Introduction
As a common disease in the gastrointestinal system,
acute pancreatitis is characterized by increasing incidence and
high mortality (1). Although there
is timely update on the guidelines for the management of acute
pancreatitis worldwide (2-4),
symptomatic treatment currently remains the predominant option for
the treatment of acute pancreatitis, including inflammation
control, inhibition of pancreatic secretion, pain management,
maintenance of stable blood circulation, and treatment of systemic
or local complications (5).
Although multiple hypotheses have been proposed, the
exact pathogenesis of acute pancreatitis remains unclear now.
Multiple factors are considered to be involved in the
pathophysiological process of acute pancreatitis, in which
pancreatic ductal obstruction and hypertension may play critical
roles (6). Endoscopic retrograde
cholangiopancreatography (ERCP), an effective treatment for acute
biliary pancreatitis, may alleviate the obstruction and edema in
the common channel of the bile duct and pancreatic duct to achieve
the indirect treatment of acute pancreatitis (7). To date, there is little knowledge on
the treatment of acute pancreatitis through alleviation of
pancreatic ductal hypertension and removal of pancreatic ductal
obstruction. However, pancreatic ductal obstruction and
hypertension is found in both biliary and non-biliary acute
pancreatitis (8). Nevertheless, ERCP
may be performed to remove intrapancreatic ductal obstruction and
reduce intrapancreatic ductal pressure. Hereby, we summarize the
experiences of successful treatment of acute pancreatitis through
the pancreatic duct decompression via ERCP in one single center
from China.
Case reports
Case 1
A 29-year-old woman was presented with complaints of
intermittent right upper abdominal pain for 10 days. She was
admitted to a local county hospital at the first onset of the pain,
and was misdiagnosed as gastritis. However, the clinical symptoms
did not relieve remarkably following acid suppressive and analgesic
therapy. Three days ago, obvious aggravation of the middle and
upper abdominal pain was found, complicated by fever. Elevated
serum bilirubin and aminotransferase levels were detected in the
local hospital, with serum amylase activity increased to 1,518 U/l.
Transabdominal color Doppler ultrasonography displayed multiple
multiple gallbladder stones, cholecystitis and pancreatic edema.
The woman was then diagnosed as acute pancreatitis, and given
conservative treatment. However, no satisfactory outcome was
achieved, and the case was then transferred to our hospital. She
denied history of alcohol addiction or other medical history of
specific diseases.
Admission physical examinations showed a body
temperature of 38.9˚C, a heart rate of 125 beats/min; tenderness
over the whole abdomen, notably in the middle and upper abdomen;
and decreased bowel sounds. Blood testing revealed a WBC count of
25.4x109/l, 50% hematocrit, 90.5% neutrophils, 1,499 U/l
LDH, 360.9 U/l AST, 1,447 U/l amylase, 1.29 mmol/l 48 h serum
calcium, and 3.3 mmol/l potassium. She had a Ranson score of 5 and
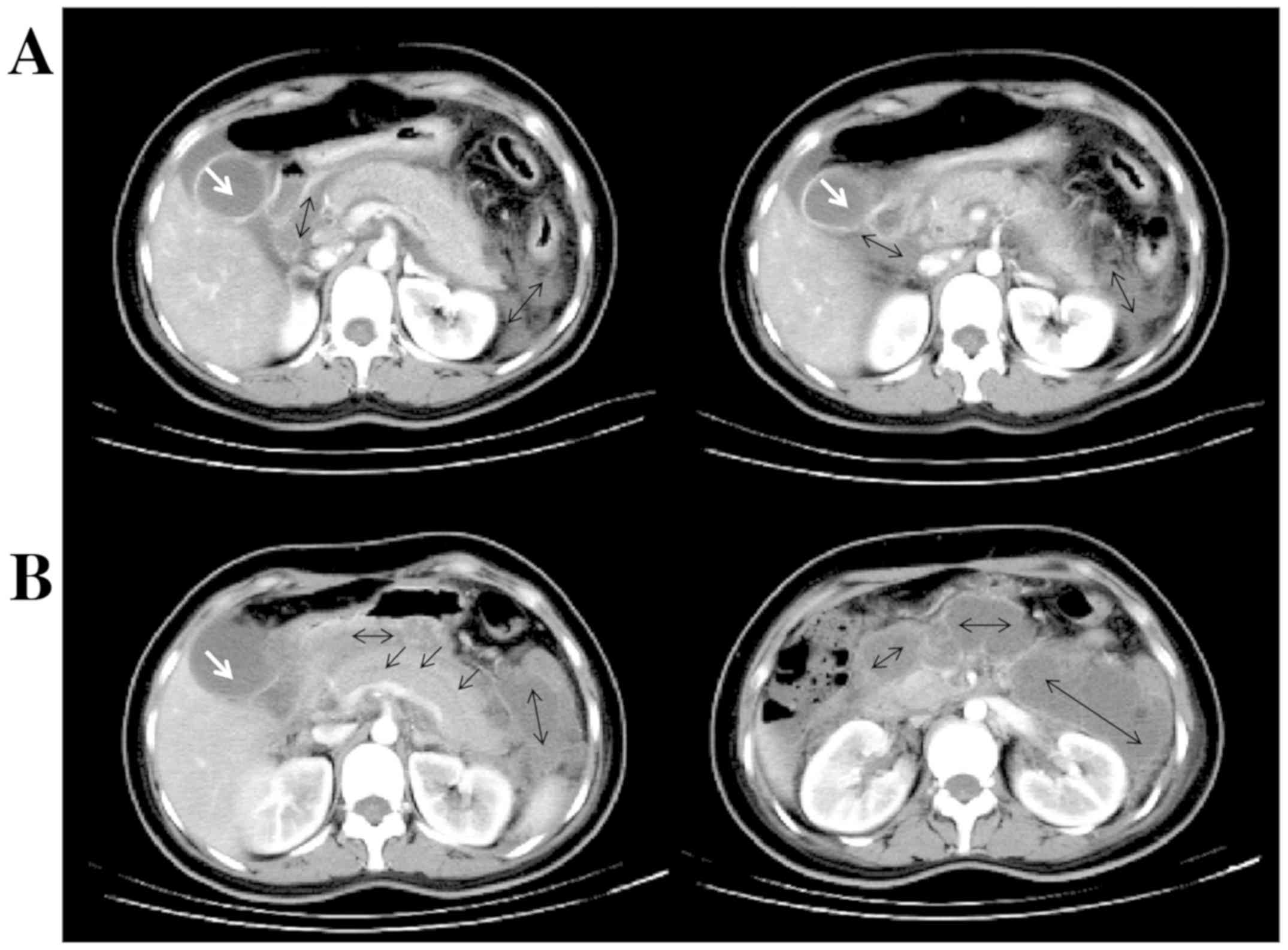
an APACHE II score of 8. Abdominal enhanced CT scan revealed
pancreatic parenchymal swelling, a little effusion surrounding the
pancreas, and grade C acute pancreatitis according to the computed
tomography severity index (Balthazar score) (Fig. 1A). Gallbladder stone and small
terminal common bile duct stones were identified based on MRCP and
transabdominal color Doppler ultrasonography; however, no
respiratory or renal insufficiency was detected. Then, the woman
was diagnosed as critical acute biliary pancreatitis.
The case was given conservative and symptomatic
treatment, and underwent ERCP 11 days post-admission. Before
treatment, she had a WBC count of 25.4x109/l, 81%
neutrophils, normal amylase, 1,092 U/l lipase, remarkable
pancreatic parenchymal enlargement, a plenty of effusions around
the pancreas and grade E acute pancreatitis at Balthazar score
(Fig. 1B). During ERCP, a guidewire
was inserted into the bile duct and cholangiography was performed.
Filling defects were seen in the upper common bile duct, while
truncation of the lower common bile duct was observed. Since the
enlarged pancreatic head was considered to compress the common bile
duct, endoscopic papillotomy (EPT) was performed. Another guidewire
was inserted into the pancreatic duct, and a 5 Fr pancreatic stent
was placed along the guidewire. A lot of thick, purulent mucus was
found coming out from the pancreatic duct (Video S1). Following insertion of
nasobiliary tube along the biliary guidewire, purulent bile was
found. Purulent pancreatic juice and bile were collected for
microbiological examinations.
On day 1 after ERCP, the case had normal body
temperature, a WBC count of 9.8x109/l, 77.3%
neutrophils, and alleviation of abdominal pain; however, turbid,
purulent bile was seen in the nasobiliary tube, and mild tenderness
was felt on the middle and upper abdomen. The woman had no
abdominal pain or tenderness on her abdomen on day 2, with normal
routine blood test and normal bile color. Bacterial culture of both
pancreatic juice and bile showed Klebsiella pneumoniae infection.
After discharge from hospital, she had no discomfort like abdominal
pain or fever. Re-examinations showed normal serum and urine
amylase activity and normal serum lipase 10 days after ERCP, and
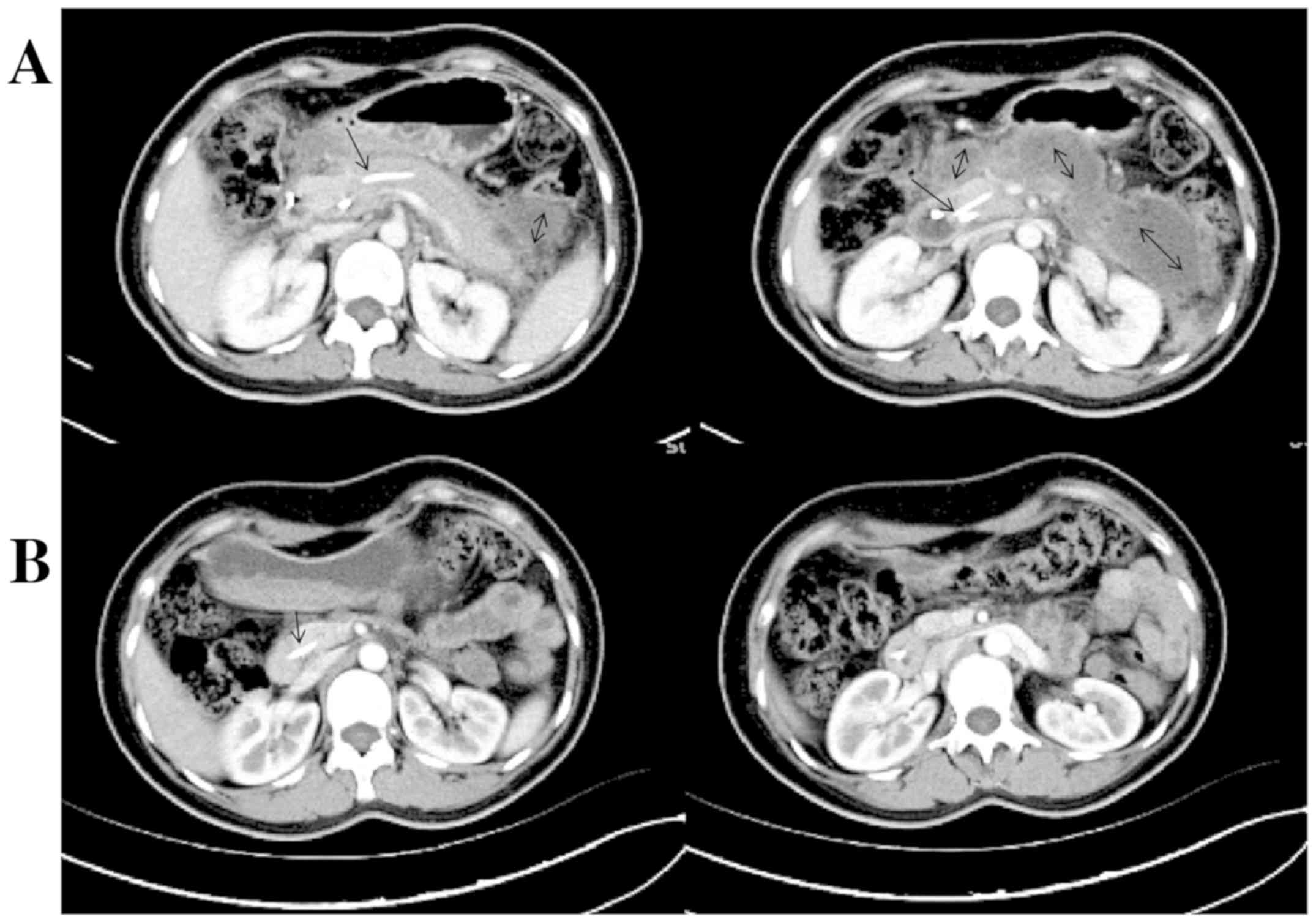
enhanced CT displayed a reduction of the pancreas size and multiple
liquid accumulations around the pancreas, with grade E pancreatitis
assessed (Fig. 2A). On day 14,
unobstructed bile duct was found, and no filling defects were seen;
and then, the nasobiliary tube was removed. On day 21, an enhanced
CT re-examination revealed that the pancreatic stent was in the
place, the pancreas size and morphology returned to normal level,
and no peri-pancreatic effusions were seen, which was classified as
grade A pancreatitis (Fig. 2B). The
case received selective cholecystectomy and pancreatic stent
removal, and no onset of acute pancreatitis was reported at a
long-term follow-up.
Case 2
A 33-year-old man was presented with complaints of
persistent descending pain on his middle and upper abdominal pain
for 8 h, complicated by discontinuation of passage of flatus and
defecation. He had no fever, and denied history of alcohol
addiction or other medical history of specific diseases. Admission
physical examinations showed 26.37 BMI, abdominal fullness,
tenderness and rebound pain over the upper abdomen, and bowel
sounds were absent. Blood testing showed 17.3x109/l WBC
count, 48.4% hematocrit, 92.7% neutrophils, 1,127 U/l serum
amylase, 604 U/l LDH, 49 µmol/l serum creatinine, and severe
hyperlipidemia. He had a Ranson score of 3 and an APACHE II score
of 3. Abdominal ultrasonography indicated no gallbladder stone, and
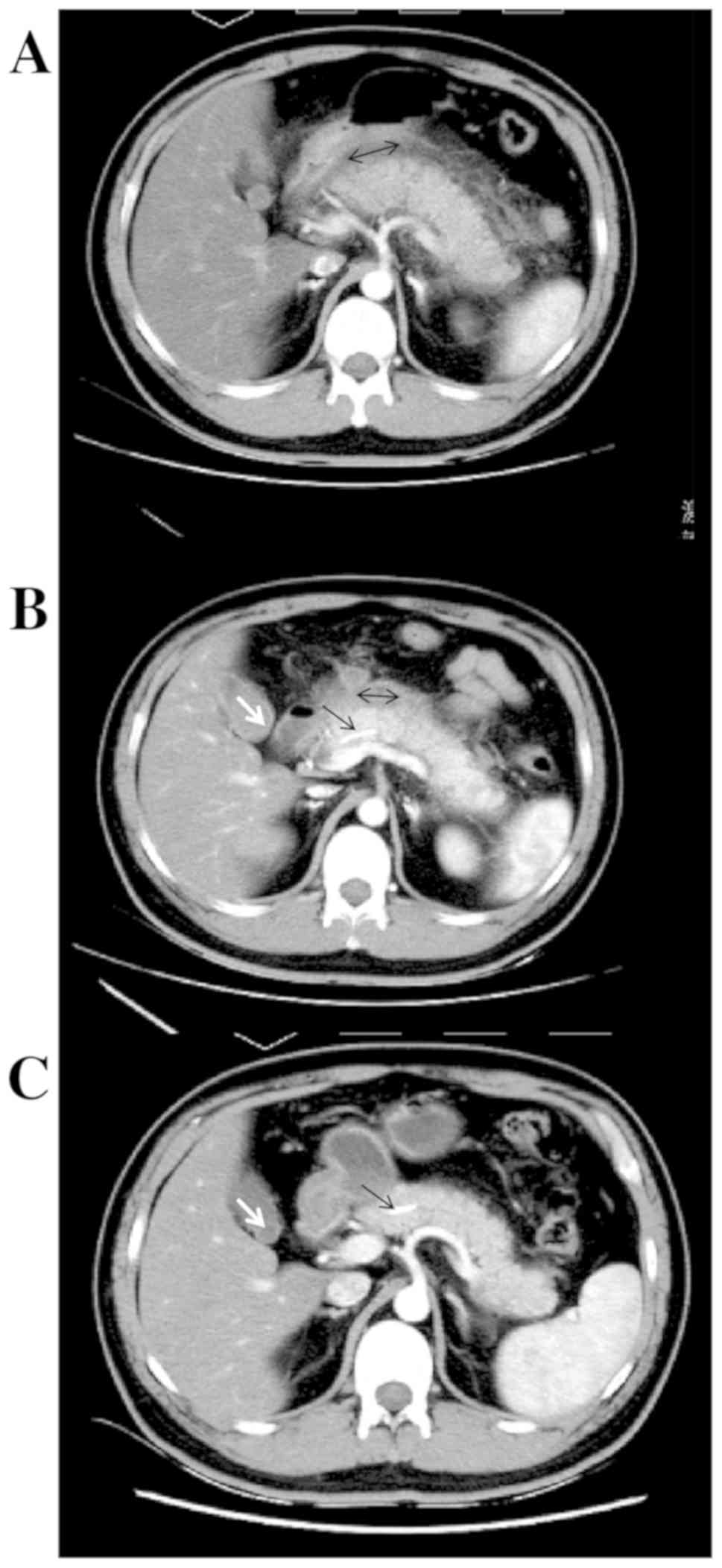
enhanced CT displayed remarkable pancreatic swelling and patchy
exudations around the pancreas, and grade C pancreatitis (Fig. 3A). He was clinically diagnosed as
moderately severe hyperlipidemic acute pancreatitis.
Following active conservative treatment, ERCP was
performed. After the guidewire was inserted into the nipple, X-ray
scan showed the insertion of the guidewire into the pancreatic
duct, and a lot of pancreatic juice and thick, purulent mucus came
out from the pancreatic duct (Video
S2). A 5 Fr pancreatic stent was placed along the guidewire,
and the bile and pancreatic juice were collected for
microbiological examinations. On day 1 after ERCP, the man had
obvious relief from abdominal pain, distending pain on his back,
mild tenderness over the middle and upper abdomen and peristalsis.
Blood testing showed 9.52x109/l, 80.3% neutrophils, and
reduced but abnormal serum and urine amylase. On day 2, he had no
obvious abdominal pain or tenderness on the abdomen, normal serum
and urine amylase and discharged from the hospital. Bacterial
culture of both pancreatic juice and bile showed Escherichia coli
infection. On day 7, enhanced CT re-examination displayed a
reduction of the pancreas size, small necrotic foci in the
pancreatic tail and liquid accumulations around the pancreas, with
grade D pancreatitis identified (Fig.
3B). No specific interventions were given since the case had no
comfort. One month after ERCP, an enhanced CT scan displayed normal
pancreas size and morphology, no peri-pancreatic effusions, with
grade A pancreatitis identified (Fig.
3C). The pancreatic stent was removed 2 months after ERCP, and
no recurrence was found until April, 2017.
Case 3
A 39-year-old man was presented with complaints of
the left upper abdominal pain complicated by nausea and vomiting
for 7 days. He was diagnosed as acute pancreatitis in a local
hospital based on abnormal elevation of serum and urine amylase
levels. Following conservative therapy, the amylase activity
restored to normal and abdominal pain relieved. However, abdominal
pain developed again after the return to normal diet, and routine
blood test showed 14.7x109/l WBC count. The man was then
transferred to our hospital.
The case had no history of alcohol addiction. He
suffered from gallbladder stone and acute cholangitis half a year
ago, and was given laparoscopic cholecystectomy and ERCP. Admission
physical examinations showed tenderness over the upper abdomen,
12.1x109/l WBC count, 42.4% hematocrit, 69.5%
neutrophils, 365 U/l LDH, normal bilirubin and aminotransferase
levels, mild elevation of serum amylase and lipase, and no
manifestations of organ dysfunctions. He had a Ranson score of 1
and an APACHE II score of 1. Abdominal enhanced CT displayed
pancreas tail enlargement and patchy exudations around the pancreas
tail, and identified grade C pancreatitis. The man was therefore
diagnosed as mild acute pancreatitis.
Following conservative treatment, including
anti-infective therapy with third-generation cephalosporins, ERCP
was performed. The duodenal papilla showed post-EPT alterations,
and no filling defects were seen in the bile duct. Another
guidewire was inserted into the pancreatic duct, and a pancreatic
stent was inserted along the guidewire, and detected flocculent,
thick, purulent mucus. On day 1 after ERCP, abdominal pain was
relieved, and normal diet was returned. After ERCP, the WBC count
continued to be abnormal and showed an increase trend (with the
greatest to 16.52x109/l); however, the case had no
fever. Bacterial culture revealed E. coli infection.
Antibiotic susceptibility testing showed resistance to most
cephalosporins, including all cephalosporins used for previous
anti-infective therapy, and carbapenems were administered. Then,
the WBC count reduced greatly, and returned to normal level after 5
day of carbapenems. The man had no rebound in the WBC count after
the cease of anti-infective therapy, and was then discharged from
the hospital. Pancreatic stent was removed 2 months after the
discharge. During the follow-up period, the man had no abdominal
pain, abnormal elevation of WBC counts or recurrence of acute
pancreatitis.
Discussion
In this study, we presented the treatment of three
cases with acute pancreatitis through removing pancreatic duct
obstruction and insertion of pancreatic duct for decompression via
ERCP. During ERCP, thick mucus was found to come out from the
pancreatic duct. Following ERCP, abdominal pain was alleviated, and
inflammation-related parameters reduced. Oral re-feeding was
advanced after the procedure. Re-examinations showed complete
absorption of peripancreatic effusions within one month after ERCP
and recovery of pancreas morphology to normal. In addition, no
recurrence of acute pancreatitis was found during the follow-up
period.
To date, management of clinical symptoms and
complications remains the predominant treatment for acute
pancreatitis (5), since the
pathogenesis of acute pancreatitis has not been fully demonstrated
(1). Pancreatic ductal obstruction
and hypertension has been considered as the primary cause of acute
biliary pancreatitis (8); however,
they also occur in non-biliary acute pancreatitis (9). In addition, it was reported that
non-biliary acute pancreatitis was not completely dependent on bile
duct obstruction and bile reflux (10). Therefore, bile duct obstruction and
bile reflux are considered to be key events of acute pancreatitis
(11). As an important approach in
the diagnosis and treatment of pancreaticobiliary duct diseases,
ERCP has been widely employed in pancreatic diseases. Notably, the
effectiveness of ERCP for the treatment of biliary acute
pancreatitis has been recognized throughout the world, and early
ERCP is recommended for biliary acute pancreatitis, which has been
proved to greatly reduce the morbidity and mortality of the
complications of acute pancreatitis. However, ERCP is performed
aiming to relieve the edema and obstruction in the common channel
of the bile duct and pancreatic duct, thereby achieving the
treatment of acute pancreatitis (7).
Currently, there is little knowledge regarding the specific
interventions targeting pancreatitis during ERCP. In our case
series, thick, purulent mucus was found in the pancreatic duct
during ERCP, suggesting both biliary and non-biliary acute
pancreatitis is associated with pancreatic duct obstruction and
hypertension. Similar findings have been obtained in animal studies
in 1990s (10), and pancreatic duct
hypertension was reported to positively correlate with the severity
of acute pancreatitis(12), and may
cause pancreatic ischemia and necrosis and aggravate pancreatitis
(13,14). In addition, the thick, purulent mucus
may aggravate pancreatic duct hypertension, resulting in the
aggravation of the disease and extension of the disease course.
After the removal of the obstruction, normal pancreatic juice came
out from the pancreatic duct, while insertion of a pancreatic stent
may smooth the pancreatic juice drainage and relieve pancreatic
duct hypertension, which may prevent pancreatitis recurrence caused
by pancreatic duct obstruction again. At the same time, pancreatic
duct decompression brought about obvious improve of inflammatory
response. Inflammation indexes such as blood leukocytes and
temperature, which was not well controlled by conservative
treatment, improved significantly after ERCP.
Pain is the most common initial symptom of acute
pancreatitis, which may cause anxiety and even respiratory distress
(15). Pain occurs across the entire
course of acute pancreatitis, and is an important factor for the
return to normal diet (2).
Therefore, pain control is an important part of acute pancreatitis
treatment. Analgesics are widely recommended to relieve the pain
due to acute pancreatitis; however, opioids, anaesthetics and
non-steroidal antiinflammatory drugs (NSAIDs) suffer from two
problems of drug-related adverse reactions and repeated
administration. Pancreatic duct hypertension has been proved to be
the primary cause of chronic pancreatitis pain, and therefore,
pancreatic duct decompression via ERCP is a common approach for the
treatment of chronic pancreatitis (16). In acute pancreatitis patients,
pancreatic duct decompression via ERCP may remarkably alleviate
abdominal pain as well. In our case series, all three cases had
obvious abdominal pain prior to ERCP, which required analgesics to
alleviate pain, and the pain was effectively alleviated after ERCP
without analgesics. All cases had their abdominal pain relief and
their normal diet returned 1 to 3 days after ERCP. For instance,
case 3 had normal serum amylase and alleviation of abdominal pain 6
days after conservative therapy; however, abdominal pain occurred
again after eating. On day 1 after ERCP, he got pain relief and
oral re-feeding. No abdominal pain was seen in all three cases
after re-feeding, suggesting that abdominal pain correlates with
pancreatic duct hypertension in acute pancreatitis patients, and
pancreatic duct via ERCP may achieve an analgesic efficacy. Early
return to normal diet facilitates the recovery of gastrointestinal
functions and prevents the development of many complications,
therefore shortens the course of acute pancreatitis.
Peripancreatic fluid accumulation is the most common
local complication of acute pancreatitis. The imaging findings of
pancreas cannot indicate the severity of acute pancreatitis.
However, if peripancreatic exudations cannot be absorbed timely,
these exudations may wall-off and even develop to peripancreatic
infection or pseudocysts, resulting in aggravation of the disease
and extension of the disease course. The outcome of local
complications is therefore an important parameter that requires
periodical monitoring during the treatment of acute pancreatitis.
In our case series, peripancreatic exudations were completely
absorbed following one-month after pancreatic decompression via
ERCP, and the pancreas morphology significantly improved regardless
of the amount of peripancreatic exudations or pancreatic
parenchymal necrosis. It is recommended to wait for the
peripancreatic fluid to be wrapped before performing interventions,
such as percutaneous drainage, endoscopic drainage or surgical
treatment (2-4).
However, the intention of endoscopic pancreatic duct decompression
and other interventions was not completely the same. Hypertension
of the pancreatic duct can lead to increased pancreatic duct
permeability and even rupture (9,17), which
was the reason why peripancreatic fluid was frequently rich in
amylase. After ERCP dredged the pancreatic duct and thus reduced
further overflow from pancreas, the effusion would also accelerate
its own absorption. The second purpose was to directly drain the
pancreatic or peripancreatic fluid for certain patients. After
pancreatic decompression, other invasive interventions may be
avoided. In addition, collecting pancreatic juice for bacterial
culture can guide the application of antibacterial drugs, as shown
in case 3. It is recommended to use further clinical application of
pancreatic decompression via ERCP as the first choice to treat
pancreatitis.
Supplementary Material
Legends for Videos
During endoscopic procedure for case
1, a great amount of thick, purulent mucus was revealed emerging
from the pancreatic duct after stenting.
During endoscopic procedure for case
2, a great amount of pancreatic juice and solid debris flowed out
from the pancreatic duct after the insertion of the guidewire.
Acknowledgements
Many thanks are expressed to the three patients and
their family members for their participation and active cooperation
in this study.
Funding
This study was supported by the Ningxia Hui
Autonomous Region Science and Technology Pillar Program (grant no.
2015KJHM40).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and QW wrote the manuscript. JS and ML conceived
and designed the study. FW and BC were responsible for the
collection and analysis of the experimental data. ZL and HC
interpreted the data and drafted the manuscript. NW, WY and LY
revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
General Hospital of Ningxia Medical University (Ningxia, China).
Patients who participated in this research, signed the informed
consent and had complete clinical data.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yokoe M, Takada T, Mayumi T, Yoshida M,
Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H, et al:
Japanese guidelines for the management of acute pancreatitis:
Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 22:405–432.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Working Group IAP/APA Acute Pancreatitis
Guidelines. IAP/APA evidence-based guidelines for the management of
acute pancreatitis. Pancreatology. 13 (Suppl 2):e1–e15.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tenner S, Baillie J, DeWitt J and Vege SS:
American College of Gastroenterology. American College of
Gastroenterology Guideline: Management of acute pancreatitis. Am J
Gastroenterol. 108:1400–15. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Janisch NH and Gardner TB: Advances in
management of acute pancreatitis. Gastroenterol Clin North Am.
45:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhatia M, Wong FL, Cao Y, Lau HY, Huang J,
Puneet P and Chevali L: Pathophysiology of acute pancreatitis.
Pancreatology. 5:132–144. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kapetanos DJ: ERCP in acute biliary
pancreatitis. World J Gastrointest Endosc. 2:25–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Delhaye M, Matos C, Arvanitakis M and
Deviere J: Pancreatic ductal system obstruction and acute recurrent
pancreatitis. World J Gastroenterol. 14:1027–1033. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Harvey MH, Wedgwood KR, Austin JA and
Reber HA: Pancreatic duct pressure, duct permeability and acute
pancreatitis. Br J Surg. 76:859–862. 1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arendt T, Nizze H, Mönig H, Kloehn S,
Stüber E and Fölsch UR: Biliary pancreatic reflux-induced acute
pancreatitis-myth or possibility? Eur J Gastroenterol Hepatol.
11:329–335. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Siqin D, Wang C, Zhou Z and Li Y: The key
event of acute pancreatitis: Pancreatic duct obstruction and bile
reflux, not a single one can be omitted. Med Hypotheses.
72:589–591. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujiwara H: Pressure measurement in
pancreatic duct and biliary duct system in dogs with acute
pancreatitis. Kobe J Med Sci. 37:47–55. 1991.PubMed/NCBI
|
|
13
|
Shi CX, Chen JW, Carati CJ, Schloithe AC,
Toouli J and Saccone GT: Effects of acute pancreatic duct
obstruction on pancreatic perfusion: Implication of acute
pancreatic duct decompression. Scand J Gastroenterol. 37:1328–1333.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arendt T: Bile-induced acute pancreatitis
in cats. Roles of bile, bacteria, and pancreatic duct pressure. Dig
Dis Sci. 38:39–44. 1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fantini L, Tomassetti P and Pezzilli R:
Management of acute pancreatitis: Current knowledge and future
perspectives. World J Emerg Surg. 1(16)2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Seicean A and Vultur S: Endoscopic therapy
in chronic pancreatitis: Current perspectives. Clin Exp
Gastroenterol. 8:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nadkarni NA, Kotwal V, Sarr MG and Swaroop
Vege S: Disconnected pancreatic duct syndrome: Endoscopic stent or
surgeon's knife? Pancreas. 44:16–22. 2015.PubMed/NCBI View Article : Google Scholar
|