Introduction
Osteoarticular tuberculosis is a chronic
inflammatory disease characterized by Mycobacterium
tuberculosis (M.tb) infection that occurs through the
respiratory tract and spreads through the blood to the bones and
joints (1). This disease is
characterized by enhanced bone and joint absorption and bone
destruction (1). Osteoarticular
tuberculosis causes limb deformity and nerve compression, greatly
affecting the quality of life of patients and imposing a global
health and socio-economic burden (2). Clinically, osteoarticular tuberculosis
accounts for ~10% of all extrapulmonary tuberculosis cases, which
is second only to pleural and lymphatic tuberculosis (3). Antituberculosis drugs have been widely
used since the mid-20th century to effectively control this
infection (4); however, to the best
of our knowledge, the pathogenesis of osteoarticular tuberculosis
has not been widely studied. In recent years, drug-resistant
tuberculosis, particularly multidrug-resistant tuberculosis, has
emerged globally (5). Additionally,
an increase in longer chemotherapy cycles and drug side effects
have presented major challenges to effectively control
osteoarticular tuberculosis (6,7).
Therefore, the pathogenic mechanism of osteoarticular tuberculosis
must be determined to develop new and effective prevention and
treatment strategies.
The earliest study of the pathogenesis of
osteoarticular tuberculosis was reported in 1997, which suggested
that heat shock protein Cpn10 in M.tb can promote chemotaxis
of osteoclasts and locally infiltrate lesions, while inhibiting
proliferation of osteoblast precursors and increasing bone
resorption. Furthermore, histopathological analysis of samples from
patients with osteoarticular tuberculosis showed that local
abnormal activation of osteoclasts lead to bone destruction
(8). In 2015 a study conducted using
an osteoarticular tuberculosis rabbit animal model confirmed that
the number of osteoclasts in the damaged spine increased, while the
number of osteoblasts decreased (9).
Therefore, increased numbers and activation of osteoclasts after
M.tb infection are key factors leading to the pathogenesis
of osteoarticular tuberculosis.
Autophagy is a basic metabolic process in cells and
involves the degradation of intracellular proteins and invading
pathogens by lysosomal pathways to maintain cell survival. This is
the most primitive innate immune mechanism used by eukaryotic cells
to clear invading pathogens (10,11). In
a rheumatoid arthritis model, tumor necrosis factor (TNF)-α caused
inflammation of the synovial membrane in the joint, increased
expression of the autophagy pathway molecules autophagy-related
protein (Atg)7 and Beclin1 in osteoclasts and promoted autophagy
(12). A previous study demonstrated
that TNF-α regulates autophagy and affects osteoclast production
(13). Autophagy is also an innate
immune mechanism by which macrophages control M.tb infection
(14). Macrophage autophagy is
activated through the action of type 1 T helper (Th1) cytokines
such as interferon-γ, leading to degradation of phagocytic
M.tb via the lysosomal pathway, preventing the proliferation
of M.tb in macrophages and avoiding cell necrosis (15). However, whether Th1 cells and their
cytokine networks activated by M.tb infection in patients
with osteoarticular tuberculosis regulate and maintain the
autophagy of infected osteoclasts remains to be elucidated.
In the present study, clinical specimens were
evaluated and cell experiments were conducted. TNF-α activated
autophagy and inhibited apoptosis of osteoclasts infected with
M.tb, thereby maintaining cell homeostasis. The results have
practical value for guiding the prevention and treatment of
osteoarticular tuberculosis.
Materials and methods
Patients and samples
Patients were divided into two groups: The
osteoarticular tuberculosis group and osteoarthritis (OA) group.
The selection criteria for the osteoarticular tuberculosis group
were as follows: Hospitalized patients from the First Hospital of
Wuhan (Wuhan, China) were diagnosed as having osteoarticular
tuberculosis according to their medical history, clinical symptoms,
signs, imaging findings, related laboratory tests, and pathological
examination. Lesions with necrotic bone and the synovium were
collected as specimens, and 30 cases were included (14 males and 16
females; age range 61-70 years). The selection criteria for the OA
group were as follows: Hospitalized cases from the First Hospital
of Wuhan were diagnosed as OA according to their medical history,
clinical symptoms, signs, imaging findings and relevant laboratory
tests. Additionally, patients with surgical indications requiring
joint replacement were included. Bone and synovial tissue removed
during the operation were collected as specimens from a total of 25
cases (11 males and 14 females; age range 63-72 years). All 55
specimens were collected from January 2017 to December 2018. All
experimental protocols were approved by the Clinical Research
Ethics Committee of Wuhan First Hospital, Tongji Medical College,
Huazhong University of Science and Technology. All patients or
their parents provided informed consent.
Isolation and culture of human
osteoclasts
The standards for healthy volunteers were as
follows: Males aged 20-30 years, no metabolic bone disease
according to blood and urine analysis, normal liver and kidney
function, no hereditary, blood, or infectious diseases and no
general infections, such as colds, within the past 1 month. Samples
were collected at Wuhan First Hospital from January 2017 to
December 2018. Informed consent was provided by the volunteers
prior to elbow vein blood collection. Healthy male volunteers were
selected, and 80-100 ml of whole blood was collected from the
venous blood. Human monocytes were separated by density gradient
centrifugation. DMEM (Gibco; Thermo Fisher Scientific, Inc.) was
mixed with equal volumes of heparin-anticoagulated whole blood.
Diatrizoate was added to a 50 ml centrifuge tube, and the mixture
was slowly added along the tube wall to ensure a clear interface
between the two samples, which were then centrifuged at 362 x g at
4˚C for 30 min. The obtained mononuclear cells were placed in two
25-mm petri dishes and cultured in 5% CO2 at 37˚C for 24
h, before being washed three times with DMEM. Unattached cells were
eluted, and the remaining adherent cells were considered as
mononuclear cells. Confluent cells were resuspended in DMEM
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100
mg/ml streptomycin, 100 U/ml penicillin, 30 ng/ml macrophage
colony-stimulating factor (PeproTech, Inc.) and 40 ng/ml receptor
activator of NF-κB ligand (PeproTech, Inc.). Cells were then
incubated at 37˚C in a humidified atmosphere with 95% (v/v) air and
5% (v/v) CO2. The culture medium was replaced every
three days for 21 days.
Tartrate-resistant acid phosphatase
(TRAP) staining
Patient-derived osteoclast droplets were placed on a
loading slide to prepare a cell smear. The slide was allowed to dry
naturally, and TRAP (Beijing Solarbio Science & Technology Co.,
Ltd.) fixative was added to fix the cells at 4˚C for 60 sec. After
washing the slides with water and slight drying, the samples were
placed in TRAP incubation solution. Slides were then incubated at
37˚C for 60 min in the dark, and then washed with water. Cells were
stained with hematoxylin for 5 min or methyl green solution for 3
min at 37˚C. The samples were washed with water, dried, and
examined using a light microscope at 40X objective.
M.tb infection and treatment
A homogenizer seal was first weighed. A pipette tip
was used to scrape the bacteria (provided by Professor Fan
Xionglin, School of Basic Medicine, Tongji Medical College,
Huazhong University of Science and Technology) from the plate and
the homogenizer was sterilized and sealed, weighing the
homogenizer. The dry weight of the bacteria was calculated, and 1
ml of complete DMEM was added to the sample. The osteoclasts were
ground and the supernatant was collected by centrifugation at 362 x
g for 5 min at 4˚C. A total of 1 ml supernatant was added to the
autophagy gene knockout type M.tb H37Rv (M.tb
H37RvΔeis) and wild-type M.tb H37Rv (M.tb H37Rv WT)
cultured complete DMEM and then mixed well. Osteoclasts not
infected with M.tb were used as a blank control group.
Following infection, cells were incubated at 37˚C for 24 h and
osteoclasts were centrifuged at 362 x g at 4˚C for 5 min and washed
twice with PBS.
For TNF-α treated experiments, infected cells were
treated with 40 ng/ml TNF-α (cat. no. 1217202; Dakewe Biotech Co.,
Ltd.) for 24 h or pre-treated with 10 µM 3-methyladenine (3-MA)
(cat. no. M9281; Sigma-Aldrich, Merch KGaA) before TNF-α
administration.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the harvested cells and
tissues using TRIzol™ reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The RNA
was denatured for 5 min at 70˚C and placed on ice for 5 min.
Denatured RNA was added to a mixture of MMLV-RT, MMLV-RT buffer,
horseradish peroxidase (HRP) RNA-RNA interaction/RNase inhibitor
and dNTPs and incubated for 60 min at 42˚C. The mixture was
inactivated by heating at 95˚C for 5 min. qPCR was performed using
Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, Inc.) on
a 7900HT thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for the PCR: Initial denaturation at 50˚C for 2 min; 40 cycles of
95˚C for 10 min, 95˚C for 30 sec and 60˚C for 30 sec; and a final
extension step at 60˚C for 30 sec. A total of 1 µg cDNA and 0.4 µl
of primer were used for the qPCR, Relative gene expression levels
were calculated using the 2-ΔΔCq method (16). GAPDH was used as an endogenous
control to normalize the level of each mRNA. The sequences of the
primers used are shown in Table I.
All experiments were performed at least in triplicate.
 | Table IList of primers used for reverse
transcription-quantitative PCR. |
Table I
List of primers used for reverse
transcription-quantitative PCR.
| | Primer sequence
(5'→3') |
|---|
| Gene | GenBank
accession | Forward | Reverse |
|---|
| Human Bax | NM_003217 |
CATATAACCCCGTCAACGCAG |
GCAGCCGCCACAAACATAC |
| Human Bcl-2 | AF089746 |
GTCTTCGCTGCGGAGATCAT |
CATTCCGATATACGCTGGGAC |
| Human Caspase
3 | NM_004346 |
CATGGAAGCGAATCAATGGACT |
CTGTACCAGACCGAGATGTCA |
| Human Atg7 | NM_001144912 |
CAGTTTGCCCCTTTTAGTAGTGC |
CCAGCCGATACTCGTTCAGC |
| Human Beclin1 | NM_017749 |
CTGGTAGAAGATAAAACCCGGTG |
AGGTAGAGCGTGGACTATCCG |
| Human GAPDH | NM_002046 |
CTGCTCCTCCTGTTCGACAGT |
CCGTTGACTCCGACCTTCAC |
Western blotting
Osteoclasts were rinsed 2-3 times with TBS. An
appropriate volume of RIPA buffer (Biosharp) was added to plates
and flasks for 3-5 min followed by incubation in an ice bath for 30
min. The supernatant was collected as a total protein solution.
Total, cytoplasmic, and mitochondrial proteins were extracted using
corresponding kits (Beyotime Institute of Biotechnology). Protein
concentration was determined using a BCA protein assay kit. A total
of 20 µg/lane extracted proteins were separated by 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore). After blocking in 5%
non-fat dried milk in TBS-Tween-20 (TBS-T) at 37˚C for 2 h, the
blots were incubated with anti-Atg7 (1:500; cat. no. Ag27914;
ProteinTech Group, Inc.), anti-Beclin1 (1:500; cat. no. 11306-1-AP;
ProteinTech Group, Inc.), anti-Bax (1:1,000; cat. no. 50599-2-Ig;
ProteinTech Group, Inc.), anti-Bcl-2 (1:1,000; cat. no. 10927-1-AP;
ProteinTech Group, Inc.), anti-cytochrome C (1:500; cat. no.
12245-1-AP; ProteinTech Group, Inc.), anti-cleaved-caspase3
(1:1,000; 19677-1-AP; ProteinTech Group, Inc.),
anti-microtubule-associated protein 1A/1B light chain 3A (LC-3;
1:500; cat. no. 18725-1-AP; ProteinTech Group, Inc.), anti-β-actin
(1:1,000; cat. no. TDY051; Beijing TDY Biotech Co., Ltd.) and
anti-voltage-dependent anion channel (1:3,000; cat. no. sc-32063;
Santa Cruz Biotechnology; Dallas, TX, USA), overnight at 4˚C.
β-actin served as an internal control. The membranes were then
washed three times with TBS-T on a bleaching shaker at room
temperature for 5 min. HRP-labeled goat anti-mouse antibody
(1:3,000; cat. no. BL002A; Biosharp) diluted with TBS-T was
incubated with the membranes for 30 min at room temperature. The
membranes were washed three times with TBS-T on a decolorizing
shaker at room temperature for 5 min. A total of 1 ml
Electrochemiluminescence A (ECLA) (cat. no. BL520A; Biosharp) and
electrochemiluminescence B (ECLB) (cat. no. BL520A; Biosharp)
reagents were mixed in a centrifuge tube. The protein side of the
PVDF membrane was brought into full contact with the mixed
solution. After 1-2 min, the residual liquid was removed and the
membrane was incubated in the dark. Protein expression was
quantified using ImageJ v1.46 (National Institutes of Health). All
experiments were performed at least in triplicate.
ELISA
The level of TNF-α in culture supernatant was
measured using ELISA kits (cat. no. E-EL-H0109c; Elabscience)
according to the manufacturer's instructions. Optical density was
detected by a microplate reader at a wavelength of 450 nm.
TUNEL
DNA fragmentation was detected using an In
Situ Cell Death Detection kit (Roche Diagnostics). After
fixation with 4% paraformaldehyde at 37˚C for 1 h, the cells were
incubated with 3% H2O2 and 0.1% Triton X-100
for 10 min and washed with PBS three times following each step. In
accordance with standard protocols, the cells were stained with
TUNEL inspection fluid (1:100) and DAPI at 37˚C for 1 h. The slides
were mounted on a mounting solution containing an anti-fluorescent
quencher (Fluoromont-G; cat. no. 0100-01; SouthernBiotech). Three
fields of view on each slide were randomly chosen for observation
with a fluorescence microscope.
Transmission electron microscopy
Medium was first decanted before cells were fixed
with 2.5% glutaraldehyde (Sinopharm Chemical Reagent Co., Ltd.) at
4˚C for 15 min. Cells were subsequently collected by centrifugation
at 362 x g and 37˚C and stored at 4˚C. Cells were rinsed three
times with 0.1 M phosphate buffer (Sinopharm Chemical Reagent Co.,
Ltd.) for 15 min, followed by dehydration in a 30, 50, 70, 80, 85,
90 and 100% ethanol gradient for 15-20 min in each alcohol
solution. The penetrant was composed of epoxy resin (Sinopharm
Chemical Reagent Co., Ltd.) and acetone (Sinopharm Chemical Reagent
Co., Ltd.). The infiltrated sample was placed in an embedding
plate, before addition of embedding epoxy resin. The samples were
embedded with epoxy resin (Hubei Xinkang Pharmaceutical Chemical
Co., Ltd.) in a 60˚C incubator for 48 h. An ultramicrotome was used
to slice the sections to 80-100 nm thickness. Uranyl acetate was
added to the sections and incubated at room temperature for 15 min.
The sections were dried overnight at room temperature and observed
under an electron microscope.
Statistical analysis
The results are presented as the mean ± SD.
Statistical analysis was performed using SPSS 20.0 software (IBM
Corp.). Two groups were compared using an independent sample
t-test, and three or more groups were compared by one-way ANOVA
followed by Dunnett's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Autophagy is enhanced in
osteoarticular tuberculosis tissues
The number of osteoclasts in 30 osteoarticular
tuberculosis pathological tissues and 25 osteoarthritis
pathological tissues was detected by TRAP staining. As shown in
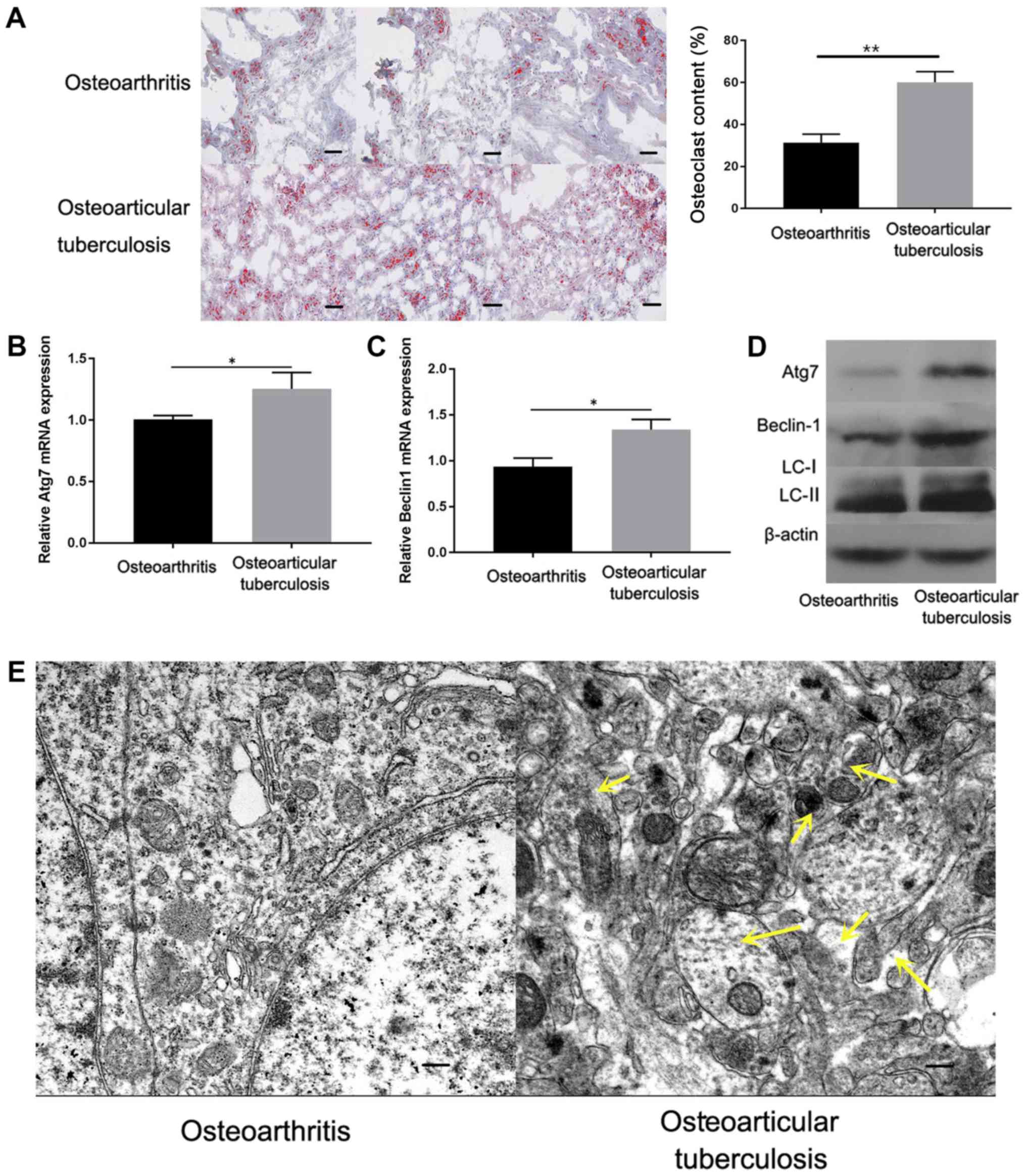
Fig. 1A, the osteoclast content was
markedly increased in osteoarticular tuberculosis pathological
tissues compared with OA samples. Autophagic activity during
osteoarticular tuberculosis was subsequently investigated. RT-qPCR
was performed to detect Atg7 and Beclin1 mRNA levels in
osteoarticular tuberculosis tissues. The results showed that Atg7
and Beclin1 mRNA levels were significantly increased in
osteoarticular tuberculosis samples compared with OA samples
(Fig. 1B and C). Additionally, the expression of Atg7,
Beclin1 and LC3-II/I protein were investigated by western blotting.
The results showed that the protein levels of Atg7, Beclin1 and
LC3-II/I were increased in osteoarticular tuberculosis pathological
tissue samples compared with OA samples (Fig. 1D). Furthermore, transmission electron
microscopy was conducted to observe autophagy. As shown in Fig. 1E, increased accumulation of
autophagosomes was observed in osteoclasts from tuberculosis
lesions, as indicated by yellow arrows. The results indicated that
autophagy was enhanced in osteoarticular tuberculosis compared with
OA samples.
Autophagy induction reduces the
toxicity of M.tb to osteoclasts
Osteoclasts were transfected with M.tb
H37Rv∆eis or M.tb H37Rv and mRNA expression of Atg7 and
Beclin1 was investigated by RT-qPCR. As shown in Fig. 2A and B, M.tb H37Rv∆eis increased the mRNA
expression levels of Atg7 and Beclin1 compared with the M.tb
H37Rv infection group and control group. Western blot analysis was
performed to detect Atg7, Beclin1, and LC3-II/I protein levels in
osteoclasts. The results showed that the expression levels of these
proteins were increased in the M.tb H37Rv∆eis infection
group compared with the control group (Fig. 2C).
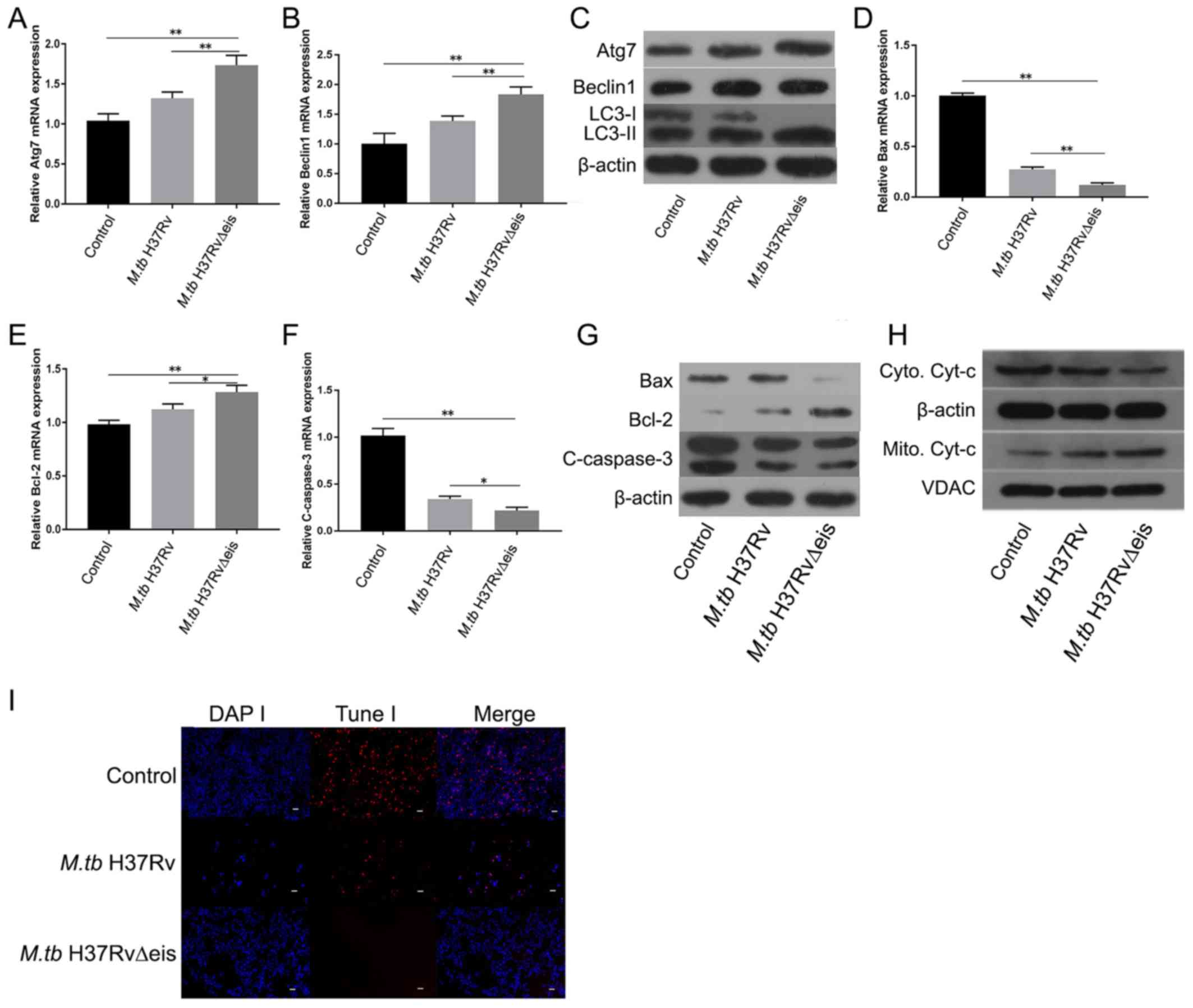 | Figure 2Induced autophagy reduces the
toxicity of M.tb to osteoclasts. (A) mRNA expression levels
of Atg7 in control, M.tb H37Rv and M.tb H37Rv∆eis
groups. (B) mRNA expression levels of Beclin1 in control,
M.tb H37Rv and M.tb H37Rv∆eis groups. (C) Protein
levels of Atg7, Beclin1, and LC3-II/I in control, M.tb H37Rv
and M.tb H37Rv∆eis groups. mRNA expression levels of (D)
Bax, (E) Bcl-2, and (F) cleaved-caspase 3 in control, M.tb
H37Rv infection and M.tb H37Rv∆eis infection groups. (G)
Protein levels of Bax, Bcl-2 and cleaved-caspase 3 in control,
M.tb H37Rv and M.tb H37Rv∆eis groups. (H) Western
blotting assay of the levels of Cyt-c in cytoplasmic and
mitochondrial extracts from control, M.tb H37Rv infection
and M.tb H37Rv∆eis infection groups. (I) TUNEL assay was
performed in osteoclasts in control, M.tb H37Rv and
M.tb H37Rv∆eis osteoclasts (Magnification, x200; scale bars,
50 µm). Data are presented as the mean ± SD. *P<0.05
and **P<0.01. M.tb H37RvΔeis, inhibition of
autophagy gene knockout type Mycobacterium tuberculosis
H37Rv; M.tb H37Rv, wild type Mycobacterium
tuberculosis H37Rv; Atg7, autophagy-related protein 7; LC-3,
microtubule-associated proteins 1A/1B light chain 3A; Cyt-c,
cytochrome-c; VDAC, voltage-dependent anion channel. |
The role of autophagy in regulating apoptosis of
osteoclasts infected with M.tb. was studied using
M.tb H37Rv∆eis- and M.tb H37Rv-infected osteoclasts.
RT-qPCR was performed to detect the apoptotic markers Bax, Bcl-2,
and cleaved-caspase 3 mRNA levels in osteoclasts. Bax and
cleaved-caspase 3 mRNA levels were significantly reduced in the
H37Rv∆eis-infected group compared with controls and H37Rv-infected
samples, whereas Bcl-2 mRNA levels significantly increased
(Fig. 2D-F). Similarly, M.tb
H37Rv∆eis infection decreased the expression of cleaved-caspase 3
and Bax protein levels and increased the expression of
anti-apoptotic Bcl-2 protein levels compared with controls and
H37Rv-infected samples (Fig. 2G). In
addition, attenuated translocation of mitochondrial cytochrome c to
the cytoplasm was detected by western blot analysis (Fig. 2H). TUNEL analysis further confirmed
that autophagy reduced osteoclast apoptosis (Fig. 2I).
TNF-α enhances autophagic activity of
osteoclasts infected with M.tb
TNF-α plays an important role in the development of
tuberculosis (17). ELISA was
performed to detect the secretion of TNF-α from osteoclasts
infected with M.tb. As shown in Fig. 3A, M.tb infection significantly
increased the levels of TNF-α compared with controls. Subsequently,
the effect of TNF-α on osteoclasts infected with M.tb was
tested. Osteoclasts infected with M.tb were treated with
TNF-α (40 ng/ml) for 24 h. Untreated osteoclasts infected with
M.tb were used as controls. RT-qPCR, western blotting and
transmission electron microscopy were performed to determine
autophagy activation. The results demonstrated that mRNA levels of
Atg7 and Beclin1 were significantly upregulated in osteoclasts
infected with M.tb and treated with TNF-α compared with
corresponding cells that were not treated with TNF-α (Fig. 3B and C). Western blotting results showed that
protein expression levels of Atg7, Beclin1 and LC3-II/I increased
following TNF-α treatment of osteoblasts infected with M.tb
(Fig. 3D). Furthermore, increased
accumulation of autophagosomes was observed in infected osteoclasts
treated with TNF-α as indicated by the arrows (Fig. 3E). The results indicated that TNF-α
enhanced the autophagy of osteoclasts infected with
M.tb.
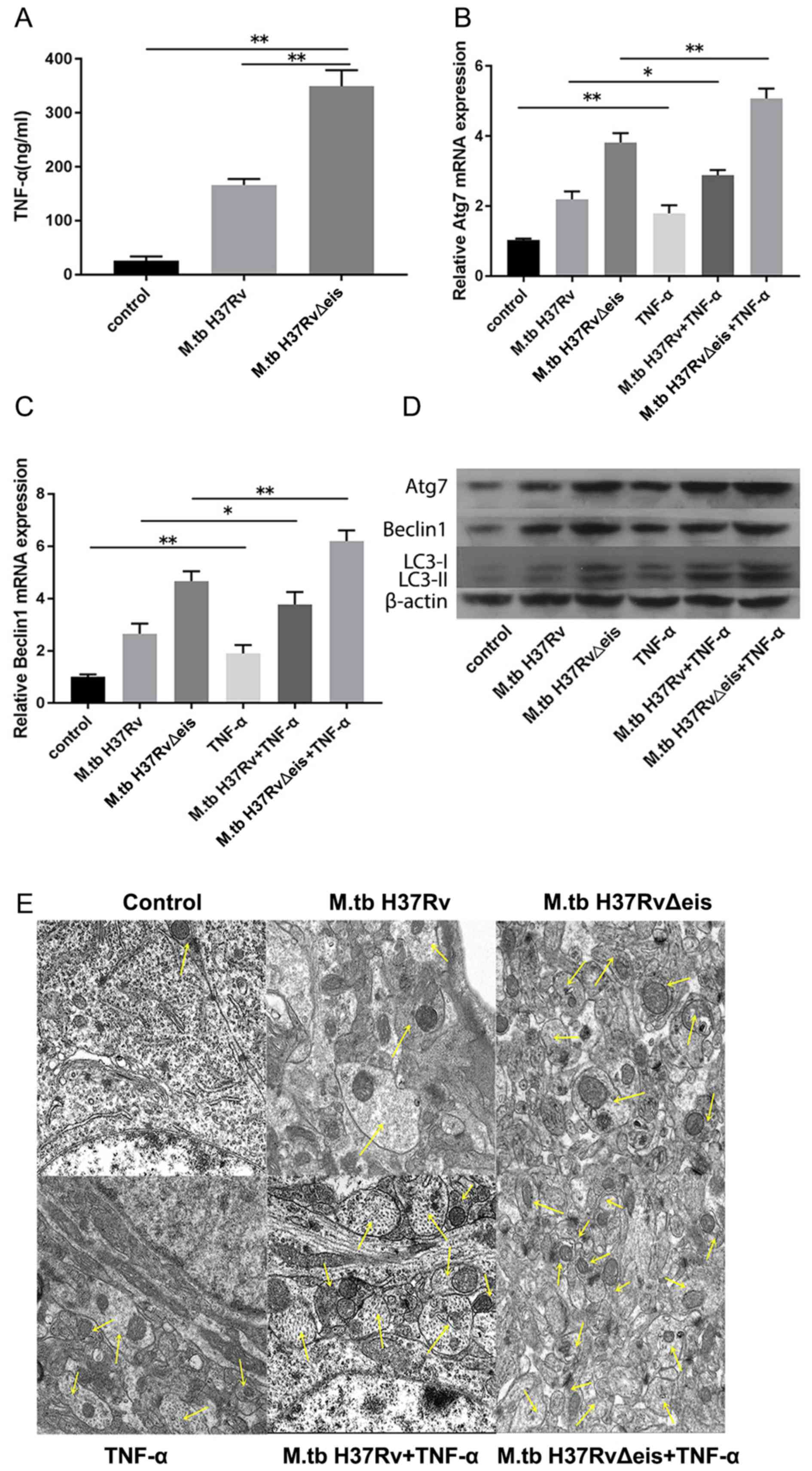 | Figure 3TNF-α enhances the autophagic
activity of osteoclasts infected with M.tb. (A) Levels of
TNF-α in control, M.tb H37Rv and M.tb H37Rv∆eis
groups. mRNA expression levels of (B) Atg7 and (C) Beclin1 in
osteoclasts infected with M.tb untreated or treated with
TNF-α. (D) Protein expression levels of Atg7, Beclin1 and LC3-II/I
in osteoclasts infected with M.tb untreated or treated with
TNF-α. (E) Autophagosomes in osteoclasts infected with M.tb
untreated or treated with TNF-α were observed by transmission
electron microscopy (magnification, x5,000). Yellow arrows indicate
autophagosomes. Scale bars, 1 µm. Data are presented as the mean ±
SD. *P<0.05 and **P<0.01. M.tb
H37RvΔeis, inhibition of autophagy gene knockout type M.tb
H37Rv; M.tbH37Rv wild type M.tb H37Rv; TNF-α, tumor
necrosis factor-α; Atg7, autophagy-related protein 7; LC-3,
microtubule-associated proteins 1A/1B light chain 3A; M.tb,
Mycobacterium tuberculosis. |
TNF-α inhibits apoptosis of
osteoclasts infected with M.tb by activating autophagy
To understand whether TNF-α affects the apoptosis of
osteoclasts infected with M.tb, M.tb H37Rv∆eis or
M.tb H37Rv was transfected into human osteoclasts before the
cells were stimulated with TNF-α. Following osteoclast treatment
with TNF-α (40 ng/ml) for 24 h, mRNA and protein expression of
apoptosis-associated factors were quantified by RT-qPCR and western
blotting. The mRNA and protein expression of pro-apoptotic factors
(cleaved-caspase 3 and Bax) in osteoclasts infected with
M.tb significantly decreased after treatment with TNF-α,
whereas the mRNA and protein expression of anti-apoptotic Bcl-2 was
significantly increased compared with the respective
TNF-α-untreated groups (Fig. 4A-D).
In addition, western blot analysis detected attenuated
translocation of mitochondrial cytochrome c in TNF-α-treated groups
when compared with corresponding TNF-α-untreated groups (Fig. 4E). Additionally, TUNEL staining
showed that TNF-α treatment decreased the apoptosis of osteoclasts
infected with M.tb (Fig.
4F).
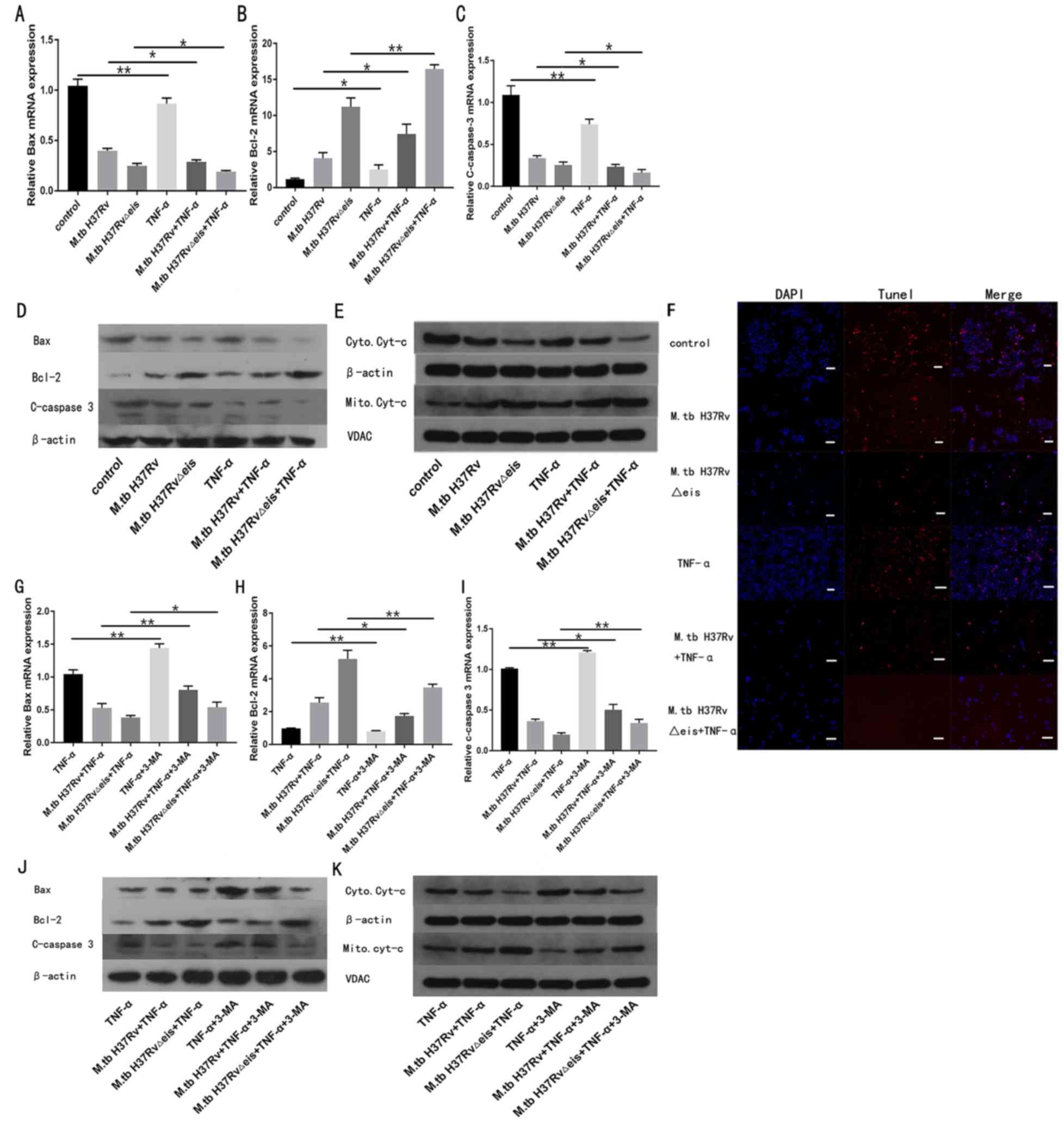 | Figure 4TNF-α inhibits apoptosis of
osteoclasts infected with M.tb by activating autophagy. mRNA
expression levels of (A) Bax, (B) Bcl-2 and (C) cleaved-caspase 3
in osteoclasts infected with M.tb untreated or treated with TNF-α.
(D) Protein levels of Bax, Bcl-2, and cleaved-caspase 3 in
osteoclasts infected with M.tb untreated or treated with TNF-α. (E)
Western blotting assay of the levels of Cyt-c in cytoplasmic and
mitochondrial extracts. (F) TUNEL assay was performed in
osteoclasts infected with M.tb untreated or treated with TNF-α.
(magnification, x200; scale bar, 50 μm). mRNA expression levels of
(G) Bax, (H) Bcl-2 and (I) cleaved-caspase 3 in osteoclasts
infected with M.tb treated with TNF-α, and untreated or treated
with 3-MA. (J) Protein levels of Bax, Bcl-2, and cleaved-caspase 3
in osteoclasts infected with M.tb treated with TNF-α, and untreated
or treated with 3-MA. (K) Western blotting assay of the levels of
Cyt-c in cytoplasmic and mitochondrial fractions of osteoclasts
infected with M.tb treated with TNF-α, and untreated or treated
with 3-MA. Data are presented as the mean ± SD. *P<0.05 and
**P<0.01. M.tbH37RvΔeis, inhibition of autophagy gene knockout
type Mycobacterium tuberculosis H37Rv; M.tbH37Rv wild type
Mycobacterium tuberculosis H37Rv; TNF-α, tumor necrosis factor-α;
Atg7, autophagy-related protein 7; 3-MA, 3-methyladenine; VDAC,
voltage-dependent anion channel. |
To investigate the beneficial effects of TNF-α on
the autophagy of osteoclasts infected with M.tb, cells were
pre-treated with the autophagy inhibitor 3-MA before TNF-α
administration. As shown in Fig.
S1, 3-MA treatment decreased the protein levels of Atg7,
Beclin1 and LC3-II/I compared with the corresponding 3-MA-untreated
groups. Pre-treatment with 3-MA significantly attenuated the
effects of TNF-α on the expression of apoptosis-related factors
compared with the respective 3-MA-untreated groups (Fig. 4G-K).
Discussion
Recent studies have shown that autophagy plays an
important role in diverse biological and pathological processes,
including cell proliferation, differentiation, apoptosis, and
carcinogenesis; however, little is known about the role of
autophagy in the development of osteoarticular tuberculosis
(18-21).
The present results demonstrated that the number of osteoclasts
increased and autophagy was enhanced in the lesions of patients
with osteoarticular tuberculosis compared with patients with OA.
Activation of autophagy inhibited the apoptosis of osteoclasts
infected with M.tb and increased the expression levels of
TNF-α. Furthermore, TNF-α enhanced the autophagic activity of
osteoclasts infected with M.tb and inhibited cell
apoptosis.
Osteoclasts are the only cells in the body that
perform bone resorption and are closely related to normal bone
metabolism and various diseases (22). The pathological changes in vertebrae
destruction in spinal tuberculosis are closely related to abnormal
activation and proliferation of osteoclasts caused by M.tb
(23). The synergistic effect of
osteoclasts and osteoblasts is crucial in bone formation and
development. Once the balance between osteoclasts and osteoblasts
is disrupted, abnormal bone diseases such as bone and joint
tuberculosis can occur (24).
Autophagy plays an important role in the formation, apoptosis and
function of osteoclasts as an important mechanism of action to
maintain cell homeostasis (25).
Both osteoclast formation and bone resorption require the
involvement of Atg5, Atg7, Atg4B and LC3. For example, Atg5 and
Atg7 promote bone resorption in vivo and in vitro,
mainly through the actin loop of osteoclasts as a target for
lysozymes (26). Atg5, Atg7, Atg4B
and LC3 are important components and regulatory molecules in
autophagy, suggesting an important role for autophagy in osteoclast
formation (19). LC3 is also
involved in osteoclast differentiation, which is modified by Atg4B
to block the absorption activity and expression of cathepsin K and
inhibit the degradation of collagen in the bone matrix (27). The present results showed that
tuberculosis infection induced autophagy, which inhibited the
apoptosis of osteoclasts, further demonstrating a key role of
autophagy in maintaining the osteoclast steady state.
TNF-α can promote granuloma formation, envelop
tubercle bacillus, limit pathogen spread and promote pathological
changes caused by tuberculosis (28). High levels of TNF-α were detected in
the serum and cerebrospinal fluid of patients with spinal
tuberculosis (29). TNF-α, a
pro-inflammatory factor, is key in the immunopathological response
of tuberculosis. TNF-α can inhibit M.tb infection and
accelerate bone destruction. It is closely related to the
occurrence, development, severity, therapeutic effect and prognosis
of the disease (30). Additionally,
Mattos et al (31) reported
that the levels of TNF-α were significantly lower after
tuberculosis chemotherapy than before, demonstrating that TNF-α may
play an important role in osteoarticular tuberculosis. Once
M.tb enters the bone through the blood or lymphatic system,
it activates mononuclear macrophages around the lesion to produce a
large amount of TNF-α, which directly acts on osteoclast precursor
cells in the bone marrow to form a large number of osteoclasts,
causing bone destruction (32).
Kobayashi et al (33)
reported that TNF-α directly induced osteoclast precursor cells
(mononuclear macrophages) in the bone marrow to differentiate into
osteoclasts, which does not depend on any other route. TNF-α was
reported to directly induce osteoclast formation in the bone
marrow, as well as induce osteoblast apoptosis, reduce the
proliferation and differentiation of osteoblasts, inhibit new bone
formation and aggravate bone destruction; thus, TNF-α inhibitors
are effective for reducing bone loss (34,35).
Autophagy is activated by the proinflammatory cytokine TNF-α in the
osteoclasts of patients with rheumatoid arthritis (13,36). The
present study showed that TNF-α enhanced autophagic activity and
inhibited apoptosis of osteoclasts infected with M.tb.
Autophagy induced osteoclast differentiation and apoptosis and
stimulated osteoclast-mediated bone resorption in vitro,
thereby highlighting autophagy as a novel mediator of TNF-α induced
bone resorption (37).
However, the present study had certain limitations.
The study preliminarily explored the relationship between TNF-α and
autophagy and did not determine the mechanism of TNF-α-activated
autophagy. Subsequent studies should be performed on
TNF-α-activated autophagy signaling pathways. The present study was
limited to the cellular level, and no animal experiments were
performed. The results of this experiment should be further
verified in animal models.
In conclusion, to the best of our knowledge, this is
the first study to show that autophagy is an important factor in
maintaining the homeostasis of osteoclasts infected with
M.tb. Additionally, TNF-α promoted the autophagy of mature
osteoclasts infected with M.tb and inhibited the apoptosis
of mature osteoclasts. These findings reveal a new osteoarticular
tuberculosis-activated cytokine network through which autophagy may
regulate bone metabolism and play an important role in the
pathogenesis of osteoarticular tuberculosis. These results provide
a foundation for the development of new drugs for treating
osteoarticular tuberculosis based on TNF-α-mediated osteoclast
autophagy.
Supplementary Material
Effect of 3-MA on autophagy protein
expression. Protein expression levels of Atg7, Beclin1 and LC3-II/I
in osteoclasts infected with M.tb, treated with TNF-α, and
untreated or treated with 3-MA. M.tb H37RvΔeis, inhibition
of autophagy gene knockout type M.tb H37Rv; M.tb
H37Rv wild type M.tb H37Rv; TNF-α, tumor necrosis factor-α;
Atg7, autophagy-related protein 7; 3-MA, 3-methyladenine; LC-3,
microtubule-associated proteins 1A/1B light chain 3A; M.tb,
Mycobacterium tuberculosis.
Acknowledgements
The authors would like to thank Dr Shuwen Jin from
the College of Acupuncture of Orthopedics, Hubei University of
Chinese Medicine, Wuhan, China for providing technical assistance.
The authors would like to thank Professor Fan Xionglin, School of
Basic Medicine, Tongji Medical College, Huazhong University of
Science and Technology, Wuhan, China for providing the bacteria
strain used in the study.
Funding
The current study is supported by grants from the
Research Project of Hubei Province Health and Family Planning
Commission (grant no. WJ2017Z022), Research Projects of Traditional
Chinese Medicine of Hubei Health Commission (grant no. ZY2019F025),
Wuhan Health and Family Planning Scientific Research Fund (grant
nos. WZ18Q05 and WX19Q11) and the Funded Research Project of Wuhan
First Hospital (grant no. 2019Y01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL contributed to study conception and design, data
collection and analysis and manuscript writing. JZ and FN
contributed to study conception and design and in critical revision
and review of the manuscript. FFP, ZWW, MH and XLZ analyzed the
data and reviewed the manuscript. LY and PFT performed the
experiments. PX and JF analyzed and interpreted the data and
contributed to final manuscript preparation. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of Wuhan First Hospital, Tongji Medical
College, Huazhong University of Science Technology. All patients or
their parents provided informed consent.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tiwari U, Ramachandran VG, Das S and Kumar
S: Interleukin-3 and interleukin-17 do not play a dynamic role in
the immunopathogenesis of osteoarticular tuberculosis. Indian J
Tuberc. 61:142–147. 2014.PubMed/NCBI
|
|
2
|
Kaufmann SH, Lange C, Rao M, Balaji KN,
Lotze M, Schito M, Zumla AI and Maeurer M: Progress in tuberculosis
vaccine development and host-directed therapies-a state of the art
review. Lancet Respir Med. 2:301–320. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Agarwal A: Acute suppurative presentation
of osteoarticular tuberculosis in children. Indian J Tuberc.
58:66–71. 2011.PubMed/NCBI
|
|
4
|
Hu P, Zhang H, Fleming J, Zhu G, Zhang S,
Wang Y, Liu F, Yi S, Chen Z, Chen Z, et al: Retrospective analysis
of false-positive and disputed rifampin resistance Xpert MTB/RIF
assay results in clinical samples from a referral hospital in
hunan, China. J Clin Microbiol. 57: pii:e01707–e01718.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu W, Lyu J, Cheng P, Cheng Y, Zhang Z, Li
L, Zheng Y and Xu J: Improvement in clinical outcome and infection
control using molecular diagnostic techniques for early detection
of MDR tuberculous spondylitis: A multicenter retrospective study.
Emerg Microbes Infect. 6(e97)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Trébucq A, Decroo T, Van Deun A, Piubello
A, Chiang CY, Koura KG and Schwoebel V: Short-course regimen for
multidrug-resistant tuberculosis: A decade of evidence. J Clin Med.
9: pii(E55)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou Y, Tan CY, Mo ZJ, Gao QL, He D, Li J,
Huang RF, Li YB, Guo CF, Guo Q, et al: Polymorphisms in the
SP110 and TNF-α gene and susceptibility to pulmonary
and spinal tuberculosis among southern chinese population. Dis
Markers. 2017(4590235)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meghji S, White PA, Nair SP, Reddi K,
Heron K, Henderson B, Zaliani A, Fossati G, Mascagni P, Hunt JF, et
al: Mycobacterium tuberculosis chaperonin 10 stimulates bone
resorption: A potential contributory factor in Pott's disease. J
Exp Med. 186:1241–1246. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu X, Jia W, Wang H, Wang Y, Ma J, Wang
H, Zhou X and Li G: Establishment of a rabbit model of spinal
tuberculosis using Mycobacterium tuberculosis strain H37Rv. Jpn J
Infect Dis. 68:89–97. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lawlor C, O'Connor G, O'Leary S, Gallagher
PJ, Cryan SA, Keane J and O'Sullivan MP: Treatment of Mycobacterium
tuberculosis-infected macrophages with Poly(Lactic-Co-Glycolic
Acid) microparticles drives NFκB and autophagy dependent bacillary
killing. PLoS One. 11(e0149167)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tateosian NL, Pellegrini JM, Amiano NO,
Rolandelli A, Casco N, Palmero DJ, Colombo MI and García VE: IL17A
augments autophagy in Mycobacterium tuberculosis-infected monocytes
from patients with active tuberculosis in association with the
severity of the disease. Autophagy. 13:1191–1204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ozeki N, Mogi M, Hase N, Hiyama T,
Yamaguchi H, Kawai R, Matsumoto T and Nakata K: Bone morphogenetic
protein-induced cell differentiation involves Atg7 and Wnt16
sequentially in human stem cell-derived osteoblastic cells. Exp
Cell Res. 347:24–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin NY, Beyer C, Giessl A, Kireva T,
Scholtysek C, Uderhardt S, Munoz LE, Dees C, Distler A, Wirtz S, et
al: Autophagy regulates TNFα-mediated joint destruction in
experimental arthritis. Ann Rheum Dis. 72:761–768. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Espert L, Beaumelle B and Vergne I:
Autophagy in Mycobacterium tuberculosis and HIV infections. Front
Cell Infect Microbio. 5(49)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gutierrez MG, Master SS, Singh SB, Taylor
GA, Colombo MI and Deretic V: Autophagy is a defense mechanism
inhibiting BCG and Mycobacterium tuberculosis survival in infected
macrophages. Cell. 119:753–766. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aydin V, Akici A, Isli F, Aksoy M, Aydin M
and Gursoz H: Relative risk of tuberculosis in patients with
rheumatic diseases managed with anti-tumour necrosis factor-alpha
therapy: A nationwide cohort study. J Clin Pharm The. 44:553–560.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo C, Wang L, Zhao Y, Jiang B, Luo J and
Shi D: BOS-93, a novel bromophenol derivative, induces apoptosis
and autophagy in human A549 lung cancer cells via PI3K/Akt/mTOR and
MAPK signaling pathway. Exp Ther Med. 17:3848–3858. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arai A, Kim S, Goldshteyn V, Kim T, Park
NH, Wang CY and Kim RH: Beclin1 modulates bone homeostasis by
regulating osteoclast and chondrocyte differentiation. J Bone Miner
Res. 34:1753–1766. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sabir N, Hussain T, Liao Y, Wang J, Song
Y, Shahid M, Cheng G, Mangi MH, Yao J, Yang L, et al: Kallikrein 12
regulates innate resistance of murine macrophages against
Mycobacterium bovis infection by modulating autophagy and
apoptosis. Cells. 8: pii(E415)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trejo-Solís C, Serrano-Garcia N,
Escamilla-Ramírez Á, Castillo-Rodríguez RA, Jimenez-Farfan D,
Palencia G, Calvillo M, Alvarez-Lemus MA, Flores-Nájera A,
Cruz-Salgado A and Sotelo J: Autophagic and Apoptotic pathways as
targets for chemotherapy in glioblastoma. Int J Mol Sci. 19:
pii(E3773)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park YE, Musson DS, Naot D and Cornish J:
Cell-cell communication in bone development and whole-body
homeostasis and pharmacological avenues for bone disorders. Curr
Opin Pharmacol. 34:21–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hoshino A, Hanada S, Yamada H, Mii S,
Takahashi M, Mitarai S, Yamamoto K and Manome Y: Mycobacterium
tuberculosis escapes from the phagosomes of infected human
osteoclasts reprograms osteoclast development via dysregulation of
cytokines and chemokines. Pathog Dis. 70:28–39. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yi L, Li Z, Jiang H, Cao Z, Liu J and
Zhang X: Gene modification of transforming growth factor β (TGF-β)
and interleukin 10 (IL-10) in suppressing Mt sonicate induced
osteoclast formation and bone absorption. Med Sci Monit.
24:5200–5207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun KT, Chen MY, Tu MG, Wang IK, Chang SS
and Li CY: MicroRNA-20a regulates autophagy related protein-ATG16L1
in hypoxia-induced osteoclast differentiation. Bone. 73:145–153.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
DeSelm CJ, Miller BC, Zou W, Beatty WL,
van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL and
Virgin HW: Autophagy proteins regulate the secretory component of
osteoclastic bone resorption. Dev Cell. 21:966–974. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xing L, Xiu Y and Boyce BF: Osteoclast
fusion and regulation by RANKL-dependent and independent factors.
World J Orthop. 3:212–222. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mootoo A, Stylianou E, Arias MA and Reljic
R: TNF-alpha in tuberculosis: A cytokine with a split personality.
Inflamm Allergy Drug Targets. 8:53–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen H, Cheng C, Li M, Gao S, Li S and Sun
H: Expression of TNF-α, IFN-γ, TGF-β and IL-4 in the spinal
tuberculous focus and its impact on the disease. Cell Biochem
Biophys. 70:1759–1764. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Patil T, Garg RK, Jain A, Goel MM,
Malhotra HS, Verma R, Singh GP and Sharma PK: Serum and CSF
cytokines and matrix metalloproteinases in spinal tuberculosis.
Inflamm Res. 64:97–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mattos AM, Almeida Cde S, Franken KL,
Alves CC, Abramo C, de Souza MA, L'Hotellier M, Alves MJ, Ferreira
AP, Oliveira SC, et al: Increased IgG1, IFN-gamma, TNF-alpha and
IL-6 responses to Mycobacterium tuberculosis antigens in patients
with tuberculosis are lower after chemotherapy. Int Immunol.
22:775–782. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng M, Shi S, Wei W, Zheng Q, Wang Y,
Ying X and Lu D: Correlation between MBL2/CD14/TNF-α gene
polymorphisms and susceptibility to spinal tuberculosis in Chinese
population. Biosci Rep. 38: pii(BSR20171140)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kobayashi K, Takahashi N, Jimi E, Udagawa
N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima
N, et al: Tumor necrosis factor alpha stimulates osteoclast
differentiation by a mechanism independent of the ODF/RANKL-RANK
interaction. J Exp Med. 191:275–286. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Osta B, Benedetti G and Miossec P:
Classical and paradoxical effects of TNF-α on bone homeostasis.
Front Immunol. 5(48)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawai VK, Stein CM, Perrien DS and Griffin
MR: Effects of anti-tumor necrosis factor α agents on bone. Curr
Opin Rheumatol. 24:576–585. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin NY, Stefanica A and Distler JH:
Autophagy: A key pathway of TNF-induced inflammatory bone loss.
Autophagy. 9:1253–1255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tong X, Gu J, Song R, Wang D, Sun Z, Sui
C, Zhang C, Liu X, Bian J and Liu Z: Osteoprotegerin inhibit
osteoclast differentiation and bone resorption by enhancing
autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell
Biochem. 2018, doi: 10.1002/jcb.27468. [Epub ahead of print].
|