Introduction
Post-infectious irritable bowel syndrome (PI-IBS) is
characterized by the onset of the symptoms mentioned in the
diagnostic criteria for irritable bowel syndrome (IBS) (the most
recent criteria being those in Rome IV) (1). They occur as a result of an episode of
acute infectious gastroenteritis (AGE) characterized by two or more
of the following symptoms: diarrhea, vomiting, fever, and a
positive result of the etiologic agent in the stool (2). A recent systematic review and
meta-analysis have shown that the risk of developing IBS increases
six times after a gastrointestinal infection and is maintained at
least 2-3 years after infection (3,4). The
data in the literature are limited regarding the risk of PI-IBS
(5). Among the first studies that
raised the suspicion of a link between IBS and intestinal infection
was 6 decades ago, the study by Stewart (6). In 1962, Chaudhary and Truelove reported
that one third of patients with a history of gastroenteritis
continued to develop symptoms of IBS. All these studies have
demonstrated an incidence or prevalence of PI-IBS between 5 and 32%
(7-21).
Unlike sporadic IBS, PI-IBS has a defined onset moment. The risk of
PI-IBS appears to correlate with the severity of acute enteric
infection (14,16).
Despite the fact that there are no reported sex
differences in the severity of the initial infectious disease or in
the immune response, the reported risk of developing PI-IBS is
higher in women than in men with a relatively adjusted risk between
1.47 and 2.86 (14,16,22-24).
One of the earliest reports of PI-IBS refers to
unexplained diarrhea and abdominal discomfort, symptoms that
started after an episode of amniotic dysentery (25). Another study conducted in Sheffield
examined 75 individuals who reported themselves to an infectious
disease unit with the diagnosis of gastroenteritis. It was observed
that 25% of them developed IBS when assessed 6 months after
infection (26). Various bacterial
pathogens such as Shigella, Salmonella, Campylobacter
and Escherichia coli (14,15,18-20,27,28)
were involved in PI-IBS development, but it remains unclear whether
all these micro-organisms give an equivalent risk. The risk of
developing Clostridium difficile community infection is
steadily rising, reaching up to 20-40% of all CDI cases (29). Two current studies have shown that
4-12% of the population may experience PI-IBS symptoms following a
Clostridium difficile infection. Another retrospective study
among military personnel revealed an incidence of PI-IBS of
5-9/100,000, years after CDI (30).
The viral etiology seems to provoke a transient form of PI-IBS
compared with bacterial etiology (4,13).
Prospective studies provide strong evidence that the development of
PI-IBS involves an interactive multifactorial etiopathogenic
process (31-33).
Probiotics have been shown to be effective in preventing or
attenuating the symptoms of acute gastroenteritis (34-36).
However, no study has yet evaluated the efficacy of interventions
that modulate intestinal flora for the prevention or treatment of
PI-IBS (4).
The objective of this study was to determine the
risk factors of developing PI-IBS following an acute AGE. We
assessed the incidence of PI-IBS by sex distribution and regarding
the etiology of the infectious gastroenteritis.
Patients and methods
The type of the study was case control. The data
collected were retrospective. The variables studied were
qualitative and quantitative.
The target population was formed by patients
admitted to a tertiary center of infectious diseases, the Clinical
Hospital of Infectious Diseases, Cluj-Napoca, Romania in a time
interval of three consecutive years (1.01.2013-31.12.2015). The
target group was divided into two subgroups.
The case group was composed by patients with an AGE
episode in which the etiological agent was isolated by direct
examination: microscopy, coproparasitological examination of the
stool specimens; bacteriological examinations: coproculture-Hektoen
enteric (HE) agar for the isolation of Shigella and
Salmonella from stool specimens, Campy CVA Agar selective
medium for the primary isolation of Campylobacter jejuni
from stool specimens, CIN (Cefsulodin, Irgasan, Novobiocin). Agar
selective differential medium for the isolation of Yersinia
enterocolitica from stool specimens; immunological
examinations: rapid Rotavirus/Adenovirus/Norovirus test
(coloured chromatographic immunoassay for the simultaneous
qualitative detection of Rotavirus, Adenovirus and
Norovirus in stool samples), Giardia lamblia antigen
(coloured chromatographic immunoassay for the qualitative detection
of Giardia in stool samples), determination of toxins A and
B for Clostridium difficile (enzyme-linked fluorescent assay
in stool samples).
The type of identification of the etiological agent
was chosen based on clinical examination and the epidemiological
data of the patient. The control group consisted of patients
admitted to the same medical service for acute upper respiratory
tract infection (URTI).
Inclusion criteria were: patients over the age of 18
with an AGE in which the infectious etiologic agent could be
determined or a URTI episode. Exclusion criteria were: patients
under the age of 18, gastroenteritis where the infectious
etiological agent could not be isolated, HIV-infected patients,
patients who died during the course of this study, and patients
previously diagnosed with IBS.
The patients filled the Rome III questionnaire for
IBS (37) and identified the stool
consistency with the Bristol Stool Form Scale (38). The questionnaires were filled in 6
months after the acute infectious episode in both groups. The
questionnaires were paper printed and directly filled in by the
subjects, after being recalled to our center to be evaluated. The
average response time was 5 min.
The results were statistically processed with the
program SPSS Statistics 24.
All procedures performed in the study involving
human participants were in accordance with the ethical standards of
the Institutional and/or National Research Committee (Committee
chiar - Felicia Loghin; members: Anca Buzoianu, Ioana Cristolțan,
Vasile Fluieraș; jurist - Luminița Gocan; reference no.
132/11.04.2014) and with the 1964 Helsinki Declaration and its
later amendments or comparable ethical standards. Informed consent
was obtained from all the patients included in the study.
Results
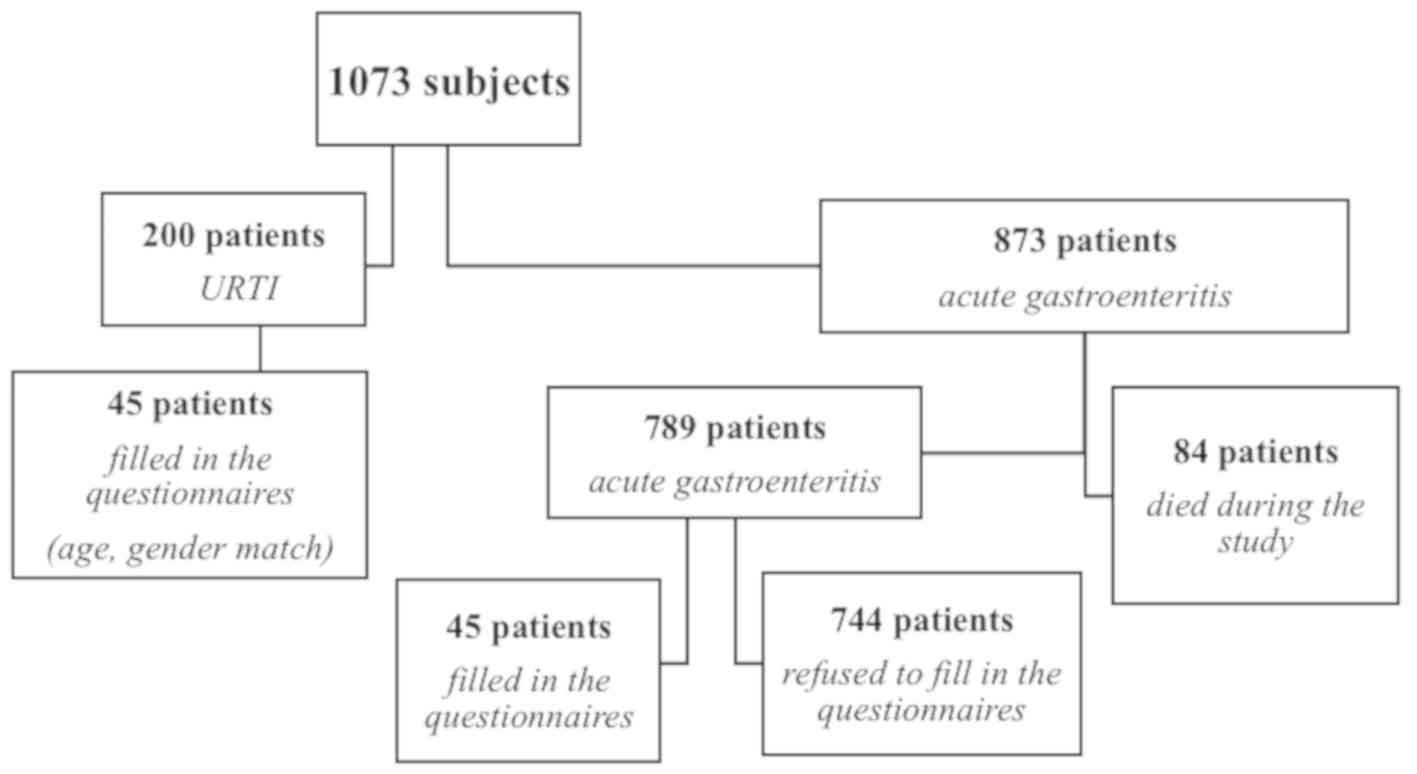
The target population consisted of 1,073 patients,
divided into two groups. The case group consisted of 873 patients
with an infectious gastroenteritis episode were the etiological
agent was isolated. In this group, 84 patients (10%) died during
the course of this study. In the case group, 45 patients filled in
the questionnaires (5%) they were aged 18-80 years (average,
57.36). The reasons for the refusal to fill in the questionnaires
in the case group were: lack of time, they did not want to be part
of the study and to disclose medical data, without interest for the
field of medical research. The sex distribution was: 17 male
patients (38%) and 28 female patients (62%). Of these, 56% were
diagnosed with irritable bowel syndrome (25 patients) and 44% were
without irritable bowel syndrome (20 patients).
In the control group, out of 200 patients admitted
for URTI, 45 patients were selected by age and sex matching; all
patients selected filled in the evaluation questionnaires. The
response rate in the control group was 100%. The reasons for
completing the questionnaires in the control group were because the
subjects wanted to take part in the study. Of the 45 patients with
URTI, 13% were diagnosed with irritable bowel syndrome (6 patients)
and 87% were without irritable bowel syndrome (39 patients)
(Fig. 1).
After statistical analysis, we observed that
patients who had an AGE were at a higher risk of developing PI-IBS
at a relative risk (RR) of 4.16 (95% CI, 1.89-9.17). By comparing
the two subgroups, statistically significant data were obtained
that are in favor of the alternative hypothesis (P=0.00002,
P<0.05), so the case group showed an increased incidence of
PI-IBS compared with the control group (Table I).
 | Table IThe incidence of PI-IBS within the
study population. |
Table I
The incidence of PI-IBS within the
study population.
| Patients
(N=90) | IBS N=31 | No IBS N=59 | RR for IBS RR (95%
CI) | P-value |
|---|
| AGE | 25 (56%) | 20 (44%) | 4.16
(1.89-9.17) | 0.00002 |
| URTI | 6 (13%) | 39 (87%) | | |
In the group of patients with AGE, after processing
the results, an increased incidence of PI-IBS was noted among
female patients compared with males, with 79% of women (22
patients) developing PI-IBS and 18% men (3 patients) developing
PI-IBS. The alternative hypothesis is valid, statistically
significant (P=0.0006, P<0.05), with RR=4.4 (95% CI,
1.56-12.65). In the group with URTI, sex distribution of PI-IBS
showed an RR=1 (95% CI, 0.20-4.85) for female patients, with
P=0.11, P>0.05, not statistically significant. There was no
higher incidence of PI-IBS in female patients in the URTI group. By
correlating the data from the case group and the control group,
intestinal infection is shown as a risk factor in increasing the
incidence of PI-IBS in female patients in the studied population
(Table II).
 | Table IIThe incidence of PI-IBS within the
study population by sex distribution. |
Table II
The incidence of PI-IBS within the
study population by sex distribution.
| Patients (N=45)
AGE | IBS N=25 | No IBS N=20 | RR for IBS RR (95%
CI) | P-value |
|---|
| Male | 3 (18%) | 14 (82%) | 4.4
(1.56-12.65) | 0.0006 |
| Female | 22 (79%) | 6 (21%) | | |
| Patients (N=45)
URTI | IBS N=6 | No IBS N=39 | RR for IBS RR (95%
CI) | P-value |
| Male | 2 (13%) | 13 (87%) | 1 (0.20-4.85) | 0.1169 |
| Female | 4 (13%) | 26 (87%) | | |
From the point of view of the subtypes of PI-IBS, a
distribution in favor of the mixed subtype was observed (Table III).
 | Table IIIPI-IBS subtype distribution in the
case group. |
Table III
PI-IBS subtype distribution in the
case group.
| AGE PI-IBS
subtype | N=25 |
|---|
| IBS-D | 8 (32%) |
| IBS-C | 5 (20%) |
| IBS-M | 10 (40%) |
| IBS-U | 2 (8%) |
Regarding the infectious etiology of the AGE, in the
case group, there were 17 patients with Clostridium
difficile (52%), 5 patients with Salmonella spp. (62%),
2 patients with Campylobacter jejuni (67%) and a patient
with Rotavirus (100%) who developed PI-IBS (Table IV).
 | Table IVPI-IBS distribution in the case group
according to the etiology of the AGE. |
Table IV
PI-IBS distribution in the case group
according to the etiology of the AGE.
| Etiological
agent | PI-IBS N=25 | No PI-IBS N=20 |
|---|
| Clostridium
difficile | 17 (52%) | 16 (48%) |
| Salmonella
spp. | 5 (62%) | 3 (38%) |
| Campylobacter
jejuni | 2 (67%) | 1 (33%) |
|
Rotavirus | 1 (100%) | 0 |
Analyzing the statistical significance test for each
of the coefficients of the logistic regression model, it was
observed that both Rotavirus (B=20.692, P=0.00001,
P<0.05) and Campylobacter jejuni (B=0.182, P=0.04) are
good predictors of the appearance of PI-IBS. The risk of IBS
depending on the bacteria is RR=0.6 for Clostridium
difficile infection (CDI) and 1.2 times higher for
Campylobacter jejuni infection than for Salmonella
spp. to which these values were reported.
Distribution of IBS subtypes depending on the
infectious etiology of the AGE revealed that in patients with
Salmonella spp. infection the incidence of IBS subtypes with
constipation predominance (IBS-C) and mixed (IBS-M) were increased.
Patients with CDI showed diarrhea predominance subtype (IBS-D) and
mixed subtype similar to patients with Campylobacter jejuni
infection. The patient with Rotavirus infection presented
the subtype ‘unsubtyped’ (IBS-U). (Table
V).
 | Table VPI-IBS subtype distribution depending
on the infectious etiology. |
Table V
PI-IBS subtype distribution depending
on the infectious etiology.
| PI-IBS subtype | Clostridium
difficile N=17 | Salmonella
spp. N=5 | Campylobacter
jejuni N=2 | Rotavirus
N=1 |
|---|
| IBS-D | 6 (35%) | 1 (20%) | 1 (50%) | 0 |
| IBS-C | 3 (18%) | 2 (40%) | 0 | 0 |
| IBS-M | 7 (41%) | 2 (40%) | 1 (50%) | 0 |
| IBS-U | 1 (6%) | 0 | 0 | 1 (100%) |
Discussion
Our study looked for risk factors for developing
PI-IBS following AGE. The etiological agent of AGE was isolated by
direct examination (microscopy, coproparasitological examination),
bacteriological examinations (coproculture) and immunological
examinations (rapid
Rotavirus/Adenovirus/Norovirus/Astrovirus test, Giardia
lamblia antigen, determination of toxins A and B for
Clostridium difficile). The type of identification of the
etiological agent was chosen based on clinical examination and the
epidemiological data of the patient.
In this study we observed that PI-IBS occurs with an
RR of 4.16 (95% CI, 1.89-9.17) compared with controls. The
prognostic factors are female sex, the etiological agent involved
in the AGE correlate with the duration of the probiotic therapy
after AGE.
The limitations of our study were the fact that not
all the patients filled in the questionnaires: 84 patients (10%)
died during the course of this study. In the case group, 45
patients filled in the questionnaires (5%) and 744 patients (85%)
refused to fill in the questionnaires. The reasons for the refusal
to fill in the questionnaires in the case group were: lack of time,
they did not want to be part of the study and to disclose medical
data, without interest for the field of medical research. Another
limitation was the fact that the evaluation was performed at 6
months after the AGE, but there was no follow-up after this.
Regarding sex distribution, until now there are 21
studies published showing an increased incidence of PI-IBS in
female patients. In a 2017 meta-analysis performed by Klem et
al (39) based on data from 11
studies with extractable data, it was revealed that female sex was
associated with a 2.2 times increase in PI-IBS (OR, 2.19; 95%, CI,
1.57-3.07). Summarizing the estimate, a substantial heterogeneity
(I2=72%) was noted. On the other hand, a study led by Litleskare
et al (40) in Norway in 2018
on the prevalence of PI-IBS after intestinal infection with
Giardia lamblia showed that female sex is also known as a
risk factor in sporadic PI-IBS.
The data in our study reveals that in the case
group, female sex had 4.4 higher risk of developing PI-IBS compare
to the control group.
Based on a meta-analysis of 45 studies, in 2017 Klem
et al (39) reported that
approximately one in 9 people (95% CI, 7-13) exposed to different
forms of infectious enteritis can develop IBS at a rate 4 times
higher than persons who are not exposed. The risk rate of
developing PI-IBS among patients in our study with an exposure to
infectious gastroenteritis was 4.16 times higher than the patients
non-exposed (95% CI, 1.89-9.17).
Infectious etiology of gastroenteritis and the
prevalence of PI IBS, in a study from Nottingham in 1996, limited
only to gastroenteritis with Campylobacter, confirmed that
9% of the 189 infected individuals developed PI-IBS (22). However, in our subgroup of patients
with C. jejuni infection, the percentage was higher, 67%,
respectively, 2 patients in 3 had PI-IBS. Mearin et al
(41) prospectively evaluated the
evolution of dyspepsia and IBS in a cohort of adult patients
affected by a Salmonella enteritidis epidemic one year after
the acute gastroenteritis episode and observed that intestinal
salmonellosis is a significant risk factor not only for IBS but
also for dyspepsia; so 1 out of 7, and 1 out of 10, subjects
developed dyspepsia, respectively, IBS. In our case, 3 out of 8
patients were diagnosed with IBS after intestinal Salmonella
infection. The risk rate to develop PI-IBS following an enteritis
with C. difficile was 52% in our study. Wadhwa et al
(42) observed in a transversal
study in which patients were contacted and evaluated by sampling
(by methods similar to those used in our study) demonstrated that
25% of patients with C. difficile infection without previous
irritable bowel syndrome developed PI-IBS; this incidence being
higher than the mean incidence of patients with PI-IBS due to
infection with other pathogens. Gastroenteritis due to viral
etiology is mostly associated with acute episodes of diarrhea and
with low risk of residual digestive symptoms. This may be
associated with a lower incidence of PI-IBS compared with infection
due to bacterial pathogens. In a viral gastroenteritis outbreak,
approximately a quarter of the patients reported symptoms of PI-IBS
3 months after the outbreak (13).
The results obtained in our study showed that the only patient
diagnosed with gastroenteritis with Rotavirus developed the
‘unsubtype-able’ form of PI-IBS. They is also an argument for the
prevention of enteral infection from the hospital admission, thus
avoiding subsequent development of long-term medical conditions
such as PI-IBS (43).
In conclusion, the risk of developing PI-IBS after
AGE infection is 4.16 higher than after URTI. The female sex is a
risk factor for PI-IBS, 79% of the female patients developed PI-IBS
after AGE. The incidence of PI-IBS is highest in patients with
Campylobacter jejuni AGE compared with the other agents.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from the Hospital of Infectious Diseases (Cluj-Napoca,
Romania); however, restrictions apply to the availability of these
data, which were used under license for the current study, and are
not publicly available. Data are however available from the authors
upon reasonable request and with the permission of the Hospital of
Infectious Diseases.
Authors' contributions
TI wrote the manuscript, provided the data for
Fig. 1 and
Tables I-V, conducted the patient interviews and performed all
statistical analyses. DLD conceived the study, participated in the
design and coordination of the study, and assisted with the
drafting of the manuscript. MSL and DFT participated in the design
of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the study involving
human participants were in accordance with the ethical standards of
the Institutional and/or National Research Committee (Committee
chiar - Felicia Loghin; members: Anca Buzoianu, Ioana Cristolțan,
Vasile Fluieraș; jurist - Luminița Gocan; reference no.
132/11.04.2014) and with the 1964 Helsinki Declaration and its
later amendments or comparable ethical standards. Informed consent
was obtained from all the patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Drossman DA and Hasler WL: Rome
IV-Functional GI Disorders: Disorders of gut-brain interaction.
Gastroenterology. 150:1257–1261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ericsson CD, Hatz C and DuPont AW:
Postinfectious irritable bowel syndrome. Clin Infect Dis.
46:594–599. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Thabane M, Kottachchi DT and Marshall JK:
Systematic review and meta-analysis: The incidence and prognosis of
post-infectious irritable bowel syndrome. Aliment Pharmacol Ther.
26:535–544. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thabane M and Marshall JK: Post-infectious
irritable bowel syndrome. World J Gastroenterol. 15:3591–3596.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sethi S, Garey KW, Arora V, Ghantoji S,
Rowan P, Smolensky M and DuPont HL: Increased rate of irritable
bowel syndrome and functional gastrointestinal disorders after
Clostridium difficile infection. J Hosp Infect. 77:172–173.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stewart GT: Post-dysenteric colitis. BMJ.
1:405–409. 1950.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim HS, Kim MS, Ji SW and Park H: The
development of irritable bowel syndrome after Shigella
infection: 3 year follow-up study. Korean J Gastroenterol.
47:300–305. 2006.PubMed/NCBI(In Korean).
|
|
8
|
Ilnyckyj A, Balachandra B, Elliott L,
Choudhri S and Duerksen DR: Post-traveler's diarrhea irritable
bowel syndrome: A prospective study. Am J Gastroenterol.
98:596–599. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rodríguez LA and Ruigómez A: Increased
risk of irritable bowel syndrome after bacterial gastroenteritis:
Cohort study. BMJ. 318:565–566. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ruigómez A, García Rodríguez LA and Panés
J: Risk of irritable bowel syndrome after an episode of bacterial
gastroenteritis in general practice: Influence of comorbidities.
Clin Gastroenterol Hepatol. 5:465–469. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Soyturk M, Akpinar H, Gurler O, Pozio E,
Sari I, Akar S, Akarsu M, Birlik M, Onen F and Akkoc N: Irritable
bowel syndrome in persons who acquired trichinellosis. Am J
Gastroenterol. 102:1064–1069. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Piche T, Vanbiervliet G, Pipau FG, Dainese
R, Hébuterne X, Rampal P and Collins SM: Low risk of irritable
bowel syndrome after Clostridium difficile infection. Can J
Gastroenterol. 21:727–731. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marshall JK, Thabane M, Borgaonkar MR and
James C: Postinfectious irritable bowel syndrome after a food-borne
outbreak of acute gastroenteritis attributed to a viral pathogen.
Clin Gastroenterol Hepatol. 5:457–460. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marshall JK, Thabane M, Garg AX, Clark WF,
Salvadori M and Collins SM: Walkerton Health Study Investigators.
Incidence and epidemiology of irritable bowel syndrome after a
large waterborne outbreak of bacterial dysentery. Gastroenterology.
131:445–450; quiz 660. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang LH, Fang XC and Pan GZ: Bacillary
dysentery as a causative factor of irritable bowel syndrome and its
pathogenesis. Gut. 53:1096–1101. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Neal KR, Hebden J and Spiller R:
Prevalence of gastrointestinal symptoms six months after bacterial
gastroenteritis and risk factors for development of the irritable
bowel syndrome: Postal survey of patients. BMJ. 314:779–782.
1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Borgaonkar MR, Ford DC, Marshall JK,
Churchill E and Collins SM: The incidence of irritable bowel
syndrome among community subjects with previous acute enteric
infection. Dig Dis Sci. 51:1026–1032. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gwee KA, Leong YL, Graham C, McKendrick
MW, Collins SM, Walters SJ, Underwood JE and Read NW: The role of
psychological and biological factors in postinfective gut
dysfunction. Gut. 44:400–406. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ji S, Park H, Lee D, Song YK, Choi JP and
Lee SI: Post-infectious irritable bowel syndrome in patients with
Shigella infection. J Gastroenterol Hepatol. 20:381–386.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McKendrick MW and Read NW: Irritable bowel
syndrome - post Salmonella infection. J Infect. 29:1–3.
1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mearin F, Badía X, Balboa A, Baró E,
Caldwell E, Cucala M, Díaz-Rubio M, Fueyo A, Ponce J, Roset M, et
al: Irritable bowel syndrome prevalence varies enormously depending
on the employed diagnostic criteria: Comparison of Rome II versus
previous criteria in a general population. Scand J Gastroenterol.
36:1155–1161. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gwee KA, Graham JC, McKendrick MW, Collins
SM, Marshall JS, Walters SJ and Read NW: Psychometric scores and
persistence of irritable bowel after infectious diarrhoea. Lancet.
347:150–153. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tuteja AK, Talley NJ, Gelman SS, Alder SC,
Thompson C, Tolman K and Hale DC: Development of functional
diarrhea, constipation, irritable bowel syndrome, and dyspepsia
during and after traveling outside the USA. Dig Dis Sci.
53:271–276. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Spence MJ and Moss-Morris R: The cognitive
behavioural model of irritable bowel syndrome: A prospective
investigation of patients with gastroenteritis. Gut. 56:1066–1071.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei X, Chen M and Wang J: The epidemiology
of irritable bowel syndrome and functional constipation of
Guangzhou residents. Zhonghua Nei Ke Za Zhi. 40:517–520.
2001.PubMed/NCBI(In Chinese).
|
|
26
|
Spiller RC: Postinfectious irritable bowel
syndrome. Gastroenterology. 124:1662–1671. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moss-Morris R and Spence M: To ‘lump’ or
to ‘split’ the functional somatic syndromes: Can infectious and
emotional risk factors differentiate between the onset of chronic
fatigue syndrome and irritable bowel syndrome? Psychosom Med.
68:463–469. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parry SD, Stansfield R, Jelley D, Gregory
W, Phillips E, Barton JR and Welfare MR: Does bacterial
gastroenteritis predispose people to functional gastrointestinal
disorders? A prospective, community-based, case-control study. Am J
Gastroenterol. 98:1970–1975. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gupta A and Khanna S: Community-acquired
Clostridium difficile infection: An increasing public health
threat. Infect Drug Resist. 7:63–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gutiérrez RL, Riddle MS and Porter CK:
Increased risk of functional gastrointestinal sequelae after
Clostridium difficile infection among active duty United
States military personnel (1998-2010). Gastroenterology.
149:1408–1414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Walker ARP and Segal I: Epidemiology of
noninfective intestinal diseases in various ethnic groups in South
Africa. Isr J Med Sci. 15:309–313. 1979.PubMed/NCBI
|
|
32
|
Buéno L, Fioramonti J and Garcia-Villar R:
Pathobiology of visceral pain: molecular mechanisms and therapeutic
implications. III. Visceral afferent pathways: a source of new
therapeutic targets for abdominal pain. Am J Physiol Gastrointest
Liver Physiol. 278:G670–G676. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Neal KR, Barker L and Spiller RC:
Prognosis in post-infective irritable bowel syndrome: A six year
follow up study. Gut. 51:410–413. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rohde CL, Bartolini V and Jones N: The use
of probiotics in the prevention and treatment of
antibiotic-associated diarrhea with special interest in
Clostridium difficile-associated diarrhea. Nutr Clin Pract.
24:33–40. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shukla G, Devi P and Sehgal R: Effect of
Lactobacillus casei as a probiotic on modulation of
giardiasis. Dig Dis Sci. 53:2671–2679. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Resta-Lenert S and Barrett KE: Live
probiotics protect intestinal epithelial cells from the effects of
infection with enteroinvasive Escherichia coli (EIEC). Gut.
52:988–997. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Drossman DA and Dumitrascu DL: Rome III:
New standard for functional gastrointestinal disorders. J
Gastrointestin Liver Dis. 15:237–241. 2006.PubMed/NCBI
|
|
38
|
Lewis SJ and Heaton KW: Stool form scale
as a useful guide to intestinal transit time. Scand J
Gastroenterol. 32:920–924. 1997.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Klem F, Wadhwa A, Prokop LJ, Sundt WJ,
Farrugia G, Camilleri M, Singh S and Grover M: Prevalence, risk
factors, and outcomes of irritable bowel syndrome after infectious
enteritis: A systematic review and meta-analysis. Gastroenterology.
152:1042–1054.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Litleskare S, Rortveit G, Eide GE, Hanevik
K, Langeland N and Wensaas KA: Prevalence of irritable bowel
syndrome and chronic fatigue 10 years after Giardia
infection. Clin Gastroenterol Hepatol. 16:1064–1072.e4.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mearin F, Pérez-Oliveras M, Perelló A,
Vinyet J, Ibañez A, Coderch J and Perona M: Dyspepsia and irritable
bowel syndrome after a Salmonella gastroenteritis outbreak:
One-year follow-up cohort study. Gastroenterology. 129:98–104.
2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wadhwa A, Al Nahhas MF, Dierkhising RA,
Patel R, Kashyap P, Pardi DS, Khanna S and Grover M: High risk of
post-infectious irritable bowel syndrome in patients with
Clostridium difficile infection. Aliment Pharmacol Ther.
44:576–582. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peterson LR, O'Grady S, Keegan M, Fisher
A, Zelencik S, Kufner B, Shah M, Lim R, Schora D, Das S, et al:
Reduced Clostridioides difficile infection in a pragmatic
stepped-wedge initiative using admission surveillance to detect
colonization. PLoS One. 15(e0230475)2020.PubMed/NCBI View Article : Google Scholar
|