Introduction
Periodontal disease (PD) is a chronic inflammatory
condition that leads to the gradual destruction of the tissues
supporting the teeth (1). The
disease occurs in patients who have subgingival bacterial biofilm
and an inadequate type and intensity of host immune response
(2). Although the bacterial
subgingival biofilm can be found in numerous patients, the disease
is triggered only in part of them, suggesting an important impact
of the interaction between the bacterial challenge and the host
immune response on the pathological mechanisms that govern this
condition (3).
During periodontal inflammation, host immune cells,
such as polymorphonuclear neutrophils (PMNs), macrophages and
lymphocytes, will produce increased levels of pro-inflammatory
mediators: cytokines, mainly interleukin-1 family (IL-1),
interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α),
prostaglandins, prostaglandin E2 (PGE2) and
enzymes, matrix-metalloproteinases (MMPs), collagenases or
gelatinases (4-6).
These pro-inflammatory mediators are intended to improve the
quality and intensity of the host immune response, but due to the
particular characteristics of periodontal inflammation, they lack
efficacy (7). Consequently, their
increased levels inside the gingival connective tissue or
epithelium result in an exacerbated chronic inflammatory reaction,
which will contribute to periodontal damage (8).
The levels of pro-inflammatory cytokines can be a
useful tool for the assessment of periodontal disease activity and
evolution (9). The inflammatory
infiltrate, found inside the gingival connective tissue, creates an
augmented osmotic pressure which increases the production of
gingival crevicular fluid (GCF) (10). This fluid contains important
inflammation indicators, such as cytokines, leucocytes, bacterial
cells and degraded periodontal tissue components (11). The cytokines from the gingival fluid
can be assessed qualitatively and quantitatively, being a useful
indicator of the periodontal inflammatory status (12). Important risk factors for the PD's
aetiology are systemic diseases and conditions (13). These can have a major impact on the
host immune response and can alter the inflammatory reaction, which
occurs during periodontal bacterial challenge, causing important
tissue damage (5,13). A large number of systemic conditions
can have oral or periodontal manifestations (13). The correlation between PD and various
systemic conditions such as diabetes mellitus (DB), cardiovascular
disease (CAD), rheumatoid arthritis (RA) and others has been
intensely studied and relevant results have been united under the
concept of ‘periodontal medicine’, which offers practitioners a
comprehensive perspective of PD pathology, diagnosis and
therapeutic strategies (14).
Chronic viral hepatitis C (CHC) occurs as a
consequence of the infection with the hepatitis C virus (HCV) that
uses hepatic or peripheral blood cells for replication (15). The spread of the infection is
considered a global health issue, as it interests more than 200
million people worldwide, and is particularly difficult to combat,
because during its initial stages, the disease has no symptoms
(15). As a result, infected persons
may be unaware and prone to infect others (15). After the acute stage of infection,
most patients will develop a chronic inflammation of the hepatic
tissue. Gradually, the hepatic function is impaired, as the hepatic
tissue is replaced with fibrotic tissue (16) and liver cirrhosis occurs. Chronic
hepatitis C patients can also develop other life-threathening
complications, such as hepatocellular carcinoma (17,18).
Being a chronic inflammatory condition, CHC could exhibit some
correlations and interactions with PD, possibly via the
pro-inflammatory mediators that are discharged into the blood flow
of HCV patients (19). Natural
killer cells (NK), which have TNF production capabilities, play an
important role in the immunological pathogenesis of CHC (20). Blood levels of certain cytokines,
such as IL-18 and IL-33, are used as an indicator of CHC disease
activity and severity, as these patients have been shown to exhibit
increased serological levels of these interleukins (21,22).
Elevated levels of interleukin-1α have also been found in serum
samples of chronic hepatitis C patients, and have been directly
associated with the severity of the disease (23,24).
Furthermore, some pro-inflammatory mediators, such as
interleukin-1β, have been shown to promote liver inflammatory
processes in chronic hepatitis C patients (25), expressing increased serum levels in
these patients (26).
The study objective was to analyse the impact that
chronic hepatitis C could have on the inflammatory status of
patients with chronic periodontal disease, who also suffer from HCV
infection, by assessing the GCF levels of interleukin-1α (IL-1α)
and interleukin-1β (IL-1β) in these patients, and comparing the
results to those of systemically healthy periodontal patients.
Higher levels of these particular interleukins, which have
considerable implications in the pathogenic processes of both PD
and CHC, could imply that chronic hepatitis C acts as an additional
risk factor for PD and that it has a negative impact on the
inflammatory status of periodontal patients, assessed by
interleukin detection.
Patients and methods
Patient selection
The patients were selected among those who addressed
the Department of Periodontology of the University of Medicine and
Pharmacy of Craiova (Craiova, Romania), for periodontal complaints
or pre-orthodontic treatment periodontal evaluation. Upon approval
from the University's Ethical Scientific Committee, the patients
who expressed their informed consent for participating in the study
were subjected to an oral and periodontal examination (27). The patients were also requested to
fill a medical questionnaire regarding their medical history and
existing systemic diagnosis or medication.
For each patient the following periodontal
parameters were recorded: The number of teeth with periodontal
pockets deeper than 4 mm (AT), the maximum periodontal probing
depth (MD) and the number of existing/remnant teeth (RT). Only
patients having at least two periodontal pockets in two distinct
teeth, with minimum 5 mm probing depth, were included in the
study.
Patients suffering from asymptomatic chronic
hepatitis C were included in the study, the diagnosis being
confirmed in the Gastroenterology Clinic of the University of
Medicine and Pharmacy of Craiova (Craiova, Romania) based on blood
tests results regarding the aspartate transaminase (AST) and
alanine transaminase (ALT) serum levels. The age of the HCV
infection diagnosis was also recorded for the chronic hepatitis C
patients.
Patients not suffering from systemic diseases, and
who were diagnosed with chronic periodontal disease, through the
periodontal examination, were included in the study, as part of the
periodontally affected, but systemically healthy group.
The study protocol and patient informed consent form
for participation in the study and collection of gingival
crevicular samples were approved by the University Ethics
Scientific Committee (University of Medicine and Pharmacy of
Craiova, Romania, no. 17/2016). Written informed consent was given
by all participating patients.
The study also comprised a group of control
patients, periodontally and systemically healthy, who were referred
to the Department of Periodontology for pre-orthodontic periodontal
evaluation.
For the unbiased evaluation of their inflammatory
status, patients were excluded from the study if they met at least
one of the following exclusion criteria: i) Anti-inflammatory
treatment in the last month; ii) antibiotic treatment in the last 3
months; iii) pregnancy; iv) decompensated or symptomatic chronic
hepatitis C; v) current smoker and vi) any diagnosed systemic
condition other than chronic hepatitis C.
After applying the inclusion and exclusion criteria,
three groups of patients were set up for the study: i) P group, 13
patients with chronic periodontal disease and no systemic disease
(6 male and 7 female, aged between 40-58 years); ii) PH group, 11
patients with chronic periodontal disease and asymptomatic chronic
hepatitis C (8 male and 3 female, aged between 36-62 years); iii) H
group, 11 control patients with no systemic and periodontal disease
(7 male and 4 female, aged between 31-43 years).
GCF sampling
Gingival crevicular fluid samples were collected
from all participating patients by using absorbent paper strips
(PerioPaper; Oraflow Inc.). The paper strips were inserted into the
gingival sulcus or periodontal pockets and kept for 30 sec. For
each participating patient two samples of gingival fluid were
collected, from two distinct teeth and placed into polypropylene
containers with 200 µl of phosphate-buffered saline (PBS) solution.
The sampling was repeated at 1 min interval from the same two teeth
and the paper strips were pooled into another container. For the
periodontitis patients, the teeth selected for gingival fluid
sampling were the two teeth with the deepest periodontal pockets,
while the same analogue teeth were used for the control group
patients. Preventive measures to avoid sample contamination were
taken. To prevent saliva contamination, cotton rolls were placed on
oral vestibule and saliva suction was used underneath the tongue,
and to prevent blood contamination, the paper strips were only
inserted into the sulcus after no clearly visible signs of gingival
bleeding were observed. Only the paper strips with no visible
stains of blood on their surface were used. To assess the quantity
of GCF absorbed by the paper strips, these were inserted into a
special volume analyzer, Periotron 8000 (Oraflow Inc.). Afterwards,
the two containers from each patient were refrigerated at -20˚C,
until completion of sample collection.
Assessment of GCF IL-1α and IL-1β
level
The enzyme-linked immunosorbent assay (ELISA) was
used for qualitative and quantitative assessment of the two
pro-inflammatory cytokines in the GCF samples. Commercial detection
kits designed for the micro-detection of the two cytokines were
used (Quantikine; R&D Systems) in accordance with the
manufacturer's indications and prescribed method. In order to
decrease optical imperfections on the reading plate, reading was
performed at 450 with 540 nm corrections. The obtained results were
expressed in pg/ml.
Statistical analysis
Data was statistically analyzed with Microsoft
Office Excel Data Analysis tool kit software (Microsoft
Corporation), the resulted mean values and standard deviations
being used as primary data (mean ± SD). The statistical
significance of the results was assessed using the Mann-Whitney U
test (P<0.05) for comparison between the different study groups.
Correlations between statistical and clinical data were made using
Pearsons's test.
Results
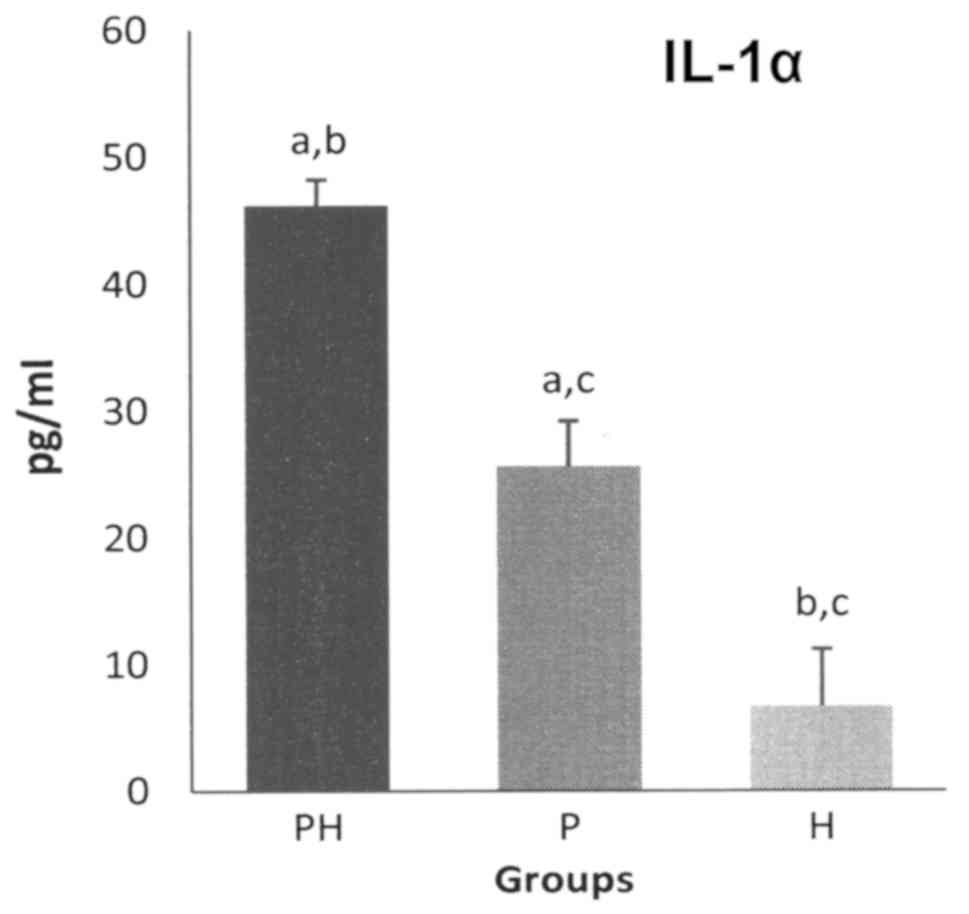
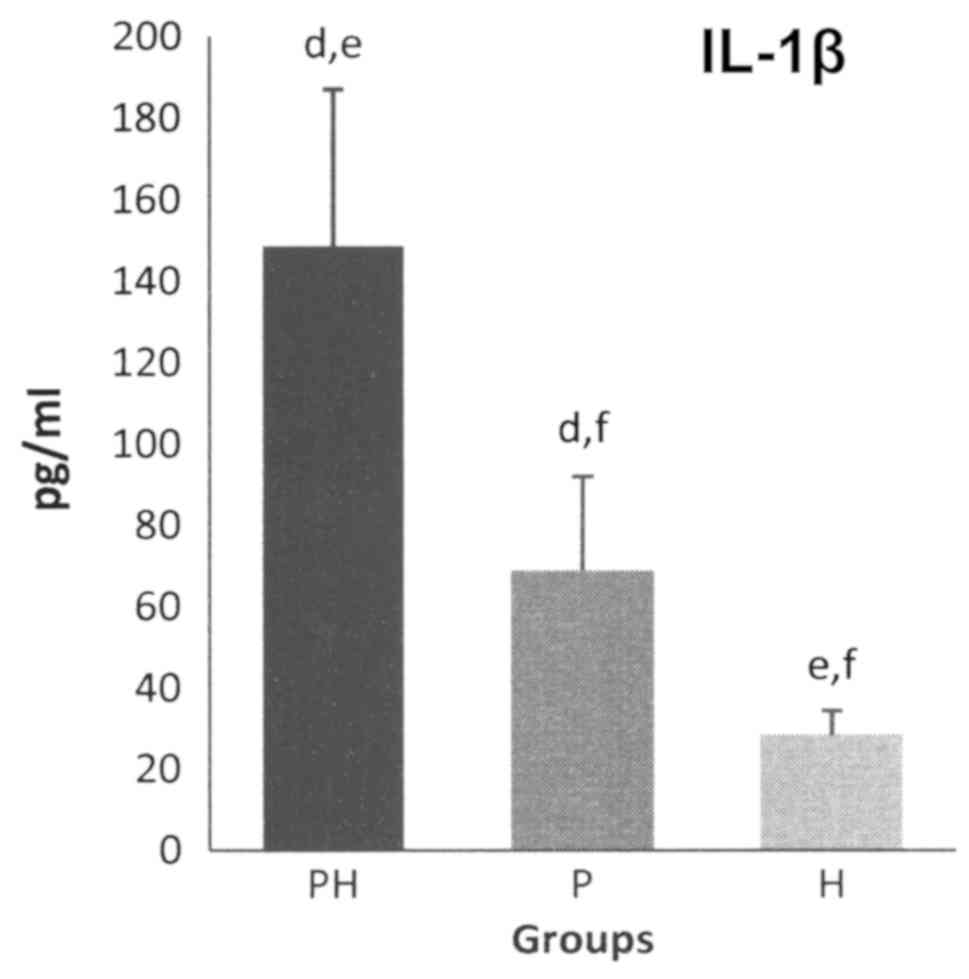
The GCF levels of pro-inflammatory cytokines, for
both IL-1α and IL-1β, were the highest for the PH group, followed
by the P group, while the lowest were those of the H group
(Figs. 1 and 2).
Statistically significant differences (P<0.05)
were recorded for GCF IL-1α levels, between the PH and P groups
(the levels for the PH group being 1.8-fold higher than that for P
group), between the PH and H groups (the levels for the PH group
being 6.9-fold higher than that for the H group) and between P and
H groups (the levels for the P group being 3.8-fold higher than
that for the H group) (Fig. 1).
The average GCF levels of IL-1β expressed
statistically significant differences (P<0.05) between the PH
and P groups (the levels for the PH group being 2.1-fold higher
than that of the P group), between the PH and H groups (the levels
for the PH group being 5.1-fold higher than that for the H group)
and between the P and H groups (the levels for the P group being
2.41-fold higher than that for the H group) (Fig. 2).
In P and PH patients, the levels of GCF cytokines
expressed correlations with certain parameters of periodontal
status (Table I). The results of the
periodontal and hepatic evaluation have been previously published
(27), expressing statistically
significant differences between the P and PH groups, regarding the
average number of teeth, the maximum periodontal probing depth and
the AST/ALT serum levels.
 | Table ICorrelations between the assessed
clinical and immunologic parameters. |
Table I
Correlations between the assessed
clinical and immunologic parameters.
| | PH Group | P Group | H Group |
|---|
| Index | IL-1α | IL-1β | IL-1α | IL-1β | IL-1α | IL-1β |
|---|
| RT |
|
r | -0.44 | -0.29 | -0.42 | -0.3 | -0.37 | -0.8 |
|
p | 0.17 | 0.37 | 0.13 | 0.3 | 0.25 | 0.02 |
| MD |
|
r | 0.23 | 0.35 | 0.21 | 0.21 | | |
|
p | 0.48 | 0.26 | 0.42 | 0.41 | | |
| AT |
|
r | 0.32 | 0.25 | 0.28 | 0.68 | | |
|
p | 0.33 | 0.37 | 0.31 | 0.01 | | |
| DG |
|
r | 0.23 | 0.29 | | | | |
|
p | 0.49 | 0.38 | | | | |
For the PH group, the MD was positively correlated
to the GCF levels of both IL-1α and IL-1β. For the P group, a
moderate positive correlation was found between the MD parameter
and the level of GCF cytokines.
As previously shown (27), the AT did not express significant
statistical difference between the PH and P groups. No significant
correlations were found between this periodontal parameter and the
GCF levels of IL-1α or IL-1β. As it was shown (27), the RT was significantly different
between the PH and P groups. This parameter expressed negative
correlations with the GCF levels of both IL-1α and IL-1β.
For the PH group, the age of the HCV infection
diagnosis expressed a slight positive correlation with the GCF
levels of IL-1α and IL-1β.
Discussion
In PD patients, an important imbalance exists
between the subgingival bacterial plaque aggression and the host
immune response, a situation that can lead to extensive damage of
the periodontal tissues, such as the periodontal ligament and
alveolar bone. Consequently, the teeth lose their supporting
structures and gain increasing mobility (28).
PD usually has a chronic evolution, when episodes of
disease activity and high tissue destruction alternate with periods
of inactivity and slight improvement of the clinical features of
periodontal inflammation (29). This
is caused either by fluctuations in bacterial aggressiveness, or by
improvements of the host immune response. Unlike other gingival
changes that do not involve deep tissue destruction, as those
occurring during orthodontic treatment (30), these events will eventually lead to
tooth loss, if left untreated or if continuous periodontal
treatment is disrupted (5).
Periodontal infection is caused by the subgingival
bacterial biofilm, with important influences from local and general
risk factors (31,32). The bacterial biofilm develops
gradually and can survive for an undetermined period of time, if
left undisrupted by mechanical and chemical means. This causes the
periodontal tissues to be exposed for a long period to bacterial
toxins [lipopolysaccharide (LPS)], enzymes (proteases) and other
noxious metabolic products (ammonia, hydrogen sulphide and butyric
acid) (33). Moreover, bacterial
cells invade the periodontal connective tissues via the internal
and junctional epithelium. All these factors act as aggressors and
cause chemical, physical and biological damage to cells.
Consequently, the inflammatory reaction is triggered and cytokine
production is elevated, in order to boost the immune response and
to uphold the bacterial challenge (5).
Elevated gingival fluid levels for IL-1, found in
periodontal patients, compared to healthy controls, have been shown
to decrease after periodontal treatment (34), a fact which further supports their
important role in periodontal disease development. The results of
the present study expressed a statistically significant difference
between the GCF cytokine levels of the PH and P groups. Since
patients in both groups were diagnosed with periodontal disease, in
similar degrees of severity and evolution, the higher GCF cytokines
levels in periodontitis patients with chronic hepatitis C, could be
explained by the additional chronic hepatic inflammation that these
patients manifest. This fact can impact the intensity of the
inflammatory periodontal reaction as well. Moreover, the GCF
cytokine level was significantly different between P and H groups
and between PH and H groups, suggesting the reliability of
cytokines as indicators of the inflammatory status.
In chronic periodontitis patients, the levels of GCF
cytokines expressed a positive correlation to the degree of
periodontal inflammation. In the present study, the clinical
indicators used for the assessment of the periodontal status (MD,
AT, RT) were correlated to the GCF levels of IL-1α and IL-1β in
periodontal patients with chronic hepatitis C. There was a moderate
positive correlation also between these clinical parameters in
periodontal patients with no systemic condition and the GCF
cytokine levels, suggesting the additional effect that systemic
chronic inflammation can have on the periodontal status of such
patients. The number of remnant teeth was statistically
significantly different between the three groups and in a negative
correlation with the GCF cytokine levels, emphasizing the important
impact that severe chronic inflammation has on the dental and
periodontal history of the patient, frequently resulting in the
loss of teeth, as a consequence of periodontal disease. Due to the
reduced number of participating patients in the study, consequent
to the numerous exclusion criteria, required in order to follow a
precise and reliable scientific method, the identified correlations
generally lacked statistical significance, despite their moderate
correlation strength. This issue motivates the extension of the
study design on a broader cohort of participating patients.
The number of teeth with periodontal pockets deeper
than 4 mm (AT) was not statistically different between the PH and P
groups and it did not correlate with the GCF cytokine levels.
However, the average maximum periodontal pocket depth (MD) was
statistically different between the PH and P groups and expressed a
positive correlation to the GCF levels of both IL-1α and IL-1β.
This fact could imply that hepatic chronic inflammation could have
a significant impact on the severity of periodontal disease and a
less important one on its extent, as in the number of affected
teeth.
In this study, there was a moderate positive
correlation between the GCF levels of cytokines of the PH group
patients and the age of their HCV infection diagnosis. This
correlation suggests that, as the chronic hepatic inflammatory
reaction progresses, it has an increasingly important negative
impact on the inflammatory periodontal status of CHC patients, who
also suffer from periodontal disease. Thus, periodontal and hepatic
chronic inflammatory reactions could influence one another, as they
may be fuelled by the same pro-inflammatory cytokines, i.e. IL-1α
and IL-1β. These correlations suggest the important negative impact
that hepatic chronic inflammation has on the periodontal status
regarding the intensity of the periodontal inflammatory
reaction.
In CHC patients, elevated levels of IL-1α and IL-1β
were showed in serum samples, in previous studies (23,24).
Levels of certain cytokines were significantly elevated when the
CHC patients were also suffering from diabetes (35). Furthermore, the incidence of type Ⅱ
diabetes among CHC patients is higher than that of the general
population (36). One explanation
for this correlation can be found in the impact of chronically
elevated systemic IL-1β levels on general homeostasis (37). It appears that, cellular insulin
resistance is closely linked to elevated levels of cytokines, which
are common in CHC patients (38-40).
In the hepatic tissue of affected patients, insulin receptor
substrate has been reported to be impaired, together with the
disruption of normal insulin signalling pathways (40). Increased cytokine expression (such as
TNF-α) could also contribute to the onset of insulin resistance in
CHC patients (40). Consequently,
the cells become insensitive to insulin action and glucose is
unable to enter inside them, in order to be metabolised. Thus,
chronic hyperglycemia occurs, the most important pathologic
manifestation of diabetes mellitus. Interestingly, insulin
resistance has also been associated with PD, while elevated levels
of IL-1β have often been recorded in chronic periodontitis patients
(41,42). The interactions between PD and liver
diseases, including CHC, could be mediated by either bacterial
elements, pro-inflammatory cytokines or oxidative stress (43). Elevated GCF levels of IL-1α and IL-1β
could explain the pathogenic mechanism that connects PD and CHC. By
changing the general homeostasis, the chronic inflammatory status
caused by PD and CHC, inflicts a modified cellular response.
Patients with CHC and PD exhibit elevated cytokine profiles,
suggesting the bi-directional impact that chronic hepatic
inflammation and periodontal disease could manifest on each other.
Insulin resistance could be one of the probable pathogenic
mechanisms to mediate the PD-CHC relationship (19,43),
motivating extended future research on the matter.
In conclusion, elevated levels of IL-1α and IL-1β,
which have considerable implications in the pathogenic processes of
both PD and CHC, could imply that chronic hepatitis C has a
negative impact on the inflammatory status of periodontal patients,
as assessed by interleukin detection.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PS, DNG, AR, LL, SIS, MVB and IR contributed to the
study design. DMP and DNG performed the clinical periodontal
assessment and collection of gingival crevicular fluid samples. MD
and IR interpreted the hepatic status of chronic hepatitis C
patients. MVB and LB carried out the immunological analysis and
acquisition of laboratory data. AMM, SS and DNG performed the
statistical data analysis. DR and DNG drafted the manuscript, which
was critically revised by PS, AR, LL, SS, SIS and LF. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocol and patient informed consent form for
participating and collection of gingival crevicular samples were
approved by the University Ethics Scientific Committee (University
of Medicine and Pharmacy of Craiova, Romania, no. 17/2016). Written
informed consent was given by all participating patients.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Benedetto A, Gigante I, Colucci S and
Grano M: Periodontal disease: linking the primary inflammation to
bone loss. Clin Dev Immunol. 2013(503754)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peyyala R and Ebersole JL: Multispecies
biofilms and host responses: ‘Discriminating the trees from the
forest’. Cytokine. 61:15–25. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Silva N, Abusleme L, Bravo D, Dutzan N,
Garcia-Sesnich J, Vernal R, Hernández M and Gamonal J: Host
response mechanisms in periodontal diseases. J Appl Oral Sci.
23:329–355. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stadler AF, Angst PDM, Arce RM, Gomes SC,
Oppermann RV and Susin C: Gingival crevicular fluid levels of
cytokines/chemokines in chronic periodontitis: A meta-analysis. J
Clin Periodontol. 43:727–745. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cekici A, Kantarci A, Hasturk H and Van
Dyke TE: Inflammatory and immune pathways in the pathogenesis of
periodontal disease. Periodontol 2000. 64:57–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
7
|
Genco RJ and Slots J: Host responses in
periodontal diseases. J Dent Res. 63:441–451. 1984.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tribble GD and Lamont RJ: Bacterial
invasion of epithelial cells and spreading in periodontal tissue.
Periodontol 2000. 52:68–83. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ghallab NA: Diagnostic potential and
future directions of biomarkers in gingival crevicular fluid and
saliva of periodontal diseases: Review of the current evidence.
Arch Oral Biol. 87:115–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Barros SP, Williams R, Offenbacher S and
Morelli T: Gingival crevicular fluid as a source of biomarkers for
periodontitis. Periodontol 2000. 70:53–64. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta G: Gingival crevicular fluid as a
periodontal diagnostic indicator - I: Host derived enzymes and
tissue breakdown products. J Med Life. 5:390–397. 2012.PubMed/NCBI
|
|
12
|
Majeed ZN, Philip K, Alabsi AM,
Pushparajan S and Swaminathan D: Identification of gingival
crevicular fluid sampling, analytical methods, and oral biomarkers
for the diagnosis and monitoring of periodontal diseases: A
systematic review. Dis Markers. 2016(1804727)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cardoso EM, Reis C and Manzanares-Céspedes
MC: Chronic periodontitis, inflammatory cytokines, and
interrelationship with other chronic diseases. Postgrad Med.
130:98–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beck JD, Papapanou PN, Philips KH and
Offenbacher S: Periodontal medicine: 100 Years of Progress. J Dent
Res. 98:1053–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wedemeyer H: Hepatitis C. In: Sleisenger
and Fordtran's Gastrointestinal and Liver Disease. Feldman M,
Friedman LS and Brandt LJ (eds). Elsevier Saunders, Philadelphia,
pp1332-1352, 2015.
|
|
16
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Gheonea DI, Streba CT, Vere CC, Şerbănescu
M, Pirici D, Comănescu M, Streba LA, Ciurea ME, Mogoantă S and
Rogoveanu I: Diagnosis system for hepatocellular carcinoma based on
fractal dimension of morphometric elements integrated in an
artificial neural network. Biomed Res Int.
2014(239706)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sandulescu L, Rogoveanu I, Gheonea IA,
Cazacu S and Saftoiu A: Real-time elastography applications in
liver pathology between expectations and results. J Gastrointestin
Liver Dis. 22:221–227. 2013.PubMed/NCBI
|
|
19
|
Gheorghe DN, Foia L, Toma V, Surdu A,
Herascu E, Popescu DM, Surlin P, Vere CC and Rogoveanu I: Hepatitis
C infection and periodontal disease: Is there a common
immunological link? J Immunol Res. 2018(8720101)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoon JC, Yang CM, Song Y and Lee JM:
Natural killer cells in hepatitis C: Current progress. World J
Gastroenterol. 22:1449–1460. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang J, Zhao P, Guo H, Sun X, Jiang Z, Xu
L, Feng J, Niu J and Jiang Y: Serum IL-33 levels are associated
with liver damage in patients with chronic hepatitis C. Mediators
Inflamm. 2012(819636)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma A, Chakraborti A, Das A, Dhiman RK
and Chawla Y: Elevation of interleukin-18 in chronic hepatitis C:
Implications for hepatitis C virus pathogenesis. Immunology. 128
(Suppl 1):e514–e522. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tawfik AK, Amin AM, Yousef M, El-Sayd NM,
Elashry H, Elkadeem M and Abd-Elsalam S: IL-1α correlates with
severity of hepatitis C virus-related liver diseases. J Inflamm
Res. 11:289–295. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vanis N, Mehmedović A and Mesihović R: Use
of serum levels of proinflammatory cytokine IL-1α in chronic
hepatitis C. Coll Antropol. 39:75–79. 2015.PubMed/NCBI
|
|
25
|
Negash AA, Ramos HJ, Crochet N, Lau DT,
Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, et
al: IL-1β production through the NLRP3 inflammasome by hepatic
macrophages links hepatitis C virus infection with liver
inflammation and disease. PLoS Pathog. 9(e1003330)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
El-Ghaffar NA, Rasheed W, Ramzy T and El
Batae H: Prognostic significance of interleukins determination in
liver diseases. Res J Med Med Sci. 3:124–131. 2008.
|
|
27
|
Gheorghe DN, Rusu D, Herascu E, Popescu
DM, Surlin P and Rogoveanu I: Evaluation of liver chemistry tests
and clinical parameters in patients with periodontal disease and
chronic hepatitis C. Rev Chim. 68:1252–1254. 2017.
|
|
28
|
Hajishengallis G: Immunomicrobial
pathogenesis of periodontitis: Keystones, pathobionts, and host
response. Trends Immunol. 35:3–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shaddox LM and Walker CB: Treating chronic
periodontitis: Current status, challenges, and future directions.
Clin Cosmet Investig Dent. 2:79–91. 2010.PubMed/NCBI
|
|
30
|
Surlin P, Silosi I, Rauten AM, Cojocaru M
and Foia L: Involvement of TSP1 and MMP9/NGAL in angiogenesis
during orthodontic periodontal remodeling. ScientificWorldJournal.
2014(421029)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim J and Amar S: Periodontal disease and
systemic conditions: A bidirectional relationship. Odontology.
94:10–21. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
AlJehani YA: Risk factors of periodontal
disease: Review of the literature. Int J Dent.
2014(182513)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kinane DF, Podmore M, Murray MC, Hodge PJ
and Ebersole J: Etiopathogenesis of periodontitis in children and
adolescents. Periodontol 2000. 26:54–91. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reis C, DA Costa AV, Guimarães JT, Tuna D,
Braga AC, Pacheco JJ, Arosa FA, Salazar F and Cardoso EM: Clinical
improvement following therapy for periodontitis: Association with a
decrease in IL-1 and IL-6. Exp Ther Med. 8:323–327. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jia HY, Du J, Zhu SH, Ma YJ, Chen HY, Yang
BS and Cai HF: The roles of serum IL-18, IL-10, TNF-alpha and
sIL-2R in patients with chronic hepatitis C. Hepatobiliary Pancreat
Dis Int. 1:378–382. 2002.PubMed/NCBI
|
|
36
|
Garcia-Compean D, Jaquez-Quintana JO,
Gonzalez-Gonzalez JA and Maldonado-Garza H: Liver cirrhosis and
diabetes: Risk factors, pathophysiology, clinical implications and
management. World J Gastroenterol. 15:280–288. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gao D, Madi M, Ding C, Fok M, Steele T,
Ford C, Hunter L and Bing C: Interleukin-1β mediates
macrophage-induced impairment of insulin signaling in human primary
adipocytes. Am J Physiol Endocrinol Metab. 307:E289–E304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kukla M, Piotrowski D, Waluga M and
Hartleb M: Insulin resistance and its consequences in chronic
hepatitis C. Clin Exp Hepatol. 1:17–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Knobler H and Schattner A: TNF-{alpha},
chronic hepatitis C and diabetes: A novel triad. QJM. 98:1–6.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bose SK and Ray R: Hepatitis C virus
infection and insulin resistance. World J Diabetes. 5:52–58.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Demmer RT, Squillaro A, Papapanou PN,
Rosenbaum M, Friedewald WT, Jacobs DR Jr and Desvarieux M:
Periodontal infection, systemic inflammation, and insulin
resistance: Results from the continuous National Health and
Nutrition Examination Survey (NHANES) 1999-2004. Diabetes Care.
35:2235–2242. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gurav AN: Periodontitis and insulin
resistance: Casual or causal relationship? Diabetes Metab J.
36:404–411. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Han P, Sun D and Yang J: Interaction
between periodontitis and liver diseases. Biomed Rep. 5:267–276.
2016.PubMed/NCBI View Article : Google Scholar
|