Introduction
Developing from the neural crest, both the brain and
the thyroid can be affected by immunological imbalances. Possible
associations of cancer with multiple sclerosis and immunomodulatory
or immunosuppressant drugs have been investigated. The successive
use of two or more disease-modifying therapies for MS patients can
increase the risk of neoplasia (1).
The results are contradictory regarding this topic, ranging between
an increased risk of respiratory cancer, urinary system neoplasia,
and nervous system cancer for MS patients and a lower risk of
cancer in the MS population (2-5).
One study found a higher incidence of thyroid cancer in MS cohort,
but it did not specify the type of thyroid neoplasm implicated
(1).
Recent studies have revealed that long-term
immunosuppressive treatment for MS increases the risk of cancer
(6,7). Thyroid neoplasia is the most frequent
endocrine cancer, accounting for ~2.1% of all malignancies
(8). Differentiated thyroid cancer,
including papillary and follicular types, evolving from
thyroglobulin-secreting follicular cells, represents ~80% of all
thyroid neoplasia cases. Medullary carcinoma, which derives from
calcitonin-producing parafollicular C cells, is relatively rare,
accounting for 5-10% (9). However,
very rare combinations of two thyroid malignancies in the same
patient have also been observed. Such cases are known as collision
tumors, a rare entity described in the literature as the
coexistence of at least two distinct tumors with different genetic
origins and histologically distinct morphologies in the same organ
and with no transition area between them. Such tumors have been
described in the colon, lungs, ovaries, skin, and thyroid gland,
with increasing incidence. Regarding the thyroid gland, the most
frequent collision is that of papillary and medullary carcinomas
(9). The role of the Epstein-Barr
virus (EBV) is often discussed in relation to both MS and thyroid
cancers, with a possible association between the two. Collision
tumors must be considered more aggressive and posing a greater risk
of recurrence compared with independent tumors. Therefore,
monitoring cases is more complicated, as the evolution and risks of
each tumor involved must be taken into account.
Case report
A 46-year-old female patient was admitted to our
clinic in 2018. Her personal medical history included arterial
hypertension with preeclampsia, with a first neurological episode
of abdominal and crural paresthesia in 2003, when she was 31 years
old, which spontaneously remitted within a month. Upon relapse in
2008 with the same symptoms, after a magnetic resonance imaging
(MRI) examination, she was diagnosed with recurrent-remitting
multiple sclerosis, with an Expanded Disability Status Scale (EDSS)
score of 1. Interferon β-1b (IFNβ-1b) treatment was initiated at
doses of 250 mg (8.0 million IU) subcutaneously every other day.
Her family medical history included breast cancer (maternal
grandmother) and diabetes mellitus type 2 (father). The course of
disease was good after 10 years of treatment, with no evidence of
disease activity (NEDA 2): clinical activity, disability
progression. In 2018, she was admitted for cervical pain, and
cervical MRI examination showed a gadolinium enhancement of the
right thyroid lobe. In October 2018, the patient was referred to
the Endocrinology Unit for evaluation. No family history of thyroid
cancer or multiple endocrine neoplasia (MEN) was reported. Her
physical examination was normal. Moderately high serum calcitonin
values (70.53 pg/ml; normal value: <9.82 pg/ml) were detected.
The patient had normal thyroid function (thyroid-stimulating
hormone [TSH)]: 1.65 µIU/ml; normal range: 0.5-4.5 µIU/ml) and no
alteration of calcium metabolism. As her serum parathormone,
fractionated plasma, and 24-h urinary total metanephrines were
normal, MEN syndrome was excluded. Her serum 25 (OH) vitamin D
level was 14.7 ng/ml (normal value: >20 ng/ml). Her
immunoglobulin G (IgG) antibodies against EB viral capsid antigen
were positive >750 U/ml (negative <20 U/ml), while her
anti-EBV nuclear antigen antibody IgG value was 476 E/ml (normal
value <5), indicating a prior EBV infection. RET and BRAF V600E
gene mutations were not detected.
Ultrasound examination revealed two nodules in the
right thyroid lobe: one in the middle third of the posterior part,
hypoechoic (14.5x16.9x8.7 mm), with irregular margins, showing
micro- and macrocalcifications, with chaotic vascularity in the
entire nodule in color Doppler evaluation; the other inferior and
lateral to it, a hypoechoic mass (11x10.2x7.9 mm) with regular
margins and moderately perinodular flow signals.
The dominant nodule was classified as highly
suspected for malignancy. Based on her serum calcitonin level, the
patient was referred for surgery. Total thyroidectomy with central
compartment cervical lymph node dissection was performed in
November 2018. Histopathological examination of the resected
specimen identified a collision tumor with a combination of
papillary carcinoma and medullary microcarcinoma within the right
lobe of the thyroid gland. Medullary thyroid microcarcinoma is a
neuroendocrine tumor derived from C cells (formerly called
parafollicular cells) of the ultimobranchial body of the neural
crest, which secrete calcitonin, usually located at the junction of
the upper and middle portions of the thyroid lobes. This tumor can
mimic any other thyroid malignancy of microscopic description.
Immunohistochemical examination showed that the patient's medullary
thyroid microcarcinoma tumor cells stained positive
weak-to-moderate for calcitonin and strong positive for generic
neuroendocrine markers (i.e., chromogranin), thyroid transcription
factor 1, and carcinoembryonic antigen (Fig. 1) (10,11).
One-year follow-up after thyroidectomy showed no evidence of
recurrent disease (structural or biochemical).
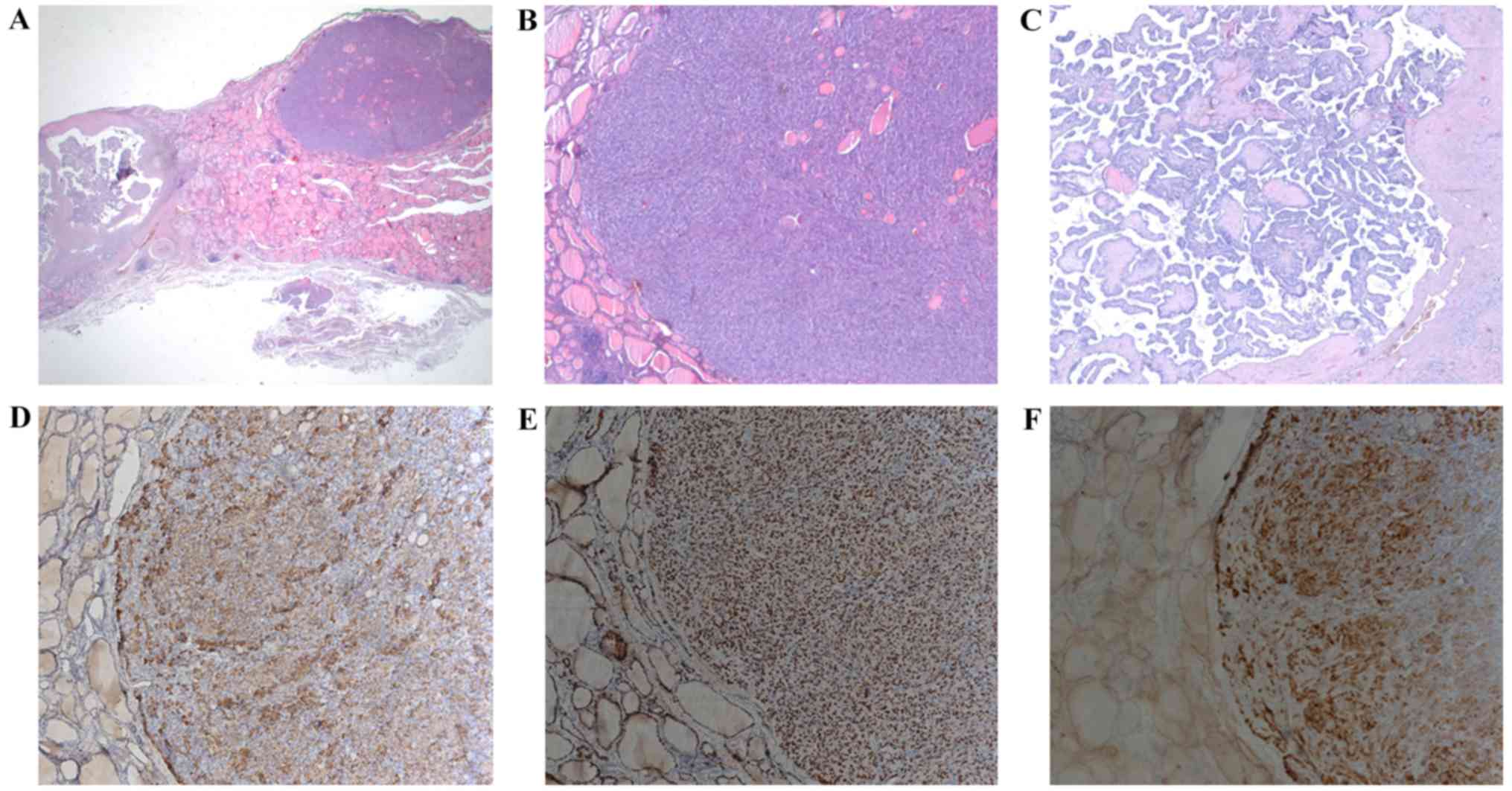 | Figure 1(A) Hematoxylin and eosin stain.
Original magnification, x10. First nodule (9x5 mm): medullary
thyroid microcarcinoma (right; pT1a N0 Mx). This type is
represented in the International Classification of Diseases for
Oncology (ICD-O) by the code 8345-3. Second marginal nodule (17
mm): papillary thyroid carcinoma (left; pT1b N0 Mx L1). This type
is represented in the International Classification of Diseases for
Oncology (ICD-O) by the code 8260-3. (B) Medullary thyroid
microcarcinoma. Hematoxylin and eosin stain. Original
magnification, x50. This type of tumor, measuring <1 cm in
diameter, exhibits a nesting, solid, and trabecular growth pattern.
The tumor cells are round, polygonal, and focal spindle-shaped,
with round nuclei with coarsely clumped chromatin and
eosinophilic-to-amphophilic granular cytoplasm due to secretory
granules. (C) papillary thyroid carcinoma. Hematoxylin and eosin
stain. Original magnification, x50. This type of infiltrating,
partially encapsulated tumor exhibits a papilliferous growth
pattern, composed of randomly oriented papillae with a complex
arborizing pattern, which are formed by a central fibrovascular
stalk covered by a neoplastic epithelial lining, together with the
presence of well-formed psammoma bodies or in an early stage of
formation. These tumor cells have typical nuclear features and
display a characteristic set of abnormalities divided into three
major categories: nuclear enlargement and overlapping,
circumferential irregularities of the nuclear membrane (garlands),
and nuclear pseudoinclusions that represent deep cytoplasmic
invaginations into the nucleus. Immunohistochemical staining shows
that medullary thyroid microcarcinoma tumor cells stain
consistently positive weak-to-moderate for calcitonin and strong
positive for generic neuroendocrine markers (i.g., chromogranin),
thyroid transcription factor 1 (TTF1), also called thyroid specific
enhancer-binding protein, and carcinoembryonic antigen (CEA). (D)
Chromogranin. Original magnification, x100. The patient's tumor
cells showed strong positivity for chromogranin. (E) TTF1. Original
magnification, x100. The patient's tumor cells showed strong
positivity for TTF1. (F) CEA. Original magnification, x100. The
patient's tumor cells showed strong positivity for CEA. |
The study was approved by the Ethics Committee of
‘Carol Davila’ Central Military Emergency University Hospital
(Bucharest, Romania) and the patient’s informed consent was
obtained.
Discussion
Due to the suspected medullary carcinoma, and
because of the preoperatively high level of calcitonin, whole
thyroidectomy with central lymph node resection was performed
(12). As a consequence of total
thyroidectomy through throat dissection, hypoparathyroidism, either
transitory or permanent (due to inadequate surgical techniques,
local hematoma, blood flow disturbances, or direct glandular
lesions) may occur. Some authors have recommended
autotransplantation of at least two parathyroid glands in such
cases (13,14). After surgery, our patient developed
laryngeal diplegia, with the left vocal cord immobilized in the
paramedian position and the right vocal cord exhibiting a discrete
adduction movement with sufficient respiratory space. The laryngeal
nerve palsy recovered within a few months.
Neurological monitoring during thyroidectomy reveals
useful information regarding the integrity and functionality of the
recurrent laryngeal nerves. In this context, two-stage
thyroidectomy is useful (15).
Another surgical procedure to preserve the recurrent laryngeal
nerve is robotic-assisted breast-axillo insufflation thyroidectomy
(RABIT), which allows a simultaneous and symmetrical visualization
and an easier approach (16). After
surgery, our patient's serum calcitonin levels returned to normal
levels (<2 pg/ml; normal value: <11.5 pg/ml), which indicated
that there was no residual tumor tissue. For the papillary cancer
with microscopic invasion into the perithyroidal soft tissue, the
patient was administered radioiodine (50 mCi of iodine-131).
Thyroid hormone withdrawal was induced six weeks prior to
radioablation. At that time, biochemical tests of the patient's
thyroid status showed the following values: TSH >47.6 µIU/ml
(normal range, 0.5-4.5), stimulated thyroglobulin 0.5 ng/ml, and
anti-thyroglobulin antibodies <1 IU/ml (normal range, 0-4).
One year after surgery and radiotherapy, the patient
was well, with hormonal levels within normal ranges and no evidence
of local recurrence on ultrasound. No clinical or imaging signs of
MS progression were detected, despite the discontinuation of
immunomodulatory treatment with IFNβ-1b. Simultaneous occurrences
of two or more cancers in the thyroid or in multiple organs have
been described in immunosuppressed patients, some with autoimmune
diseases, previously administered immunosuppressive treatments
(17,18).
Exposure to IFNβ-1b is not associated with an
increased risk of neoplasia (19).
It is estimated that infective agents are implicated in 2 million
cancers yearly, 10% of which are due to EBV (20). EBV, also known as human herpesvirus 4
(HH4), is found in over 95% of the general population, transmitted
through saliva. EBV infects the B lymphocytes, reducing gene
expressions from ~100 to just 9 proteins, and has the ability to
hide and remain in a latent state for years (21). While the incorporation of viral DNA
in that of the host cell is well known, the oncogenesis mechanism
has not yet been identified (22).
EBV genome examination by polymerase chain reaction detected EBV
DNA in 71.9% of a thyroid cancer patient cohort. Other authors
reported similar findings (23,24).
Another study, however, did not confirm the association between
thyroid tumors and the presence of EBV (25). EBV involvement in MS etiopathogenesis
has been extensively studied (26).
B lymphocytes hosting EBV are involved in triggering aberrant
immune responses in multiple sclerosis, associated with genetic
predisposition and environmental factors as a background. The EBV
involvement in MS etiopathogenesis have been revealed by
serological studies and the detection of the virus in patients'
brains (27).
However, vitamin D deficiency, confirmed in our case
also correlates with thyroid cancers and MS. Meta-analyses have
associated an optimum level of 25 (OH) vitamin D with a low risk of
thyroid cancer (28,29). Although vitamin D levels are
associated with increased MS, the role of supplement doses should
be further investigated (30).
Difficulties in supporting differential diagnosis, or in the
medical or surgical approach of patients with chronic neoplastic
conditions are inherent (31-34),
but early detection and intervention increase survival and quality
of life.
In conclusion, the coexistence of thyroid cancers in
MS patients could be explained by an immune-mediated inflammation
involved in both pathologies. Although EBV is not the only agent
responsible for the development of MS or thyroid cancers, it could
be considered a contributory factor in our case. Simultaneous onset
of medullary and papillary thyroid carcinoma is rare. Treatment and
follow-up strategies should be individualized according to the
aggressiveness of tumors. Further research on EBV involvement in
the occurrence of simultaneous immune pathologies in various organs
is needed to confirm these data.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMSi, CAS, LE, AMSo, MCG and FIR were involved in
the conception of the study. AMSi, CAS and LE contributed equally
to the acquisition of the data and the drafting of the manuscript.
AMSo, MCG and FIR contributed equally to the critical revisions of
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Carol Davila’ Central Military Emergency University Hospital
(Bucharest, Romania).
Patient consent for publication
The patient's informed consent was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Amico E, Chisari CG, Arena S, Zanghì A,
Toscano S, Lo Fermo S, Maimone D, Castaing M, Sciacca S, Zappia M,
et al: Cancer risk and multiple sclerosis: Evidence from a large
italian cohort. Front Neurol. 10(337)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Etemadifar M, Jahanbani-Ardakani H,
Ghaffari S, Fereidan-Esfahani M, Changaei H, Aghadoost N, Jahanbani
Ardakani A and Moradkhani N: Cancer risk among patients with
multiple sclerosis: A cohort study in Isfahan, Iran. Caspian J
Intern Med. 8:172–177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gaindh D, Kavak KS, Teter B, Vaughn CB,
Cookfair D, Hahn T and Weinstock-Guttman B: New York State Multiple
Sclerosis Consortium. Decreased risk of cancer in multiple
sclerosis patients and analysis of the effect of disease modifying
therapies on cancer risk. J Neurol Sci. 370:13–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grytten N, Myhr KM, Celius EG, Benjaminsen
E, Kampman M, Midgard R, Vatne A, Aarseth JH, Riise T and
Torkildsen Ø: Risk of cancer among multiple sclerosis patients,
siblings, and population controls: A prospective cohort study. Mult
Scler: Oct 1, 2019 (Epub ahead of print). doi:
10.1177/1352458519877244.
|
|
5
|
Moisset X, Perié M, Pereira B, Dumont E,
Lebrun-Frenay C, Lesage FX, Dutheil F, Taithe F and Clavelou P:
Decreased prevalence of cancer in patients with multiple sclerosis:
A case-control study. PLoS One. 12(e0188120)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lebrun C and Rocher F: Cancer risk in
patients with multiple sclerosis: Potential impact of
disease-modifying drugs. CNS Drugs. 32:939–949. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ragonese P, Aridon P, Vazzoler G, Mazzola
MA, Lo Re V, Lo Re M, Realmuto S, Alessi S, D'Amelio M, Savettieri
G, et al: Association between multiple sclerosis, cancer risk, and
immunosuppressant treatment: A cohort study. BMC Neurol.
17(155)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grimm D: Current knowledge in thyroid
cancer-from bench to bedside. Int J Mol Sci.
18(1529)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thomas VP and George R: Collision tumors
of the thyroid: Review of literature and report of a case of
papillary-follicular collision tumor. Thyroid Res Pract. 15:60–64.
2018.
|
|
10
|
Lloyd RV, Osamura RY, Kloppel G and Rosai
J: WHO Classification of Tumours of Endocrine Organs. 4th edition.
IARC, Lyon, pp65-114, 2017.
|
|
11
|
Rosai J, BeLellis RA, Carcangiu ML, Frable
WJ and Tallini G: Tumors of the thyroid and parathyroid glands. In:
AFIP Atlas of Tumor Pathology. ARP, Maryland, pp103-130, 2014.
|
|
12
|
Park YM, Kim JR, Oh KH, Cho JG, Baek SK,
Kwon SY, Jung KY and Woo JS: Comparison of functional outcomes
after total thyroidectomy and completion thyroidectomy:
Hypoparathyroidism and postoperative complications. Auris Nasus
Larynx. 46:101–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ritter K, Elfenbein D, Schneider DF, Chen
H and Sippel RS: Hypoparathyroidism after total thyroidectomy:
Incidence and resolution. J Surg Res. 197:348–353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Teshima M, Otsuki N, Morita N, Furukawa T,
Shinomiya H, Shinomiya H and Nibu KI: Postoperative
hypoparathyroidism after total thyroidectomy for thyroid cancer.
Auris Nasus Larynx. 45:1233–1238. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Christoforides C, Papandrikos I, Polyzois
G, Roukounakis N, Dionigi G and Vamvakidis K: Two-stage
thyroidectomy in the era of intraoperative neuromonitoring. Gland
Surg. 6:453–463. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nayak SP, Sadhoo A, Gangadhara B, Reddy S,
Khan A, Munisiddaiah D and Ramakrishnan A: Robotic-assisted breast-
axillo insufflation thyroidectomy (RABIT): A retrospective case
series of thyroid carcinoma. Int J Clin Oncol. 25:439–445.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Milosevic Z, Tanic N, Bankovic J,
Stankovic T, Buta M, Lavrnic D, Milovanovic Z, Pupic G, Stojkovic
S, Milinkovic V, et al: Genetic alterations in quadruple
malignancies of a patient with multiple sclerosis: Their role in
malignancy development and response to therapy. Int J Clin Exp
Pathol. 7:1826–1833. 2014.PubMed/NCBI
|
|
18
|
Roshini AP, Ramesh R and Rajalakshmi T:
HATRICK-synchronous triple primary tumors of thyroid. Indian J Surg
Oncol. 9:592–594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kingwell E, Evans C, Zhu F, Oger J,
Hashimoto S and Tremlett H: Assessment of cancer risk with
β-interferon treatment for multiple sclerosis. J Neurol Neurosurg
Psychiatry. 85:1096–1102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Young LS, Yap LF and Murray PG:
Epstein-Barr virus: More than 50 years old and still providing
surprises. Nat Rev Cancer. 16:789–802. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fugl A and Andersen CL: Epstein-Barr virus
and its association with disease - a review of relevance to general
practice. BMC Fam Pract. 20(62)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pyzik A, Grywalska E, Matyjaszek-Matuszek
B, Ludian J, Kiszczak-Bochyńska E, Smoleń A, Roliński J and Pyzik
D: Does the Epstein-Barr virus play a role in the pathogenesis of
Graves' disease? Int J Mol Sci. 20(3145)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bychkov A and Keelawat S: Epstein-Barr
virus and thyroid cancer: The controversy remains. J Endocrinol
Invest. 40:891–892. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Moghoofei M, Mostafaei S, Nesaei A,
Etemadi A, Sadri Nahand J, Mirzaei H, Rashidi B, Babaei F and
Khodabandehlou N: Epstein-Barr virus and thyroid cancer: The role
of viral expressed proteins. J Cell Physiol. 234:3790–3799.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu ST, Ge JN, Li RC, Wei ZG, Sun BH, Jiang
YM, Luo JY, Liu H and Lei ST: Is Epstein-Barr Virus infection
associated with thyroid tumorigenesis? a southern China cohort
study. Front Oncol. 9(312)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guan Y, Jakimovski D, Ramanathan M,
Weinstock-Guttman B and Zivadinov R: The role of Epstein-Barr virus
in multiple sclerosis: From molecular pathophysiology to in vivo
imaging. Neural Regen Res. 14:373–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bar-Or A, Pender MP, Khanna R, Steinman L,
Hartung HP, Maniar T, Croze E, Aftab BT, Giovannoni G and Joshi MA:
Epstein-Barr virus in multiple sclerosis: Theory and emerging
immunotherapies. Trends Mol Med. 26:296–310. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu MJ, Zhang Q, Liang L, Wang SY, Zheng
XC, Zhou MM, Yang YW, Zhong Q and Huang F: Association between
vitamin D deficiency and risk of thyroid cancer: A case-control
study and a meta-analysis. J Endocrinol Invest. 41:1199–1210.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim D: The role of vitamin D in thyroid
diseases. Int J Mol Sci. 18(1949)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sintzel MB, Rametta M and Reder AT:
Vitamin D and multiple sclerosis: A comprehensive review. Neurol
Ther. 7:59–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tomescu D, Cobilinschi C, Tincu RC, Totan
A, Neagu TP, Diaconu CC, Tiglis M, Bratu OG and Macovei RA: Changes
of thyroid hormonal status in organophosphate exposure. A
systematic literature review. Rev Chim. 69:3364–3366. 2018.
|
|
32
|
Marcu DR, Ionita Radu F, Iorga LD, Manea
M, Socea B, Scarneciu I, Isvoranu G, Costache R, Diaconu CC and
Bratu OG: Vascular involvement in primary retroperitoneal tumors.
Rev Chim. 70:445–448. 2019.
|
|
33
|
Marcu RD, Diaconu CC, Constantin T, Socea
B, Ionita-Radu F, Mischianu DL and Bratu OG: Minimally invasive
biopsy in retroperitoneal tumors. Exp Ther Med. 18:5016–5020.
2019.PubMed/NCBI View Article : Google Scholar : (Review).
|
|
34
|
Bratu OG, Diaconu CC, Mischianu DL,
Constantin T, Stanescu AM, Bungau SG, Ionita-Radu F and Marcu RD:
Therapeutic options in patients with biochemical recurrence after
radical prostatectomy. Exp Ther Med. 18:5021–5025. 2019.PubMed/NCBI View Article : Google Scholar
|















