Introduction
Cerebrovascular diseases are one of the major health
problems in the world (1). According
to Love and Miners (2), there were
~1.6 million patients with the diseases worldwide in 2016, most of
whom were middle-aged and elderly. According to Kamat et al
(3), increasing number of young
people are developing the diseases, so it is predicted that young
and middle-aged patients will account for 35% of patients of all
ages by 2030. Cerebrovascular diseases are extremely sudden, so
many patients become disabled and die due to untimely rescue
(4). These diseases are a research
hotspot in clinical practice because of their high incidences and
mortality. Main diseases among cerebrovascular diseases are
cerebral ischemic disease with the infarction of middle cerebral
artery or branch artery, accounting for ~75% of all patients
(5). Therefore, the timely and
effective opening of occluded vessels as well as the recovery of
cerebral tissue perfusion are crucial to treat acute intracranial
arterial occlusion (AIAO). According to Powers et al
(6), intravenous thrombolysis (IVT)
is the first choice for the treatment of AIAO, but its therapeutic
effect is unsatisfactory due to its low recanalization rate.
Therefore, finding an effective therapeutic method for AIAO is very
important.
Vascular intervention is the product of the
iterative design and development process of evolving concepts in
vascular biology and engineering (7). According to the study by Rogers and
Edelman, the delivery of drugs near the implanted device may make
the drugs exert their best effects (8). IVT, recommended by guidelines
worldwide, is the first choice for the treatment of AIAO, but
whether its combination with vascular intervention can treat the
disease still need further study. Therefore, the efficacy and
safety of the combination in the treatment of AIAO were explored in
the present study, to provide accurate basis for the future
treatment of the disease.
Patients and methods
Clinical data
In this retrospective study, 92 patients with AIAO
treated in People's Hospital of Tongchuan (Tongchuan, Chna) from
January 2014 to February 2016 were enrolled. Forty-two patients
were treated with vascular intervention (the control group),
including 29 males and 13 females, aged 35-70 years with an average
age of 52.6±10.3 years. Fifty patients were treated with vascular
intervention combined with IVT (study group), including 34 males
and 16 females, aged 36-72 years, with an average age of 53.1±10.6
years. The study was approved by the Ethics Committee of the
People’s Hospital of Tongchuan. Patients who participated in this
study signed an informed consent and had complete clinical
data.
Inclusion and exclusion criteria
Inclusion criteria
Patients diagnosed and treated in People's Hospital
of Tongchuan; patients with complete general information; patients
aged 35-75 years; patients with the educational background of
primary school or above; patients who cooperated in the
investigation; patients without intracranial hemorrhage detected by
head CT; patients who or whose immediate family members signed the
informed consent form.
Exclusion criteria
Patients who died during the treatment; patients
complicated with other tumors; patients with physical disability;
patients transferred to other hospitals; patients resistant or
allergic to the drugs used in this study; patients with a history
of intracranial hemorrhage including suspected subarachnoid
hemorrhage; patients with a history of head injury in the past 3
months; patients with mental disorders, language dysfunction, or
diseases affecting the results of this study.
Therapeutic schemes
Patients in the control group were treated with
vascular intervention. After placed in a supine position, the
patients were locally anesthetized and then intravenously injected
with heparin (2-3 mg/kg), once every 2 h (half of the last dose).
The lowest dose was maintained at 10 mg/h. The arterial sheath was
inserted through femoral artery using Seldinger method, and the 5F
catheter was used for brain angiography (iohexol was used as a
contrast agent). The angiography was repeatedly performed to select
the best work position. After the microcatheter was passed through
the occluded blood vessel using directed acyclic graph and under
the guidance of the micro-guide wire, the 4x15 mm Solitaire™ AB
stent [no. SFDA(I) 20133465291] was placed in the occluded artery
via the microcatheter and then released. After opened and
maintained for more than 10 sec, the stent was retracted together
with the microcatheter. The angiography after thrombectomy was
performed to check whether the blood vessel was unobstructed, and
multiple thrombectomy was conducted if necessary. The sheath was
pulled out at 6 h after the operation. Patients in the study group
were treated with vascular intervention combined with IVT. The
steps of vascular intervention were as above, and alteplase
(Boehringer Ingelheim Pharma GmbH & Co. KG, item no.
RK20180329n) and isotonic saline (100 IU+100 ml in total) were
intravenously dripped for 30 min. Changes in blood pressure and
heart rate during the treatment were closely monitored.
Scoring criteria
The National Institute of Health Stroke Scale
(NIHSS) was used for scoring (9). At
3 months after treatment, the modified Rankin Scale (mRS) score was
used to assess the neurological function recovery of the patients
to assess their prognoses (10). The
Mini-Mental State Examination (MMSE) score was used to assess
patients' cognitive function before treatment and 3 months after
treatment.
Efficacy evaluation
Markedly effective outcome: symptoms disappeared and
the patient's nerve function returned to normal. Effective outcome:
Symptoms disappeared and the patient's nerve function improved. No
effect, no improvement in the above indicators.
Follow-up
The patients were followed up for 3 months after
operation, and their adverse reactions were recorded by telephone
and outpatient medical records.
Outcome measures
Main outcome measures: The improvement of clinical
efficacy after treatment was observed. Comparison of complications
after treatment was carried out. Secondary outcome measures: The
NIHSS score after treatment was recorded. The mRS score at 3 months
after treatment and the MMSE score at 3 months after treatment were
recorded.
Statistical analysis
In this study, SPSS 20.0 was used to statistically
analyze the collected data. GraphPad 7 was used to plot figures.
K-S test was used to analyze the distribution of measurement data.
The data that conformed to normal distribution were expressed as
mean ± standard deviation (mean ± SD), analyzed by parametric
tests, and represented by Z. The comparison between groups was
conducted by independent samples t-test, and the comparison within
groups was conducted by paired t-test. Count data were expressed by
rate (%), analyzed by Chi-square test, and represented by
χ2. P<0.05, was accepted as a statistically
significant difference.
Results
Clinical data
There was no statistically significant difference
between the study and control groups in age, sex, body mass index
(BMI), marital status, ethnicity, place of residence, smoking,
NIHSS score (points) at admission, and MMSE score (points) at
admission, which indicated comparability (P>0.05) (Table I).
 | Table IClinical data [n (%), mean ± SD]. |
Table I
Clinical data [n (%), mean ± SD].
| Features | Study group
(n=50) | Control group
(n=42) | χ2 or t
value | P-value |
|---|
| Age (years) | 53.1±10.6 | 52.6±10.3 | 0.228 | 0.820 |
| Sex | | | 0.012 | 0.914 |
|
Male | 34 (68.00) | 29 (69.05) | | |
|
Female | 16 (32.00) | 13 (30.95) | | |
| BMI
(kg/m2) | 22.26±0.37 | 22.21±0.25 | 0.744 | 0.459 |
| Marital status | | | 0.068 | 0.794 |
|
Married | 47 (94.00) | 40 (95.24) | | |
|
Unmarried | 3 (6.00) | 2 (4.75) | | |
| Ethnicity | | | 0.076 | 0.782 |
|
Han | 37 (74.00) | 30 (71.43) | | |
|
Ethnic
minorities | 13 (26.00) | 12 (28.57) | | |
| Place of
residence | | | 0.007 | 0.934 |
|
City | 29 (58.00) | 24 (57.14) | | |
|
Countryside | 21 (42.00) | 18 (42.86) | | |
| History of
smoking | | | 0.024 | 0.877 |
|
Yes | 27 (54.00) | 22 (52.38) | | |
|
No | 23 (46.00) | 20 (47.62) | | |
| NIHSS score
(points) | 13.03±4.21 | 13.01±3.08 | 0.026 | 0.980 |
| MMSE score
(points) | 18.01±4.02 | 18.03±3.01 | 0.027 | 0.979 |
Clinical efficacy
Compared with those in the control group, patients
in the study group had statistically significantly higher marked
effectiveness and statistically significantly lower ineffectiveness
(P=0.018), without statistically significant difference in
effectiveness between the two groups (P=0.224). The overall
effective rate in the study group was statistically significantly
higher than that in the control group (P=0.042) (Table II).
 | Table IIEfficacy. |
Table II
Efficacy.
| | Efficacy [n (%)] | |
|---|
| Groups | No. of cases | Markedly
effective | Effective | Ineffective | Effective rate
(%) |
|---|
| Study group | 50 | 37 (74.00) | 11 (22.00) | 2 (4.00) | 96.00 |
| Control group | 42 | 21 (50.00) | 14 (33.33) | 7 (16.67) | 83.00 |
| χ2
value | | 5.643 | 1.482 | 4.150 | 4.150 |
| P-value | | 0.018 | 0.224 | 0.042 | 0.042 |
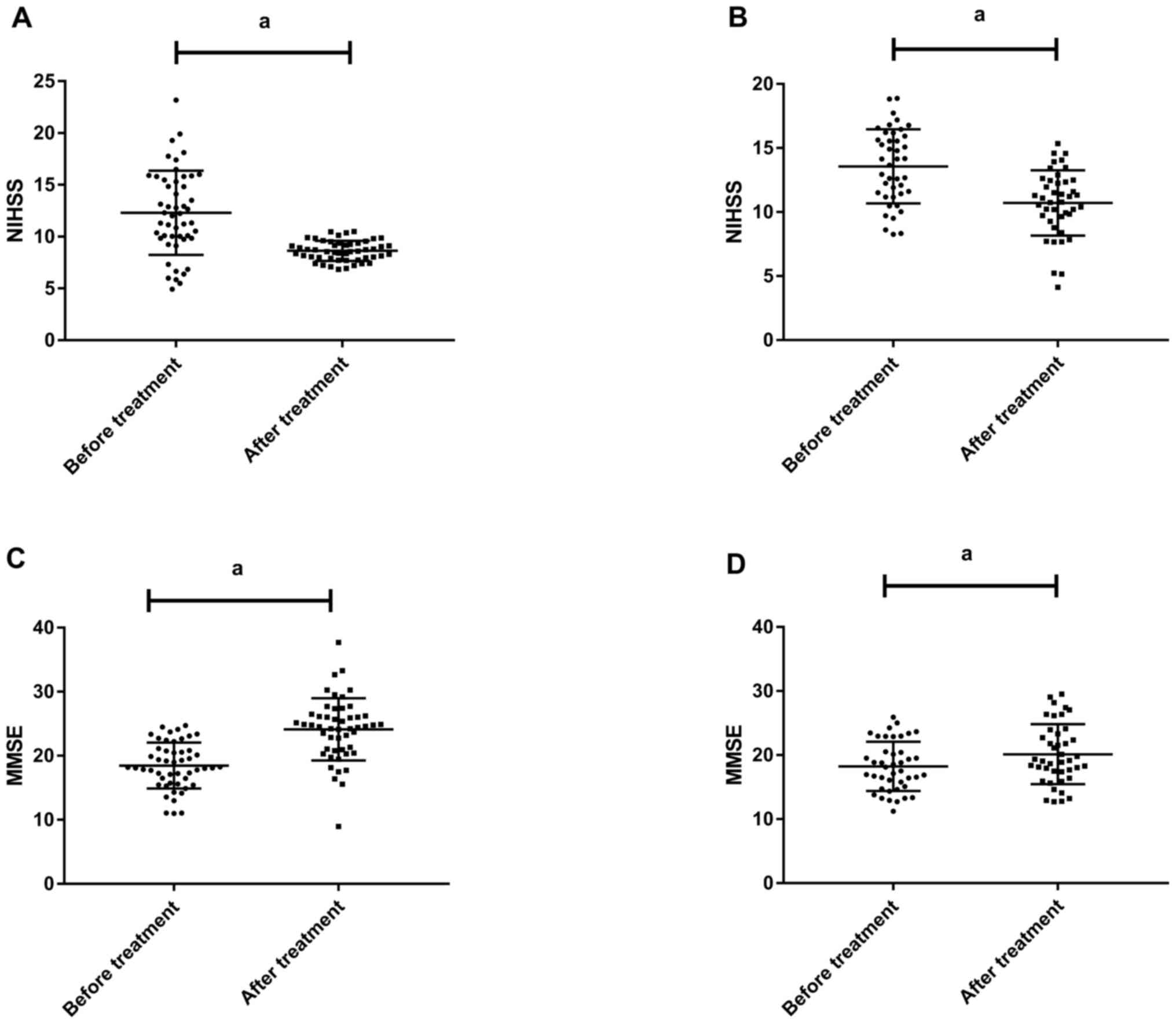
NIHSS score after treatment
Before treatment, the NIHSS score was 13.03±4.21
points in the study group and 13.01±3.08 points in the control
group. After treatment, the score was 9.01±1.22 points in the study
group and 10.85±2.71 points in the control group. There was no
statistically significant difference between the two groups in the
score before treatment (P=0.980). After treatment, the score in the
study group was statistically significantly lower than that in the
control group (P=0.001) (Fig.
1).
mRS score at 3 months after
treatment
The patients were followed up at 3 months after
treatment. There was a statistically significant difference between
the study and control groups in the mRS score at 3 months after
treatment (Z=8.764, P>0.05) (Table
III).
 | Table IIIComparison of the mRS score after
treatment. |
Table III
Comparison of the mRS score after
treatment.
| Groups | No. of cases | 0 point | 1 point | 2 points | 3 points | 4 points | 5 points | 6 points |
|---|
| Study group | 50 | 0 | 37 | 10 | 1 | 2 | 0 | 0 |
| Control group | 42 | 0 | 21 | 6 | 5 | 3 | 7 | 0 |
MMSE score at 3 months after
treatment
Before treatment, the MMSE score was 18.01±4.02
points in the study group and 18.03±3.01 points in the control
group. After treatment, the score was 24.15±5.03 points in the
study group and 20.35±5.12 points in the control group. There was
no statistically significant difference between the two groups in
the score before treatment (P=0.179). After treatment, the score in
the study group was statistically significantly higher than that in
the control group (P=0.001) (Fig.
1).
Complications after treatment
After treatment, the study group had 1 case of
intracranial hypertension, 2 cases of dysphagia, 1 case of urinary
incontinence, and 1 case of abnormal body temperature, while the
control group had 3 cases of intracranial hypertension, 4 cases of
dysphagia, 3 cases of urinary incontinence, and 2 cases of abnormal
body temperature. The total incidence of postoperative
complications in the study group was statistically significantly
lower than that in the control group (P=0.022) (Table IV).
 | Table IVComplications after treatment [n
(%)]. |
Table IV
Complications after treatment [n
(%)].
| Complications | Study group
(n=50) | Control group
(n=42) | χ2
value | P-value |
|---|
| Intracranial
hypertension | 1 (2.00) | 3 (7.14) | | |
| Pneumonia | 0 (0.00) | 0 (0.00) | | |
| Dysphagia | 2 (4.00) | 4 (9.52) | | |
| Urinary
incontinence | 1 (2.00) | 3 (7.14) | | |
| Acute renal
failure | 0 (0.00) | 0 (0.00) | | |
| Cardiac damage | 0 (0.00) | 0 (0.00) | | |
| Abnormal body
temperature | 1 (2.00) | 2 (4.76) | | |
| Total | 5 (10.00) | 12 (28.56) | 5.226 | 0.022 |
Discussion
The incidence of cerebrovascular diseases among the
elderly is the highest, however, the pathophysiological mechanism
of brain responding to cerebral ischemia in the elderly is still
poorly understood (11). Old age is
a major risk factor for the diseases and is associated with their
increasing incidence and mortality (12,13). In
most developed countries, ~87% of strokes are ischemic and
originate from intracranial arterial occlusion (IAO), being the
major cause of patient death and adult disability (14,15).
Therefore, it is urgent for clinicians to improve the condition of
patients with IAO.
The pathogenesis of IAO is based on atherosclerotic
plaque formation, Takayasu's arteritis, and vascular fibromuscular
dysplasia (16). At present,
patients with IAO are mainly treated by IVT and vascular
intervention (17-19).
According to Bracard et al (20), IVT with alteplase (a commonly used
thrombolytic drug in clinical practice) alone cannot achieve the
best therapeutic effect. Therefore, mechanical thrombectomy and IVT
are used for the treatment to improve the patients' functional
independence and to reduce their mortality. In this study, patients
with AIAO were treated with vascular intervention combined with
IVT, and the improvement of clinical efficacy and the safety of
treatment were observed, to provide reference for the clinical
treatment.
In this study, the clinical efficacy after treatment
was first compared between the two groups. The results showed that
compared with those in the control group, patients in the study
group had statistically significantly higher marked effectiveness,
statistically significantly lower ineffectiveness, and a
statistically significantly higher overall effective rate. This
shows that vascular intervention combined with IVT can increase the
effective rate of treatment. NIHSS, one of the scales for
evaluating the neurological function of patients with
cerebrovascular diseases, can reflect the patients' neurological
impairment and accurately determine their prognoses (21). There are currently few comparative
studies on scoring systems for evaluating the severity and
prognosis of neurological impairment in the patients. In our study,
before treatment, there was no statistically significant difference
between the two groups in the NIHSS score, while the score in the
study group was statistically significantly lower than that in the
control group after treatment. This suggests that vascular
intervention combined with IVT can reduce the patients'
neurological deficits. The mRS can measure patients' neurological
function recovery (10), and
psychological and physical factors affecting the recovery are
included. The scale includes 6 grades (from 0 to 5), with 0
indicating no symptom and 5 points indicating severe disability.
The patients in this study were followed up at 3 months after
treatment, and the mRS score was used to judge the neurological
function recovery. The mRS score in the study group was
statistically significantly better than that in the control group.
Additionally, there was no difference between the two groups in the
MMSE score before treatment, but the score in the study group was
higher than that in the control group after treatment. This reveals
that vascular intervention combined with IVT can better improve the
prognosis than IVT alone. Finally, the complications at 3 months
after treatment were observed. Patients in the two groups had no
pneumonia, acute renal failure, or cardiac damage, while the
incidence rate of intracranial hypertension, dysphagia, urinary
incontinence, and abnormal body temperature in the study group was
lower than those in the control group; the total incidence of
complications in the study group was also lower than that in the
control group. This well illustrates the prognostic effect and
safety of vascular intervention combined with IVT.
In the present study, through the above research,
the clinical efficacy of vascular intervention combined with IVT in
the treatment of patients with AIAO was preliminarily proven.
However, there are still limitations. Firstly, whether the
treatment rate can be increased by changing the dosage was not
fully investigated. Secondly, rat experiments and long-term
follow-up were not conducted. We will carry out more in-depth
experimental analysis and long-term follow-up as soon as
possible.
In conclusion, vascular intervention combined with
IVT has good efficacy and high safety in the treatment of AIAO, and
the combination can statistically significantly improve the
patients' quality of life, so it has a good clinical application
value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ wrote the manuscript. DZ and QW conceived and
designed the study. QW and WZ were responsible for the collection
and analysis of the experimental data. CL and LX interpreted the
data and drafted the manuscript. SL was responsible for the
efficacy evaluation and the patients’ follow-up. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the People's Hospital of Tongchuan (Tongchuan, China). Patients who
participated in this study signed an informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP,
Fullerton HJ, et al: Writing Group Members; American Heart
Association Statistics Committee; Stroke Statistics Subcommittee:
Heart disease and stroke statistics-2016 update: A report from the
American Heart Association. Circulation.
133(e38-e360)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Love S and Miners JS: Cerebrovascular
disease in ageing and Alzheimer's disease. Acta Neuropathol.
131:645–658. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kamat PK, Vacek JC, Kalani A and Tyagi N:
Homocysteine induced cerebrovascular dysfunction: A link to
Alzheimer's disease etiology. Open Neurol J. 9:9–14.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lahousse L, Tiemeier H, Ikram MA and
Brusselle GG: Chronic obstructive pulmonary disease and
cerebrovascular disease: A comprehensive review. Respir Med.
109:1371–1380. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Drandley TD and Floras JS (eds): Sleep
Apnea: Implications in Cardiovascular and Cerebrovascular Disease.
CRC Press, Florida. 2016.
|
|
6
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: American Heart Association Stroke Council. 2018
Guidelines for the early management of patients with acute ischemic
stroke: a guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke.
49(e46-e110)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nakamura K, Keating JH and Edelman ER:
Pathology of endovascular stents. Interv Cardiol Clin. 5:391–403.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rogers C and Edelman ER: Endovascular
stent design dictates experimental restenosis and thrombosis.
Circulation. 91:2995–3001. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ortiz GA and Sacco R: National Institutes
of Health Stroke Scale (nihss). Wiley Statistics Reference Online.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sulter G, Steen C and De Keyser J: Use of
the Barthel index and modified Rankin scale in acute stroke trials.
Stroke. 30:1538–1541. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Di Napoli M and Shah IM: Neuroinflammation
and cerebrovascular disease in old age: A translational medicine
perspective. J Aging Res. 2011(857484)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Modrego PJ, Pina MA and Lerín FJ: The
impact of ageing on stroke subtypes, length of stay and mortality:
Study in the province of Teruel, Spain. Acta Neurol Scand.
108:435–442. 2003. View Article : Google Scholar
|
|
13
|
Saposnik G, Cote R, Phillips S, Gubitz G,
Bayer N, Minuk J and Black S: Stroke Outcome Research Canada
(SORCan) Working Group. Stroke outcome in those over 80: A
multicenter cohort study across Canada. Stroke. 39:2310–2317.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feigin VL: Stroke epidemiology in the
developing world. Lancet. 365:2160–2161. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feigin V and Hoorn SV: How to study stroke
incidence. Lancet. 363:1920–1921. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
DeBaun MR, Armstrong FD, McKinstry RC,
Ware RE, Vichinsky E and Kirkham FJ: Silent cerebral infarcts: A
review on a prevalent and progressive cause of neurologic injury in
sickle cell anemia. Blood. 119:4587–4596. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Emberson J, Lees KR, Lyden P, Blackwell L,
Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al:
Stroke Thrombolysis Trialists' Collaborative Group. Effect of
treatment delay, age, and stroke severity on the effects of
intravenous thrombolysis with alteplase for acute ischaemic stroke:
A meta-analysis of individual patient data from randomised trials.
Lancet. 384:1929–1935. 2014. View Article : Google Scholar
|
|
18
|
Guillan M, Alonso-Canovas A,
Garcia-Caldentey J, Sanchez- Gonzalez V, Hernandez-Medrano I,
Defelipe-Mimbrera A, Matute MC, Alonso-Arias MA, Alonso de Leciñana
M and Masjuan J: Off-label intravenous thrombolysis in acute
stroke. Eur J Neurol. 19:390–394. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Göthe F, Enache D, Wahlund LO, Winblad B,
Crisby M, Lökk J and Aarsland D: Cerebrovascular diseases and
depression: Epidemiology, mechanisms and treatment. Panminerva Med.
54:161–170. 2012.PubMed/NCBI
|
|
20
|
Bracard S, Ducrocq X, Mas JL, Soudant M,
Oppenheim C, Moulin T and Guillemin F: THRACE investigators.
Mechanical thrombectomy after intravenous alteplase versus
alteplase alone after stroke (THRACE): A randomised controlled
trial. Lancet Neurol. 15:1138–1147. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goldstein LB, Bertels C and Davis JN:
Interrater reliability of the NIH stroke scale. Arch Neurol.
46:660–662. 1989.PubMed/NCBI View Article : Google Scholar
|