Introduction
Epilepsy, a neurological disease with seizure
susceptibility, has a negative impact on 0.6% of the population in
developed countries and 1.6% in developing countries (1,2).
Refractory epilepsy (RE) is a kind of drug-resistant epilepsy,
which is defined as refractory because it has no successful
therapeutic response to a variety of antiepileptic drugs (3). Ten to twenty percent (%) of epileptic
children progress to RE, and ~470,000 children suffer from
epilepsy, which means there are tens of thousands of RE children
(4,5). At present, antiepileptic drugs (AEDs)
are the first-line therapy for RE, and the second-line is surgery,
diet therapy, and vagus nerve stimulation (6). Although the treatment for RE has been
continuously updated, the exploration of high-efficacy AED
combinations is still ongoing (7).
Our study explored the clinical efficacy and safety of AED regimens
for RE children, which is of great value to improve their quality
of life.
Sodium valproate (SV) is a first-line anti-epilepsy
drug that can be applied to various seizure types in children, but
it may also induce teratogenicity, neurocognitive impairment and
other side effects (8). Studies have
shown that SV plays a neuroprotective role by inhibiting
endoplasmic reticulum stress and reducing neuronal apoptosis in
epilepsy models induced by experiments (9). SV, an anticonvulsant through regulating
neuronal pathways, has a close molecular structure with
neurotransmitter γ-aminobutyric acid (GABA), resulting in GABA
synergism, which inhibits the occurrence of epileptic onsets and
epileptic states (10,11). Lamotrigine (LTG) is a
second-generation AED after SV, and also has the function of
resisting depression and stabilizing mood (12,13). It
is applicable for children and adolescents with various seizure
types and syndromes due to its good anticonvulsant, tolerance,
broad spectrum activity, and safety (14). SV plus LTG, the most effective AED
combination for RE, plays a synergistic role in pharmacodynamics
and reduces the seizure frequency of children (15).
At present, there are few studies on the efficacy
and safety of SV plus LTG in RE children. Therefore, we evaluated
the clinical promotion value of these two AEDs by comparing the
efficacy and clinical response.
Patients and methods
General data
A total of 110 RE children admitted to Xuzhou
Children's Hospital, Xuzhou Medical University (Xuzhou, China) from
February 2018 to March 2019 were enrolled. Patients treated with SV
alone served as the control group, and those treated with SV plus
LTG as the study group. There were 35 males and 16 females in the
control group, aged 3-11 years, with an average age of 6.12±1.05
years, and 34 males and 25 females in the study group, aged 3-12
years, with an average age of 6.18±1.13 years. The study was
approved by the Ethics Committee of the Affiliated Xuzhou
Children's Hospital of Xuzhou Medical University. The guardians
were all informed and signed a fully informed consent form.
Inclusion and exclusion criteria
Inclusion criteria: conforming to the guidelines of
RE developed by the International League Against Epilepsy (ILAE)
(16); diagnosed by imaging
examinations (17); receiving at
least two AEDs in the past 6 months to one year; reaching maximum
blood drug concentration, and the attacks reduced by at least half;
aged 3-12 years. Exclusion criteria: complicated with malignant
tumor or severe heart, lung, kidney and liver dysfunction; allergic
to the drugs in this study; with incomplete clinicopathological
data; pregnant women; not cooperating with this study.
Treatment methods
The patients in the control group were treated with
SV alone (J65363, Jinsui Biotechnology Co., Ltd.). If SV was taken
and the blood drug concentration range was 50-100 µg/ml, other
drugs were gradually discontinued. For patients who had not taken
SV, the initial dose was 20 mg/kg/day, which was gradually
increased until the blood drug concentration was in the range of
50-100 µg/ml. The patients in the study group were treated with SV
plus LTG (Jinsui Biotechnology Co., Ltd., J34775). If SV was taken
and the blood drug concentration range was 50-100 µg/ml, LTG at
0.15 mg/kg was taken once a day, with a weekly increase of 0.20
mg/kg/day in the first month, 0.30 mg/kg/day in the second month
and 0.50-1.00 mg/kg/day in the third month. If the frequency of RE
attacks and related symptoms were controlled or the total dose of
LTG reached 10.00 mg/kg/day, LTG had to be stopped. If SV was not
used before, the initial dose of SV was first taken, then LTG. The
specific administration was as above.
Efficacy assessment
By comparing the average monthly seizure frequency
after treatment with the first three months of treatment, the
efficacy was quantified. Reduction of seizure frequency by 100%,
i.e., no seizures, was considered as control; Reduction of seizure
frequency by 75-99% was considered as markedly effective; Reduction
of seizure frequency by 50-74% was considered as considered
effective; Reduction of seizure frequency by no more than 49% was
considered as ineffective; Increase of seizure frequency by at
least 25% was considered as deterioration. The total effective rate
= (control+markedly effective+effective)/total number of cases
x100%.
Outcome measures
The seizure frequency in the two groups in the 3
months before treatment, and 3 and 6 months after treatment was
observed and compared to assess the efficacy. The incidence of
adverse reactions, serum brain derived neurotrophic factor (BDNF),
nerve growth factor (NGF) concentration changes, and the expression
of serum neuron-specific enolase (NSE) and central nervous system
specific S100β protein (S100β) in effectively and ineffectively
treated children were compared.
Detection methods
Elbow venous blood (5 ml) was drawn from the
subjects before treatment from 8:00 to 9:00 a.m. 4 weeks after
treatment, and placed in a vacuum tube without anticoagulant, then
centrifuged at 1,500 x g and 4˚C for 10 min. Sera were collected in
an Eppendorf (EP) tube and stored at -60˚C. After taken from the
freezer, the sera were dissolved in a refrigerator at 4˚C, and then
placed at room temperature for complete dissolution. The expression
of BDNF, NGF, NSE, and S100β in serum was detected by enzyme-linked
immunosorbent assay (ELISA) (18) in
strict accordance with the instructions of the kits (Keshun
Biotechnology Co., Ltd., KS017148, KS018187, KS015255, KS13441).
Sample, standard and blank wells were set up. Test sample (50 µl)
and standard (50 µl) were added to the sample well and standard
well, respectively, no treatment for the blank well. The sample and
standard wells were each added with 100 µl of horseradish
peroxidase labeled antibody, sealed and incubated at 37˚C for 60
min. The liquid was removed, the wells were dried and washed 5
times. Substrates A and B were fully mixed (1:1) and added to all
wells (100 µl each well). Afterwards, the plate was sealed,
incubation was carried out at 37˚C for 15 min, and 50 µl of
termination solution was added to each well. The optical density
(OD) value at 450 nm of each well was read by a multifunctional
ELISA analyzer (Shanghai Flash Spectrum Biotechnology Co., Ltd.,
SuPerMax 3000FL), and the concentrations of BDNF, NGF, NSE, and
S100β were calculated.
Statistical analysis
This figures were visualized by GraphPad Prism 6
(GraphPad Software). Counting data were expressed by
cases/percentage [n (%)], and Chi-square (χ2) test was
used for comparison between groups. The measurement data were
expressed by mean ± SD, and independent sample t-test was used for
the comparison between the two groups. Receiver operating
characteristic (ROC) curve was employed to assess the value of
serum NSE and S100β in predicting the efficacy in patients. A value
of P<0.05 was considered to be statistically significant.
Results
Baseline data
There was no significant difference between the two
groups in sex, average age, average course of disease, systolic
blood pressure (SBP), diastolic blood pressure (DBP), seizure type,
medication history, ADE combination, family history of RE,
residence (P>0.05) (Table I).
 | Table IComparison of baseline data [n (%),
mean ± SD]. |
Table I
Comparison of baseline data [n (%),
mean ± SD].
| Classification | n | Control group
(n=51) | Study group
(n=59) | χ2/t | P-value |
|---|
| Sex | | | | 1.416 | 0.234 |
|
Male | 69 | 35 (68.63) | 34 (57.63) | | |
|
Female | 41 | 16 (31.37) | 25 (42.37) | | |
| Average age
(years) | 110 | 6.12±1.05 | 6.18±1.13 | 0.287 | 0.775 |
| Average course of
disease (years) | 110 | 3.01±0.45 | 3.06±0.52 | 0.535 | 0.594 |
| SBP (mmHg) | 110 | 110.24±4.82 | 109.56±5.06 | 0.718 | 0.474 |
| DBP (mmHg) | 110 | 75.02±4.45 | 74.86±5.10 | 0.174 | 0.862 |
| Seizure type | | | | 0.124 | 0.989 |
|
Partial
seizure | 66 | 31 (60.78) | 35 (59.32) | | |
|
Generalized
seizure | 19 | 9 (17.65) | 10 (16.95) | | |
|
Secondarily
generalized seizure | 13 | 6 (11.76) | 7 (11.86) | | |
| Lennox Gastaut
syndrome | 12 | 5 (9.81) | 7 (11.87) | | |
| Medication
history | | | | - | - |
|
Carbamazepine | 46 | 21 (-) | 25 (-) | | |
|
SV | 56 | 26 (-) | 30 (-) | | |
|
Topiramate | 30 | 17 (-) | 13 (-) | | |
|
Valnromide | 8 | 5 (-) | 3 (-) | | |
|
Phenytoin
sodium | 6 | 3 (-) | 3 (-) | | |
|
Gabapentin | 6 | 2 (-) | 4 (-) | | |
| AED
combination | | | | 2.100 | 0.552 |
|
2 | 67 | 30 (58.82) | 37 (62.71) | | |
|
3 | 33 | 18 (35.29) | 15 (25.42) | | |
|
4 | 6 | 2 (3.92) | 4 (6.78) | | |
|
5 | 4 | 1 (1.97) | 3 (5.09) | | |
| Family history of
RE | | | | 0.283 | 0.595 |
|
No | 95 | 45 (88.24) | 50 (84.75) | | |
|
Yes | 15 | 6 (11.76) | 9 (15.25) | | |
| Residence | | | | 0.294 | 0.588 |
|
Rural | 33 | 14 (27.45) | 19 (32.20) | | |
|
Urban | 77 | 37 (72.55) | 40 (67.80) | | |
Comparison of efficacy
Efficacy in the control group: the number of cases
of control, markedly effective, effective, ineffective and
deterioration were 15, 10, 6, 14 and 6, respectively, with a total
effective rate of 60.78%. Efficacy in the study group: the number
of cases of control, markedly effective, effective, ineffective and
deterioration were 23, 14, 11, 9 and 2, respectively, with a total
effective rate of 81.35%. The total effective rate in the study
group was significantly higher than that in the control group
(P<0.001) (Table II).
 | Table IIComparison of efficacy [n (%)]. |
Table II
Comparison of efficacy [n (%)].
| Group | n | Control | Markedly
effective | Effective | Ineffective | Deterioration | Total effective
rate |
|---|
| Control group | 51 | 15 (29.41) | 10 (19.61) | 6 (11.76) | 14 (27.45) | 6 (11.77) | 60.78 |
| Study group | 59 | 23 (38.98) | 14 (23.73) | 11 (18.64) | 9 (15.25) | 2 (3.39) | 81.35 |
| χ2
value | - | - | - | - | - | - | 18.888 |
| P-value | - | - | - | - | - | - | <0.001 |
Comparison of seizure frequency
There was no significant difference in seizure
frequency between the study group and the control group 3 months
before treatment (P<0.05). At 3 and 6 months after treatment,
the frequency in the study group was significantly lower than that
in the control group (P<0.05) (Table III).
 | Table IIIComparison of seizure frequency (mean
± SD). |
Table III
Comparison of seizure frequency (mean
± SD).
| Group | n | 3 months before
treatment | 3 months after
treatment | 6 months after
treatment |
|---|
| Control group | 51 | 15.43±2.29 | 10.43±2.29 | 6.97±1.15 |
| Study group | 59 | 15.89±1.04 | 7.89±1.04 | 1.88±0.60 |
| t value | - | 1.387 | 7.659 | 29.660 |
| P-value | - | 0.168 | <0.001 | <0.001 |
Incidence of adverse reactions
After treatment, RE children may present with loss
of appetite, hyperactivity, hair loss, lower limb soreness,
dizziness, rash and other adverse reactions, and loss of appetite,
hyperactivity, lower limb soreness are the main ones. The incidence
rate of adverse reactions in the study group was significantly
lower than that in the control group (Table IV).
 | Table IVAdverse reactions [n (%)]. |
Table IV
Adverse reactions [n (%)].
| Classification | Control group
(n=51) | Study group
(n=59) | χ2
value | P-value |
|---|
| Loss of
appetite | 4 (7.84) | 3 (5.08) | 0.349 | 0.555 |
| Hyperactivity | 4 (7.84) | 2 (3.39) | 1.052 | 0.305 |
| Hair loss | 2 (3.92) | 1 (1.69) | 0.511 | 0.475 |
| Lower limb
soreness | 3 (5.88) | 2 (3.39) | 0.531 | 0.392 |
| Dizziness | 2 (3.92) | 1 (1.69) | 0.511 | 0.475 |
| Rash | 1 (1.96) | 0 (0.00) | 1.167 | 0.280 |
| Total | 16 (31.37) | 9 (15.25) | 4.047 | 0.044 |
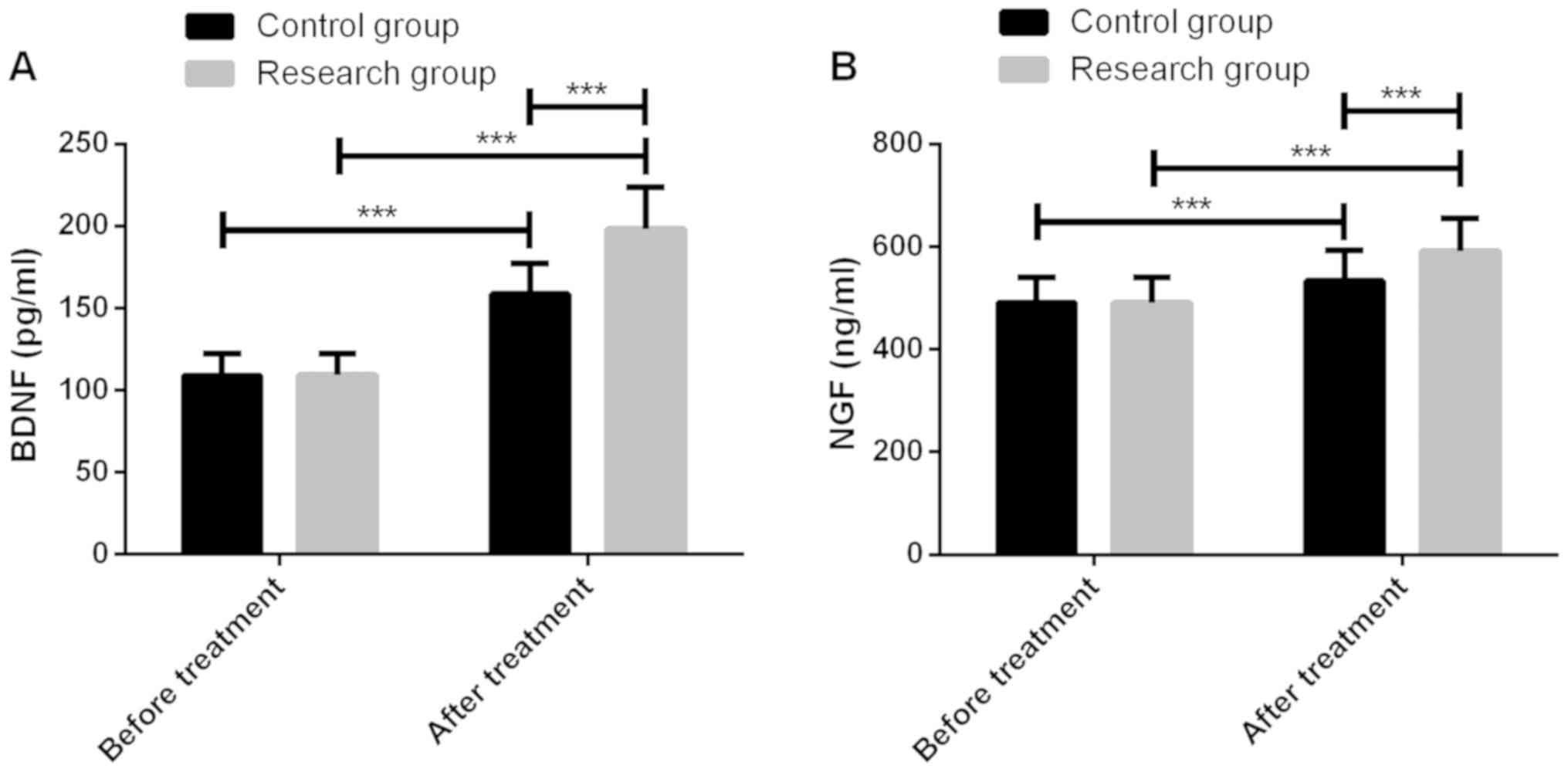
Comparison of neurotrophic
indexes
Before treatment, the neurotrophic indexes BDNF and
NGF were not significantly different between the two groups
(P<0.05), which were significantly increased after treatment
(P<0.001), and in the study group they were significantly higher
than the control group (P<0.001) (Fig. 1).
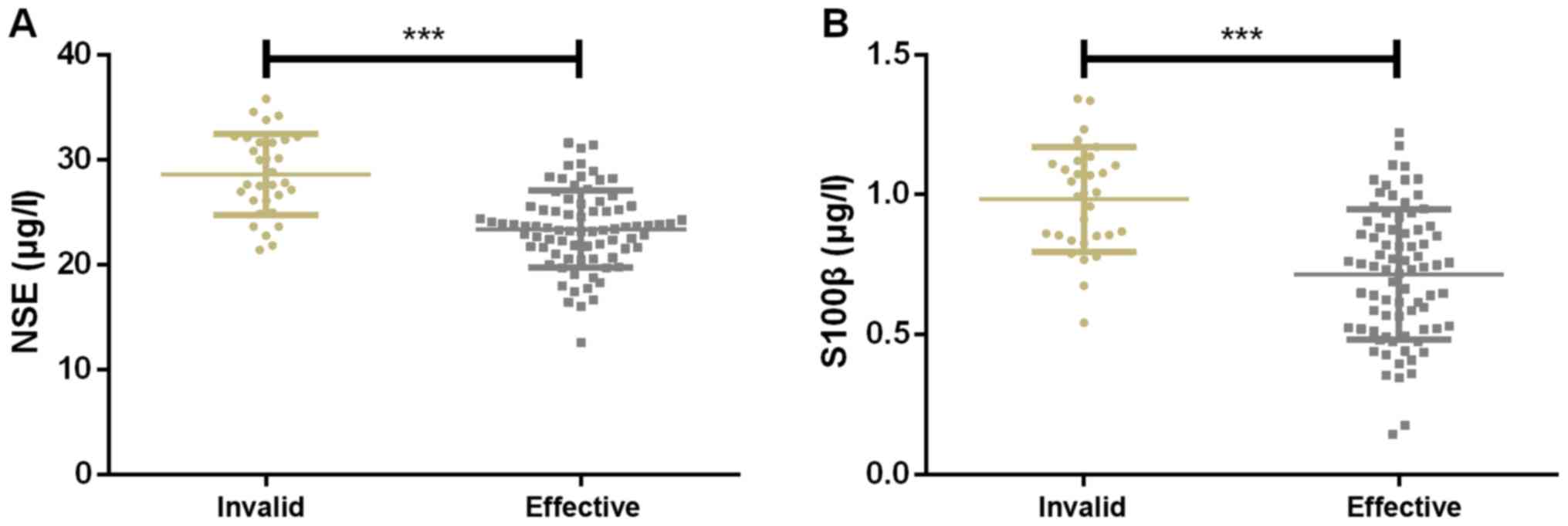
Expression of serum NSE and S100β
The treatment was effective in 79 children and
ineffective in 31 children. The expression of serum NSE was
28.47±3.99 and 21.03±3.18 µg/l in ineffectively treated and
effectively treated children, respectively, while that of serum
S100β were 0.97±0.23 and 0.65±0.26 µg/l, respectively. Therefore,
the expression of serum NSE and S100β in ineffectively treated
children were significantly higher than that in effectively treated
ones (Fig. 2).
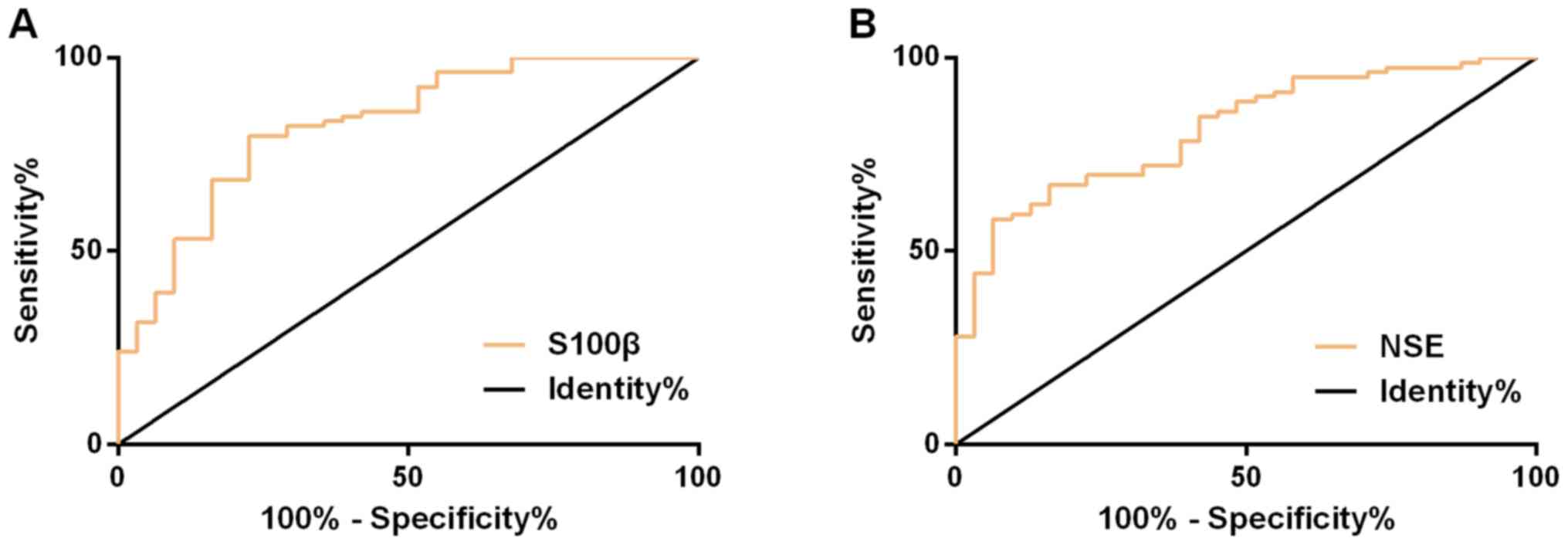
Serum NSE and S100β in assessing the
efficacy
ROC curve demonstrated that the AUC, cut-off,
sensitivity, and specificity of serum NSE in assessing the efficacy
were 0.828 (95% CI, 0.742-0.914), 26.05, 79.75%, and 77.42%,
respectively; while those of serum S100β were 0.814 (95% CI,
0.731-0.896), 0.77, 58.23 and 93.55%, respectively (Fig. 3 and Table
V).
 | Table VPredictive value of serum NSE and
S100β on efficacy assessment. |
Table V
Predictive value of serum NSE and
S100β on efficacy assessment.
| Group | AUC | 95%CI | S.E | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| NSE | 0.828 | 0.742-0.914 | 0.044 | 26.05 | 79.75 | 77.42 |
| S100β | 0.814 | 0.731-0.896 | 0.042 | 0.77 | 58.23 | 93.55 |
Discussion
RE is a chronic and debilitating disease of the
nervous system with epileptic seizure caused by accidental
discharge of cerebral neurons, which may lead to stigma in patients
(19-21).
Therefore, appropriate inhibition of neuronal excitability is the
key in selection of AEDs in RE children (22). SV has been proved to alleviate
neuronal apoptosis in a kainic acid model of epilepsy by enhancing
phosphorylation of PKC-dependent GABA A R γ2 Serine 327 (23,24). LTG
acts as glutamate antagonist to exert anticonvulsant and sedative
functions through its pharmacological mechanism affecting sodium
and calcium channels. It can also disturb the pathogenesis of
hyperactivity via regulating excitatory neurotransmitters (25). In this study, hair loss,
hyperactivity, and lower limb soreness were the main adverse
reactions of patients. The seizure frequency in the study group was
significantly lower than that in the control group, and the total
effective rate and safety of treatment were significantly higher
than those in the control group. Therefore, SV plus LTG has high
efficacy and safety on RE children and has better inhibitory effect
on the seizure frequency compared with SV alone.
We screened two neurotrophic indexes, BDNF and NGF,
to compare the improvement of neurotrophic level of RE children
treated with drugs that have inhibitory effects on neuronal
excitability. BDNF mediates survival, growth, and regeneration of
neurons and participates in the regulation of neural plasticity,
playing an important role in the healthy brain development, and
being of high diagnostic and prognostic value for brain injury
(26,27). Tan et al pointed out that BDNF
protected neurons by inhibiting the secretion of excitatory amino
acids, maintaining calcium homeostasis in neurons, as well as
inhibiting the high expression of oxygen free radicals. Moreover,
low BDNF level generally indicated the decline of cognitive
function in epileptic patients (28). NGF, a typical representative of
neurotrophic factors, is responsible for the growth, survival, and
differentiation of mature neurons. In addition to being active in a
wide array of non-nervous system cells, it is also synthesized by
various cell types (29). NGF has a
protective effect on basal forebrain cholinergic neurons and may
reduce the susceptibility to generalized seizures (30,31).
BDNF and NGF were reported to be closely related to epilepsy and
involved in the occurrence and progression of focal RE (32). Our findings showed that BDNF and NGF
levels in the study group were more significantly increased,
indicating that SV combined with LTG was more useful than SV alone
in improving neurotrophic levels of RE children.
Finally, we assessed the predictive value of serum
NSE and S100β on the efficacy in RE children. NSE and S100β are
brain-derived proteins whose high expression is related to the
increase of brain injury (33).
Shaik et al found that serum NSE of patients with convulsion
showed abnormally high expression, suggesting that it was a marker
of epilepsy-related neuron injury (34). Another study demonstrated that serum
S100β protein level of patients with focal epilepsy was
significantly higher than that in healthy controls, which can be a
biomarker for neuronal damage in patients with focal RE (35). In this study, the effectively treated
children had a significantly lower expression of serum NSE and
S100β than those with ineffective treatment, so serum NSE and S100β
gradually recovered to normal levels in RE children after
treatment. From the ROC curve, we obtained AUC of serum NSE and
S100β and the efficacy was 0.828 and 0.814, respectively, which
showed that they had better predictive value in efficacy assessment
for RE children.
This study confirmed that SV plus LTG has higher
efficacy and fewer adverse reactions in the treatment of RE.
However, statistics of various attack types and the efficacy of
treatment in RE children need to be recorded to know which RE type
of children treated by SV plus LTG achieves the highest curative
effect.
In conclusion, SV combined with LTG is better and
safer than SV alone in the treatment of RE in children, which is
more worthy of clinical promotion. Serum NSE and S100β are of high
value in predicting the efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ wrote the manuscript. DZ and LQ conceived and
designed the study. YZ and YS were responsible for the collection
and analysis of the experimental data. NZ and XL interpreted the
data and drafted the manuscript. LQ and YS revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou,
China). Patients who participated in this research, had complete
clinical data. Signed informed consents were obtained from the
parents or the guardians of the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armeno M, Verini A, Del Pino M, Araujo MB,
Mestre G, Reyes G and Caraballo RH: A prospective study on changes
in nutritional status and growth following two years of ketogenic
diet (KD) therapy in children with refractory epilepsy. Nutrients.
11(1596)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McGovern RA, Banks GP and McKhann GM:
Responsive stimulation in the management of medically refractory
epilepsy. In: Epilepsy Surgery and Intrinsic Brain Tumor Surgery.
Fountas K and Kapsalaki E (eds). Springer, Cham, pp205-211,
2019.
|
|
3
|
Sirven JI, Pedley TA and Wilterdink JL:
Evaluation and management of drug-resistant epilepsy. UpToDate.
https://www.uptodate.com/contents/evaluation-and-management-of-drug-resistant-epilepsy.
Accessed August 13, 2018.
|
|
4
|
Mishra S: Refractory epilepsy in children:
A short review. South Asian Res J Med Sci. 1:24–29. 2019.
|
|
5
|
Nigro SE: The efficacy of neurofeedback
for pediatric epilepsy. Appl Psychophysiol Biofeedback. 44:285–290.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moosa ANV: Antiepileptic drug treatment of
epilepsy in children. Continuum (Minneap Minn). 25:381–407.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Golyala A and Kwan P: Drug development for
refractory epilepsy: The past 25 years and beyond. Seizure.
44:147–156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Balagura G, Iapadre G, Verrotti A and
Striano P: Moving beyond sodium valproate: Choosing the right
anti-epileptic drug in children. Expert Opin Pharmacother.
20:1449–1456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fu J, Peng L, Wang W, He H, Zeng S, Chen
TC and Chen Y: Sodium valproate reduces neuronal apoptosis in acute
pentylenetetrazole-induced seizures via inhibiting ER stress.
Neurochem Res. 44:2517–2526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brown C and Smith C: Sodium valproate.
Pract Diabetes. 35:186–187. 2018.
|
|
11
|
Çavuş I, Romanyshyn JC, Kennard JT,
Farooque P, Williamson A, Eid T, Spencer SS, Duckrow R, Dziura J
and Spencer DD: Elevated basal glutamate and unchanged glutamine
and GABA in refractory epilepsy: Microdialysis study of 79 patients
at the yale epilepsy surgery program. Ann Neurol. 80:35–45.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Johannessen Landmark C and Patsalos PN:
Drug interactions involving the new second- and third-generation
antiepileptic drugs. Expert Rev Neurother. 10:119–140.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reimers A: Lamotrigine, bipolar disorder,
and the pill-free week. Bipolar Disord. 21:372–373. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yasam VR, Jakki SL, Senthil V,
Eswaramoorthy M, Shanmuganathan S, Arjunan K and Nanjan MJ: A
pharmacological overview of lamotrigine for the treatment of
epilepsy. Expert Rev Clin Pharmacol. 9:1533–1546. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Poolos NP, Castagna CE, Williams S, Miller
AB and Story TJ: Association between antiepileptic drug dose and
long-term response in patients with refractory epilepsy. Epilepsy
Behav. 69:59–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lanteaume L, Guedj E, Bastien-Toniazzo M,
Magalahaes A, Mundler O and Bartolomei F: Cognitive and metabolic
correlates of emotional vulnerability in patients with temporal
lobe epilepsy. J Neurol Neurosurg Psychiatry. 83:522–528.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ollenberger GP, Byrne AJ, Berlangieri SU,
Rowe CC, Pathmaraj K, Reutens DC, Berkovic SF, Scheffer IE and
Scott AM: Assessment of the role of FDG PET in the diagnosis and
management of children with refractory epilepsy. Eur J Nucl Med Mol
Imaging. 32:1311–1316. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hornbeck PV: Enzyme-linked immunosorbent
assays. Curr Protoc Immunol. 110:2.1.1–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ramaratnam S, Panebianco M and Marson AG:
Lamotrigine add-on for drug-resistant partial epilepsy. Cochrane
Database Syst Rev: CD001909, 2016.
|
|
20
|
Verrotti A, Iapadre G, Di Donato G, Di
Francesco L, Zagaroli L, Matricardi S, Belcastro V and Iezzi ML:
Pharmacokinetic considerations for anti-epileptic drugs in
children. Expert Opin Drug Metab Toxicol. 15:199–211.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pagura JR and Alessi R: Epilepsy and
seizures. In: The Sports Medicine Physician. Piedade SR, Imhoff AB,
Clatworthy M, Moises Cohen M and Espregueira-Mendes J (eds).
Springer Cham, pp235-240, 2019.
|
|
22
|
Hernan AE and Holmes GL: Antiepileptic
drug treatment strategies in neonatal epilepsy Elsevier. Prog Brain
Res. 226:179–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Q, Li QQ, Jia JN, Cao S, Wang ZB, Wang
X, Luo C, Zhou HH, Liu ZQ and Mao XY: Sodium valproate ameliorates
neuronal apoptosis in a kainic acid model of epilepsy via enhancing
PKC-dependent GABAAR γ2 Serine 327 phosphorylation. Neurochem Res.
43:2343–2352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rosenberg EC, Patra PH and Whalley BJ:
Therapeutic effects of cannabinoids in animal models of seizures,
epilepsy, epileptogenesis, and epilepsy-related neuroprotection.
Epilepsy Behav. 70B:319–327. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han SA, Yang EJ, Song MK and Kim SJ:
Effects of lamotrigine on attention-deficit hyperactivity disorder
in pediatric epilepsy patients. Korean J Pediatr. 60:189–195.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Korley FK, Diaz-Arrastia R, Wu AHB, Yue
JK, Manley GT, Sair HI, Van Eyk J, Everett AD, Okonkwo DO, Valadka
AB, et al: TRACK-TBI investigators: Circulating brain-derived
neurotrophic factor has diagnostic and prognostic value in
traumatic brain injury. J Neurotrauma. 33:215–225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bathina S and Das UN: Brain-derived
neurotrophic factor and its clinical implications. Arch Med Sci.
11:1164–1178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tan XH, Song ZB, Wang H, Wang Q and He JL:
Influence of adjuvant levetiracetam therapy on serum nerve
cytokines and apoptosis molecules in patients with refractory
partial epileptic seizure. Hainan Yixueyuan Xuebao. 23:145–149.
2017.(In Chinese).
|
|
29
|
Skaper SD: Nerve growth factor: A
neuroimmune crosstalk mediator for all seasons. Immunology.
151:1–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tuszynski MH, Yang JH, Barba D, U HS,
Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, et al:
Nerve growth factor gene therapy: Activation of neuronal responses
in Alzheimer disease. JAMA Neurol. 72:1139–1147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Silveira DC, Holmes GL, Schachter SC,
Geula C and Schomer DL: Increased susceptibility to generalized
seizures after immunolesions of the basal forebrain cholinergic
neurons in rats. Brain Res. 878:223–227. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang F, Lin Y, Kang D, Chen F, Lin K and
Su X: Distribution and expression of brain-derived neurotrophic
factor, nerve growth factor, and neurotrophic factor-3 in
refractory epilepsy-associated focal cortical dysplasia. Clin
Neuropathol. 36:233–239. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Alpdemir M, Özcan O, Alpdemir MF, Şeneş
M, Azak A, Duranay M and Yücel M: Serum neuron specific enolase
and S-100B levels in hemodialysis and peritoneal dialysis patients.
Eur Arch Med Res. 35:83–87. 2019.
|
|
34
|
Shaik AJ, Reddy K, Mohammed N, Tandra SR,
Rukmini Mridula Kandadai and Baba Kss S: Neuron specific enolase as
a marker of seizure related neuronal injury. Neurochem Int.
131(104509)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Calik M, Abuhandan M, Kandemir H, Güzel B,
Solmaz A, Celik H, Taskin A and Iscan A: Interictal serum S-100B
protein levels in intractable epilepsy: A case-control study.
Neurosci Lett. 558:58–61. 2014.PubMed/NCBI View Article : Google Scholar
|