Introduction
Adenomyosis (AM) is a common gynecological disorder
defined by the presence of heterotopic endometrial glands and stroma
in the myometrium with adjacent smooth muscle hyperplasia (1,2).
Patients with AM often complain of menorrhagia, dysmenorrhea, and
subfertility (3). Despite the
widespread prevalence of AM, the precise etiology and pathogenesis
of AM remains poorly understood (4).
Although AM is recognized as a hormone-sensitive disorder, the use
of progestogenic agents for treatment has failed to show
satisfactory efficacy, indicating the possibility of progesterone
resistance (5). Other therapeutic
options include gonadotrophin-releasing hormone agonists (GnRH-a),
as anti-oestrogenic therapeutic agent, can inhibit AM progression
(6); however, the use of GnRH-a is
limited to a short-term treatment due to its side effects and
recurring symptoms after discontinuation of GnRH-a therapy
(7,8). The management of chronic AM is
currently a major challenge, with hysterectomy or adenomyomectomy
being the frequent treatment of choice (9). Surgery is not a preferred treatment
option, especially for patients who wish to remain fertile
(10). However, despite the clinical
importance of AM, there is little evidence on which to base
treatment decisions (11).
Therefore, numerous patients with AM turn to alternative treatment
modalities, such as Traditional Chinese Medicine (TCM).
Shi Xiao San (SXS), a classical Chinese herbal
formula, originated in the Song Dynasty. Based on the fundamental
theories of Chinese medicine and clinical experience, SXS is
prescribed by herbalists to relieve clinical symptoms resulting
from blood stasis, including irregular menstruation, pelvic pain,
progressive dysmenorrhea, and postpartum lochiorrhea. Furthermore,
patients are able to tolerate long-term SXS therapy (12-14).
In Chinese medicine, ‘blood stasis’ or ‘blood stagnation’ is
considered the main pathogenesis of AM, causing the formation of
adenomyotic lesions leading to endometriosis (15,16);
therefore, it is suggested that enhancing blood circulation and
removing stasis is the basic treatment approach for AM (17,18).
However, the therapeutic effect of SXS on AM remains unclear.
To date several animal models of AM have been
developed. The exposure of neonatal ICR mice to tamoxifen yields
the classical model of AM (19-21).
Feeding neonatal mice with tamoxifen is a much simpler method
compared with using ectopic grafting of pituitary glands, as it
requires no surgical skills and has a high induction success rate
(22). Furthermore, the classic AM
mouse model has an aggravated pathogenic condition with age,
including increased myometrial infiltration depth and progressive
generalized hyperalgesia (23-25).
Finally, this model is not associated with trauma and mice have
relatively normal sex hormone levels in their blood; however, this
may cause endometrial hormone imbalance (26). Although this is not caused by trauma,
it is important for adenomyosis pain research, as it may affect the
accuracy of efficacy studies.
The clinical profiles of several premenopausal women
with AM, who underwent a hysterectomy, have shown that dysmenorrhea
is the most common side effect, often accompanied by menorrhagia
(27). It is reported that a higher
myometrial infiltration depth is associated with more severe
dysmenorrhea in AM (28). Other
studies have reported that AM-associated symptoms are variable,
non-specific, and related to other associated pathological
conditions such as dysfunctional uterine bleeding, leiomyomas and
endometriosis (11,29). The factors that determine the
frequency and severity of dysmenorrhea in AM remain unknown. No
reliable biomarkers for dysmenorrhea or its severity in AM have
been identified. A previous study reported that immunoreactivity to
both the oxytocin receptor (OXTR) and the transient receptor
potential vanilloid type 1 (TRPV1) were positively correlated with
the severity of dysmenorrhea and were significant independent
predicators for the severity of dysmenorrhea (30). Subsequent research indicated that
overexpression of OXTR protein in myometrial smooth muscle cells,
that were derived from uterine tissues of patients with AM may be
responsible for increased uterine contractibility and AM-associated
dysmenorrhea (31). Hence, the
specific inhibition of OXTR and Trpv1 may yield a promising
therapeutic treatment in the near future.
A previous case-control study found that women with
AM were more likely to have a history of depression, or were at
higher risk for depression, and therefore were also taking
antidepressants (32). Chronic
stress is a primary cause of depression and it is well-known that
pain is a physical stress factor (33). Therefore, AM-induced chronic pain may
result in an elevated physiological arousal that is often
associated with the release of stress hormones, such as cortisol in
humans or corticosterone (CORT) in rodents. Furthermore, AM has
been shown to cause elevated systemic CORT levels in conjunction
with generalized hyperalgesia, which was suggestive of increased
stress (19).
In the present study, a model of AM was established
in ICR mice and the effect of SXS was evaluated on the development
of AM to validate our hypothesis that SXS could delay the
myometrial infiltration of endometrial implants, alleviate
generalized hyperalgesia, and lower stress levels in mice with AM.
The current study will be conducive to the development and
promotion of SXS as a clinical treatment for AM.
Materials and methods
Chemicals
Tamoxifen citrate was purchased from Fudan Forward
Pharmaceutical Co., Ltd. Danazol capsules were purchased from
Jiangsu Lianhuan Pharmaceutical Co., Ltd. Danazol (200 mg) was
suspended in phosphate-buffered saline (pH 7.2) at a concentration
of 10 mg/ml and was homogenized using ultrasound in an ice bucket
at 400 w for 30 min (9 sec on, 5 sec off) before intragastric
administration.
SXS preparation
The composition of SXS is listed in Table I. All medicinal plants used to
prepare the formula were provided and identified by the Department
of Pharmacy at the Affiliated Hospital of Integrated Traditional
Chinese and Western Medicine, Nanjing University of Chinese
Medicine. Individual herbs were deposited in the herbarium for
preservation at the Laboratory of Cellular and Molecular Biology,
Jiangsu Province Academy of Traditional Chinese Medicine (Nanjing,
JiangSu, China). The method used for the preparation of the SXS
decoction was as follows: The Chinese medicinal herb, Faeces
Trogopterori, was first ground into a fine powder using rice
wine (Sha Zhou You Huang; Zhangjiagang Wine Co., Ltd.) and
Pollen Typhae Angustifoliae was processed via stir-frying.
This was followed by preparation of high-dose SXS, where equal
parts of Faeces Trogopterori and Pollen Typhae
Angustifoliae were mixed and soaked in 182 ml rice wine for 3
days at room temperature. Meanwhile, low-dose SXS was prepared by
diluting the high-dose SXS with an equal volume of rice wine
(high-dose SXS volume: rice wine, 1:1). The SXS and its
preparations were standardized, regulated, and quality controlled
(QC) according to the guidelines defined by the Chinese State Food
and Drug Administration (standard no. WS3-B-1912-95). The QC
consists of a regular quality check of the herbal medicines, which
is performed according to the product specifications for identity,
purity, and content of characterizing compounds, thereby ensuring
the safety, efficacy, and quality of herbal medicines and their
preparations (34).
 | Table IThe composition of Shi Xiao San. |
Table I
The composition of Shi Xiao San.
| Ingredients (Latin
name) | Chinese name | Medicinal
parts | Origin | Grams | % |
|---|
| Pollen Typhae
Angustifoliae | Puhuang | Pollen | Jiangsu, China | 9 | 50 |
| Faeces
Trogopterori | Wulingzhi | Feces | Hebei, China | 9 | 50 |
| Total amount | N/A | N/A | N/A | 18 | 100 |
SXS analysis using high performance
liquid chromatography (HPLC)
Faeces Trogopterori (1.5 g) and Pollen Typhae
(1.5 g) were extracted twice (2 h/time) under reflux with 150 ml of
yellow rice wine. The extracted solutions were combined,
concentrated, and dried at 50˚C in a vacuum. The dried extract was
resuspended in 25 ml methanol then subsequently filtered through a
0.22 µm membrane and 20 µl of the filtrate was analyzed using HPLC.
The internal standards were protocatechuic acid, typhaneoside-
reference material, amentoflavone and isorhamnetin.
The HPLC analysis was performed on a serial system
(Agilent 1100; Agilent Technologies, Inc.). Briefly, 10 µl samples
were separated on a reverse-phase analytical column (Zorbax
XDB-C18; 4.6x150 mm, 5 mm; Agilent Technologies, Inc.). The mobile
phase consisted of solvents A (acetonitrile) and B (0.1% aqueous
acetic acid, v/v) with a linear gradient elution as follows: B,
0-25 min at 3-10%; B, 25-40 min at 10-15%; A, 40-65 min at 30%; B,
65-70 min at 100%. The flow rate was 1 ml/min with a column
temperature of 30˚C. The experiment was performed on a serial
System (LC-20AT HPLC system; Shimadzu Corporation) with an SPD-20A
detector (Shimadzu Corporation) using validated methods for
linearity and quantification. A Shimadzu diode array detector
(Shimadzu Corporation) was set at 280 nm to detect the constituents
of SXS.
Animals and treatments
A total of 15 separate litters of newborn (day 1
after birth; born at the same time point) ICR mice and their mother
were purchased from Shanghai Laboratory Animal Corporation. The
mother mouse together with their respective offspring were housed
in the same cage under the following controlled conditions: ~25˚C,
12:12 light/dark cycle with lights on at 6:00 in the morning and
had ad libitum access to chow and fresh water (20). A total of 1 mg/kg tamoxifen suspended
in a peanut oil/lecithin/condensed milk mixture [2:(0.2):3, by
volume] was orally administered to female neonatal mice at 5 µl/g
body weight (bw) from days 2 to 5 after birth. A total of 10
randomly designated control female neonatal mice were fed
similarly, however they were not administered with tamoxifen. At 3
weeks, offspring were weaned and separated from their mothers. A
total of 60 days after birth, 10 treated and 10 untreated mice were
used to detect the incidence of AM using hematoxylin and eosin
(H&E) staining. All experiments were approved by the
Institutional Experimental Animals review board of the Affiliated
Hospital of Integrated Traditional Chinese and Western Medicine,
Affiliated to Nanjing University of Chinese Medicine Ethics Review
Board.
Experimental protocol
Female neonatal mice were given 1 mg/kg tamoxifen
orally, from days 2 to 5 after birth. A total of 60 days after
birth, 10 randomly selected mice were used for model identification
via histopathological examination, while 10 neonatal mice, which
were treated similarly with the solvent alone, served as controls.
The remaining mice treated with tamoxifen were randomly divided
into five, equal-sized groups, with each receiving a different
treatment via daily intragastric administration for 2 months, after
the AM model was successfully established. Successful establishment
was confirmed via HE examination of uterine tissue sections, which
exhibited endometrial infiltration into the muscle layer in mice.
The five groups were as follows: The model (M) group (n=10)
received the vehicle only (sterile water); the positive (P) group
(n=10) received danazol (1 mg/20 g bw) treatment; the low-dose (L)
group (n=10) received a low-dose (55 mg/kg bw/day) SXS treatment;
the high-dose (H) group (n=10) received a high-dose (110 mg/kg
bw/day) SXS treatment; and the control (C) group (n=10) consisted
of neonatal mice treated vehicle alone (PBS) and served as
controls. The doses of the drugs used in the present study were
determined based on the doses used in humans converted to that used
in mice, using the formula given in previous reports (22,35,36).
Following 2 months of SXS treatment, the hotplate and tail-flick
test were performed in all groups. Mice were randomly selected for
hyperalgesia measurements. Hotplate test and tail-flick tests were
separately performed with a >30 min interval. The final body
weight was measured and peripheral blood was collected subsequent
to all mice being euthanized using cervical dislocation. Mice were
weighed prior to sacrifice, and uterine weight was subsequently
recorded during dissection to calculate the ratio of the uterine
wet weight to total body weight (U/B). Blood collection from
orbital venous plexus was performed before sacrifice, both uterine
horns were harvested, and the uterine weight was recorded. The
contour of the uterus was examined immediately with the naked eye
and adenomyotic nodules extruding from the surface of the uterus
were counted under a magnifier (magnification, x50 and x200). The
left uterine horn was snap-frozen in liquid nitrogen and stored at
-80˚C until further analysis, and the right uterine horn was fixed
in 4% paraformaldehyde and embedded in paraffin at room temperature
for <1 week for subsequent histopathological studies.
Subsequently, H&E staining was performed to evaluate the depth
of myometrial infiltration of the ectopic endometrium, according to
the standard of Mori's classification, which grades the progression
of adenomyosis. The grading system was as follows: Grade 0, normal
uterus; grade 1, uterus with endometrial stromal cell invasion into
the inner layer of the myometrium; grade 2, uterus with endometrial
stromal and gland cell invasion into the inner layer of the
myometrium; grade 3, uterus with endometrial stromal and gland cell
invasion into the connective tissue space between the inner and
outer myometrial layers; grade 4, uterus with cystic hyperplasia of
the endometrial glands and small nodules beneath the serosa; grade
5, uterus with cystic hyperplasia of the endometrial glands and a
large number of subserosal nodules (37).
Histopathological examination
H&E staining was performed as described in our
previous report (38). Briefly,
serial 4-µm sections were obtained from each paraffin-embedded
tissue block, and subsequently three randomly selected sections
were deparaffinized for 15 min, and rehydrated in descending
alcohol series (100, 95, and 70%) for 5 min each at room
temperature. The sections were washed briefly with distilled water,
stained in hematoxylin solution for 8 min at room temperature, and
washed again with running tap water for 5 min. The sections were
then differentiated in 1% acid alcohol for 30 sec and
counterstained with eosin solution for 30 sec. Following
dehydration using 95% alcohol for 5 min, the sections were
air-dried and mounted. All images were obtained with an Olympus
BX51 microscope (magnification, x50 and x200) fitted with a digital
camera (Olympus Corporation). For histological examination, three
randomly selected sections of each mouse were chosen for H&E
staining to confirm the pathological diagnosis. If the endometrial
stroma with or without gland was observed to be displaced in the
myometrium, the diagnosis of AM was considered to be confirmed.
Sections were scored by two blinded pathologists at the Department
of Pathology, Affiliated Hospital of Integrated Traditional Chinese
and Western Medicine, Nanjing University of Chinese Medicine
(Jiangsu, China). The H&E scoring was performed in accordance
with previously published studies (39-41).
Hotplate and tail-flick tests
The hotplate test is a classic method for measuring
the latent period preceding pain and nociception in rodents, for
the evaluation of analgesic drugs. It is also a test of the
response threshold to thermal stimuli, representing a form of acute
thermal pain. In the present study, the hotplate test was performed
with the Hot Plate Analgesia Meter (BME-480; Jiangsu Province
Academy of Traditional Chinese Medicine, China) consisting of a
metal plate (25x25 cm) with a plastic cylinder (20 cm in diameter;
18 cm in height). The surface of the plate can be heated to a
constant temperature of 50.0 (±0.1)˚C as measured using a built-in
digital thermometer and a plastic cylinder. Mice were brought to
the testing room and allowed to acclimatize for 5 min prior to the
test. The response latency to a noxious thermal stimulus is defined
as the time(s) elapsed from the moment when the mouse was inserted
inside the cylinder to the point when it licked its hind paws.
The tail-flick test was evaluated with a commercially
available tail-flick Analgesia Meter (33T; NatureGene Corporation).
Thermal stimulation was provided using a beam of high-intensity
light focused 1-2 cm distal to the tail end. The time between the
start of the stimulation and tail withdrawal was measured as the
tail-flick latency. The intensity of the beam was adjusted to
produce a mean control reaction time within 60 sec prior to the
initiation of the test, after which the heat source was held
constant (59˚C). Mice were then placed in a metallic cage with
their tails extended through a slot located at the rear of the cage
and allowed to acclimatize for 10 min prior to the test. Withdrawal
of the tail exposed to the light instantly turned off the thermal
stimulus, automatically stopped the internal timer, and the latency
time was automatically displayed. For the hotplate and tail-flick
tests, mice were tested only once per session, separately. The
latency was calculated as the mean of three recorded readings at 12
h intervals.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The mRNA levels of OXTR and Trpv1 were determined
using RT-qPCR analysis. Total RNA from uterine tissues was isolated
with TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). Complementary DNA (cDNA) was synthesized from 1 µg RNA using
the Maxima first-strand cDNA synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
RT-qPCR was performed using a 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 2X Maxima SYBR
Green/ROX qPCR master mix (Fermentas; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. The primers used
were as follows: OXTR forward, 5'-CAGACGCAGAGTGGTGCTAA-3' and
reverse, 5'-CCTTTCAGCCCTCGTTCTCA-3'; Trpv1 forward,
5'-CCACTCTTCTCCCACACGAG-3' and reverse, 5'-GGCAGGTGTCCTTTTGGAGT-3';
GAPDH forward, 5'-CTCCCACTCTTCCACCTTCG-3' and reverse,
5'-CCACCACCCTGTTGCTGTAG-3'. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 5 min; followed by 15 sec
at 95˚C and 30 sec at 60˚C for 35 cycles. The relative
transcription level was calculated using 2-∆∆Cq
(35,42,43)
taking GAPDH as the internal standard control. Five samples from
each group were analyzed in triplicate.
Measurement of plasma CORT level using
ELISA
The CORT ELISA kit (cat. no. ml037564) was purchased
from Shanghai Enzyme-linked Biotechnology Co., Ltd. with a
sensitivity limit of 2 µg/ml for CORT and measurements were
performed following the manufacturer's protocols. Briefly, the
harvested blood samples were collected in sterile tubes containing
liquid EDTA, which were kept on ice. The samples were centrifuged
at 2,500 x g for 10 min at 4˚C and with use of an anticoagulation
tube with 1 mg/ml EDTA, the plasma was harvested from the orbital
venous plexus and stored at -80˚C until use. When ELISA assay was
performed, the absorbance was read immediately using a microplate
reader (Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm
and the mean optical density was converted into concentration. Five
samples from each group were evaluated in triplicate. The
coefficients of variation were <5% and r2 of the
standard curve for the assay was >0.98.
Statistical analysis
SPSS 19.0 software (IBM Corp.) and GraphPad Prism
5.01 (GraphPad Software Inc.) were used for statistical analysis.
The data are presented as the mean ± standard error of mean or
medians unless otherwise indicated. Differences among multiple
groups were evaluated via one-way analysis of variance with Tukey's
post hoc test to identify the significantly different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Successful induction of AM in ICR
mice
Hematoxylin and eosin staining revealed that AM was
successfully induced in all mice treated with tamoxifen and was
absent in untreated mice, consistent with previous reports
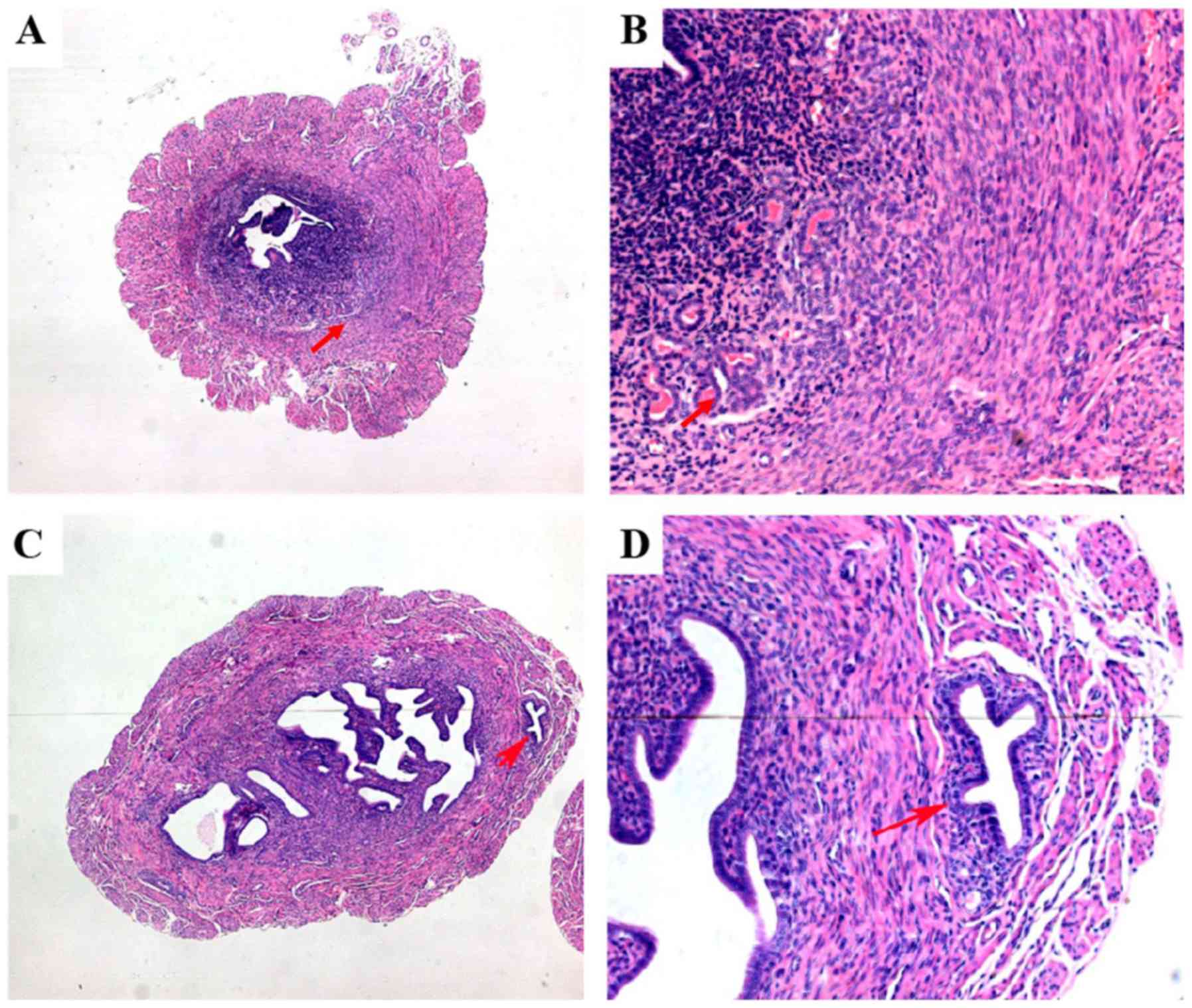
(20,21). As indicated in Fig. 1C and D, ectopic endometrial tissues invading the
myometrium and reaching the uterine serosa appeared in the
adenomyotic uteri of mice treated with tamoxifen. In mice without
AM, a distinct border between the basal layer of the endometrium
and the adjacent myometrium was observed (Fig. 1A and B). At 60 days after birth, the H&E
staining scores in tamoxifen-treated mice and controls were 1.6±0.3
and 0.0±0.0, respectively.
Effect of SXS treatment on myometrial
infiltration in AM-induced mice
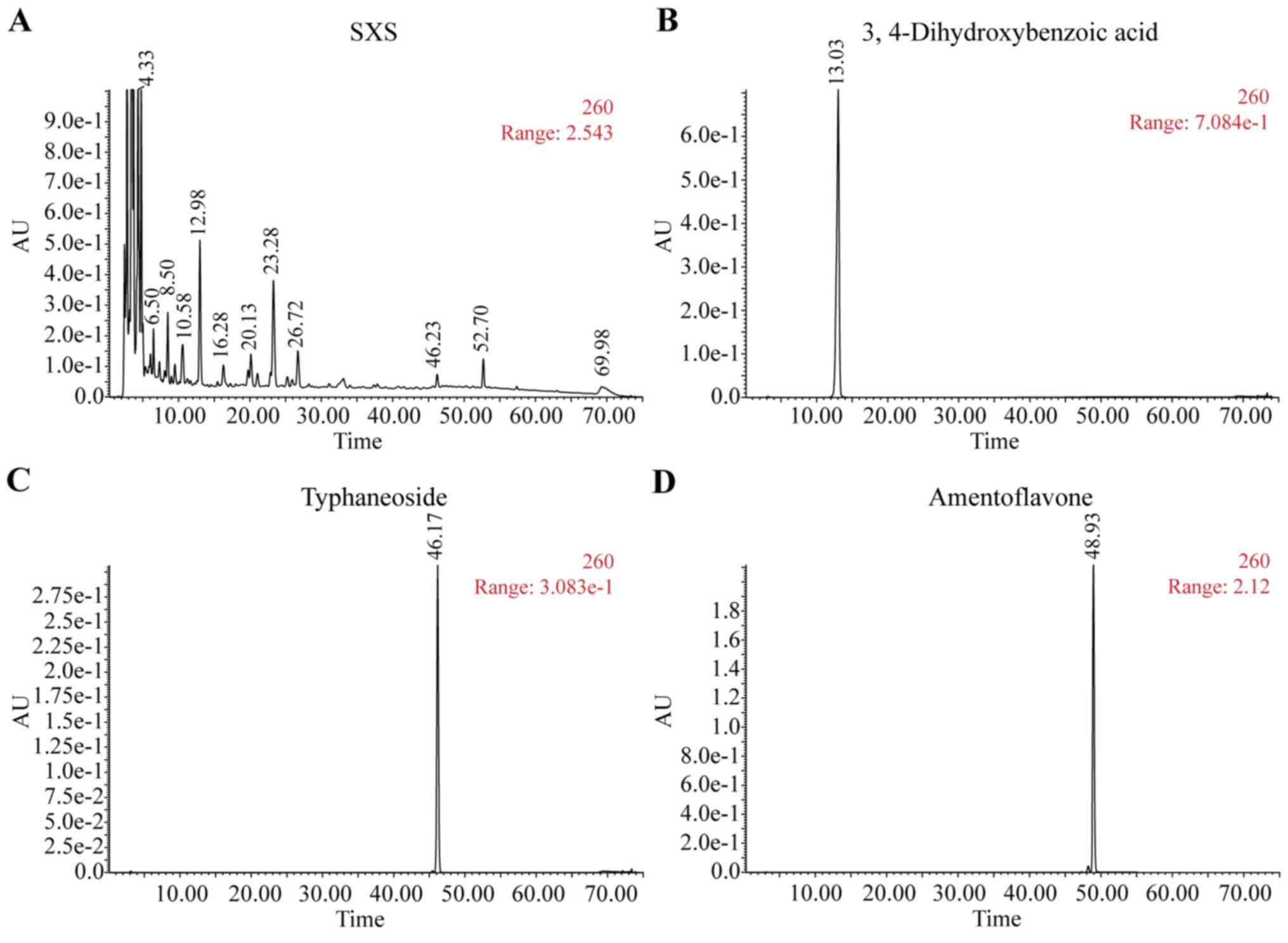
HPLC fingerprint spectra for SXS and the main
standard components are presented in Fig. 2. The content of standard material in
SXS (Fig. 2A), including
3,4-Dihydroxybenzoic acid (Fig. 2B),
typhaneoside (Fig. 2C),
amentoflavone (Fig. 2D),
isorhamnetin (Fig. 2E) complied with
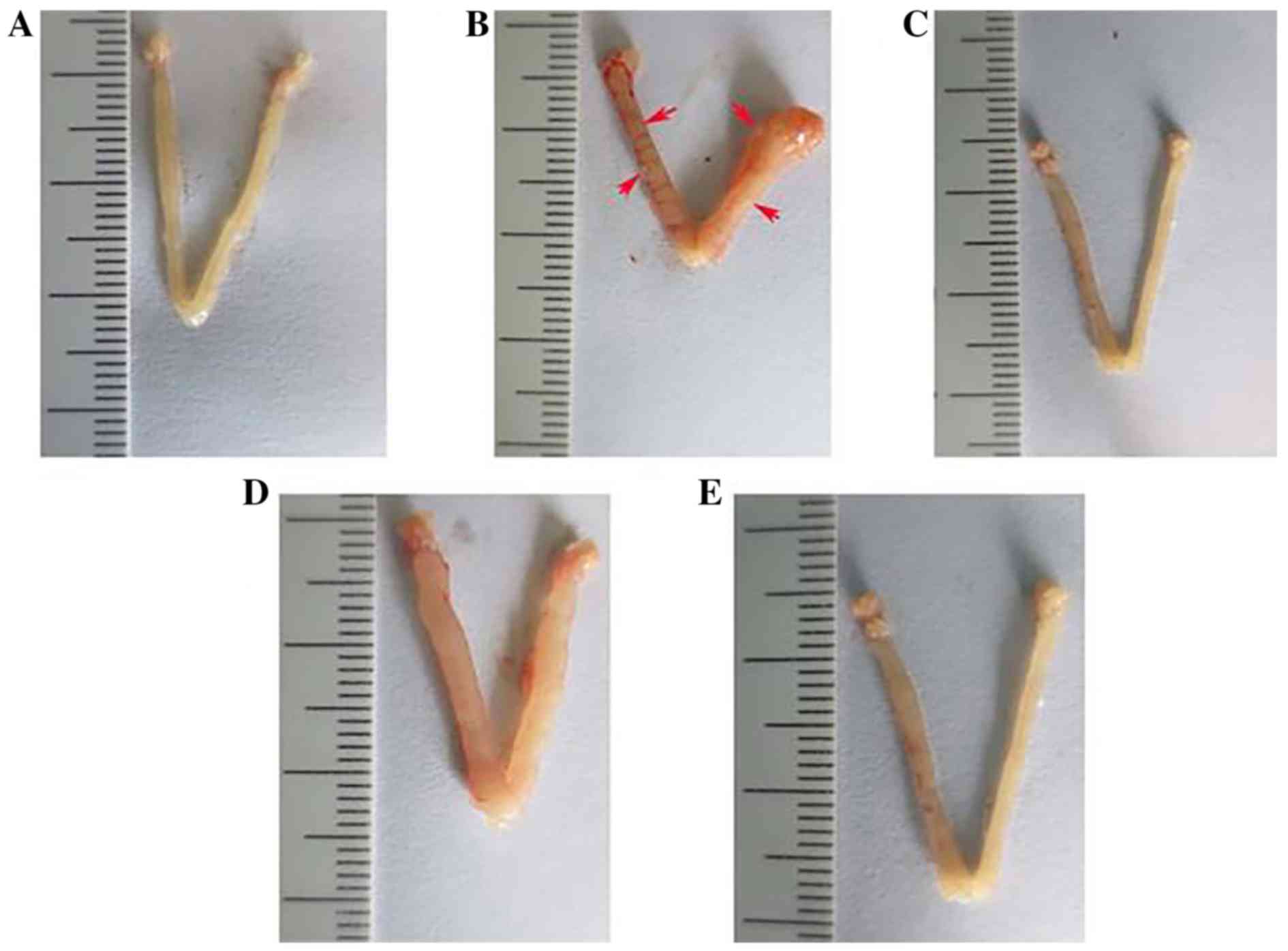
Chinese Pharmacopoeia standards. The uteri of the mice in the
control group showed normal contours and normal blood flow without
noticeable redness (Fig. 3A). The
uteri of mice in group M showed distorted contours and adenomyotic
nodules that clearly extruded from the serosal surface (Fig. 3B). The uteri of mice in group P
showed improved uterine contours with no obvious adenomyotic
nodules extruding from the serosal surface (Fig. 3C). The uteri from mice in groups H
and L showed improved contours and less congestion compared with
that in group M, with changes occurring in a dose-dependent manner.
The uterus of the low-dose group is presented in Fig. 3D and the uterus of the high-dose
group is demonstrated in Fig.
3E.
As indicated in Table
II, the uterine wet weight and the final body weight of the mice
in the control and treatment groups were not significantly
different. However, the ratio of the uterine wet weight to the
total body weight (U/B) was significantly lower in the model group
and group L compared with that in the control group (both
P<0.001). In addition no significant differences were observed
between the control group and group P (P=0.664) or H (P=0.152). In
addition, the U/B ratio of group H was comparable to that of group
P (P=0.313).
 | Table IIComparisons of the uterine wet
weight, final body weight, adenomyosis nodules, and H&E score
among the controls and the treated groups. |
Table II
Comparisons of the uterine wet
weight, final body weight, adenomyosis nodules, and H&E score
among the controls and the treated groups.
| Group | Mean uterine wet
weight ± SEM, mg | Mean final bw ±
SEM, g | Ratio, U/B | Mean number of
nodules ± SEM, n | Mean H&E score
± SEM |
|---|
| C | 120.0±3.0 | 35.7±1.5 | 2.2±0.0 | N/A | 0.0±0.0 |
| M | 129.8±3.5 | 39.5±0.8 |
2.6±0.1a | 11.2±0.9 | 3.3±0.4 |
| P | 123.0±4.0 | 36.4±1.6 | 2.2±0.1 | 4.4±0.5 | 1.5±0.2 |
| L | 128.7±3.7 | 37.6±1.6 |
2.4±0.0a | 5.2±0.6 | 2.1±0.2 |
| H | 124.0±6.2 | 36.9±1.7 | 2.3±0.0 | 3.4±0.4 | 1.7±0.2 |
The number of AM nodules and the H&E scores in
groups M and P were 11.2±0.9, 3.3±0.4; and 4.4±0.5, 1.5±0.2,
respectively (Table II). SXS
treatment reduced the number of AM nodules and the H&E scores
in a dose-dependent manner. The decrease in the number of AM
nodules and H&E scores was significant in Group L (P<0.001
and P=0.002, for AM nodules and H&E scores, respectively) and H
(P<0.001, P<0.001, for AM nodules and H&E scores,
respectively) compared with that in the model group. In addition,
the number of AM nodules in groups H and L was compared to the
number of AM nodules in group P (P=0.794 and P=0.041, for group H
and L, respectively). H&E scores of group P were not
significant when compared with that in group H (P=0.38) however,
they were significant compared with that in group L (P=0.44).
Effect of SXS on the thermal response
latency
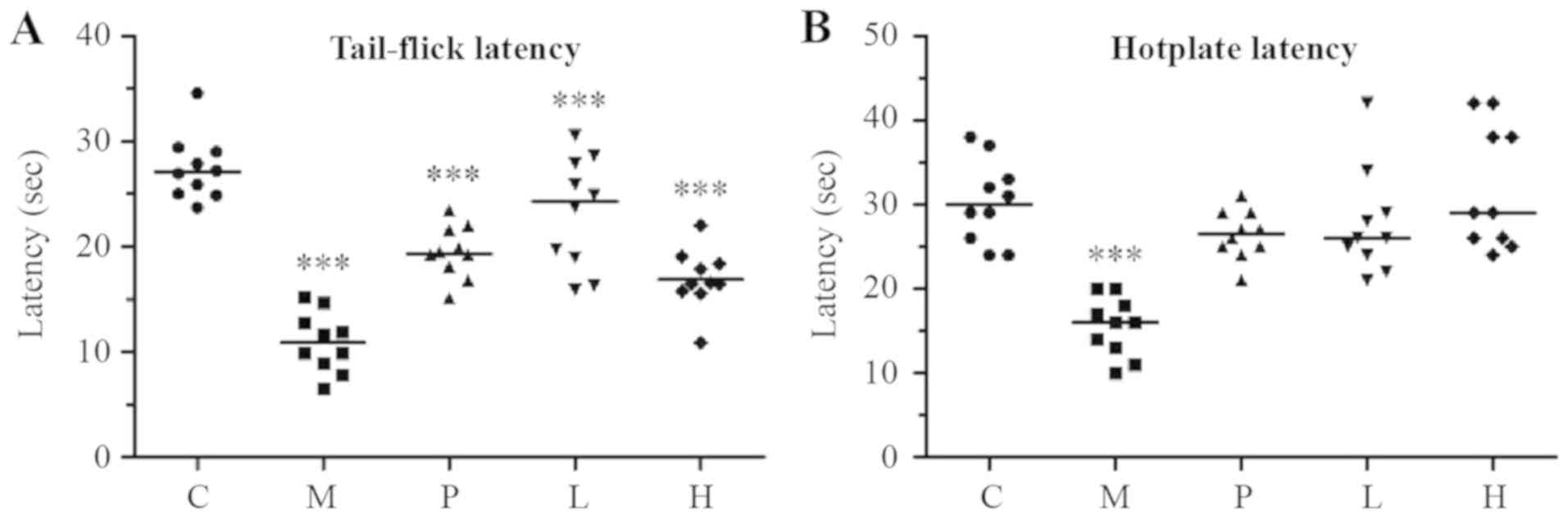
Using the tail-flick test, the present study assessed
the response to noxious thermal stimuli in mice after 2 months of
SXS treatment (Fig. 4A). In mice
treated with danazol (group P) and the low- and high-dose SXS
(groups L and H, respectively), the mean response latency was
significantly lower (P<0.001) compared with that in group C,
suggesting that the treatment did not completely restore the
response latency to a normal level.
The present study also assessed the response latency
in mice using the hotplate test. Unlike the tail-flick latency test,
the treatment completely restored the response latency to a normal
level in mice treated with danazol, low-dose SXS, and high-dose SXS
(P=0.055, P=0.196, and P=0.960, for groups P, L, and H,
respectively) compared with that in the control group.
SXS modulates the expression of genes
associated with AM-induced pain
Using RT-qPCR, the present study analyzed the
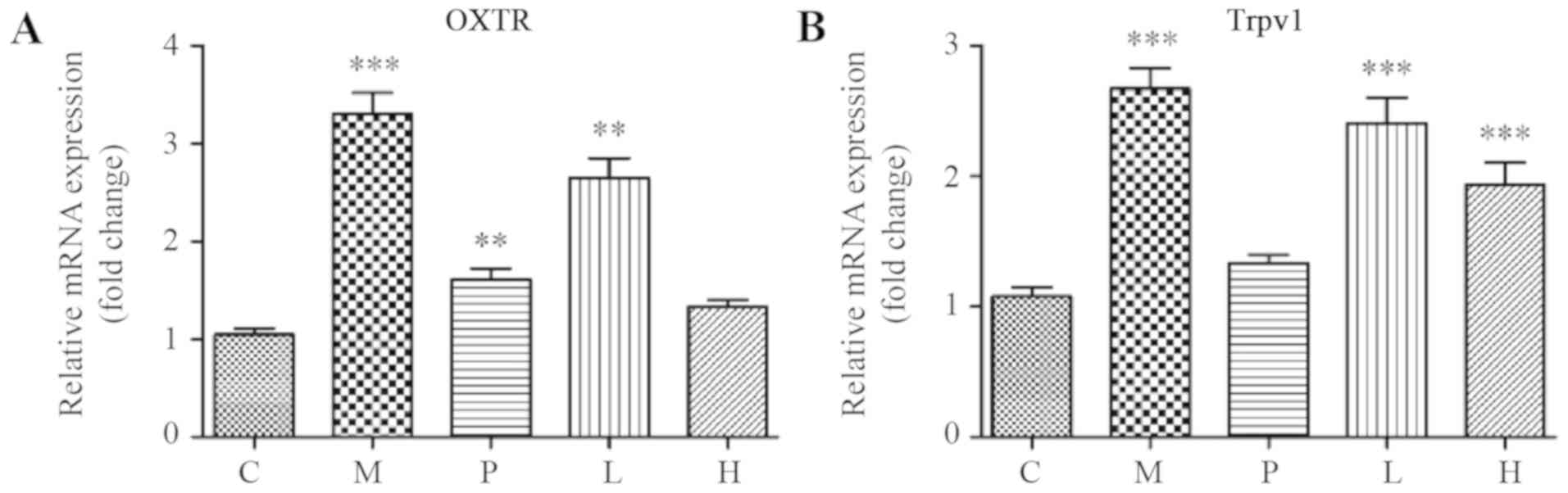
expression of genes associated with AM-induced pain. As indicated
in Fig. 5A and B, the expression of OXTR and Trpv1 was
significantly higher in the uteri of mice in group M compared with
that in group C (P<0.001). As shown in Fig. 5A, both low- and high-dose SXS
treatment downregulated the mean mRNA expression level of OXTR in
the uteri in a dose-dependent manner compared to that in group M.
Furthermore, the OXTR expression level in group H was similar with
the control group (P=0.301). In addition, SXS treatment also
reduced the mRNA expression level of Trpv1 in a dose-dependent
manner, and this decrease in Trpv1 expression was significantly
lower in groups L and H compared with that in group M (P=0.001 and
P<0.001, for groups L and H, respectively). However, the mRNA
expression level of Trpv1 in both groups L (P<0.001) and H
(P=0.010) remained significantly higher compared with that in group
C.
Effect of SXS on CORT plasma
levels
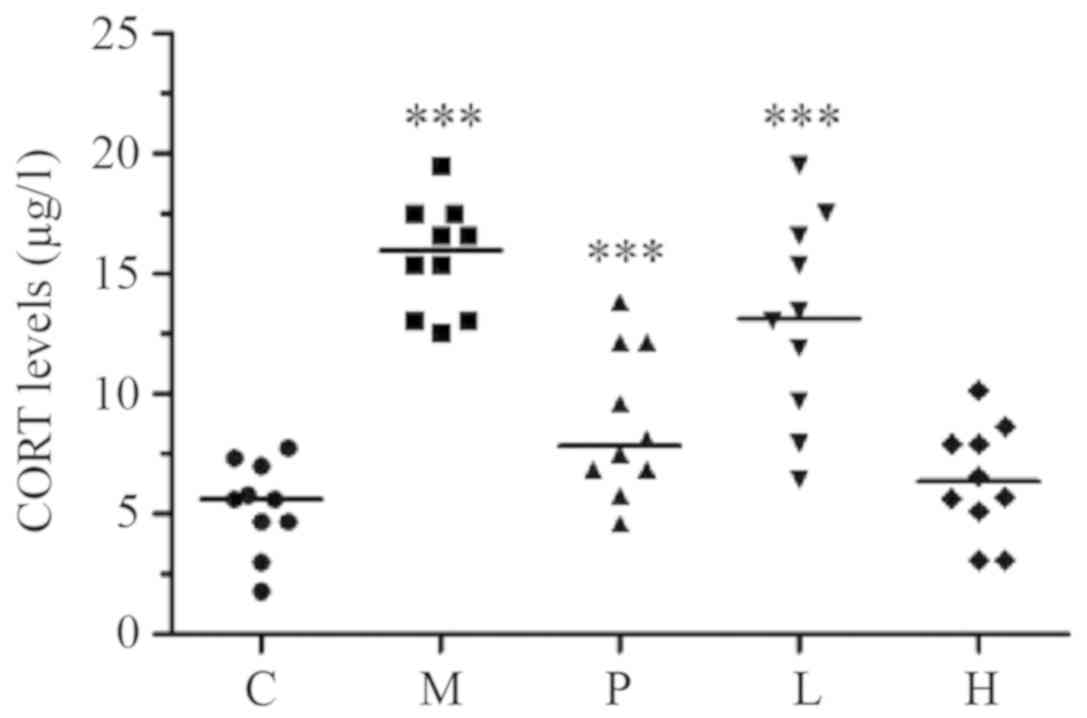
As indicated in Fig.
6, the present study found significant differences in the
plasma CORT levels among the five mice groups
(P<1.0x10-4). In particular, group M had a
significantly elevated CORT expression level when compared with
that in the control group (P<0.001). The mean CORT level in
group H was similar to that in the control group (P=0.613), however
it was significantly lower compared with that in group P (P=0.010).
In contrast, the mean CORT level in group L was similar to that in
group P (P=0.912) however, it was significantly higher compared
with that in the control group (P=0.010). Furthermore, unlike the
model group, both low- and high-dose SXS lowered plasma CORT levels
in mice with induced AM, in a dose-dependent manner.
Discussion
The use of TCM for disease treatment is considered
to have low side effects and to be relatively cost-efficient;
therefore, TCM is being used more frequently worldwide (44). SXS was designed based on TCM syndrome
typing, and followed the rules for drug synergism and compatibility
to resolve blood stasis (12-14).
In the present study, it was found that SXS could attenuate the
development of AM in mice with experimentally-induced AM in a
dose-dependent manner.
In previous studies, the area and depth of the
ectopic endometrium in the uterus of mice with induced AM
continuously increased with age (45-48).
Consistent with the aforementioned, the present study conducted a
comparison of H&E staining scores of tamoxifen-treated mice at
60 and 120 days following birth. The results revealed a progressive
infiltration of the endometrium. SXS treatment showed a
dose-dependent suppression of myometrial infiltration. Based on a
comparison of AM nodules and H&E scores for groups H and P
(danazol treatment), it can be concluded that SXS treatment and
danazol oral administration had almost equivalent efficacy in this
model.
The induction of AM in ICR mice results in
progressive generalized hyperalgesia, similar to that in humans,
along with elevated amplitude and increased irregularity of uterine
contractions (19-21).
The present study evaluated the response of mice to thermal stimuli
using the hotplate and tail-flick tests after 2 months of SXS
treatment, which confirmed the results of previous reports that
exposure of neonatal ICR mice to tamoxifen could induce generalized
hyperalgesia (19-21,43,49).
Furthermore, it was demonstrated that treatment with SXS improved
the generalized hyperalgesia, although the response latency in the
tail-flick test was not completely restored to the normal level.
Nevertheless, the effect of SXS appeared to be dose-dependent in
both the hotplate and tail-flick tests. In brief, these results
indicated that SXS was a promising compound for the treatment of
AM-induced hyperalgesia, especially considering the low incidence
of side effects and the cost-efficiency of treatment.
OXTR expression has previously been reported to be
increased in the epithelial, however, not in the stromal cells and
in the smooth muscle cells in endometriotic lesions (42). TRPV1 is known to be a key molecule in
sensory nerves in peripheral nociception integrating multiple
pain-producing stimuli (43). A
previous study revealed that immunoreactivity of OXTR and TRPV1 was
significantly higher in the ectopic endometrium of women with AM
compared with women with a healthy endometrium, and that both OXTR
and TRPV1 immunoreactivity positively correlated with the severity
of dysmenorrhea; therefore, they are considered significant
predictors of AM (30). TRPV1
activation may trigger the release of neuropeptides, such as
substance P and calcitonin gene-related peptide, causing increased
blood flow and hyperalgesia and perhaps dysmenorrheal (50). Therefore, TRPV1 could be an important
therapeutic target for adenomyosis-associated dysmenorrhea in light
of the recent development in TRPV1 antagonists (51).
The data from the present study suggests that SXS
was a potent inhibitor of OXTR and Trpv1. However, owing to
technical limitations, the expression of genes associated with
AM-induced pain from the entire uterus could be investigated. It is
expected that the response of the endometrial, stromal, and
myometrial components will differ. If proteins are only expressed
in different cell types, the extent of the changes in these
proteins may be larger compared with that identified from analyzing
the whole organ. Techniques, such as laser capture microdissection
are required to examine cell-specific responses to SXS.
Irrespective of the cause, the AM-induced elevation
of plasma CORT levels observed in the present study is consistent
with several studies, which have reported that chronic pain
conditions, such as rheumatoid arthritis, induce stress in patients
and are associated with dysfunction of the
hypothalamic-pituitary-adrenal axis. In turn, this may exacerbate
the symptoms of chronic pain (52,53). The
results from the present study, together with a previous report
that pre-induction stress exacerbates AM (54), has raised the possibility that stress
and AM may be mutually facilitative, thereby perpetuating
AM-induced pain. Furthermore, SXS treatment was found to be
effective in reducing systemic CORT levels in the present
study.
Aside from abnormal uterine bleeding, dysmenorrhea
is the most prevalent symptom of AM, and is arguably the most
debilitating symptom (17). The data
from the present study suggests that SXS alleviated generalized
hyperalgesia in the AM mouse model through the regulation of the
expression of pain-associated genes and stress levels. However, it
remains to be elucidated whether the ability to regulate these
genes can be considered a primary or a secondary therapeutic target
for AM.
Collectively, the present study has demonstrated
that treatment with SXS reduced myometrial infiltration, alleviated
generalized hyperalgesia, and decreased plasma CORT levels in mice
with induced AM. In addition, SXS tolerance, demonstrated in the AM
mouse model, was consistent with that of long-term SXS therapy for
patients with AM in the clinical setting (12-14).
From the perspective of Chinese medicine, SXS is a promising
formula for the treatment of AM, owing to the favorable associated
costs and side-effect profile (55,56).
However, caution should be exercised, as a successful therapy for
animals does not guarantee an effective therapy for humans. Thus,
further research is required to allow a comprehensive examination
of the potential benefits of SXS.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81674013), Jiangsu
Province's Outstanding Leader Program of Traditional Chinese
Medicine, and the Natural Science Foundation of Jiangsu Province
(grant no. BK20161607) and Science and technology project of
Jiangsu Provincial Administration of traditional Chinese Medicine
(grant no. YB2015049).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH, PC and JuY designed the research. FZ, DW, ZW,
ZP, JiY and HY performed the experiments. XC, MC and JL analyzed
the data. JuY wrote the manuscript. All authors read and approved
the final version of this manuscript.
Ethics approval and consent to
participate
This study was approved by the Hospital of
Integrated Traditional Chinese and Western Medicine Affiliated to
Nanjing University of Chinese Medicine Ethics Review Board
(approval no. 2018LWKYS-06).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Munro MG: Classification and reporting
systems for adenomyosis. J Minim Invasive Gynecol. 27:296–308.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yu O, Schulze-Rath R, Grafton J, Hansen K,
Scholes D and Reed SD: Adenomyosis incidence, prevalence and
treatment: United States population-based study 2006-2015. Am J
Obstet Gynecol: doi:10.1016/j.ajog.2020.01.016.
|
|
3
|
Tomassetti C, Meuleman C, Timmerman D and
D'Hooghe T: Adenomyosis and subfertility: Evidence of association
and causation. Semin Reprod Med. 31:101–108. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Koike N, Tsunemi T, Uekuri C, Akasaka J,
Ito F, Shigemitsu A and Kobayashi H: Pathogenesis and malignant
transformation of adenomyosis (review). Oncol Rep. 29:861–867.
2013.PubMed/NCBI View Article : Google Scholar : (review).
|
|
5
|
Jichan Nie, Xishi Liu and Guo SW: Promoter
hypermethylation of progesterone receptor isoform B (PR-B) in
adenomyosis and its rectification by a histone deacetylase
inhibitor and a demethylation agent. Reprod Sci. 17:995–1005.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Morelli M, Rocca ML, Venturella R,
Mocciaro R and Zullo F: Improvement in chronic pelvic pain after
gonadotropin releasing hormone analogue (GnRH-a) administration in
premenopausal women suffering from adenomyosis or endometriosis: A
retrospective study. Gynecol Endocrinol. 29:305–308.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bergeron C, Amant F and Ferenczy A:
Pathology and physiopathology of adenomyosis. Best Pract Res Clin
Obstet Gynaecol. 20:511–521. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grow DR and Filer RB: Treatment of
adenomyosis with long-term GnRH analogues: A case report. Obstet
Gynecol. 78:538–539. 1991.PubMed/NCBI
|
|
9
|
Saremi A, Bahrami H, Salehian P, Hakak N
and Pooladi A: Treatment of adenomyomectomy in women with severe
uterine adenomyosis using a novel technique. Reprod Biomed Online.
28:753–760. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pepas L, Deguara C and Davis C: Update on
the surgical management of adenomyosis. Curr Opin Obstet Gynecol.
24:259–264. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taran FA, Stewart EA and Brucker S:
Adenomyosis: Epidemiology, risk factors, clinical phenotype and
surgical and interventional alternatives to hysterectomy.
Geburtshilfe Frauenheilkd. 73:924–931. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu SH, Cao BL, Liu X, et al: Drug use law
of traditional chinese medicine in treating endometriosis. West J
Tradit Chin Med. 31:87–90. 2018.(In Chinese).
|
|
13
|
Wang Y and Wei SB and Wei SB: The second
stage therapy of TCM in treating 120 cases of adenomyosis of
stagnation pattern of heat-dampness. West J Tradit Chin Med.
30:1–3. 2017.(In Chinese).
|
|
14
|
Liu Z, Li YH, Sun J, et al: Study of
usages of Shixiao powder by ancient literature. J Ethnomedicine
Ethnopharmacy. 27:7–10. 2018.
|
|
15
|
Zhou J and Qu F: Treating gynaecological
disorders with traditional Chinese medicine: A review. Afr J Tradit
Complement Altern Med. 6:494–517. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Flower A, Liu JP, Lewith G, Little P and
Li Q: Chinese herbal medicine for endometriosis. Cochrane Database
Syst Rev. 5(CD006568)2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng C, Gui T, Huang MH, et al: Treatment
of adenomyosis patients by bushen huoxue sanyu decoction: A
clinical study. CJITWM. 34(11):1302–1305. 2014.PubMed/NCBI
|
|
18
|
Zhang L and Shi YP: Efficacy observation
of ultrasonic conductometric acupoint penetration of Wenhua Zhitong
recipe for adenomyosis patients. CJITWM. 36(1):54–58.
2016.PubMed/NCBI(In Chinese).
|
|
19
|
Chen Y, Zhu B, Zhang H, Liu X and Guo SW:
Epigallocatechin-3-gallate reduces myometrial infiltration, uterine
hyperactivity, and stress levels and alleviates generalized
hyperalgesia in mice induced with adenomyosis. Reprod Sci.
20:1478–1491. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mao X, Wang Y, Carter AV, Zhen X and Guo
SW: The retardation of myometrial infiltration, reduction of
uterine contractility, and alleviation of generalized hyperalgesia
in mice with induced adenomyosis by levo-tetrahydropalmatine
(l-THP) and andrographolide. Reprod Sci. 18:1025–1037.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu X and Guo SW: Valproic acid alleviates
generalized hyperalgesia in mice with induced adenomyosis. J Obstet
Gynaecol Res. 37:696–708. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mehasseb MK, Bell SC and Habiba MA: The
effects of tamoxifen and estradiol on myometrial differentiation
and organization during early uterine development in the CD1 mouse.
Reproduction. 138:341–350. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Yuan H, Deng L, Hu F, Ma J and
Lin J: Evaluation of the efficacy of a danazol-loaded intrauterine
contraceptive device on adenomyosis in an ICR mouse model. Hum
Reprod. 23:2024–2030. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kida H: Histological analysis of
spontaneous adenomyosis-like changes in recombinant inbred mouse
uterus (SMXA mouse)--a novel animal model for adenomyosis. Nihon
Sanka Fujinka Gakkai Zasshi. 46(4):323–330. 1994.PubMed/NCBI(In Japanese).
|
|
25
|
Yamada H, Shimada S and Fujimoto S:
Endometriosis-adenomyosis mouse model induced by transvaginal
pituitary transplantation. Nihon Rinsho. 59 (Suppl 1):221–224.
2001.PubMed/NCBI(In Japanese).
|
|
26
|
Li Y, Zhang SF, Zou SE, Xia X and Bao L:
Accumulation of nerve growth factor and its receptors in the uterus
and dorsal root ganglia in a mouse model of adenomyosis. Reprod
Biol Endocrinol. 9(30)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Liu X and Guo SW: Clinical profiles
of 710 premenopausal women with adenomyosis who underwent
hysterectomy. J Obstet Gynaecol Res. 40:485–494. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cirpan T, Yeniel O, Ulukus M, Ozbal A,
Gundem G, Ozsener S, Mete Itil I and Zekioglu O: Clinical symptoms
and histopathological findings in subjects with adenomyosis uteri.
Clin Exp Obstet Gynecol. 35:48–53. 2008.PubMed/NCBI
|
|
29
|
Shrestha A and Sedai LB: Understanding
clinical features of adenomyosis: A case control study. Nepal Med
Coll J. 14:176–179. 2012.PubMed/NCBI
|
|
30
|
Nie J, Liu X and Guo SW: Immunoreactivity
of oxytocin receptor and transient receptor potential vanilloid
type 1 and its correlation with dysmenorrhea in adenomyosis. Am J
Obstet Gynecol. 202:346. e1–8. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo SW, Mao X, Ma Q and Liu X:
Dysmenorrhea and its severity are associated with increased uterine
contractility and overexpression of oxytocin receptor (OTR) in
women with symptomatic adenomyosis. Fertil Steril. 99:231–240.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Taran FA, Weaver AL, Coddington CC and
Stewart EA: Understanding adenomyosis: A case control study. Fertil
Steril. 94:1223–1228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chapman CR, Tuckett RP and Song CW: Pain
and stress in a systems perspective: Reciprocal neural, endocrine,
and immune interactions. J Pain. 9:122–145. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li SP, Zhao J and Yang B: Strategies for
quality control of Chinese medicines. J Pharm Biomed Anal.
55:802–809. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ascher SM, Imaoka I and Lage JM:
Tamoxifen-induced uterine abnormalities: The role of imaging.
Radiology. 214:29–38. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mori T, Yamasaki S, Masui F, Matsuda M,
Sasabe H and Zhou YF: Suppression of the development of
experimentally induced uterine adenomyosis by a novel matrix
metalloproteinase inhibitor, ONO-4817, in mice. Exp Biol Med
(Maywood). 226:429–433. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gui T, Chen C, Zhang Z, Tang W, Qian R, Ma
X, Cao P and Wan G: The disturbance of TH17-Treg cell balance in
adenomyosis. Fertil Steril. 101:506–514. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yan Li, Shaofen Zhang, Xian Xia and Enshi
Zhou: Establishment of an ICR mouse model of adenomyosis by oral
administration of Tamoxifen. Acta laboratorium animalis scientia
sinica. 17(5):345–350. 2009.(In Chinese).
|
|
40
|
Bird CC, McElin TW and Manalo-Estrella P:
The elusive adenomyosis of the uterus--revisited. Am J Obstet
Gynecol. 112:583–593. 1972.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Emge LA: The elusive adenomyosis of the
uterus Its historical past and its present state of recognition. Am
J Obstet Gynecol. 83:1541–1563. 1962.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mechsner S, Bartley J, Loddenkemper C,
Salomon DS, Starzinski-Powitz A and Ebert AD: Oxytocin receptor
expression in smooth muscle cells of peritoneal endometriotic
lesions and ovarian endometriotic cysts. Fertil Steril. 83 (Suppl
1):1220–1231. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Szallasi A: Vanilloid (capsaicin)
receptors in health and disease. Am J Clin Pathol. 118:110–121.
2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lu AP, Jia HW, Xiao C and Lu QP: Theory of
traditional Chinese medicine and therapeutic method of diseases.
World J Gastroenterol. 10:1854–1856. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Garcia L and Isaacson K: Adenomyosis:
Review of the literature. J Minim Invasive Gynecol. 18:428–437.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Taran FA, Weaver AL, Coddington CC and
Stewart EA: Characteristics indicating adenomyosis coexisting with
leiomyomas: A case-control study. Hum Reprod. 25:1177–1182.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Levgur M, Abadi MA and Tucker A:
Adenomyosis: Symptoms, histology, and pregnancy terminations.
Obstet Gynecol. 95:688–691. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Greaves P and White IN: Experimental
adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20:503–510.
2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhu B, Chen Y, Zhang H, Liu X and Guo SW:
Resveratrol reduces myometrial infiltration, uterine hyperactivity,
and stress levels and alleviates generalized hyperalgesia in mice
with induced adenomyosis. Reprod Sci. 22:1336–1349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Moriyama T, Higashi T, Togashi K, Iida T,
Segi E, Sugimoto Y, Tominaga T, Narumiya S and Tominaga M:
Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive
mechanism of prostaglandins. Mol Pain. 17(1:3)2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nie J and Liu X: Quercetin alleviates
generalized hyperalgesia in mice with induced adenomyosis. Mol Med
Rep. 16:5370–5376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kurtais Y, Tur BS, Elhan AH, Erdogan MF
and Yalçin P: Hypothalamic-pituitary-adrenal hormonal responses to
exercise stress test in patients with rheumatoid arthritis compared
to healthy controls. J Rheumatol. 33(8):1530–1537. 2006.PubMed/NCBI
|
|
53
|
Otake T, Ashihara M, Nishino J, Kato K,
Fukaya S and Yoshida S: Stressors and rheumatoid arthritis: Changes
in stressors with advances in therapeutic agents. Rheumatol Int.
33:887–891. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cuevas M, Flores I, Thompson KJ,
Ramos-Ortolaza DL, Torres-Reveron A and Appleyard CB: Stress
exacerbates endometriosis manifestations and inflammatory
parameters in an animal model. Reprod Sci. 19:851–862.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ji GQ, Xu M, Yin H, et al: Study on
Shixiaosan Granule on Toxicity in Rats. Acta Chinese Medicine.
6:985–988. 2017.
|
|
56
|
Li Q, Xie P, Bai CX, Cui JY, Jiang C and
Wu YS: Mechanisms of Fufang Shixiao formula for experimental
primary dysmenorrhea. Zhongguo Zhong Xi Yi Jie He Za Zhi.
36:1087–1090. 2016.PubMed/NCBI
|