses the blood-retinal barrier,
leading to a more concentrated dose of steroids for a longer period
of time. Considering the autoimmune nature of uveitis, patients
should be tested for associated diseases before administering
intraocular steroids (8-12).
In order to exclude an infectious cause of uveitis, general
examination and blood tests should be performed (13-18).
Triamcinolone acetonide (TA) belongs to the
glucocorticoid family. It is a synthetic steroid with a fluorine in
the ninth position and it is the most used steroid agent for the
treatment of several retinal conditions (19). TA has an anti-inflammatory potency
five times higher than hydrocortisone, with a tenth of the
sodium-retaining potency. Its presentation form is a white colored
crystalline powder insoluble in water, which explains its prolonged
duration of action (20). Its
therapeutic effects last approximately three months after 4 mg
intravitreal TA injection (21). A
longer anti-inflammatory effect can be obtained with slow-release
intravitreal dexamethasone implants, but it is also associated with
a higher complication rate (cataract formation, elevated
intraocular pressure, retinal detachment) (22). In some cases, the intraocular
pressure can become refractory to treatment and very difficult to
manage (23,24).
Case reports
The authors report three cases where TA induced
presumed sterile endophthalmitis in three eyes with intermediate
uveitis. All three patients presented decreased visual acuity,
blurry vision and floaters in the right eye. A baseline clinical
examination was performed, including best-corrected visual acuity
(BCVA), biomicroscopy of the anterior pole, intraocular pressure
(IOP) and ocular ultrasound. Fundus examination and optical
coherence tomography could not be performed because of the vitreous
haze.
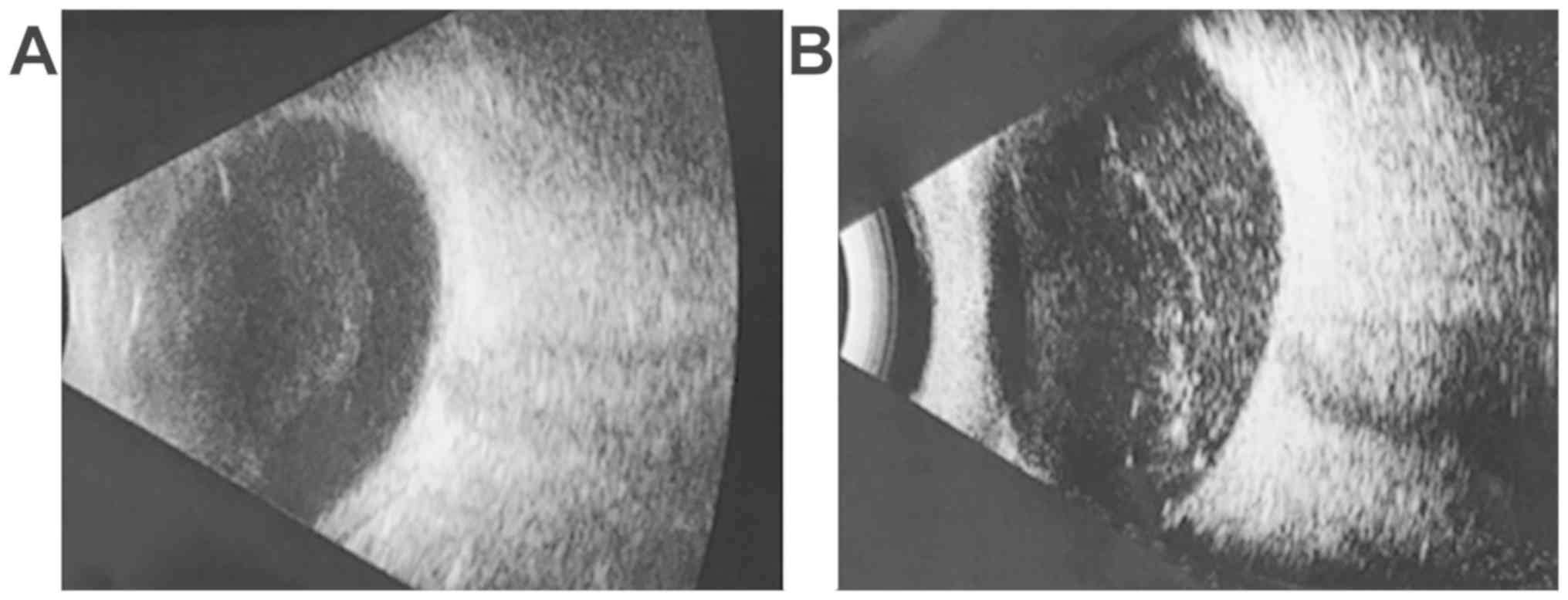
Case 1: 18-year-old female, BCVA right eye = 0.4
(20/50), left eye = 1 (20/20), IOP = 16 mmHg, normal aspect of the
anterior pole, vitreous haze evenly localized of 3+, corresponding
to moderate inflammation (25). The
inflammatory reaction was observed on the ocular ultrasound
(Fig. 1A).
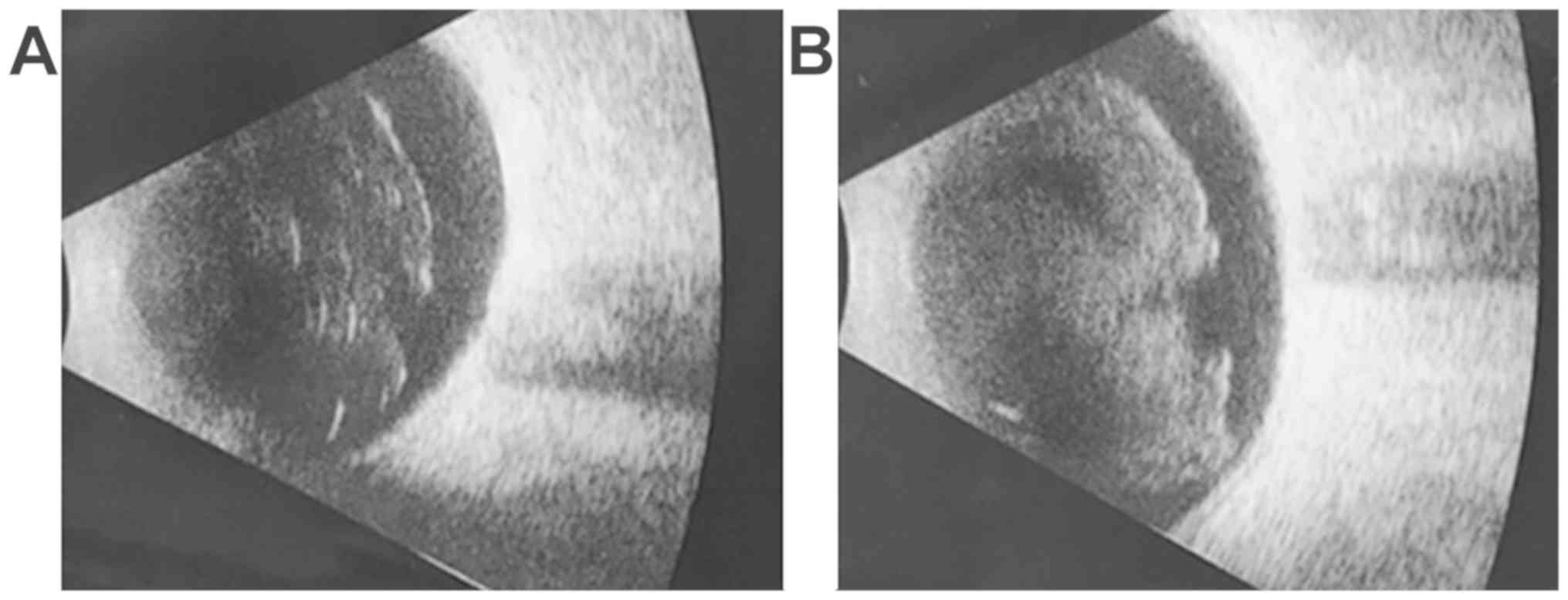
Case 2: 35-year-old male, BCVA right eye = 0.3
(20/63), left eye = 1 (20/20), IOP = 18 mmHg, normal aspect of the
anterior pole, vitreous haze evenly localized of 4+, corresponding
to marked inflammation (25). The
inflammatory reaction was observed on the ocular ultrasound
(Fig. 2A).
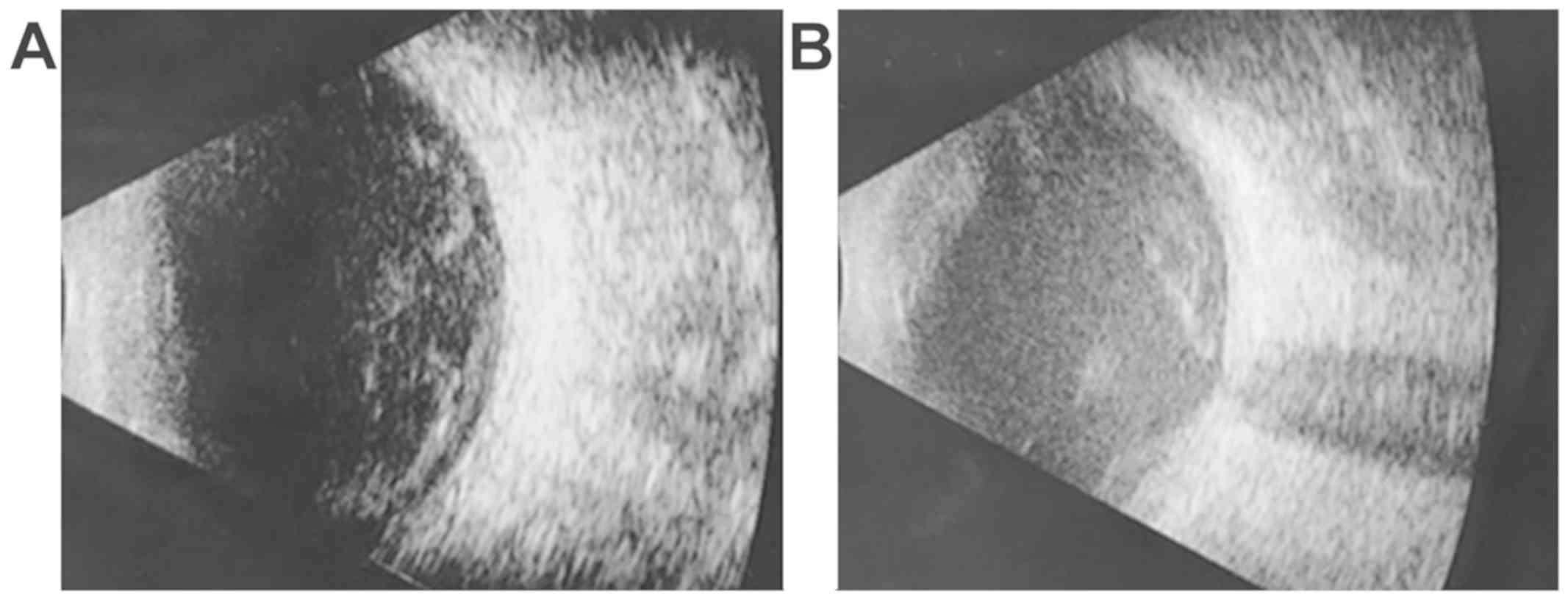
Case 3: 42-year-old male, BCVA right eye = 0.1
(20/200), left eye = 1 (20/20), IOP = 12 mmHg, normal aspect of the
anterior pole, vitreous haze evenly localized of 4+, corresponding
to marked inflammation (25). The
ultrasound revealed the inflammatory reaction (Fig. 3A).
Each patient received a single intravitreal
injection of 4 mg TA. All injections were performed in the
operating theatre. After topical disinfection with povidone-iodine,
the sterile field and the lid speculum were applied. Local
anesthesia with 0.4% oxybuprocaine drops was performed and local
antibiotic drops were spread on. Injections were performed using 30
gauge needles through the inferotemporal pars plana, 4 mm from the
limbus.
The present study was approved by the local Ethics
Committee of the ‘Centrul Oftalmologic Prof. Dr. Munteanu’ Clinic
(Timisoara, Romania). Signed written informed consents were
obtained from the patients. All patients expressed in writing,
prior to the treatment, their informed consent to receive
intraocular treatment with triamcinolone acetonide.
Results and Discussion
After 24 h, each of the patients came to our clinic
and reported a decline in their visual acuity. After performing the
ocular examinations, we concluded that all three patients had
developed an acute sterile inflammatory reaction to TA, called
sterile endophthalmitis, in their right eye.
Case 1: 24 h after the injection, BCVA right eye =
0.1 (20/200), left eye = 1 (20/20), normal IOP, anterior chamber
flare 2+, vitreous haze evenly localized of 4+, corresponding to
marked inflammation (25). The
increased inflammatory reaction was also observed on the ultrasound
(Fig. 1B).
Case 2: 24 h after the injection, BCVA right eye =
counting fingers, left eye = 1 (20/20), normal IOP, anterior
chamber cells 3+, vitreous haze evenly localized of 5+,
corresponding to severe inflammation (25). The increased inflammatory reaction
was also observed on the ocular ultrasound (Fig. 2B).
Case 3: 24 h after the injection, BCVA right eye =
hand motion, left eye = 1 (20/20), normal IOP, anterior chamber 0.2
mm pseudo-hypopyon, vitreous haze evenly localized of 5+,
corresponding to severe inflammation (25). The increased inflammatory reaction
was also noted on the ultrasound (Fig.
3B).
All patients received local treatment with topical
antibiotics, prednisolone acetate and cycloplegic eye drops; the
vitreous inflammation resolved within 3 weeks in the first case and
within 4 weeks in the other two cases. Our conclusion that these
were cases of sterile, rather than infectious endophthalmitis was
based on the resolution of the inflammation without the use of
intravitreal antibiotics.
The causes of sterile endophthalmitis are not
entirely understood. Some authors have suggested that the
contamination of triamcinolone vials with endotoxins might be a
possible cause, but studies performed on vials of triamcinolone
showed no endotoxins (26,27). Other researchers have mentioned a
toxic effect of the triamcinolone itself, as well as the
preservatives present in the vial (benzyl alcohol, polysorbate 80
and carboxymethylcellulose sodium) (28).
In a previous report, Lam et al (29) debated the issue of whether sterile
endophthalmitis after intravitreal triamcinolone injection is a
result of the preservatives contained in the triamcinolone
suspension. In both cases described in this study, patients were
injected with preservative-free triamcinolone and they developed
presumed non-infectious endophthalmitis despite the absence of
preservatives. The situation was similar to ours, since we used
preservative-free triamcinolone in all eyes. Allergic reactions to
triamcinolone have been described, but they were most likely due to
preservatives (30).
There are two kinds of potential complications of
intravitreal corticosteroid treatment: one is steroid-related and
the other one is injection-related adverse effects. Cataract
formation and an intraocular pressure increase refer to
steroid-related side effects. Injection-related adverse effects
include infectious endophthalmitis, sterile endophthalmitis and
retinal detachment (31).
In conclusion, the severe inflammatory reaction,
named sterile endophthalmitis, which appears after the intravitreal
administration of triamcinolone in patients with uveitis, seems to
occur mainly in the context of the off-label use of
anti-inflammatory drugs that have not been approved for
intravitreous use, most cases presenting a painless and acute
vision loss. If the ophthalmologist is not sure about the sterile
origin of the inflammation, this complication must be treated as an
acute endophthalmitis because of the severe visual outcome of this
intraocular infection without antibiotic therapy. The aetiology of
sterile endophthalmitis, regardless of the intravitreous drug used,
remains uncertain and a multifactorial origin must be
considered.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: Series E no. 0048).
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
MCȘ conceived and designed the study, and was
responsible for the acquisition of the data. OLK, NB and SS were
involved in the design of the study and revised the manuscript. OM,
CR, IY and DMD were also involved in the conception and design of
the study, and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the local Ethics Committee
of the ‘Centrul Oftalmologic Prof. Dr. Munteanu’ Clinic (Timisoara,
Romania).
Patient consent for publication
Signed written informed consents were obtained from
the patients. All patients expressed in writing, prior to the
treatment, their informed consent to receive intraocular treatment
with triamcinolone acetonide.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh-i K, Keino H, Goto H, Yamakawa N,
Murase K, Usui Y, Kezuka T, Sakai J, Takeuchi M and Usui M:
Intravitreal injection of Tacrolimus (FK506) suppresses ongoing
experimental autoimmune uveoretinitis in rats. Br J Ophthalmol.
91:237–242. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stanca HT, Suvac E, Munteanu M, Jianu DC,
Motoc AGM, Roşca GC and Boruga O: Giant cell arteritis with
arteritic anterior ischemic optic neuropathy. Rom J Morphol
Embryol. 58:281–285. 2017.PubMed/NCBI
|
|
3
|
Nachod GR: ACTH and cortisone in ocular
disease. J Am Med Womens Assoc. 6:453–455. 1951.PubMed/NCBI
|
|
4
|
Sarao V, Veritti D, Boscia F and Lanzetta
P: Intravitreal steroids for the treatment of retinal diseases.
ScientificWorldJournal. 2014(989501)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Umland SP, Nahrebne DK, Razac S, Beavis A,
Pennline KJ, Egan RW and Billah MM: The inhibitory effects of
topically active glucocorticoids on IL-4, IL-5, and interferon-γ
production by cultured primary CD4+ T cells. J Allergy
Clin Immunol. 100:511–519. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Floman N and Zor U: Mechanism of steroid
action in ocular inflammation: Inhibition of prostaglandin
production. Invest Ophthalmol Vis Sci. 16:69–73. 1977.PubMed/NCBI
|
|
7
|
Machemer R, Sugita G and Tano Y: Treatment
of intraocular proliferations with intravitreal steroids. Trans Am
Ophthalmol Soc. 77:171–180. 1979.PubMed/NCBI
|
|
8
|
Munteanu M, Giuri S, Roșca C, Boruga O and
Creţu O: Multifocal choroidal metastases from thyroid carcinoma: A
case report. Chirurgia (Bucur). 108:268–272. 2013.PubMed/NCBI
|
|
9
|
Munteanu M, Munteanu G, Zolog I, Giuri S,
Coviltir V, Stanca H and Cretu O: Ocular decompression retinopathy
after combined deep sclerectomy and trabeculotomy. Klin Monbl
Augenheilkd. 229:830–831. 2012.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
10
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ilie MA, Caruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging
of skin inflammation: Clinical applications and research
directions. Exp Ther Med. 17:1004–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ilie MA, Caruntu C, Tampa M, Georgescu SR,
Matei C, Negrei C, Ion RM, Constantin C, Neagu M and Boda D:
Capsaicin: Physicochemical properties, cutaneous reactions and
potential applications in painful and inflammatory conditions. Exp
Ther Med. 18:916–925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stanca S, Ulmeanu CE, Stanca HT and
Iovanescu G: Clinical features in toxic coma in children. Exp Ther
Med. 18:5082–5087. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stanca HT, Munteanu M, Jianu DC, Motoc
AGM, Tăbăcaru B, Stanca S, Ungureanu E, Boruga VM and Preda MA: New
perspectives in the use of laser diode transscleral
cyclophotocoagulation. A prospective single center observational
cohort study. Rom J Morphol Embryol. 59:869–872. 2018.PubMed/NCBI
|
|
15
|
Boruga O, Balasoiu AT, Giuri S, Munteanu
M, Stanca HT, Iovanescu G and Preda MA: Caruncular late-onset
junctional nevus: Apropos of an anatomo-clinical observation. Rom J
Morphol Embryol. 58:1461–1464. 2017.PubMed/NCBI
|
|
16
|
Stanca HT, Petrović Z and Munteanu M:
Transluminal Nd: YAG laser embolysis -a reasonable method to
reperfuse occluded branch retinal arteries. Vojnosanit Pregl.
71:1072–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
18
|
Boda D, Negrei C, Nicolescu F and Badalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
19
|
Sarao V, Veritti D and Lanzetta P:
Triamcinolone Acetonide for the treatment of diabetic macular
oedema. Eur Ophthalmic Rev. 6:28–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Beer PM, Bakri SJ, Singh RJ, Liu W, Peters
GB III and Miller M: Intraocular concentration and pharmacokinetics
of triamcinolone acetonide after a single intravitreal injection.
Ophthalmology. 110:681–686. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Inoue M, Takeda K, Morita K, Yamada M,
Tanigawara Y and Oguchi Y: Vitreous concentrations of triamcinolone
acetonide in human eyes after intravitreal or subtenon injection.
Am J Ophthalmol. 138:1046–1048. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Munteanu M and Rosca C: Repositioning and
follow-up of intralenticular dexamethasone implant. J Cataract
Refract Surg. 39:1271–1274. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Preda MA, Popa G, Karancsi OL, Musat O,
Popescu SI, Munteanu M and Popa Z: Effectiveness of subconjunctival
bevacizumab associated with a laser-based procedure in the
treatment of neovascular glaucoma. Farmacia. 66:621–626. 2018.
|
|
24
|
Preda MA, Karancsi OL, Munteanu M and
Stanca HT: Clinical outcomes of micropulse transscleral
cyclophotocoagulation in refractory glaucoma - 18 months follow-up.
Lasers Med Sci: Jan 14, 2020 (Epub ahead of print).
|
|
25
|
Zierhut M, Deuter C and Murray PI:
Classification of uveitis - current guidelines. Eur Ophthalmic Rev:
77-78, 2011. http://doi.org/10.17925/EOR.2007.00.00.77.
|
|
26
|
Jonisch J, Lai JC, Deramo VA, Flug AJ and
Fastenberg DM: Increased incidence of sterile endophthalmitis
following intravitreal preserved triamcinolone acetonide. Br J
Ophthalmol. 92:1051–1054. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roth DB, Chieh J, Spirn MJ, Green SN,
Yarian DL and Chaudhry NA: Noninfectious endophthalmitis associated
with intravitreal triamcinolone injection. Arch Ophthalmol.
121:1279–1282. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yeung CK, Chan KP, Chan CKM, Pang CP and
Lam DSC: Cytotoxicity of triamcinolone on cultured human retinal
pigment epithelial cells: Comparison with dexamethasone and
hydrocortisone. Jpn J Ophthalmol. 48:236–242. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lam A, Garg SJ, Spirn MJ, Fineman MS and
Sivalingam A: Sterile endophthalmitis following intravitreal
injection of preservative-free triamcinolone acetonide. Retin Cases
Brief Rep. 2:228–230. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Montoro J, Valero A, Elices A, Rubira N,
Serra-Baldrich E, Amat P and Malet A: Anaphylactic shock after
intra-articular injection of carboxymethylcellulose. Allergol
Immunopathol (Madr). 28:332–333. 2000.PubMed/NCBI
|
|
31
|
Scott IU and Flynn HW Jr: Reducing the
risk of endophthalmitis following intravitreal injections. Retina.
27:10–12. 2007.PubMed/NCBI View Article : Google Scholar
|