Introduction
Although extensively researched for hundreds of
years, urinary tract infections (UTI) continue to represent the
most common infectious disease in women, a leading cause of
morbidity and health associated expenditures, a reason for
diminished quality of life and an important social burden (1-7).
Based on clinical and biochemical criteria, UTIs are classified
into lower (cystitis) and upper (pyelonephritis) infections.
Empirical antibacterial therapeutic approach depends on the
severity of disease (e.g. uncomplicated vs. complicated UTIs), the
spectrum of the possible pathogens and the local resistance
patterns (8). The most frequent
pathogens involved are from Enterobacteriaceae group, mainly
E. coli and Klebsiella pneumonia (9). The continuous increasing frequency of
multidrug-resistant Enterobacteriaceae, even among
community-acquired infections, plays an essential role in
antibacterial treatment outcome (10).
As the prevalence of recurrences after a first
episode of UTI is rated at 25-50% in different analyzes, depending
on the diagnosis criteria and the methodology used for detection,
there is a growing concern about the adverse effects of the classic
antimicrobial treatment of the acute UTI and also of the recurrence
prophylaxis (11-13).
Apart from the antimicrobial resistance which is a real threat of
the modern era, prolonged antibiotic treatments (especially with
fluoroquinolones, aminopenicillins or cephalosporins) lead to
disruptions of the normal bacterial flora defined as ‘collateral
damage’ phenomenon and the development of Clostridium
difficile infections with increasing spreading worldwide
(14-16).
In these circumstances, there is a lot of interest
regarding non-antimicrobial types of UTI treatment, including
urinary antiseptics, urine pH changers, bacterial
adherence-inhibitors, immunity enhancers, probiotics and vaccines
(17-20).
There is an increasing offer of these products on the
pharmaceutical market, but adequate information on their exact role
in prophylaxis of UTI or in the treatment of acute episode are
elusive (21).
Both cranberry extracts and D-mannose have been
proven active in inhibiting the adherence of uropathogens to
urinary tract epithelium and reducing bacterial colonization. There
are evidence-based recommendations for the use of D-mannose or/and
cranberry extracts in the prophylaxis of UTI recurrences (22,23), but
few studies approached their effect for the management of acute
episodes of UTI.
The primary aim of the current study was to compare
the effectiveness of trimethoprim-sulfamethoxazole (TMP-SMX) alone
vs. TMP-SMX combined with Uro-Care with CranActin® (a
supplement containing cranberry extracts and D-mannose) in the
treatment of the acute phase of lower uncomplicated UTI in women;
the secondary objective was to evaluate the influence of Uro-Care
with CranActin® in the early UTI persistence and
recurrence prevalence.
Patients and methods
Study design
This single-center randomized study was conducted
over 18 months in the Department of Nephrology and Dialysis, ‘St.
John’ Emergency Clinical Hospital, Bucharest, Romania.
Ethics
The study protocol and the patient information
sheet(s) were reviewed and approved by the Ethics Committee of the
‘St. John’ Emergency Clinical Hospital (no. 26067/27.12.2017). The
study was performed in accordance with Good Clinical Practice and
ethical standards of Declaration of Helsinki (revised 2014).
Written informed consent was obtained from all participants.
Patient selection
One hundred and twenty participants, all women aged
18-60 years diagnosed with uncomplicated urinary tract infection
were assessed for eligibility starting from January 2018.
Patients with complicated UTIs, gynecological
disorders and also with diagnosis of neoplasia in the previous 5
years were not eligible for the study. In addition, patients with
history of any antibiotic treatment within 4 weeks previous to the
enrollment, and pregnant or lactating women were excluded.
Treatment
The study was split in two stages. In the first
stage, patients were allocated to TMP-SMX alone or in combination
to the investigated product containing cranberry extract plus
D-mannose (Uro-Care with CranActin®, further named as
the study medication) for 7 days. Patients who completed the first
phase were subsequently included in a second double-blind,
placebo-controlled stage of the study, when patients were
randomized in a 1:1 ratio to placebo or cranberry extract plus
D-mannose administered for 21 days. The composition for placebo
capsules was a mixture of glucose and pharmacologically inert
vegetal extract; the study medication contained 1000 mg D-mannose
and 400 mg Cranberry CranActin AF® incorporated in
vegetal capsules (manufactured by Solaray®). According
to guidelines for lower uncomplicated UTI, antibiotic treatment was
initiated before obtaining, and regardless, the results of urine
culture and susceptibility tests (23). Antimicrobial therapy was administered
in accordance to Summary of Product Characteristics. The
investigated product, cranberry extract plus D-mannose and placebo
were administered as two capsules daily. The entire duration of
treatment within the study was 28 days.
Outcome assessment
Medical history, clinical examination, and a list of
complaints were recorded at the baseline visit. The characteristic
UTI symptoms were noted on a questionnaire with 7 items (dysuria,
increased urinary frequency/pollakiuria, urinary urgency,
hematuria, hypogastric pain, lumbar pain, vesical tenesmus) and 3
degrees of intensity (absent, moderate, severe). For excluding
upper/complicated UTI, the C-reactive protein serum level was
analyzed. Urine culture and susceptibility tests were performed for
all patients.
Patients were evaluated after first 7 days of
treatment (at the end of first stage) and also after another 21
days (which comprised the second stage of the study). The same
questionnaire was used 3 times to evaluate clinical manifestations.
All the patients were encouraged to announce any increase in the
severity of clinical symptoms anytime along the study period.
Endpoints
The primary endpoint of the study was to assess the
efficacy of cranberry extract plus D-mannose, when added to
TMP-SMX, based on the reduction in the severity of each clinical
manifestation and based on the cure rate after 7 days of
treatment.
Cure was considered when all the symptoms were
remitted.
The second endpoint of the study was to determine
the effect of cranberry extract plus D-mannose on persistence and
recurrence of UTI, when administered for 21 days after the acute
treatment.
Occurrence of any adverse event during the study was
noted for safety analysis.
Statistical analysis
The demographic and clinical data were summarized in
frequency/percentage and distribution tables for the individual
variables (mean, standard deviation).
Analysis was performed using Levene's test and the
95% confidence intervals were calculated. A paired sample t-test
has been used in order to evaluate if there is a difference in
symptoms score and clinical signs between day 0, 7 and 28. P-values
<0.05 were considered significant. Statistical analysis was
performed using SPSS statistical package, version 20.0 (SPSS,
Inc.).
Results
Of the 120 patients screened, 26 patients were
excluded. One patient in the treatment arm withdrew her consent
because of a mild skin rash after the first day (self-limited after
the treatment discontinuation). A number of 93 patients completed
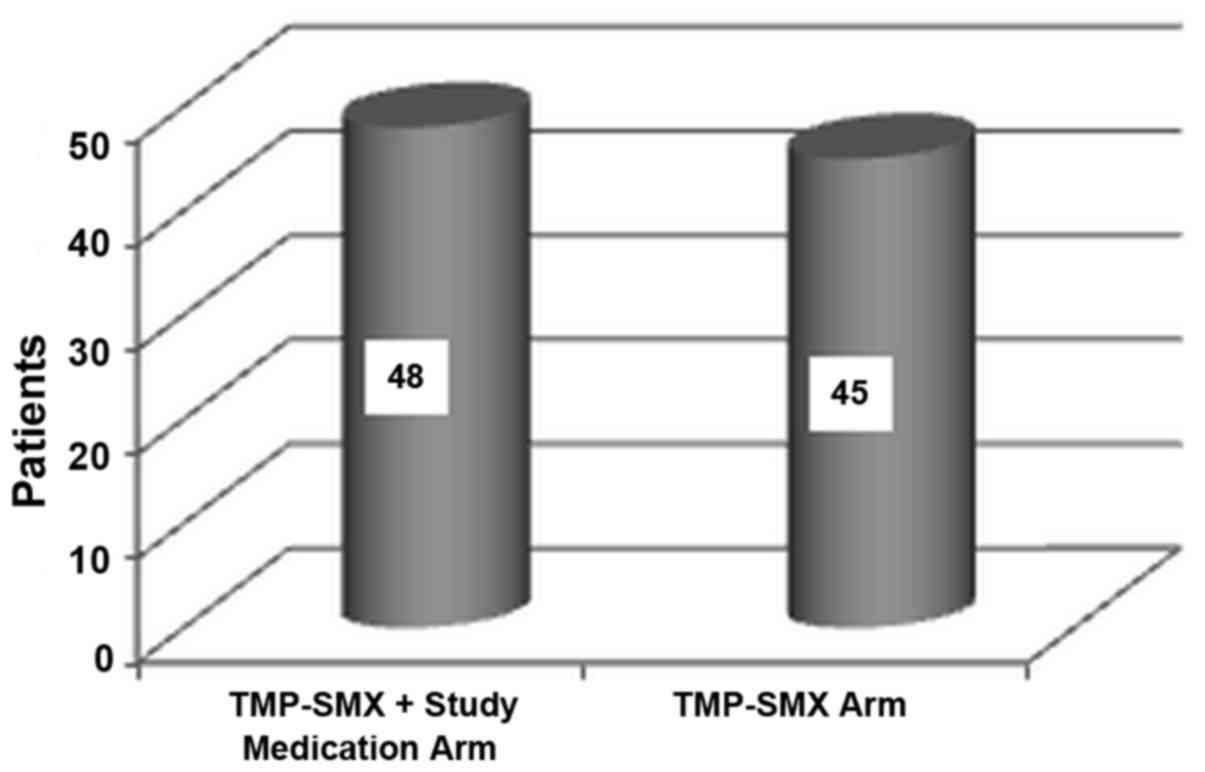
first stage of the study (48 treated with TMP-SMX plus study
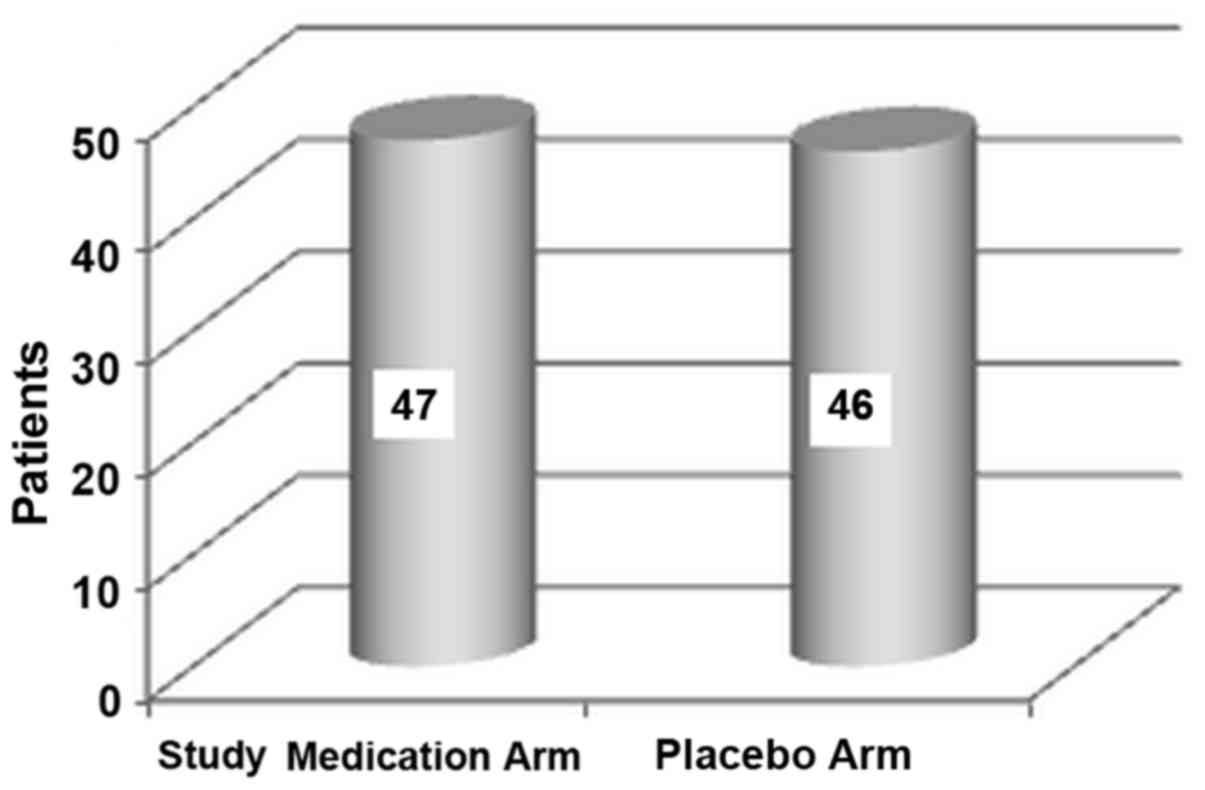
medication and 45 treated with TMP-SMX alone; Fig. 1) and were allocated to placebo (46
patients) or the investigated product (47 patients) in the second
stage (Fig. 2). All 93 patients
completed the study and were included in the statistical
analysis.
The demographic and baseline characteristics of the
participants (as randomized in the first stage of the study) are
shown in Table I.
 | Table IBaseline characteristics and
demographics. |
Table I
Baseline characteristics and
demographics.
| Characteristics | Standard treatment +
study medication arm | Standard treatment
arm |
|---|
| Age |
|
Mean
(SD) | 40.77 (10.59) | 38.78 (10.13) |
|
Median (min;
max) | 41 (21;60) | 39 (23;59) |
| Sex |
|
Female | 48 | 45 |
|
Male | 0 | 0 |
| Urine culture |
|
E.
coli | 48 | 45 |
| C-reactive
protein |
|
Mean
(SD) | 0.31 (0.77) | 0.23 (0.45) |
|
Median (min;
max) | 0.15 (0;0.5) | 0.20 (0;0.3) |
The majority of women reported moderate or severe
frequency/pollakiuria (83.87% cases), urgency (84.94%) and
suprapubic pain (78.49%), and 58.06% had dysuria at baseline
clinical evaluation. Hematuria was absent in 83.87% of the cases
(Table II).
 | Table IIBaseline symptomatology per treatment
arm. |
Table II
Baseline symptomatology per treatment
arm.
| | Standard treatment +
Study medication arm | Standard
treatment |
|---|
| Symptom type | Severe | Moderate | None | Severe | Moderate | None |
|---|
| Dysuria (n) | 5 | 24 | 19 | 9 | 16 | 20 |
| Pollakiuria
(n) | 14 | 27 | 7 | 9 | 28 | 8 |
| Urinary urgency
(n) | 7 | 36 | 5 | 6 | 30 | 9 |
| Vesical tenesmus
(n) | 4 | 31 | 13 | 5 | 34 | 6 |
| Hypogastric pain
(n) | 4 | 33 | 11 | 7 | 29 | 9 |
| Lumbar pain
(n) | 0 | 4 | 44 | 0 | 5 | 40 |
| Hematuria (n) | 3 | 2 | 43 | 4 | 6 | 35 |
After 7 days of treatment, statistically significant
improvements (P<0.005) were recorded in all patients for all
symptoms, mainly for urgency (2.15% moderate/severe cases),
hypogastric pain (2.15% moderate/severe cases), but also for
dysuria (3.22% cases with moderate/severe score) and
frequency/pollakiuria (5.37% moderate/severe cases) (Tables III and IV).
 | Table IIISymptomatology per treatment arm at
day 7. |
Table III
Symptomatology per treatment arm at
day 7.
| | Standard treatment
+ Study medication arm | Standard
treatment |
|---|
| Symptom type | Severe | Moderate | None | Severe | Moderate | None |
|---|
| Dysuria (n) | 0 | 0 | 48 | 0 | 3 | 42 |
| Pollakiuria
(n) | 0 | 1 | 47 | 0 | 4 | 41 |
| Urinary urgency
(n) | 0 | 0 | 48 | 1 | 1 | 43 |
| Vesical tenesmus
(n) | 0 | 1 | 47 | 1 | 0 | 44 |
| Hypogastric pain
(n) | 0 | 0 | 48 | 0 | 2 | 43 |
| Lumbar pain
(n) | 0 | 1 | 47 | 0 | 1 | 44 |
| Hematuria (n) | 0 | 0 | 48 | 0 | 0 | 45 |
 | Table IVTreatment evolution and
symptomatology from day 0 to 7 by paired t-test. |
Table IV
Treatment evolution and
symptomatology from day 0 to 7 by paired t-test.
| Paired samples
test |
|---|
| | Paired
differences | |
|---|
| | 95% confidence
interval of the difference | |
|---|
| Symptom | Mean | Std. deviation | Std. error
mean | Lower | Upper | t | df | Sig.
(two-tailed) |
|---|
| Dysuria | -0.688 | 0.675 | 0.070 | -0.827 | -0.549 | -9.827 | 92 | <0.001 |
| Pollakiuria | -1.032 | 0.650 | 0.067 | -1.166 | -0.898 | -15.308 | 92 | <0.001 |
| Urinary
urgency | -0.957 | 0.530 | 0.055 | -1.066 | -0.848 | -17.418 | 92 | <0.001 |
| Vesical
tenesmus | -0.860 | 0.563 | 0.058 | -0.976 | -0.744 | -14.729 | 92 | <0.001 |
| Hipogastric
pain | -0.882 | 0.549 | 0.057 | -0.995 | -0.769 | -15.497 | 92 | <0.001 |
| Lumbar pain | -0.075 | 0.303 | 0.031 | -0.138 | -0.013 | -2.392 | 92 | 0.019 |
| Hematuria | -0.237 | 0.579 | 0.060 | -0.356 | -0.117 | -3.943 | 92 | <0.001 |
At the end of the first phase, the cure rate was
88.17% in the entire group, with 84.44% in the TMP-SMX alone arm
and 91.66% in the TMP-SMX plus investigated product arm (Table IV).
There were not statistically significant differences
between groups with or without co-administration of cranberry
extract plus D-mannose added to antibiotic therapy, except for
urinary urgency/pollakiuria: P=0.024 with a 95% CI (0.198-0.015)
(Table V).
 | Table VStatistical analysis by treatment
group. |
Table V
Statistical analysis by treatment
group.
|
Independent
samples test |
|---|
| | Levene's test for
equality of variances | t-test for equality
of means |
|---|
| | 95% confidence
interval of the difference |
|---|
| Symptoms at day
7 | F | Sig. | t | df | Sig.
(two-tailed) | Mean
difference | Std. error
difference | Lower | Upper |
|---|
|
Dysuriaa | 4.134 | 0.045 | -0.995 | 91 | 0.322 | -0.042 | 0.042 | -0.126 | 0.042 |
|
Dysuriab | | | -1.000 | 75.362 | 0.321 | -0.042 | 0.042 | -0.126 | 0.042 |
|
Pollakiuriaa | 27.618 | <0.001 | -2.315 | 91 | 0.023 | -0.106 | 0.046 | -0.198 | -0.015 |
|
Pollakiuriab | | | -2.340 | 46.000 | 0.024 | -0.106 | 0.045 | -0.198 | -0.015 |
|
Urgencya | 0.784 | 0.378 | -0.433 | 91 | 0.666 | -0.021 | 0.048 | -0.116 | 0.075 |
|
Urgencyb | | | -0.436 | 68.383 | 0.665 | -0.021 | 0.048 | -0.116 | 0.075 |
| Hipogastric
paina | 7.697 | 0.007 | -1.339 | 91 | 0.184 | -0.064 | 0.048 | -0.159 | 0.031 |
| Hipogastric
painb | | | -1.353 | 46.000 | 0.183 | -0.064 | 0.047 | -0.159 | 0.031 |
| Lumbar
paina | 0.001 | 0.976 | 0.015 | 91 | 0.988 | 0.000 | 0.030 | -0.060 | 0.061 |
| Lumbar
painb | | | 0.015 | 90.904 | 0.988 | 0.000 | 0.030 | -0.060 | 0.061 |
|
Hematuriaa | 0.001 | 0.976 | 0.015 | 91 | 0.988 | 0.000 | 0.030 | -0.060 | 0.061 |
|
Hematuriab | | | 0.015 | 90.904 | 0.988 | 0.000 | 0.030 | -0.060 | 0.061 |
In total 24 cases showed strains resistant to
TMP-SMX (36% cases in the investigated product group). Nine of
these patients experienced persistence or aggravation of the
symptoms during the first 7 days of treatment (47.06% in the
TMP-SMX group and 11% in the combined group); antibiotic was
replaced according to susceptibility tests.
In the second phase of the study, after 21 days of
administration of investigated product or placebo, sporadic
persistence/recurrence were noted for dysuria and pollakiuria
(6.45% cases with moderate score), urgency (2.15% moderate cases),
hypogastric pain (1.07% moderate cases), but not for lumbar pain
[P=0.057, 95% CI (0.131-0.002)]. One case of hematuria was noted at
day 28 (Tables VI and VII).
 | Table VISymptomatology per treatment arm at
day 28. |
Table VI
Symptomatology per treatment arm at
day 28.
| | Study medication
arm | Placebo arm |
|---|
| Symptom type | Severe | Moderate | None | Severe | Moderate | None |
|---|
| Dysuria (n) | 0 | 2 | 45 | 0 | 4 | 42 |
| Pollakiuria
(n) | 0 | 2 | 45 | 0 | 4 | 42 |
| Urinary urgency
(n) | 0 | 0 | 47 | 0 | 2 | 44 |
| Vesical tenesmus
(n) | 0 | 0 | 47 | 0 | 0 | 46 |
| Hypogastric pain
(n) | 0 | 0 | 47 | 0 | 1 | 45 |
| Lumbar pain
(n) | 1 | 0 | 46 | 0 | 1 | 45 |
| Hematuria (n) | 0 | 0 | 47 | 0 | 1 | 45 |
 | Table VIITreatment evolution and
symptomatology from day 0 to 28 by paired t-test. |
Table VII
Treatment evolution and
symptomatology from day 0 to 28 by paired t-test.
| Paired samples
test |
|---|
| | Paired
differences | |
|---|
| | 95% confidence
interval of the difference | |
|---|
| Symptoms evolution
from day 0 to 28 | Mean | Std. deviation | Std. error
mean | Lower | Upper | t | df | Sig.
(two-tailed) |
|---|
| Dysuria | -0.666 | 0.727 | 0.075 | -0.816 | -0.516 | -8.840 | 92 | <0.001 |
| Pollakiuria | -1.021 | 0.691 | 0.071 | -1.163 | -0.879 | -14.252 | 92 | <0.001 |
| Urinary
urgency | -0.967 | 0.560 | 0.058 | -1.083 | -0.852 | -16.650 | 92 | <0.001 |
| Vesical
renesmus | -0.892 | 0.540 | 0.056 | -1.003 | -0.781 | -15.909 | 92 | <0.001 |
| Hipogastric
pain | -0.892 | 0.579 | 0.060 | -1.011 | -0.773 | -14.845 | 92 | <0.001 |
| Lumbar pain | -0.064 | 0.323 | 0.033 | -0.131 | 0.002 | -1.925 | 92 | 0.057 |
| Hematuria | -0.215 | 0.568 | 0.058 | -0.332 | -0.098 | -3.650 | 92 | <0.001 |
Statistical analysis did not identify significant
differences between placebo and active treatment (Table VIII).
 | Table VIIIStatistical analysis by treatment
group. |
Table VIII
Statistical analysis by treatment
group.
|
Independent
samples test |
|---|
| | Levene's test for
equality of variances | t-test for equality
of means |
|---|
| | 95% confidence
interval of the difference |
|---|
| Symptoms at day
28 | F | Sig. | t | df | Sig.
(two-tailed) | Mean
difference | Std. error
difference | Lower | Upper |
|---|
|
Dysuriaa | 13.497 | <0.001 | 1.725 | 91 | 0.088 | 0.087 | 0.050 | -0.013 | 0.188 |
|
Dysuriab | | | 1.713 | 63.181 | 0.092 | 0.087 | 0.051 | -0.014 | 0.189 |
|
Pollakiuriaa | 13.497 | <0.001 | 1.725 | 91 | 0.088 | 0.087 | 0.050 | -0.013 | 0.188 |
|
Pollakiuriab | | | 1.713 | 63.181 | 0.092 | 0.087 | 0.051 | -0.014 | 0.189 |
|
Urgencya | 9.177 | 0.003 | 1.446 | 91 | 0.152 | 0.043 | 0.030 | -0.016 | 0.103 |
|
Urgencyb | | | 1.430 | 45.000 | 0.160 | 0.043 | 0.030 | -0.017 | 0.104 |
| Hipogastric
paina | 4.090 | 0.046 | -0.989 | 91 | 0.325 | -0.021 | 0.021 | -0.064 | 0.021 |
| Hipogastric
painb | | | -1.000 | 46.000 | 0.323 | -0.021 | 0.021 | -0.064 | 0.021 |
| Lumbar
paina | 8.055 | 0.006 | 1.369 | 91 | 0.175 | 0.065 | 0.047 | -0.029 | 0.159 |
| Lumbar
painb | | | 1.354 | 45.000 | 0.183 | 0.065 | 0.048 | -0.031 | 0.162 |
|
Hematuriaa | 9.177 | 0.003 | 1.446 | 91 | 0.152 | 0.043 | 0.030 | -0.016 | 0.103 |
|
Hematuriab | | | 1.430 | 45.000 | 0.160 | 0.043 | 0.030 | -0.017 | 0.104 |
Cure rate was calculated as a percentage of patients
who had no symptoms at day 28 out of the total patients in each
arm. The cure rate was higher in the cranberry extract plus
D-mannose arm (93.75%) compared with placebo arm (86.67%). However,
there was no statistically significant difference between the two
groups (P=0.794).
Discussion
Since early 90's many trials have studied the
insights of beneficial effects of cranberry products and extracts
on UTI prophylaxis and treatment. One of the first proposed
mechanism is that the reduction of the urine pH through the
phenolic content of cranberries increases the bacteriostatic
properties of the urine (24,25).
Furthermore, urine acidification can enhance the non-enzymatic
production of nitric oxide through nitrate-reductase released by
uropathogenic germs; nitric oxide has a potent antimicrobial action
(26,27). A more accepted mechanism is based on
the anti-adhesion properties of proanthocyanidins (PACs) in the
cranberry extracts, acting as inhibitors for type P pili that help
bacteria to attach to the uroepithelial cells (28,29).
The benefic effects of the PACs are well documented,
but the problem is the standardization of the cranberry products
used for UTI cure and/or prophylaxis (30).
The exact mechanism of action of D-mannose utilized
for treatment or prevention of UTI is not completely elucidated.
However, in vitro studies have demonstrated that D-mannose
could be considered a primary bladder cell receptor site for
uropathogenic E. coli since the first step in adhesion
involves the mannose-sensitive binding of FimH (the adhesin present
at the tip of type 1 pili) to bladder epithelium (31,32).
Both cranberry extract and D-mannose have been investigated in
several clinical studies, but the majority of the studies were
uncontrolled (33). We investigated
in this study the effectiveness of a supplement containing both
cranberry and D-mannose added to standard antibiotic therapy in
acute uncomplicated urinary tract infections. Moreover, we assessed
the efficiency of the phytochemical combination in early
prophylaxis of UTI recurrences. This study included female patients
with acute uncomplicated UTI. The diagnosis was symptom-based,
according to guideline recommendations stipulating that presence of
3 or more symptoms suggestive for UTI in women does not require
urine culture for a positive diagnosis (22,23).
Urine culture was performed only to determine the susceptibility of
the pathogens to TMP-SMX, but it did not influence the treatment
decision. In all cases of UTIs, E. coli was identified as
the pathogen. The CRP was assessed to differentiate the lower from
the upper UTI and to exclude the patients with pyelonephritis.
Empirical treatment was initiated with TMP- SMX as recommended by
guidelines, but the duration of treatment according to the protocol
was 7 days instead 3-5 days as recommended by guidelines in
uncomplicated cystitis, in order to gain time for the study
treatment combination to act, especially in TMP-SMX-resistant cases
(22,23).
Based on susceptibility tests, antimicrobial
resistance of the pathogens involved to TMP-SMX was identified in
24 cases (36% of the total number of cases in cranberry extract
plus D-mannose group). Nine patients, all of them with
TMP-SMX-resistant strains, experienced persistence or aggravation
of the symptoms during the first 7 days of treatment (47.06% in the
TMP-SMX alone group and only 11% in the combined therapy group). In
these cases antibiotic treatment was replaced according to
susceptibility tests. After the treatment completion they were also
randomized for the second step of the study. As expected, a
significant decrease in severity of clinical manifestations was
noted in the total study population after TMP-SMX treatment with or
without study medication. Analyzing the particular sub-group of
TMP-SMX-resistant patients, the percentage of resolution was low in
the TMP-SMX-alone arm (37.5%), as expected, but in the TMP-SMX
associated with cranberry extract plus D-mannose arm it was similar
with TMP-SMX-sensitive patients at ~90%. These results may have two
explanations. One possible reason is that the combination of
cranberry and D-mannose may have sufficient power to eradicate
lower uncomplicated UTI, supposingly in accordance with data from
other studies (33,34). Another motivation for the high
healing percentage in TMP-SMX-resistant patients with
double-treatment prescription may be the capacity of PACs+D-mannose
to increase germ sensitivity to TMP-SMX's effect. Recent research
has proven that bacteria become more sensitive to antimicrobials
when PACs are administered simultaneously, due to a complex
inhibiting effect on antibiotic resistance mechanism: they increase
the bacterial cell permeability to antibiotics and diminish the
activity of the multidrug efflux pumps (responsible for removing
the antimicrobial from the cell); for TMP-SMX, the authors revealed
a significant decrease of the minimum inhibitory concentration
(MIC) when PACs were associated to antibiotic therapy, thus a
decreased dose is necessary to inactivate several germs, including
E. coli (33,35). The role of cranberry extract for the
prevention of recurrent UTI remains under debate. There are several
studies suggesting that PACs are ineffective for this prophylaxis.
A meta-analysis of 24 studies with a total of 4,473 participants
concluded that cranberry juice is not efficient for the prevention
of UTIs (36). In this study,
cranberry extract plus D-mannose significantly improved clinical
manifestations after one month. Similar results were reported by
Genovese et al (37) who
conducted a study with a longer follow-up period and found that
combination of PACs and D-mannose was associated with a lower rate
of recurrences at 24 weeks. The main limitation, similar to this
pilot-study, was the insufficient length of the second phase for a
better assessment of the effectiveness of investigated product on
prophylaxis of UTIs recurrences.
The recurrence rate was 4.17% when cranberry extract
plus D-mannose was added to standard antibiotic treatment, lower
than in placebo arm 11.76%, though without statistically
significant differences between groups (P=0.228). On the other
hand, in the second phase of the study, in the TMP-SMX resistant
group, the persistence of symptom remission was registered in
93.33% of the cases treated with cranberry extract plus D-mannose
vs. only 55% of the cases treated with placebo.
As far as we know, this is the first clinical trial
to confirm the newly discovered effects of proanthocyanidins
contained in cranberry extract on antibiotic resistance of
gram-negative bacteria when administered to women with
uncomplicated UTIs. The association of D-mannose to cranberry
extract has not previously been studied regarding antimicrobial
susceptibility.
In conclusion, the association of D-mannose with
cranberry extract showed a promising adjuvant effect on
empirically-treated uncomplicated UTI episodes. The cure rate in
patients with resistance to the antibiotic of choice was
significant when the study medication was associated. Consistent
with data demonstrated by recent studies, the association of
D-mannose with cranberry extract may exert an
antibiotic-potentiating effect and enhance the sensitivity of
uropathogens to the antimicrobial treatment used for acute episodes
of UTI. Further trials with larger number of patients and various
types of antimicrobials are needed in order to confirm these
findings.
Acknowledgements
Not applicable.
Funding
This study was supported by SECOM Company, who
delivered the study product, placebo capsules, and the antibiotic
for standard treatment. All investigators received study fees. The
sponsor had no involvement in the collection, analysis, and
interpretation of data. SECOM Company had no contribution in the
writing of the report; there were no restrictions regarding the
submission of the report for publication. We had full access to all
of the data in this study and we take complete responsibility for
the integrity of the data and the accuracy of the data analysis. CD
received speaker honoraria from SECOM company.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DR and CD conceived the study and drafted the
manuscript. IAV, FLT, PP and DMS collected, analyzed and
interpreted the patients data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol and the patient information
sheet(s) were reviewed and approved by the Ethics Committee of the
‘St. John’ Emergency Clinical Hospital (Bucharest, Romania) (no.
26067/27.12.2017). The study was performed in accordance with Good
Clinical Practice and ethical standards of Declaration of Helsinki
(revised 2014). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nickel JC: Management of urinary tract
infections: historical perspective and current strategies: Part 1 -
Before antibiotics. J Urol. 173:21–26. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Medina M and Castillo-Pino E: An
introduction to the epidemiology and burden of urinary tract
infections. Ther Adv Urol. 11(1756287219832172)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Flores-Mireles AL, Walker JN, Caparon M
and Hultgren SJ: Urinary tract infections: Epidemiology, mechanisms
of infection and treatment options. Nat Rev Microbiol. 13:269–284.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kranz J, Schmidt S, Lebert C, Schneidewind
L, Mandraka F, Kunze M, Helbig S, Vahlensieck W, Naber K,
Schmiemann G, et al: The 2017 update of the German clinical
guideline on epidemiology, diagnostics, therapy, prevention, and
management of uncomplicated urinary tract infections in adult
patients: Part 1. Urol Int. 100:263–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tandogdu Z and Wagenlehner FM: Global
epidemiology of urinary tract infections. Curr Opin Infect Dis.
29:73–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rădulescu A, Mădan V, Aungurenci A, Bratu
O, Farcaş C, Dinu M and Mischianu D: Antibiotic resistant urinary
tract infections in an urology ward. Rom J Mil Med. 118:20–22.
2015.
|
|
7
|
Zaha DC, Bungau S, Aleya S, Tit DM, Vesa
CM, Popa AR, Pantis C, Maghiar OA, Bratu OG, Furau C, et al: What
antibiotics for what pathogens? The sensitivity spectrum of
isolated strains in an intensive care unit. Sci Total Environ.
687:118–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vata A, Hunea IM, Dorneanu O, Harja-Alexa
IA, Plesca C, Leonte-Enache G, Ciocan A, Ghiciuc C, Esanu I,
Manolache M, et al: Biochemical changes and risk factors in the
prognosis of antibiotics susceptibility in urinary tract
infections. Rev Chim Buchar. 70:1822–1825. 2019.
|
|
9
|
Mihai IF, Lacatusu G, Filip-Ciubotaru F,
Dorobat C, Romanescu C and Manciuc C: Major trends in the microbial
etiology of urinary tract infection. GARJM. 8:35–37. 2019.
|
|
10
|
Miftode E, Dorneanu O, Leca D, Teodor A,
Mihalache D, Filip O and Luca V: Antimicrobial resistance profile
of E. coli and Klebsiella spp. from urine in the
Infectious Diseases Hospital Iaşi. Rev Med Chir Soc Med Nat Iasi.
112:478–482. 2008.PubMed/NCBI(In Romanian).
|
|
11
|
Anger J, Lee U, Ackerman AL, Chou R,
Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman
MR, et al: Recurrent uncomplicated urinary tract infections in
women: AUA/CUA/SUFU Guideline. J Urol. 202:282–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Malik RD, Wu YR and Zimmern PE: Definition
of recurrent urinary tract infections in women: Which one to adopt?
Female Pelvic Med Reconstr Surg. 24:424–429. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Geerlings SE: Clinical presentations and
epidemiology of urinary tract infections. Microbiol Spectr: Oct 4,
2016 (Epub ahead of print). doi:
10.1128/microbiolspec.UTI-0002-2012.
|
|
14
|
Luc MC, Roșu F, Hurmuzache M, Luca AS,
Leca D, Iancu L and Ochiuz L: Updates in the assesment of risk
factors in Clostridium Difficile infection in patients with
infectious diseases. Farmacia. 64:112–115. 2016.
|
|
15
|
Paterson DL: ‘Collateral damage’ from
cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 38
(Suppl 4):S341–S345. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Schito GC, Naber KG, Botto H, Palou J,
Mazzei T, Gualco L and Marchese A: The ARESC study: An
international survey on the antimicrobial resistance of pathogens
involved in uncomplicated urinary tract infections. Int J
Antimicrob Agents. 34:407–413. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scottish Intercollegiate Guidelines
Network (SIGN): Management of Suspected Bacterial Urinary Tract
Infection in Adults. Edinburgh: SIGN (SIGN publication no. 88),
pp1-46, 2012. Available from URL: http://www.sign.ac.uk.
Accessed November 10, 2019.
|
|
18
|
Glover M, Moreira CG, Sperandio V and
Zimmern P: Recurrent urinary tract infections in healthy and
nonpregnant women. Urol Sci. 25:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chwa A, Kavanagh K, Linnebur SA and Fixen
DR: Evaluation of methenamine for urinary tract infection
prevention in older adults: A review of the evidence. Ther Adv Drug
Saf. 10(2042098619876749)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Eells SJ, Bharadwa K, McKinnell JA and
Miller LG: Recurrent urinary tract infections among women:
Comparative effectiveness of 5 prevention and management strategies
using a Markov chain Monte Carlo model. Clin Infect Dis.
58:147–160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ratiu MP, Purcarea I, Popa F, Purcarea VL,
Purcarea TV, Lupuleasa D and Boda D: Escaping the economic turn
down through performing employees, creative leaders and growth
driver capabilities in the Romanian Pharmaceutical Industry.
Farmacia. 59:119–129. 2011.
|
|
22
|
National Institute for Health and Care
Excellence NICE: NICE guideline NG15. Antimicrobial stewardship:
systems and processes for effective antimicrobial medicine use.
pp1-25, 2015. Available from URL: https://www.nice.org.uk/guidance/ng15.
Accessed November 10, 2019.
|
|
23
|
Grabe M, Baroletti R, Bjerklund Johansen
TE, Cai T, Çek M, Köves B, Naber KG, Pickard RS, Tenke P,
Wagenlehner F, et al: Guidelines on Urological Infections. European
Association of Urology. 2015. Available from URL: https://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
Accessed November 10, 2019.
|
|
24
|
Gopalakrishna P, Bonnie D, Richard Z and
Lacie D: Cranberry for the prevention and treatment of
non-nomplicated urinary tract infections. SOJ Pharm Sci. 6:1–9.
2018.
|
|
25
|
Vasileiou I, Katsargyris A, Theocharis S
and Giaginis C: Current clinical status on the preventive effects
of cranberry consumption against urinary tract infections. Nutr
Res. 33:595–607. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350.
1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rhee KY and Charles M: Antimicrobial
mechanisms of cranberry juice. Clin Infect Dis.
39(877)2004.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Di Martino P, Agniel R, David K, Templer
C, Gaillard JL, Denys P and Botto H: Reduction of Escherichia
coli adherence to uroepithelial bladder cells after consumption
of cranberry juice: A double-blind randomized placebo-controlled
cross-over trial. World J Urol. 24:21–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lavigne JP, Bourg G, Combescure C, Botto H
and Sotto A: In-vitro and in-vivo evidence of dose-dependent
decrease of uropathogenic Escherichia coli virulence after
consumption of commercial Vaccinium macrocarpon (cranberry)
capsules. Clin Microbiol Infect. 14:350–355. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guay DR: Cranberry and urinary tract
infections. Drugs. 69:775–807. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hung CS, Bouckaert J, Hung D, Pinkner J,
Widberg C, DeFusco A, Auguste CG, Strouse R, Langermann S, Waksman
G, et al: Structural basis of tropism of Escherichia coli to
the bladder during urinary tract infection. Mol Microbiol.
44:903–915. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hickling DR and Nitti VW: Management of
recurrent urinary tract infections in healthy adult women. Rev
Urol. 15:41–48. 2013.PubMed/NCBI
|
|
33
|
Vicariotto F: Effectiveness of an
association of a cranberry dry extract, D-mannose, and the two
microorganisms Lactobacillus plantarum LP01 and Lactobacillus
paracasei LPC09 in women affected by cystitis: A pilot study. J
Clin Gastroenterol. 48 (Suppl 1):S96–S101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Panchev P, Slavov Ch, Mladenov D, Georgiev
M, Yanev K, Paskalev E, Simeonov P, Gerassi R, Bogov B and Saltirov
I: A multicenter comparative observation on the effectiveness and
the rapidness of the effect of Cystostop Rapid vs. antibiotic
therapy in patients with uncomplicated cystitis. Akush Ginekol
(Sofiia). 51:49–55. 2012.PubMed/NCBI(In Bulgarian).
|
|
35
|
Maisuria VB, Okshevsky M, Déziel E and
Tufenkji N: Proanthocyanidin interferes with intrinsic antibiotic
resistance mechanisms of gram-negative bacteria. Adv Sci (Weinh).
6(1802333)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jepson RG, Williams G and Craig JC:
Cranberries for preventing urinary tract infections. Cochrane
Database Syst Rev. 10(CD001321)2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Genovese C, Davinelli S, Mangano K,
Tempera G, Nicolosi D, Corsello S, Vergalito F, Tartaglia E,
Scapagnini G and Di Marco R: Effects of a new combination of plant
extracts plus d-mannose for the management of uncomplicated
recurrent urinary tract infections. J Chemother. 30:107–114.
2018.PubMed/NCBI View Article : Google Scholar
|