Introduction
Bronchial asthma results from chronic allergic
inflammation of the airway, characterized by airway
hyperresponsiveness, airflow limitation and decreased lung function
(1). Airway inflammation and
remodeling are the main pathological features of bronchial asthma
(2). With the rapid development of
the economy, environmental pollution has become increasingly
serious and the incidence of bronchial asthma in children has been
increasing each year (3). Airway
remodeling occurs not only in severe or late-stage asthma, but also
during the early stages of mild asthma and childhood asthma
(4). Therefore, it has been
suggested that airway remodeling may be a cause of refractory
asthma (5). The mechanism of airway
remodeling during the early stages of bronchial asthma is an
important prognostic factor for children with bronchial asthma
(6).
Airway remodeling primarily refers to changes in the
size or number of tissue components during airway development or
occurs as a result of injury and/or inflammation (7,8).
Pathological changes in airway remodeling include epithelial cell
exfoliation, inflammatory cell infiltration, extracellular matrix
deposition, reticular basement membrane thickening, submucosal
goblet cell proliferation, fibroblast proliferation, smooth muscle
proliferation and hypertrophy, angiogenesis and airway wall
thickening (9-11).
Previous studies have reported that a large number of lung
fibroblasts exist in lung tissue and these cells maintain the
integrity of the lung tissue structure and function by synthesizing
extracellular matrix and cytokines (12,13).
When lung tissues are damaged, fibroblasts become dysfunctional and
can be transformed into extracellular matrix or lung fibroblasts
with strong cytokine synthesis ability, ultimately leading to
pulmonary inflammation and fibrotic processes (14,15).
Interleukin (IL)-22 is a cytokine that belongs to
the IL-10 family (16,17). IL-22 is primarily synthesized and
secreted by macrophages, dendritic cells, natural killer T (NKT)
cells and natural killer (NK) cells, and can bind to the IL-22
receptor 1 (IL-22R1) and IL-10 receptor 2 (IL-10R2) to activate the
mitogen-activated protein kinase (MAPK) or JAK/STAT signaling
pathways (18). IL-22 plays an
important role in multiple sclerosis, arthritis and wound tissue
repair (19-21).
However, it is unclear whether the IL-22/IL-22R1 signaling pathway
plays a role in the regulation of airway subepithelial fibrosis
caused by fibroblasts. Inflammatory factors such as tumor necrosis
factor (TNF)-α and IL-37 play a role in airway remodeling as they
can activate various signaling pathways, such as STAT3 and NF-κB
and regulate the biological functions of cells. IL-22 has been
reported to activate the JAK/STAT3 signaling pathway. Furthermore,
STAT3 participates in airway remodeling (22). Therefore, the present study
investigated the relationship between airway remodeling and the
IL-22/IL-22R1 signaling pathway in bronchial asthma.
Materials and methods
Subjects
A total of 41 children with bronchial asthma who had
received treatment at the Department of Pediatrics, Nanchong
Central Hospital between May 2015 and December 2017 were included
in the experimental group (27 male patients and 14 female patients;
mean age, 10.6±2.6 years). All subjects in the experimental group
met the diagnostic criteria detailed in the Guidelines for the
Prevention and Treatment of Bronchial Asthma formulated by the
Asthma Group of the Society of Respiratory Diseases of the Chinese
Medical Association (23).
Additionally, 12 healthy children who had undergone physical
examinations at the Physical Examination Center of Nanchong Central
Hospital between May 2015 and December 2017 were included in the
control group (8 male patients and 4 female patients; mean age,
12.2±1.8 years). The inclusion criteria for all the subjects were:
i) No recent history of acute infection; ii) no immune diseases,
for example ulcerative colitis, kidney disease and rheumatoid
arthritis; and iii) no administration of hormone drugs orally or
intravenously in the past month. Peripheral blood (10 ml) was
collected from median cubital veins of all the participants and was
stored evenly between two blood tubes (5 ml each). One tube was
used for the separation of serum by centrifugation at 600 x g and
4˚C for 5 min, and the other tube was used for the separation of
mononuclear lymphocytes. To obtain peripheral blood mononuclear
cells (PBMCs), the mixture of heparin-anticoagulant venous blood
and equal amount of serum-free Iscove's modified Dulbecco's medium
(v/v, 1:1; Stemcell Technologies, Inc.) was gently added onto
lymphocyte separation medium before centrifugation at 25˚C and 400
x g for 30 min. Following centrifugation, the middle layer was
aspirated and mixed with 20 ml Hank's Balanced Salt Solution before
centrifugation at 25˚C and 300 x g for 10 min. After washing the
cells twice, the cells were counted and diluted to a density of
1x106/ml. Finally, 3x106 cells were seeded
onto a round culture plate with a bottom area of 9 cm2,
followed by incubation at 37˚C and 5% CO2 for 1-2 h. The
cells that attached on the bottom were PBMCs.
All the procedures were approved by the Ethics
Committee of North Sichuan Medical College (approval no.
NS-CE-017-03). Written informed consent was obtained from the
guardians of all participants.
Cells
The human embryonic lung fibroblast cell line MRC-5
was purchased from the Cell Bank of Type Culture Collection,
Chinese Academy of Sciences. Prior to transfection, MRC-5 cells
(2x105) in the logarithmic growth phase were seeded into
24-well plates and cultured in antibiotic-free RPMI-1640 medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) at room temperature
until 70% confluency was reached. In the first vial, 1.5 µl
small-interfering (si)RNA of IL-22R1 (siR-IL-22R1; 50 pmol/µl;
5'-GGTCTACAGCATCGAGTAT-3'; Hanbio Biotechnology Co., Ltd.) or
negative control (si-NC; cat. no. siN0000001-1-10; Guangzhou
RiboBio Co., Ltd.) was mixed with 50 µl Opti Mem medium (Thermo
Fisher Scientific, Inc.). In the second vial, 1 µl
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
mixed with 50 µl Opti Mem medium. After 5 min, the two vials were
combined for 20 min at room temperature. Subsequently, the mixtures
(500 µl) were added onto cells in siR-NC and siR-IL-22R1 groups,
respectively. After incubation at 37˚C for 6 h, the medium was
replaced with RPMI-1640 medium containing 10% FBS. After a 48-h
incubation at 37˚C, the cells were collected for further analysis.
To evaluate the effect of patient serum on fibroblasts, serum from
children with asthma or healthy children was mixed with complete
medium (Thermo Fisher Scientific, Inc.) at a ratio of 1:1 to make
conditioned medium for the culture of MRC-5 cells for 24 h. MRC-5
cells that were not incubated with serum was used as a control
group. For rescue experiments, 1 µg IL-22 antibody (1:1,000; cat.
no. ab18498; Abcam) was added into the asthma serum group of MRC-5
cells, which were incubated at 37˚C and 5% CO2 for 12 h
as the rescue group.
Transfection of cells with pcDNA-3.1-IL-22R1 mimics
(0.5 µg/well; Hanbio Biotechnology Co., Ltd.) or empty pcDNA-3.1
vector (0.5 µg/well; NC; Hanbio Biotechnology Co., Ltd.) was
performed according to the same protocol as siRNA transfection. To
inhibit the JAK/STAT3 signaling pathway, the JAK/STAT3 signaling
pathway inhibitor stattic (2 µM; MedChemExpress) was added to MRC-5
cells and incubated at 37˚C and 5% CO2 for 2 h following
stimulation by IL-22.
Mononuclear lymphocytes (1x106/well) were
added onto wells containing MRC-5 cells, and incubated in DMEM
(Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.) at 37˚C and 5%
CO2 for 12 h.
ELISA
The IL-22 ELISA kit (cat. no. ab216170; Abcam) was
used to determine the concentration of IL-22 in patient-derived
serum according to the manufacturer's protocol. In microplates,
IL-22 standards (100 µl) and serum samples (100 µl) were added into
pre-defined wells, while blank wells were left empty. In the wells
containing standards or samples, horseradish peroxidase-labeled
conjugates (100 µl) were added before sealing the plates for
incubation at 37˚C for 1 h. After washing the plates with PBS five
times, substrates A (50 µl) and B (50 µl) were added into each
well. After incubation at 37˚C for 15 min, stop solution (50 µl)
was added into each well, and the absorbance of each well was
measured at a wavelength of 450 nm. Each experiment was repeated
three times.
Reverse transcription-quantitative PCR
(RT-qPCR)
MRC-5 cells were cultured in six-well plates
(1x106 cells/well) and lysed using 1 ml TRIzol reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Total RNA was extracted using the phenol chloroform
method. SDS-PAGE (10%) was used to detect the integrity of RNA
bands (28, 18 and 5S). The concentration and quality of RNA were
measured by ultraviolet spectrophotometry using a Nanodrop ND2000
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
obtained by reverse transcription of 1 µg RNA using the TIANScript
II cDNA First Strand Synthesis kit (Tiangen BioTech Co., Ltd.)
according to the manufacturer's protocol and was subsequently
stored at -20˚C until further analysis.
The SuperReal PreMix (SYBR Green) qPCR kit (Tiangen
BioTech Co., Ltd.) was used to detect mRNA expression of IL-22
according to the manufacturer's protocol. The reaction system (20
µl) was composed of 10 µl SYBR Premix EXTaq, 0.5 µl forward primer,
0.5 µl reverse primer, 2 µl cDNA and 7 µl ddH2O. The
following primer sequences were used: IL-22 forward,
5'-CTCTGCAGCACACTACCCTC-3' and reverse, 5'-CGTTTGGGGCATAGGACAGT-3'
and GAPDH forward, 5'-CAATGACCCCTTCATTGACC-3' and reverse,
5'-GACAAGCTTCCCGTTCTCAG-3'. The thermocycling conditions used were
as follows: Initial denaturation at 95˚C for 10 min; 40 cycles of
denaturation at 95˚C for 1 min, annealing at 60˚C for 30 sec and
elongation at 72˚C for 30 sec. qPCR was performed on an iQ5 system
(Bio-Rad Laboratories, Inc.). The 2-ΔΔCq method
(24) was used to calculate the
relative expression of IL-22 mRNA against GAPDH. Each sample was
tested in triplicate.
CCK-8 assay
To examine proliferation, MRC-5 cells were seeded at
a density of 2x103 cells/well in 96-well plates. At 0,
24, 48 and 72 h following incubation at 37˚C and 5% CO2,
20 µl CCK-8 reagent (Beyotime Institute of Biotechnology) was added
to each well. At the end of the incubation period (24, 48 or 72 h),
150 µl CCK-8 reaction solution was added into each well and the
cells were incubated at 37˚C for 2 h. Subsequently, the absorbance
of each well was measured at a wavelength of 490 nm. Each group was
tested in three replicate wells to calculate an average value.
Flow cytometry
At 24 h post-transfection, 1x106 MRC-5
cells or 1x106 patient-derived mononuclear lymphocytes
were washed twice with precooled PBS. Subsequently, the DNA Reagent
kit (cat. no. 340242; BD Biosciences) was used to determine the
cell cycle distribution of cells, according to the manufacturer's
protocol. Briefly, cells were incubated with 200 µl liquid A at
room temperature for 10 min, then with 150 µl liquid B at room
temperature for 10 min and finally with 120 µl liquid C in the dark
at room temperature for 10 min. Subsequently, the cells were
analyzed using a flow cytometer and ModFit software (v3.2; Verity
Software House, Inc.). Each experiment was repeated three
times.
To assess the ratio of IL-22+ mononuclear
lymphocytes, 1x106/ml mononuclear lymphocytes were
incubated with 250 µl BD Cytofix/Cytoperm permeabilization solution
(BD Biosciences) at 4˚C in the dark for 20 min. The
permeabilization was then stopped by addition of 1 ml PBS.
Following centrifugation at 25˚C and 1,200 x g for 10 min, the
cells were mixed with intranuclear factor (IL22+) antibody (1:20;
cat. no. IC7821P-025; R&D Systems, Inc.) before incubation at
room temperature in the dark for 30 min. Following washing and
resuspension in PBS, the cells were examined by flow cytometry, and
data were analyzed with FlowJo v10 software (FlowJo LLC).
Western blotting
MRC-5 cells (1x106) were lysed with cold
RIPA buffer (600 µl; Beyotime Institute of Biotechnology) for 30
min on ice. The mixture was then centrifuged at 12,000 x g at 4˚C
for 10 min. The protein concentration of the supernatant was
determined using a bicinchoninic acid protein concentration
determination kit [cat. no. RTP7102; Real-Times (Beijing)
Biotechnology Co., Ltd.]. The samples were then mixed with 5X
sodium dodecyl sulfate loading buffer before denaturation in a
water bath at 100˚C for 10 min. Subsequently, the samples (5 µg)
were subjected to 10% SDS-PAGE gel electrophoresis at 100 V. The
resolved proteins were transferred to PVDF membranes on ice (250
mA; 1 h) and blocked with 5% skimmed milk at room temperature for 1
h. Subsequently, the membranes were incubated with the following
monoclonal primary mouse anti-human antibodies at 4˚C overnight:
IL-22R1 (1:1,000; cat. no. ab5984; Abcam), collagen type I α1 chain
(COL1α1; 1:1,000; cat. no. ab64883; Abcam), collagen type I α2
chain (COL1α2; 1:1,000; cat. no. ab196619; Abcam) and GAPDH
(1:4,000; cat. no. ab8245; Abcam). After washing with PBS and 0.1%
Tween 20 three times for 15 min, the membranes were incubated with
goat anti-mouse horseradish peroxidase-conjugated secondary
antibody (1:4,000; cat. no. ab205719; Abcam) for 1 h at room
temperature. Subsequently, the membranes were washed with PBS and
0.1% Tween 20 three times for 15 min. The membranes were then
developed using an ECL kit (Abcam). Image Lab software (version
3.0; Bio-Rad Laboratories, Inc.) was used to acquire and analyze
imaging signals. The relative contents of target proteins were
expressed against GAPDH. Each experiment was repeated three
times.
Statistical analysis
Data were analyzed using SPSS software (version
19.0; IBM, Corp.). Data are presented as the mean ± SD. Data were
tested for normality. Data containing multiple groups were analyzed
using one-way ANOVA followed by the relevant post hoc test. In
cases of homogeneity of variance, Least Significant Difference or
Student-Newman-Keuls post hoc tests were used. In cases of
heterogeneity of variance, Tamhane's T2 or Dunnett's T3 post hoc
tests were used. Comparisons between two groups were performed
using unpaired Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of IL-22 is elevated in
peripheral blood from children with asthma, and this promotes the
proliferation of MRC-5 cells possibly via upregulating the levels
of COL1α1 and COL1α2
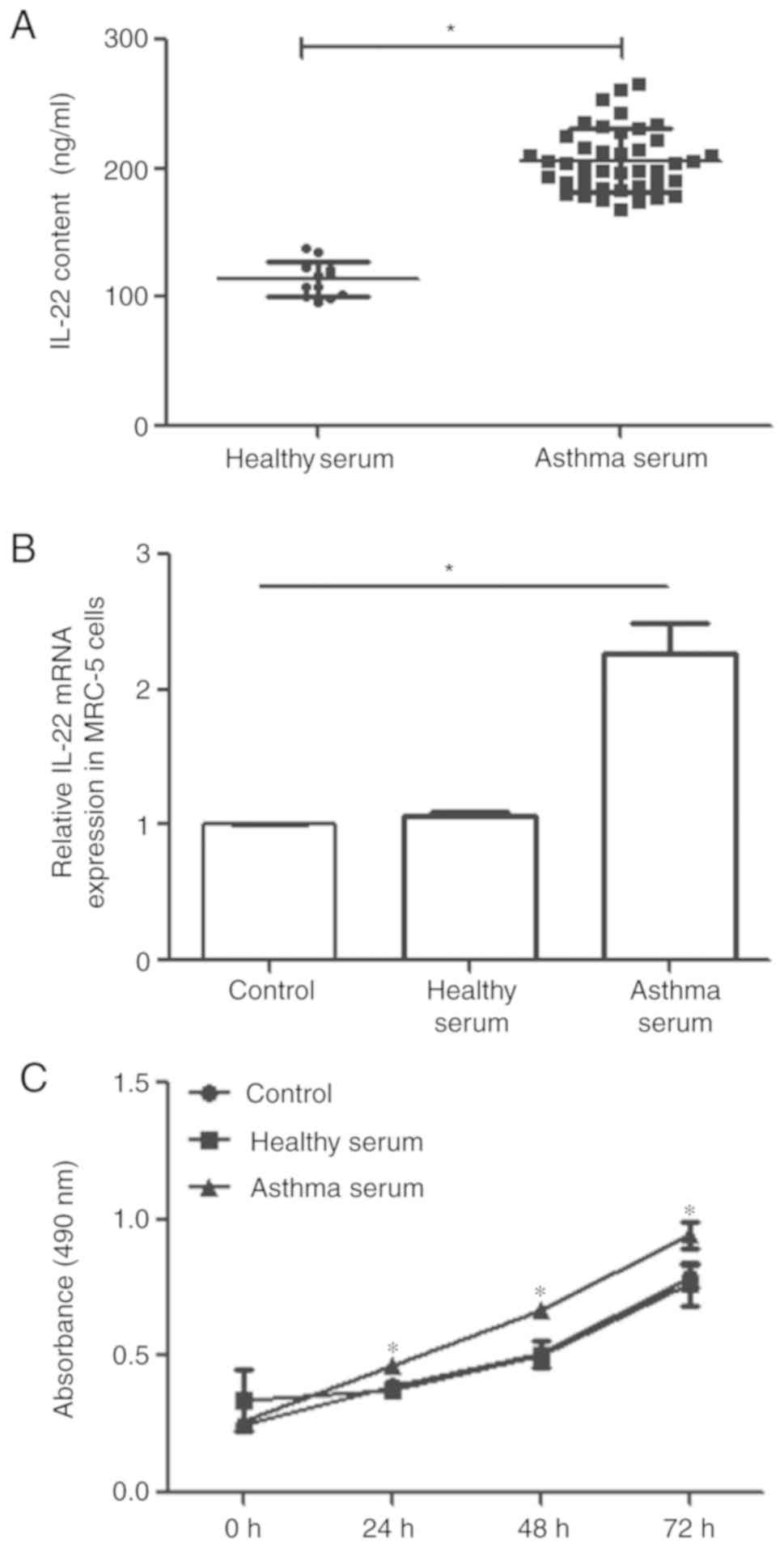
The IL-22 content of serum derived from children
with asthma (asthma serum) and healthy children (healthy serum) was
determined by ELISA. The IL-22 content of asthma serum (104.5±14.7
ng/µl) was significantly higher than that of healthy serum
(23.5±6.3 ng/µl; P<0.05; Fig.
1A). Following a 24-h incubation with healthy or asthma serum,
the level of IL-22R1 mRNA in MRC-5 cells was determined by RT-qPCR.
IL-22R1 mRNA expression in MRC-5 cells treated with asthma serum
was significantly higher than that of untreated MRC-5 cells
(P<0.05; Fig. 1B), and the
expression in the control group was not statistically significant
compared with the healthy serum group (Fig. 1B). The proliferation of MRC-5 cells
treated with asthma serum was significantly higher than that of
untreated MRC-5 cells at 24, 48 and 72 h post-treatment (P<0.05;
Fig. 1C), and proliferation in the
control group was not statistically significant compared with the
healthy serum group (Fig. 1C). The
proportion of cells in the G1 phase was significantly
lower in MRC-5 cells treated with asthma serum than in the control
(P<0.05; Fig. 1D), and the
proportion of G1 cells in the control group was not
statistically significant compared with the healthy serum group
(Fig. 1D). However, the proportion
of cells in the S phase was higher in MRC-5 cells treated with
asthma serum than that in the control (P<0.05; Fig. 1D), and that in control group was not
different from that in healthy serum group (P>0.05; Fig. 1D). Furthermore, the protein
expression levels of IL-22R1, COL1α1 and COL1α2 in MRC-5 cells
treated with asthma serum were significantly higher than in the
control (P<0.05; Fig. 1E), and
protein expression in the control group was not statistically
significant compared with the healthy serum group (Fig. 1E). To further investigate the
involvement of IL-22 in regulating the biological functions of
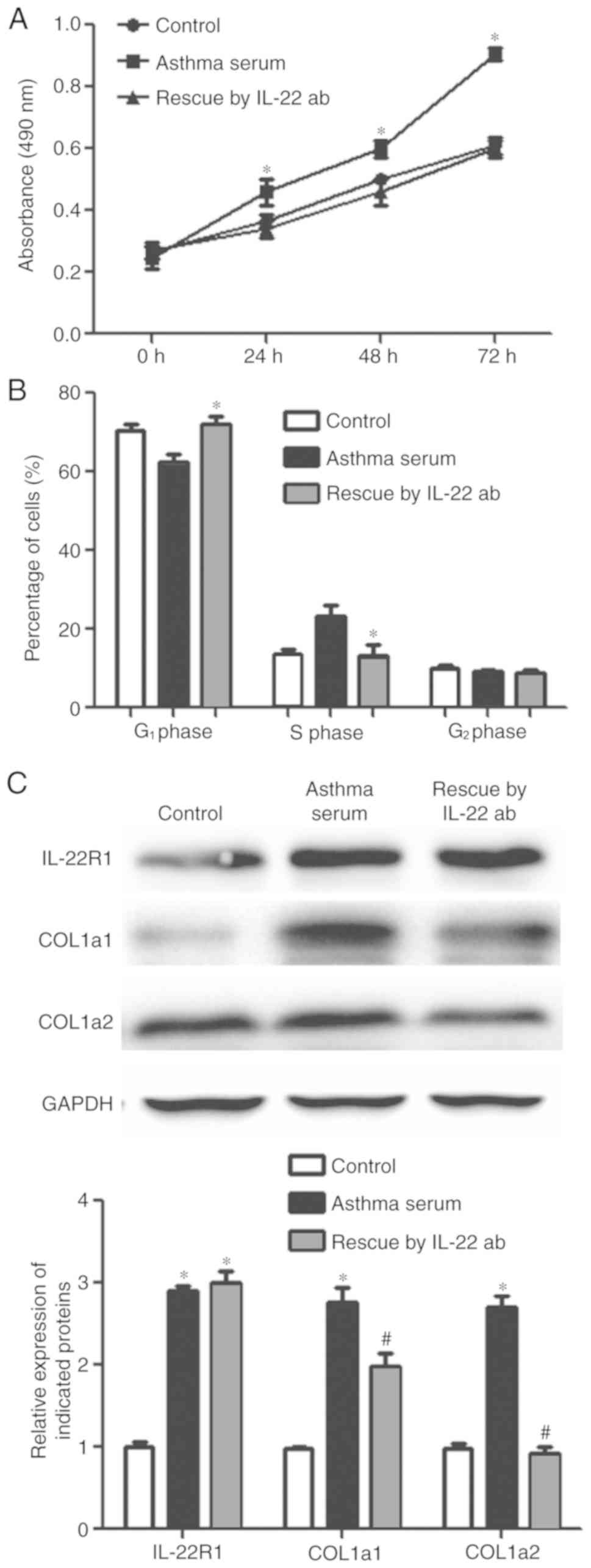
MRC-5 cells, rescue experiments using an IL-22 antibody were
performed. After the addition of IL-22 antibody, the proliferation
of MRC-5 cells treated with asthma serum was reduced compared with
the asthma serum-only group, to a level similar to the control
group (Fig. 2A). Moreover, the
transition of cells from the G1 to S phase of the cell
cycle was inhibited in the IL-22 antibody group compared with the
asthma serum-only group, to levels similar to the control group
(Fig. 2B). IL-22R1 protein
expression in the IL-22 antibody group was significantly higher
than that in the control group (P<0.05; Fig. 2C) and was similar to that of the
asthma serum-only group. Furthermore, COL1α1 and COL1α2 protein
expression in the IL-22 antibody group was significantly lower than
that of the asthma serum-only group (P<0.05; Fig. 2C). Collectively, these results
suggested that IL-22 expression was elevated in the peripheral
blood of children with asthma, and that the increased proliferative
ability of MRC-5 cells may occur via upregulation of COL1α1 and
COL1α2 expression.
IL-22 exerts its biological functions
via IL-22R1
The transfection efficiency of siR-IL22R1 and
IL-22R1 is presented in Fig. S1. To
test whether IL-22 transmits intracellular signals via IL-22R1,
IL-22R1 was knocked down or overexpressed in MRC-5 cells and the
cells were subsequently stimulated with IL-22. The expression of
IL-22R1, COL1α1 and COL1α2 in the siR-IL-22R1 group was
significantly lower than that in the siR-NC group (P<0.05).
Furthermore, the expression of IL-22R1, COL1α1 and COL1α2 in the
IL-22R1 overexpression group was significantly higher than that in
the NC group (P<0.05; Fig. 3A).
These results indicated that the transfection experiments were
successful. IL-22-induced proliferation was inhibited in the
siR-IL-22R1 group, but was increased in the IL-22R1 overexpression
group compared with the siR-NC and NC groups, respectively
(P<0.05; Fig. 3B). The proportion
of cells in the S phase in the siR-IL-22R1 group was reduced, while
that in the IL-22R1 overexpression group was increased compared
with the siR-NC and NC groups, respectively (P<0.05; Fig. 3C). The results indicated that IL-22
exerted its biological functions via IL-22R1.
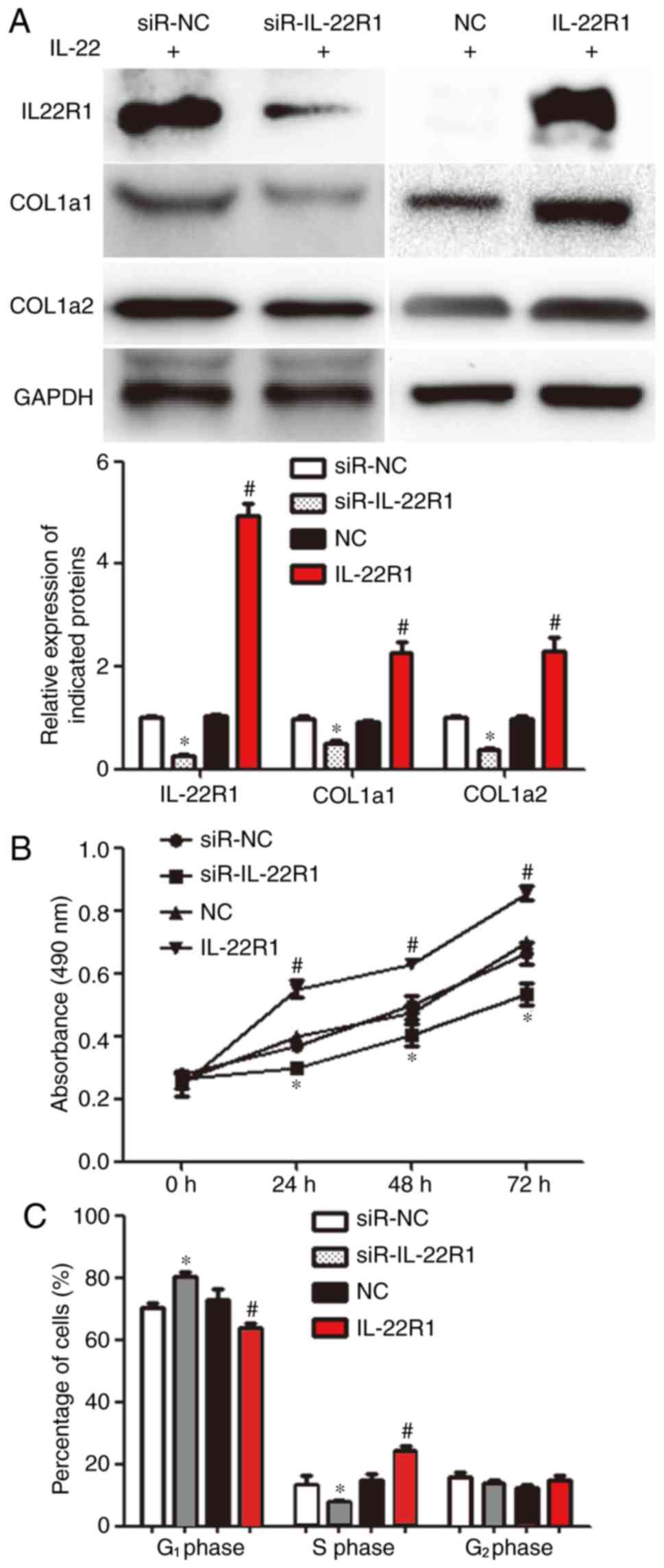 | Figure 3Effect of IL-22R1 knockdown or
overexpression on the proliferation and collagen synthesis of MRC-5
cells. (A) Expression of IL-22R1, COL1α1 and COL1α2 proteins in
MRC-5 cells transfected with siR-IL-22R1, siR-NC, an IL-22R1
overexpression plasmid or an NC plasmid, incubated with serum from
children with asthma (asthma serum). Western blotting was performed
to determine the levels of protein expression.
*P<0.05 vs. siR-NC; #P<0.05 vs. NC. (B)
Proliferation of MRC-5 cells transfected with siR-IL-22R1, siR-NC,
an IL-22R1 overexpression plasmid or an NC plasmid, incubated with
asthma serum. The Cell Counting Kit-8 assay was performed to study
cell proliferation. *P<0.05 vs. siR-NC;
#P<0.05 vs. NC. (C) Cell cycle distribution analysis
of MRC-5 cells transfected with siR-IL-22R1, siR-NC, an IL-22R1
overexpression plasmid or an NC plasmid, incubated with asthma
serum. Flow cytometry was performed to assess the cell cycle
distribution of MRC-5 cells. *P<0.05 vs. siR-NC of
the same cell cycle phase; #P<0.05 vs. NC of the same
cell cycle phase. IL, interleukin; IL-22R1, IL-22 receptor 1;
COL1α1, collagen type I α1 chain; COL1α2, collagen type I α2 chain;
siR, small-interfering RNA; NC, negative control. |
IL-22/IL-22R1 regulates the
proliferation and expression of COL1α1 and COL1α2 in MRC-5 cells
via the JAK/STAT3 signaling pathway
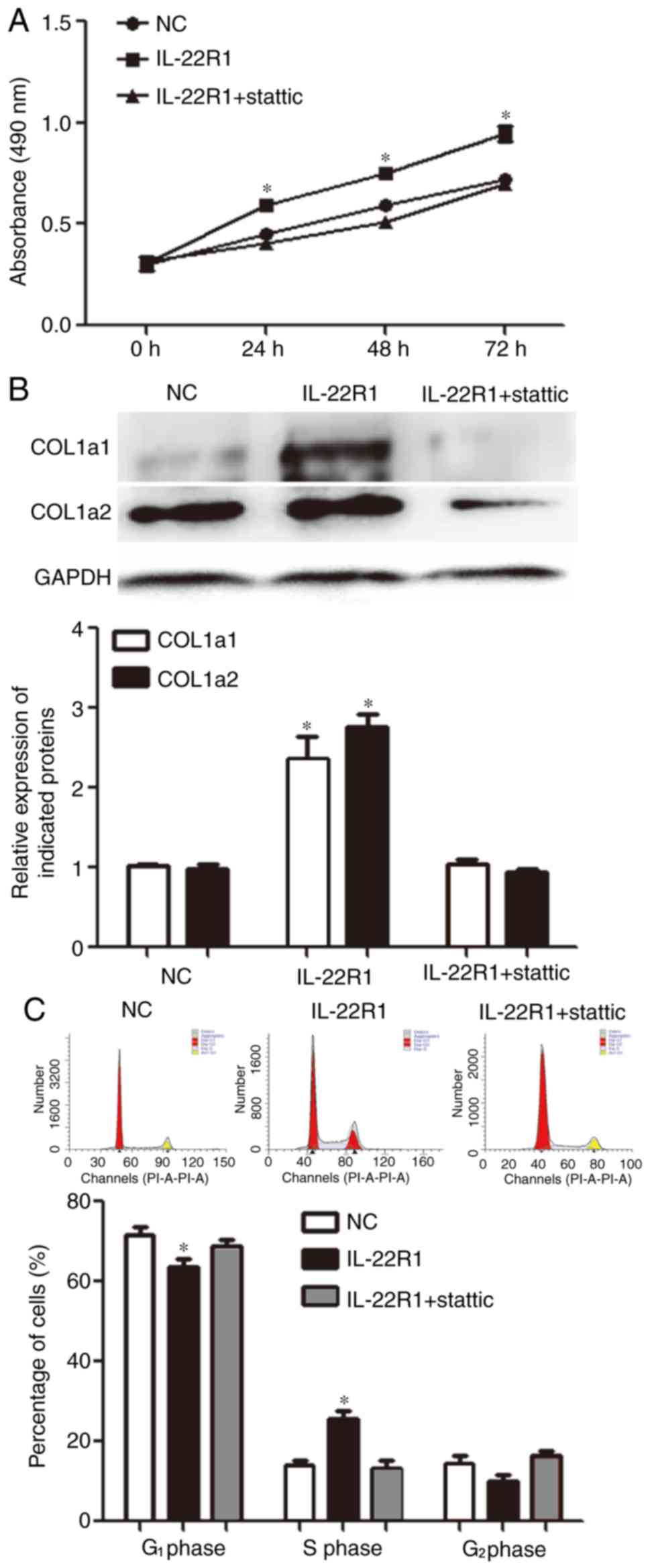
To further study the mechanism by which
IL-22/IL-22R1 regulated the biological functions of fibroblasts,
the present study investigated the effect of the JAK/STAT3
signaling pathway inhibitor stattic (2 µM) on MRC-5 cells. The
addition of stattic reduced IL-22-induced proliferation of MRC-5
cells in the IL-22R1 overexpression group (P<0.05; Fig. 4A). Furthermore, the addition of
stattic limited the effect of IL-22 on the expression of COL1α1 and
COL1α2 (P<0.05; Fig. 4B).
Additionally, flow cytometry analysis suggested that the addition
of static decreased the effect of IL-22 on the proportion of cells
transitioning from the G1 to the S phase (P<0.05;
Fig. 4C). Overall, these results
suggested that IL-22 regulated the proliferation and expression of
COL1α1 and COL1α2 proteins in MRC-5 cells, potentially via the
JAK/STAT3 signaling pathway.
Mononuclear lymphocytes from children
with asthma stimulate the proliferation and secretory function of
fibroblasts via secretion of IL-22
To analyze the origin of IL-22, mononuclear
lymphocytes isolated from the peripheral blood of healthy children
and children with asthma were assessed by flow cytometry. The ratio
of IL-22+ mononuclear lymphocytes in the asthma group
was significantly higher than that in the healthy group (P<0.05;
Fig. 5A). Then, mononuclear
lymphocytes from children with asthma were co-cultured with MRC-5
cells. MRC-5 cell proliferation was significantly higher in the
co-cultured group than in the NC group (P<0.05; Fig. 5B). Alternatively, incubation of
co-cultured MRC-5 cells with IL-22 antibody reduced proliferation
to a level similar to that of the NC group (P>0.05; Fig. 5B). Furthermore, the proportion of
cells transitioning from G1 to S phase was higher in the
co-cultured group than in the NC group (P<0.05; Fig. 5C), while incubation of the
co-cultured cells with IL-22 antibody significantly reduced the
proportion of cells transitioning from G1 to S phase
compared with the co-culture group (P<0.05; Fig. 5C). The expression of COL1α1 and
COL1α2 in the co-cultured group was higher than that in the NC
group (P<0.05; Fig. 5D), and
incubation of the co-cultured cells with IL-22 antibody reduced
COL1α1 and COL1α2 protein expression to levels similar to the NC
group (P>0.05; Fig. 5D). These
results indicated that mononuclear lymphocytes derived from
children with asthma stimulated the proliferation and secretory
function of fibroblasts via secretion of IL-22.
Discussion
Subepithelial lung fibroblast proliferation,
transformation to myofibroblasts and synthesis of extracellular
matrix are the main mechanisms leading to subepithelial fibrosis in
patients with asthma (25,26). IL-22 is an immune-related cytokine
that was discovered in previous years and plays a role in the
occurrence and development of psoriasis, arthritis and cancer
(22,27,28).
Previously, it was reported that IL-22 promotes allergic airway
inflammation in sensitized mice in vivo (17). In addition, IL-22 promotes the
occurrence and development of KRAS-mutant lung cancer by inducing
pre-cancerous immune responses and preserving stem cell
characteristics (29). These studies
suggest that IL-22 has a role in pulmonary inflammation and tumor
formation. In the present study, the expression of IL-22 in the
peripheral serum of children with asthma was significantly
increased, compared with healthy children. In vitro
co-culture experiments suggested that asthma serum could promote
the proliferation of MRC-5 cells. After co-culture with asthma
serum, the expression of COL1α1 and COL1α2, the main components of
type I collagen, was upregulated. In addition, asthma serum
enhanced the expression of IL-22R1 in MRC-5 cells after co-culture,
suggesting that IL-22 regulated MRC-5 cells by binding to IL-22R1.
Interestingly, the addition of IL-22 antibody reduced the
proliferation of MRC-5 cells and their ability to synthesize
collagen. IL-22R1 knockdown and overexpression in MRC-5 cells
suggested that the effects of IL-22 stimulation were decreased or
increased, respectively, suggesting that IL-22 regulated the
proliferation of MRC-5 cells and their ability to synthesize
collagen via IL-22R1.
The IL-22/IL-22R1 signaling pathway has been
reported to play a role in immunoregulation and tumorigenesis
(30,31). Furthermore, it has been reported that
the IL-22/IL-22R1 signaling pathway can transmit extracellular
signals via the JAK/STAT3 signaling pathway, thus regulating
various biological processes (32,33). For
example, IL-22 promotes stem cell characteristics and tumorigenesis
of pancreatic cancer via the JAK/STAT3 signaling pathway (32). Additionally, upregulation of IL-10R2
can activate the IL-22/STAT3 signaling pathway and promote the
occurrence and development of colon cancer (33). In the present study, it was suggested
that the IL-22/IL-22R1 signaling pathway activated the JAK/STAT3
signaling pathway. The application of a STAT3 inhibitor blocked the
effects of the IL-22/IL-22R1 signaling pathway on MRC-5
proliferation and collagen synthesis. These results suggested that
IL-22/IL-22R1 may promote fibroblast proliferation and collagen
synthesis via the JAK/STAT3 signaling pathway during pulmonary
fibrosis. Previous studies have shown that IL-22 is secreted by
various immune cells such as type 1 T helper cells, as well as
certain cancer cells such as pancreatic cancer and bladder cancer
cells, including mononuclear lymphocytes (29,34). The
present study suggested that the expression of IL-22 in peripheral
mononuclear lymphocytes of children with asthma was significantly
upregulated compared with healthy children. Therefore, mononuclear
lymphocytes may activate the IL-22/IL-22R1 and JAK/STAT3 signaling
pathways when migrating into the lungs. The mechanism of this
process is not completely understood and requires further
investigation.
In conclusion, the present study suggested that the
synthesis and secretion of IL-22 by peripheral blood mononuclear
lymphocytes in children with asthma was enhanced. In addition, the
IL-22/IL-22R1 signaling pathway promoted MRC-5 cell proliferation
and collagen synthesis, potentially via the JAK/STAT3 signaling
pathway and thus, may regulate airway subepithelial fibrosis. The
present study only focused on the function of IL-22, but several
other cytokines are present in peripheral serum and were not
analyzed. Therefore, further investigation of the expression
profiles of various other cytokines in peripheral serum is
required.
Supplementary Material
Transfection efficiency of siR-IL22R1
and IL-22R1. The relative expression of IL-22R1 in cells
transfected with (A) siR-NC, siR-IL22R1, (B) NC or IL22R1.
*P<0.05. siR, small interfering RNA; IL22R1,
interleukin 22 receptor 1; NC, negative control.
Acknowledgements
The authors would like to thank Dr Li Jiang of
Nanchong Central Hospital, The Second Clinical Medical College,
North Sichuan Medical College for her valuable suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL designed the study. JL, BS and JB performed the
experiments. JL and BS analyzed the data. All authors collaborated
to interpret the results and write the manuscript. All authors read
and approved the final manuscript.
Ethical approval and consent to
participate
All procedures performed in the present study were
approved by the Ethics Committee of North Sichuan Medical College
(approval no. NS-CE-017-03). Written informed consent was obtained
from all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reese SE, Xu CJ, den Dekker HT, Lee MK,
Sikdar S, Ruiz-Arenas C, Merid SK, Rezwan FI, Page CM, Ullemar V,
et al: Epigenome-wide meta-analysis of DNA methylation and
childhood asthma. J Allergy Clin Immunol. 143:2062–2074.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saglani S and Custovic A: Childhood
asthma: Advances using machine learning and mechanistic studies. Am
J Respir Crit Care Med. 199:414–422. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eldeirawi K, Kunzweiler C, Zenk S, Finn P,
Nyenhuis S, Rosenberg N and Persky V: Associations of urban
greenness with asthma and respiratory symptoms in Mexican American
children. Ann Allergy Asthma Immunol. 122:289–295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim YM, Kim J, Cheong HK, Jeon BH and Ahn
K: Exposure to phthalates aggravates pulmonary function and airway
inflammation in asthmatic children. PLoS One.
13(e0208553)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Biagini Myers JM, Schauberger E, He H,
Martin LJ, Kroner J, Hill GM, Ryan PH, LeMasters GK, Bernstein DI,
Lockey JE, et al: A pediatric asthma risk score to better predict
asthma development in young children. J Allergy Clin Immunol.
143:1803–1810.e2. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lezmi G and de Blic J: Assessment of
airway inflammation and remodeling in children with severe asthma:
The next challenge. Pediatr Pulmonol. 53:1171–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fehrenbach H, Wagner C and Wegmann M:
Airway remodeling in asthma: What really matters. Cell Tissue Res.
367:551–569. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kumari A, Singh DK, Dash D and Singh R:
Intranasal curcumin protects against LPS-induced airway remodeling
by modulating toll-like receptor-4 (TLR-4) and
matrixmetalloproteinase-9 (MMP-9) expression via affecting MAP
kinases in mouse model. Inflammopharmacology. 27:731–748.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin SC, Chou HC, Chen CM and Chiang BL:
Anti-thymic stromal lymphopoietin antibody suppresses airway
remodeling in asthma through reduction of MMP and CTGF. Pediatr
Res. 86:181–187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou J, Bai W, Liu Q, Cui J and Zhang W:
Silencing of ADAM33 restrains proliferation and induces apoptosis
of airway smooth muscle cells in ovalbumin-induced asthma model. J
Cell Biochem, Nov 18, 2018 (Epub ahead of print).
|
|
11
|
Cao L, Liu F, Liu Y, Liu T, Wu J, Zhao J,
Wang J, Li S, Xu J and Dong L: TSLP promotes asthmatic airway
remodeling via p38-STAT3 signaling pathway in human lung
fibroblast. Exp Lung Res. 44:288–301. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yadav SK, Shah SD and Penn RB: Give me a
fork: Can autophagy research solve the riddle of airway remodeling
in asthma? Am J Respir Cell Mol Biol. 60:494–496. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaczmarek KA, Clifford RL and Knox AJ:
Epigenetic changes in airway smooth muscle as a driver of airway
inflammation and remodeling in asthma. Chest. 155:816–824.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rodrigues APD, Bortolozzo ASS,
Arantes-Costa FM, Saraiva-Romanholo BM, de Souza FCR, Brüggemann
TR, Santana FPR, de Brito MV, Bonturi CR, Nunes NNDS, et al: A
plant proteinase inhibitor from enterolobium contortisiliquum
attenuates airway hyperresponsiveness, inflammation and remodeling
in a mouse model of asthma. Histol Histopathol. 34:537–552.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McAlinden KD, Deshpande DA, Ghavami S,
Xenaki D, Sohal SS, Oliver BG, Haghi M and Sharma P: Autophagy
activation in asthma airways remodeling. Am J Respir Cell Mol Biol.
60:541–553. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brunner PM, Pavel AB, Khattri S, Leonard
A, Malik K, Rose S, Jim On S, Vekaria AS, Traidl-Hoffmann C, Singer
GK, et al: Baseline IL-22 expression in patients with atopic
dermatitis stratifies tissue responses to fezakinumab. J Allergy
Clin Immunol. 143:142–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leyva-Castillo JM, Yoon J and Geha RS:
IL-22 promotes allergic airway inflammation in epicutaneously
sensitized mice. J Allergy Clin Immunol. 143:619–630.e7.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Geng H, Bu HF, Liu F, Wu L, Pfeifer K,
Chou PM, Wang X, Sun J, Lu L, Pandey A, et al: In inflamed
intestinal tissues and epithelial cells, interleukin 22 signaling
increases expression of H19 long noncoding RNA, which promotes
mucosal regeneration. Gastroenterology. 155:144–155.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen E, Cen Y, Lu D, Luo W and Jiang H:
IL-22 inactivates hepatic stellate cells via downregulation of the
TGF-β1/Notch signaling pathway. Mol Med Rep. 17:5449–5453.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Almolda B, Costa M, Montoya M, González B
and Castellano B: Increase in Th17 and T-reg lymphocytes and
decrease of IL22 correlate with the recovery phase of acute EAE in
rat. PLoS One. 6(e27473)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pollock RA, Zaman L, Chandran V and
Gladman DD: Epigenome-wide analysis of sperm cells identifies IL22
as a possible germ line risk locus for psoriatic arthritis. PLoS
One. 14(e0212043)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
He Y, Yang Y, Xu J, Liao Y, Liu L, Deng L
and Xiong X: IL22 drives cutaneous melanoma cell proliferation,
migration and invasion through activation of miR-181/STAT3/AKT
axis. J Cancer. 11:2679–2687. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bao Y: Guidelines for the diagnosis and
treatment of bronchial asthma in children (2016 edition). Data
Collection of the 20th National Pediatric Academic Conference on
Integration of Traditional Chinese and Western Medicine: 57-71,
2016.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Campa CC, Silva RL, Margaria JP, Pirali T,
Mattos MS, Kraemer LR, Reis DC, Grosa G, Copperi F, Dalmarco EM, et
al: Inhalation of the prodrug PI3K inhibitor CL27c improves lung
function in asthma and fibrosis. Nat Commun. 9(5232)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun Q, Fang L, Tang X, Lu S, Tamm M, Stolz
D and Roth M: TGF-β upregulated mitochondria mass through the
SMAD2/3→C/EBPβ→PRMT1 signal pathway in primary human lung
fibroblasts. J Immunol. 202:37–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reeder KM, Mackel JJ, Godwin MS, Dunaway
CW, Blackburn JP, Patel RP and Steele C: Role of common γ-Chain
cytokines in lung interleukin-22 regulation after acute exposure to
aspergillus fumigatus. Infect Immun. 86:e00157–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Trevejo-Nunez G, Elsegeiny W, Conboy P,
Chen K and Kolls JK: Critical role of IL-22/IL22-RA1 signaling in
pneumococcal pneumonia. J Immunol. 197:1877–1883. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khosravi N, Caetano MS, Cumpian AM, Unver
N, De la Garza Ramos C, Noble O, Daliri S, Hernandez BJ, Gutierrez
BA, Evans SE, et al: IL22 promotes kras-mutant lung cancer by
induction of a protumor immune response and protection of stemness
properties. Cancer Immunol Res. 6:788–797. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rompré-Brodeur A, Shinde-Jadhav S, Ayoub
M, Piccirillo CA, Seuntjens J, Brimo F, Mansure JJ and Kassouf W:
PD-1/PD-L1 immune checkpoint inhibition with radiation in bladder
cancer: In situ and abscopal effects. Mol Cancer Ther. 19:211–220.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou Y, Hou W, Xu K, Han D, Jiang C, Mou
K, Li Y, Meng L and Lu S: The elevated expression of Th17-related
cytokines and receptors is associated with skin lesion severity in
early systemic sclerosis. Hum Immunol. 76:22–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
He W, Wu J, Shi J, Huo YM, Dai W, Geng J,
Lu P, Yang MW, Fang Y, Wang W, et al: IL22RA1/STAT3 signaling
promotes stemness and tumorigenicity in pancreatic cancer. Cancer
Res. 78:3293–3305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Khare V, Paul G, Movadat O, Frick A,
Jambrich M, Krnjic A, Marian B, Wrba F and Gasche C: IL10R2
overexpression promotes IL22/STAT3 signaling in colorectal
carcinogenesis. Cancer Immunol Res. 3:1227–1235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eggenhofer E, Sabet-Rashedi M, Lantow M,
Renner P, Rovira J, Koehl GE, Schlitt HJ, Geissler EK and Kroemer
A: RORγt(+) IL-22-producing NKp46(+) cells protect from hepatic
ischemia reperfusion injury in mice. J Hepatol. 64:128–134.
2016.PubMed/NCBI View Article : Google Scholar
|