Introduction
As a new minimally invasive treatment,
interventional therapy has been widely used in the treatment of
various diseases (1,2), including cardiovascular disease
(3,4). Among them, femoral artery puncture is a
common approach for cardiovascular disease diagnosis and treatment
(5). However, the complications
related to femoral artery puncture receive little attention,
especially retroperitoneal hematoma (RPH) (6). RPH is an extremely rare, but the most
serious and potentially fatal complication of percutaneous
intervention (7-10).
Recently, with the increasing use of interventional therapy, the
morbidity and mortality caused by RPH continues to rise (11). As described in a number of previous
reports, the incidence of RPH ranged from 0.15 to 6% in the world
(10,12,13) and
the mortality rate reached 4% (14).
Generally, because the retroperitoneal space could
accommodate large amounts of blood, the early clinical
manifestations of RPH are relatively indistinct until hypovolemia
occurs (15). Therefore, RPH is
often delayed in diagnosis, thus increasing the risk of mortality
and leading to a potentially fatal outcome. To date, clinical
characteristics of RPH after percutaneous intervention have not
been systematically reported. Moreover, the optimal treatment of
patients with RPH after intervention has not been well defined.
Though the characteristics, management, and outcomes of RPH have
been previously reported (16),
little evidence has focused on RPH after percutaneous intervention.
Most related publications were single case reports or small
case-based series, which only reported RPH after percutaneous
coronary intervention (PCI) (7,10).
Moreover, the above studies were reported several years ago and few
Chinese cases were studied. Therefore, it is essential to describe
and summarize the clinical characteristics of RPH for its diagnosis
in China.
A retrospective cross-sectional study of patients
with RPH in the past 12 years was conducted at the China National
Center for Cardiovascular Diseases. The incidence, clinical
characteristics and management of patients with RPH after various
percutaneous interventions were analyzed. The potential risk
factors of RPH were also explored.
Materials and methods
Study population
The retrospective cross-sectional study was
performed at the China National Center for Cardiovascular Diseases
(Beijing, China). A total of 42 patients with RPH after various
interventional therapies were recruited from January 2007 to
September 2018. Clinical data were retrospectively assessed by
searching hospital diagnosis records and surgical and imaging
databases. All patients were diagnosed by imaging and followed up
after 1 year to evaluate the outcomes. The research was approved by
the Ethics Committee of Fuwai Hospital, National Center for
Cardiovascular Diseases, Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China). All written consents
from patients were waived due to the retrospective nature of the
current study.
All patients with RPH were divided into three groups
based on methods of interventional therapy. The groups were as
follows: PCI group, peripheral artery intervention (PAI) group and
other methods (OM) group. OM included atrial fibrillation ablation
(AFA), percutaneous transluminal septal myocardial ablation
(PTSMA), atrial septal defect closure (ASDC), intra-aortic balloon
pump (IABP) and coronary angiography (CA).
Data collection
Detailed characteristics and clinical data of all
patients were retrospectively collected from medical records,
imaging, diagnosis records and surgical records. The recorded data
included patient demographics, admission time, history of disease,
methods of interventional therapy, laboratory values, clinical
presentation, medical and surgical management, in-hospital clinical
events and prognosis.
Baseline data, including age, sex, weight, height,
body mass index (BMI), history of disease, intervention methods and
puncture site were analyzed to evaluate the relationship between
patients' characteristics and RPH occurrence and to explore the
risk factors of RPH occurrence. Laboratory values, including
creatinine levels, ejection fraction (EF), average heart rate,
blood pressure and hemoglobin (HB) concentration were collected
from diagnosis records to evaluate the renal and cardiac function,
and other functional indicators. Among them, the lowest HB
concentration was defined as the lowest detection value from
multiple re-examinations of whole blood cell counting during
hospitalization. Clinical presentation information was collected
for analysis of typical RPH symptoms. The time course of RPH,
patient managements and outcome were collected to investigate the
optimal approach for RPH diagnosis and treatment.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc.). Quantitative data are presented as the mean
± SD. Comparisons of quantitative data among three groups were
performed by one-way ANOVA followed by LSD test. Qualitative data
were presented as numbers or percentages and compared by
χ2 or Mann-Whitney U test, as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Distribution of patients
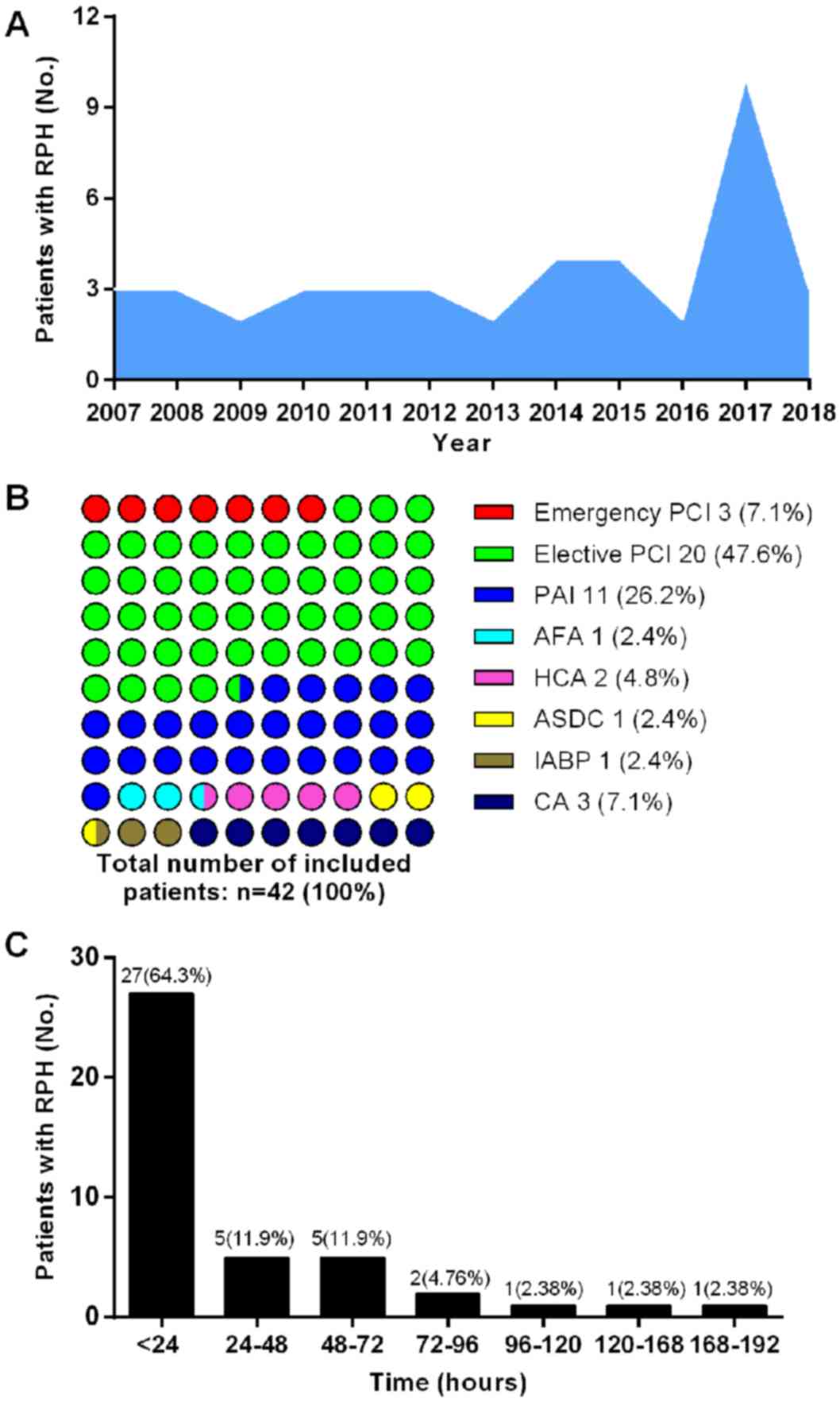
A total of 42 patients were enrolled in the
cross-sectional study between 2007 and 2019. During three of those
12 years, relatively more patients were enrolled, 10 of which were
recruited in 2017, 4 in 2014 and 4 in 2015 (Fig. 1A). The number of patients with RPH
varied with the type of intervention. As shown in Fig. 1B, RPH occurred in 23 patients after
PCI, accounting for 54.7% of all patients. Among them, three cases
(7.1%) received emergency PCI and 20 cases (47.6%) received
elective PCI. After PAI, 11 patients were diagnosed with RPH,
accounting for 26.2% of all cases. In addition, 8 patients (19.0%)
who underwent OM presented RPH complications (AFA, one case; PTSMA,
two cases; ASDC, one case; IABP, one case; CA, three cases).
Baseline demographics
Baseline demographics of patients are listed in
Table I.
 | Table IBaseline demographics of patients. |
Table I
Baseline demographics of patients.
| Index | Total (n=42) | PCI (n=23) | PAI (n=11) | OM (n=8) |
|---|
| Sex (n, %) | | | | |
|
Female | 23 (54.8) | 11 (47.8) | 7 (30.4) | 5 (21.7) |
|
Male | 19 (45.2) | 12 (63.2) | 4 (21.1) | 3 (21.1) |
| Age (years) | 63.1±2.5 | 64.3±2.4 | 59.9±8.0 | 64.6±7.8 |
| Height (cm) | 163.7±9.6 | 162.1±9.3 | 167.2±10.6 | 163.4±8.9 |
| Body weight (kg) | 66.2±1.6 | 66.5±2.4 | 65.1±2.7 | 66.6±3.0 |
| BMI
(kg/m2) | 24.6±0.5 | 25.0±0.7 | 23.4±1.0 | 25.0±0.8 |
| Smoking (n, %) | | | | |
|
No | 25 | 11 (44.0) | 8 (32.0) | 6 (24.0) |
|
Yes | 17 | 12 (70.6) | 3 (17.7) | 2 (11.8) |
| STEMI (n, %) | | | | |
|
No | 38 | 20 (52.6) | 11 (29.0) | 7 (18.4) |
|
Yes | 4 | 3 (75.0) | 0 (0.0) | 1 (25.0) |
| NSTEMI (n, %) | | | | |
|
No | 38 | 19 (50.0) | 11 (29.0) | 8 (21.0) |
|
Yes | 4 | 4 (100.0) | 0 (0.0) | 0 (0.0) |
| Angina pectoris (n,
%) | | | | |
|
No | 20 | 12 (60.0) | 6 (30.0) | 2 (10.0) |
|
Yes | 22 | 11 (50.0) | 5 (22.7) | 6 (27.3) |
| Hypertension (n,
%) | | | | |
|
No | 14 | 4 (28.6) | 7 (50.0) | 3 (21.4) |
|
Yes | 28 | 19 (67.9) | 4 (14.3) | 5 (17.9) |
| Diabetes (n,
%) | | | | |
|
No | 30 | 16 (53.3) | 8 (26.7) | 6 (20.0) |
|
Yes | 12 | 7 (58.3) | 3 (25.0) | 2 (16.7) |
| Hyperlipidemia (n,
%) | | | | |
|
No | 9 | 4 (44.4) | 3 (33.3) | 2 (22.2) |
|
Yes | 33 | 19 (57.6) | 8 (24.2) | 6 (18.2) |
| PAD (n, %) | | | | |
|
No | 27 | 16 (59.3) | 6 (22.2) | 5 (18.5) |
|
Yes | 15 | 7 (46.7) | 5 (33.3) | 3 (20.0) |
| Peripheral disease
(n, %) | | | | |
|
No | 27 | 16 (59.3) | 6 (22.2) | 5 (18.5) |
|
Yes | 15 | 7 (46.7) | 5 (33.3) | 3 (20.0) |
| OMI (n, %) | | | | |
|
No | 39 | 22 (56.4) | 10 (25.6) | 7 (18.0) |
|
Yes | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Renal insufficiency
(n, %) | | | | |
|
No | 39 | 22 (56.4) | 9 (23.1) | 8 (20.5) |
|
Yes | 3 | 1 (33.3) | 2 (66.7) | 0 (0.0) |
| Atrial fibrillation
(n, %) | | | | |
|
No | 40 | 22 (55.0) | 11 (27.5) | 7 (17.5) |
|
Yes | 2 | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| PCI postoperative
(n, %) | | | | |
|
No | 36 | 20 (55.6) | 9 (25.0) | 7 (19.4) |
|
Yes | 6 | 3 (50.0) | 2 (33.3) | 1 (16.7) |
| CABG postoperative
(n, %) | | | | |
|
No | 38 | 20 (52.6) | 11 (29.0) | 7 (18.4) |
|
Yes | 4 | 3 (75.0) | 0 (0.0) | 1 (25.0) |
| Hypothyroidism (n,
%) | | | | |
|
No | 41 | 22 (53.7) | 11 (26.8) | 8 (19.5) |
|
Yes | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| OCI (n, %) | | | | |
|
No | 38 | 19 (50.0) | 11 (28.9) | 8 (21.1) |
|
Yes | 4 | 4 (100.0) | 0 (0.0) | 0 (0.0) |
| Cardiogenic shock
(n, %) | | | | |
|
No | 41 | 22 (53.7) | 11 (26.8) | 8 (19.5) |
|
Yes | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Left main coronary
artery (n, %) | | | | |
|
No | 35 | 18 (51.4) | 9 (25.7) | 8 (22.9) |
|
Yes | 7 | 5 (71.4) | 2 (28.6) | 0 (0.0) |
| Cardiac functional
grading (n, %) | | | | |
|
I | 41 | 22 (53.7) | 11 (26.8) | 8 (19.5) |
|
IV | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Coronary artery
lesions (n, %) | | | | |
|
0 | 20 | 10 (50.0) | 7 (35.0) | 3 (15.0) |
|
1 | 4 | 3 (75.0) | 0 (0.0) | 1 (25.0) |
|
2 | 6 | 3 (50.0) | 2 (33.3) | 1 (16.7) |
|
3 | 12 | 7 (58.3) | 2 (16.7) | 3 (25.0) |
| Puncture site (n,
%) | | | | |
| Without
radiography | 16 | 9 (56.3) | 4 (25.0) | 3 (18.8) |
| Transradial
approach | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Above the inguinal
ligament | 5 | 2 (40.0) | 3 (60.0) | 0 (0.0) |
| Below the inguinal
ligament | 20 | 11 (55.0) | 4 (20.0) | 5 (25.0) |
| Creatinine
(µmol/l) | 77.7±4.0 | 74.6±4.5 | 84.5±11.3 | 77.5±5.9 |
| Ejection Fraction
(%) | 59.2±0.9 | 58.3±1.3 | 62.4±1.2 | 57.4±1.7 |
| Heart rate
(bpm) | 72.0±2.0 | 68.6±2.6 | 77.5±4.0 | 74.0±4.8 |
| SBP at admission
(mmHg) | 130.4±4.2 | 132.1±6.3 | 133.6±8.4 | 121.1±5.1 |
| DBP at admission
(mmHg) | 73.5±2.1 | 72.3±2.9 | 75.9±4.5 | 73.8±3.5 |
Epidemiology
All patients had a mean age of 63.1±2.5 years and a
BMI of 24.6±0.5 kg/m2, with 54.8% female patients (n=23)
and 45.2% male patients (n=19). The PCI group included 11 (47.8%)
females and 12 (52.2%) males, with a mean age of 64.3±2.4 years and
a BMI of 25.0±0.7 kg/m2. The PAI group comprised 7
(63.6%) females and 4 (36.4%) males. The mean age and BMI were
59.9±8.0 years and 23.4±1.0 kg/m2, respectively. For the
OM group, 8 patients (5 females and 3 males) showed a mean age of
64.6±7.8 years and a BMI of 25.0±0.8 kg/m2.
Medical history
Of the 42 patients with RPH, >50% patients had
angina pectoris (n=22; 52.4%), hypertension (n=28; 66.7%),
hyperlipidemia (n=33; 78.6%) and coronary artery lesions (n=22;
52.4%). Patients who underwent PCI were more likely to have angina
pectoris. Patients with ST-segment elevation or non-ST-segment
elevation myocardial infarction were more likely to receive
PCI.
Laboratory values
The mean value of creatinine levels in 42 patients
was 77.7±4.0 µmol/l. The values in three groups were 74.6±4.5
µmol/l (PCI group), 84.5±11.3 µmol/l (PAI group) and 77.5±5.9
µmol/l (OM group).
Data regarding cardiac function were available in
all patients with an overall ejection fraction of 59.2±0.9%. Mean
values were 58.3±1.3% (PCI group), 62.4±1.2% (PAI group) and
57.4±1.7% (OM group). The mean heart rate was 72.0±2.0 bpm, ranging
from 68.6 to 77.5 bpm between the three groups. Mean systolic and
diastolic pressure was 130.4±4.2 and 73.5±2.1 mmHg,
respectively.
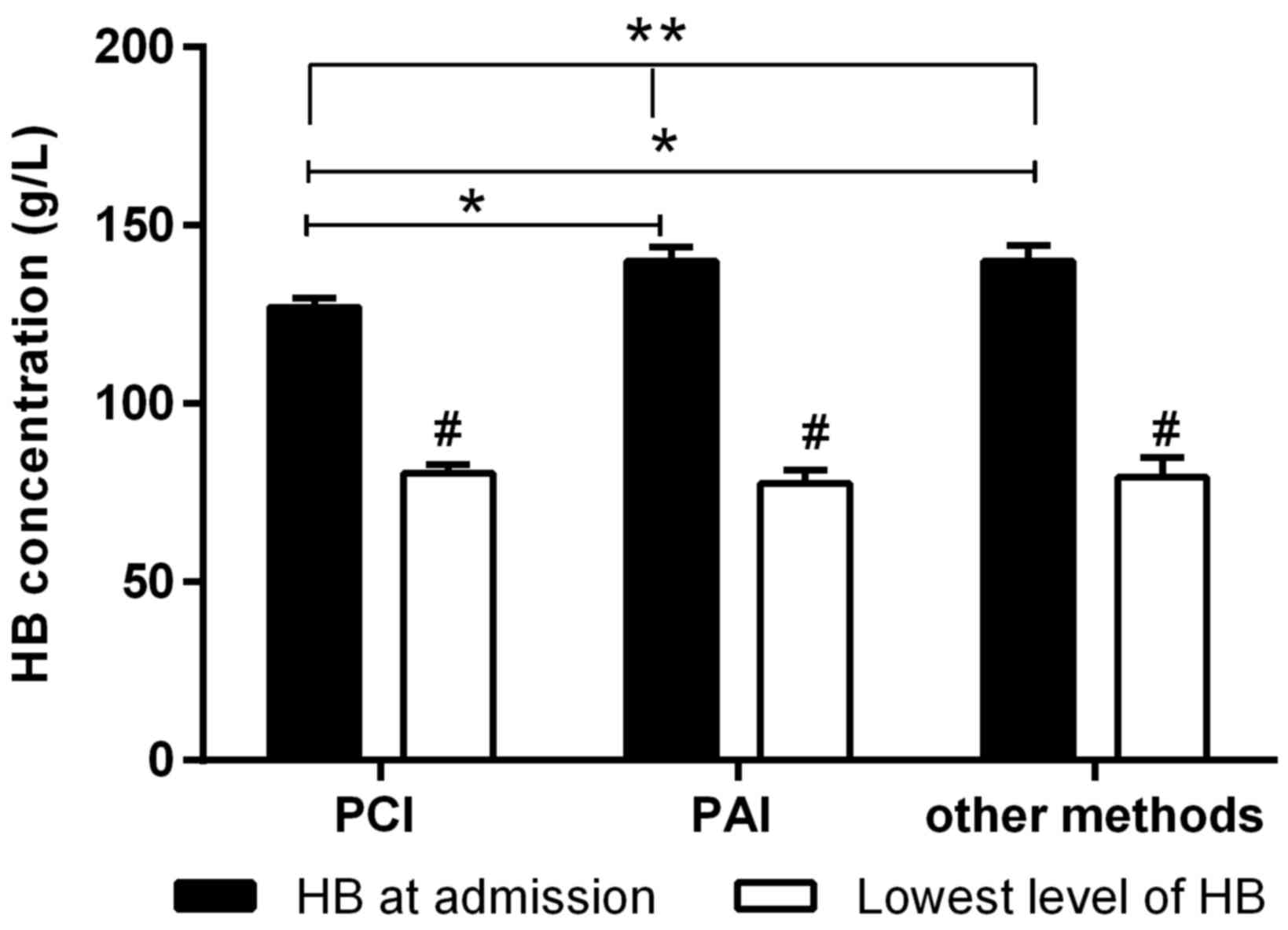
There was no significant difference in laboratory
values among the three groups (Table
I), except for HB concentration at admission (Fig. 2). HB concentration at admission in
the PCI group was significantly lower compared with PAI group
(P=0.009, Fig. 2) and OM group
(P=0.018, Fig. 2). However, no
significant difference in HB concentration at admission was
observed between the PAI and OM groups (P=0.856, Fig. 2). Notably, the lowest HB
concentrations in the three groups were all significantly decreased
(all P<0.001, Fig. 2) compared
with HB concentration at admission. No significant difference was
observed at the lowest HB concentrations among three groups.
Puncture site
Data regarding puncture site were available in 26
patients. A total of 20 cases had punctures in the femoral arterial
access under inguinal ligament (PCI, n=11; PAI, n=4; OM, n=5) and 5
cases had punctures above the inguinal ligament (PCI, n=2; PAI,
n=3). One case punctured through a transradial approach was
diagnosed with spontaneous RPH.
Time course of RPH and clinical
presentation
The time course of RPH is shown in Fig. 1C. RPH occurred in 27 (64.3%) patients
within 24 h after intervention, followed by 5 cases during
postoperative 24-48 h (11.9%) and five cases during postoperative
48-72 h (11.9%). Overall, ~40% patients presented RPH after
postoperative 24 h. The incidence of RPH decreased with the
prolongation of postoperative time.
As shown in Table
II, the most common symptom of RPH was pain (42.9%), with
abdominal pain recorded in 8 (19.0%) cases, followed by leg pain in
5 (11.9%) cases, waist pain in 4 (9.5%) cases and back pain in 1
(2.4%) case. Another primary symptom was blood pressure reduction,
which occurred in 12 (28.6%) cases. In addition, ~15% of patients
presented with sweating, HB reduction and nausea. Other
non-specific symptoms included unconsciousness (9.5%), dizziness
(7.1%) and fever (11.9%).
 | Table IISigns and symptoms presented by
patients. |
Table II
Signs and symptoms presented by
patients.
| Index | Total (n=42) | PCI (n=23) | PAI (n=11) | OM (n=8) |
|---|
| Leg pain (n,
%) | | | | |
|
No | 37 (88.1) | 21 (91.3) | 9 (81.8) | 7 (87.5) |
|
Yes | 5 (11.9) | 2 (8.7) | 2 (18.2) | 1 (12.5) |
| Waist pain (n,
%) | | | | |
|
No | 38 (90.5) | 20 (87.0) | 11 (100.0) | 7 (87.5) |
|
Yes | 4 (9.5) | 3 (13.0) | 0 (0.0) | 1 (12.5) |
| Abdominal pain (n,
%) | | | | |
|
No | 34 (81.0) | 18 (78.3) | 10 (90.9) | 6 (75.0) |
|
Yes | 8 (19.0) | 5 (21.7) | 1 (9.1) | 2 (25.0) |
| Back pain (n,
%) | | | | |
|
No | 41 (97.6) | 23 (100.0) | 10 (90.9) | 8 (100.0) |
|
Yes | 1 (2.4) | 0 (0.0) | 1 (9.1) | 0 (0.0) |
| Abdominal
distension (n, %) | | | | |
|
No | 38 (90.5) | 20 (87.0) | 10 (90.9) | 8 (100.0) |
|
Yes | 4 (9.5) | 3 (13.0) | 1 (9.1) | 0 (0.0) |
| Nausea | | | | |
|
No | 36 (85.7) | 20 (87.0) | 10 (90.9) | 6 (75.0) |
|
Yes | 6 (14.3) | 3 (13.0) | 1 (9.1) | 2 (25.0) |
| Sweating (n,
%) | | | | |
|
No | 35 (83.3) | 19 (82.6) | 10 (90.9) | 6 (75.0) |
|
Yes | 7 (16.7) | 4 (17.4) | 1 (9.1) | 2 (25.0) |
| Unconsciousness (n,
%) | | | | |
|
No | 38 (90.5) | 20 (87.0) | 10 (90.9) | 8 (100.0) |
|
Yes | 4 (9.5) | 3 (13.0) | 1 (9.1) | 0 (0.0) |
| Dizziness (n,
%) | | | | |
|
No | 39 (92.9) | 21 (91.3) | 10 (90.9) | 8 (100.0) |
|
Yes | 3 (7.1) | 2 (8.7) | 1 (9.1) | 0 (0.0) |
| Blood pressure
reduction (n, %) | | | | |
|
No | 30 (71.4) | 16 (69.6) | 7 (63.6) | 7 (87.5) |
|
Yes | 12 (28.6) | 7 (30.4) | 4 (36.4) | 1 (12.5) |
| HB reduction at
admission (n, %) | | | | |
|
No | 35 (83.3) | 19 (82.6) | 9 (81.8) | 7 (87.5) |
|
Yes | 7 (16.7) | 4 (17.4) | 2 (18.2) | 1 (12.5) |
| Fever (n, %) | | | | |
|
No | 37 (88.1) | 19 (82.6) | 10 (90.9) | 8 (100.0) |
|
Yes | 5 (11.9) | 4 (17.4) | 1 (9.1) | 0 (0.0) |
The common symptoms were generally consistent for
different interventional therapies. In the PCI group, 43.5%
patients (n=10) presented with pain, including leg pain, waist pain
and abdominal pain, followed by 7 (43.5%) with blood pressure
reduction, 4 (7.6%) with sweating, 4 (7.6%) with HB reduction, and
4 (7.6%) with fever. Three (7.1%) PCI patients presented with
abdominal distension, unconsciousness and nausea. In the PAI group,
the most common symptom of RPH was also pain (4 cases; 36.4%),
including abdominal pain (1 case), back pain (1 case) and leg pain
(2 cases). Four (36.4%) PAI patients presented with blood pressure
reduction and 2 (9.1%) patients presented with HB reduction.
Additionally, each of the patients presented with other symptoms
(Table II), including abdominal
distension (1/11, 9.1%), sweating (1/11, 9.1%), unconsciousness
(1/11, 9.1%), dizziness (1/11, 9.1%) and fever (1/11, 9.1%).
Similarly, the most common symptom of RPH after OM was also pain (4
cases, 50.0%), followed by nausea (25.0%) and sweating (25.0%). No
OM patients presented with abdominal distension, unconsciousness,
dizziness or fever.
Pre-procedural treatments
A majority of the patients (38/42, 90.5%) received
preoperative anticoagulation or antiplatelet therapy prior to PCI,
PAI and OM. Among all patients, 37 (88.1%) patients were
administrated with oral aspirin, 32 (76.2%) were administered with
plavix and 15 (35.7%) were administered with low molecular heparin.
A small number of patients were administrated with ticagrelor (5
cases; 11.9%) and rivaroxaban (15 cases; 2.4%). The pre-operative
anticoagulation or antiplatelet therapies for patients are shown in
Table III.
 | Table IIIPreoperative anticoagulant therapy
for patients with RPH. |
Table III
Preoperative anticoagulant therapy
for patients with RPH.
| Index | Total (n=42) | PCI (n=23) | PAI (n=11) | OM (n=8) |
|---|
| Low molecular
heparin (n, %) | | | | |
|
No | 27 | 12 (44.4) | 8 (29.6) | 7 (25.9) |
|
Yes | 15 | 11 (73.3) | 3 (20.0) | 1 (6.7) |
| Aspirin (n, %) | | | | |
|
No | 5 | 1 (20.0) | 2 (40.0) | 2 (40.0) |
|
Yes | 37 | 22 (59.5) | 9 (24.3) | 6 (16.2) |
| Plavix (n, %) | | | | |
|
No | 10 | 3 (30.0) | 4 (40.0) | 3 (30.0) |
|
Yes | 32 | 20 (62.5) | 7 (21.9) | 5 (15.6) |
| Ticagrelor (n,
%) | | | | |
|
No | 37 | 20 (54.1) | 10 (27.0) | 7 (18.9) |
|
Yes | 5 | 3 (60.0) | 1 (20.0) | 1 (20.0) |
| Rivaroxaban (n,
%) | | | | |
|
No | 41 | 23 (56.1) | 11 (26.8) | 7 (17.1) |
|
Yes | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) |
Management
Patient management for RPH cases is shown in
Table IV. Among all patients with
RPH, 81.0% of patients (n=34) received conservative medical
treatment and blood transfusion. Regardless of the type of
intervention received, the most common management was conservative
medical treatment, including supplement of Ringer's solution, the
administration of dopamine, norepinephrine or other vasopressive
agents (PCI, 82.6%; PAI, 72.7%; OM, 87.5%) and blood transfusion
(PCI, 78.3%; PAI, 90.9%; OM, 75.0%). A total of 3 cases with RPH
required emergency surgery, including 1 case from the PCI group and
2 cases from the PAI group. Additionally, elective operation and
balloon compression were performed in 2 cases. A spring ring was
placed in 1 case in the OM group.
 | Table IVClinical treatment for patients with
RPH. |
Table IV
Clinical treatment for patients with
RPH.
| Treatment | Total (n=42) | PCI (n=23) | PAI (n=11) | OM (n=8) |
|---|
| Emergency surgery
(n, %) | | | | |
|
No | 39 | 22 (56.4) | 9 (23.1) | 8 (20.5) |
|
Yes | 3 | 1 (33.3) | 2 (66.7) | 0 (0.0) |
| Elective operation
(n, %) | | | | |
|
No | 40 | 21 (52.5) | 11 (27.5) | 8 (20.0) |
|
Yes | 2 | 2 (100.0) | 0 (0.0) | 0 (0.0) |
| Balloon compression
(n, %) | | | | |
|
No | 40 | 22 (55.0) | 10 (25.0) | 8 (20.0) |
|
Yes | 2 | 1 (50.0) | 1 (50.0) | 0 (0.0) |
| Spring ring (n,
%) | | | | |
|
No | 41 | 23 (56.1) | 11 (26.8) | 7 (17.1) |
|
Yes | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Conservative
medical management (n, %) | | | | |
|
No | 8 | 4 (50.0) | 3 (37.50) | 1 (12.5) |
|
Yes | 34 | 19 (55.9) | 8 (23.53) | 7 (20.6) |
| Blood
transfusion | | | | |
|
No | 8 | 5 (62.5) | 1 (12.5) | 2 (25.0) |
|
Yes | 34 | 18 (52.9) | 10 (29.4) | 6 (17.6) |
In-hospital outcomes
The prognosis of patients with RPH is summarized in
Table V. The overall incidence of
nosocomial infection was 38.1%. The incidence of nosocomial
infection in patients with RPH after PCI, PAI and OM was 68.8, 18.8
and 12.5%, respectively. The overall mortality of RPH patients was
7.1%. Among them, 2 cases received PAI treatment and one case
received OM. A total of 38 (90.5%) patients showed improvement and
were discharged. One case following PCI was transferred to a
general hospital.
 | Table VAdverse events and prognosis of
patients with RPH. |
Table V
Adverse events and prognosis of
patients with RPH.
| Index | Total (n=42) | PCI (n=23) | PAI (n=11) | OM (n=8) |
|---|
| Nosocomial
infection (n, %) | | | | |
|
No | 26 | 12 (46.2) | 8 (30.8) | 6 (23.1) |
|
Yes | 16 | 11 (68.8) | 3 (18.8) | 2 (12.5) |
| Prognosis (n,
%) | | | | |
|
Death | 3 | 0 (0.0) | 2 (66.7) | 1 (33.3) |
|
Transfer | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
|
Discharge | 38 | 22 (57.9) | 9 (23.7) | 7 (18.4) |
Discussion
Although RPH is a rare complication of invasive
intervention, it is often associated with high mortality (1,15), which
makes prompt diagnosis and treatment essential. However, clinical
manifestations vary from pain to shock, thereby the indistinct
early manifestations of RPH often lead to delayed treatment
(12). To the best of our knowledge,
only a few studies about RPH have been reported, whilst no studies
involving the Chinese population have been previously carried out.
Therefore, it is particularly important to provide more clinical
evidence for the diagnosis of RPH among the Chinese population. In
the current study, 42 patients who received invasive intervention
in the past 12 years were retrospectively and continuously
studied.
The current study demonstrated that RPH is an
uncommon complication from interventions, which is in accordance
with previous reports (7,17). Notably, intervention-related RPH
appears to have a high mortality rate of 7.1%. This is partly due
to the loss of intravascular volume caused by massive bleeding in
the retroperitoneum. Another reasoning behind this is that the
increase in abdominal pressure may lead to intra-abdominal organ
injury (18,19).
The majority of patients with RPH in the present
study after intervention were the elderly (age range, 64-80 years),
similar with the majority of cases with spontaneous RPH (16). One possible explanation is that age
is a risk factor for bleeding during treatment with anticoagulants
such as heparin (20). In the
current study, although no significant difference was found, the
number of female patients with RPH included in the present study
was higher compared with males. Previous reports revealed that
female gender was identified as an independent predictor of RPH
(12,14). This suggests that additional
attention should be focused on the risk of postoperative RPH in
female patients. Although several hypotheses have been proposed,
the reason for this association remains unclear. Previous reports
hypothesized that different arterial mechanical properties, smaller
common femoral artery dimensions and estrogen-related arterial
structures in females may increase the need for multiple arterial
punctures and risk of bleeding (9,17). One
of the aforementioned reasons may have led to the difference in RPH
occurrence between sexes. In summary, the findings indicated that
age and sex may be potential risk factors for RPH.
RPH mostly occurred within 24 h after intervention
and in patients with hypertension. Although the correlation has not
been clarified, these results indicated that patients receiving
interventional therapy should be closely monitored for the
occurrence of RPH within 24 h, particularly patients with a history
of hypertension. Notably, several patients did not develop RPH
until several days after intervention. Thus, patients should also
be alert to the occurrence of RPH within one week after
intervention if they feel uncomfortable. Farouque et al
(12) previously reported the time
course of RPH; however, only short-term RPH incidence within a few
hours after intervention was reported. Approximately 75% of cases
presented with RPH within the first 3 h after conclusion of the
procedure. Therefore, the present study demonstrated that RPH might
also occur after a number of post-procedural days.
Another important finding was that 20 patients with
RPH had punctures in the femoral arterial access under the inguinal
ligament while only 5 patients had punctures above the inguinal
ligament. The result indicated that RPH might occur in patients who
underwent a femoral artery puncture either below or above the
inguinal ligaments, which was inconsistent with results from
previous reports (10,12,21).
Farouque et al (12)
indicated that a higher femoral artery puncture site was a
significant risk factor for RPH. Similarly, Levine et al
(10) and Selivanov et al
(21) found an increased risk of
vascular complications with femoral access at or above the most
inferior border of the inferior epigastric artery. However, the
present study demonstrated that punctures under the inguinal
ligament could also cause RPH. Similarly in a previous study, 45%
of patients with RPH had a sheath insertion in the common femoral
artery well below the inguinal ligament (12). The occurrence of RPH may be due to
the spread of bleeding from the femoral artery puncture site under
the inguinal ligament to the anatomic fascial planes, leading the
bleeding into the retroperitoneum. Thus, low femoral artery
punctures cannot eliminate the risk of RPH. More importantly, the
femoral vascular structures at this location are accessible to
effective manual compression, hence manual compression is advised
to prevent the occurrence of presumed RPH. More studies with
long-term follow-up periods are needed to verify the above
hypothesis.
Symptoms of RPH were usually non-specific and
atypical, however back and waist discomfort was found to be the
most common symptom (16). Among the
42 cases of the present study, abdominal pain was the most common
symptom. Moreover, other symptoms such as leg pain, waist pain and
abdominal distension also frequently occurred in some patients.
These symptoms were also described by Kent et al (8), Farouque et al (12) and Sajnani et al (22), therefore they may be identified as
specific signs of RPH. Thus, the present study suggested that the
possibility of RPH should be considered in any patients receiving
interventional therapy who present with abdominal pain and
distension or leg pain. The remaining symptoms of RPH were
non-specific clinical manifestations, including nausea, sweating,
blood pressure reduction, unconsciousness and dizziness, which were
most likely due to reduced volume of blood in the circulatory
system as a result of RPH (16). In
the present study, common symptoms were generally consistent for
the different interventional therapies (PCI, PAI or OM).
Subclinical hematoma was not observed after any interventional
therapies (17). Additionally,
patients with RPH showed lower HB concentrations after percutaneous
intervention compared with at admission, suggesting that a
reduction in HB concentration may be the most important, sensitive
and common manifestation of RPH. Thus, low HB concentration may be
a risk factor that predisposed patients to RPH. It is worth noting
that seven patients showed decreased HB concentration, but no
symptoms of discomfort. Chan-Tack (23) reported that decreasing HB
concentration was a sign of RPH in a patient with fatal spontaneous
RPH induced by enoxaparin use. Additionally, in a large series
study of RPH following cardiac catheterization, a reduction in HB
concentration was detected in 96% of patients (1). One patient with RPH resulting from dual
testicular and intra-renal arterial injury also presented lower HB
concentrations compared with normal range (24). Whilst the above results suggested
that a reduction in HB concentration might be a sign of RPH, this
still warrants further verification. Therefore, changes in HB
concentration must be serially monitored in real-time in patients
undergoing femoral artery puncture. The HB concentration at
admission in the PAI group patients was higher compared with the
PCI group, but the lowest level was similar. This fact suggested
that patients in the PAI group showed a more significant decrease
in HB. Thus, for PAI recipients, HB concentrations should be more
closely monitored. Overall, RPH has an extraordinarily pleiomorphic
presentation. The present findings have provided evidence for the
diagnosis of RPH.
Currently, to the best of our knowledge, no studies
have focused on optimal therapeutic approaches for patients with
RPH, and review of the literature did not provide clear guidance.
Thereby, clinicians are required to choose and adjust appropriate
treatment solutions according to patient status. To date, treatment
strategies for RPH are mainly based on small cohort series or
isolated case reports. Sajnani et al (22) reported two patients treated by
balloon tamponade who demonstrated improved hemodynamics in a
controlled environment. Serruys et al (3) reported a case of life-threatening RPH
that was successfully treated with balloon occlusion and catheter
delivery of thrombin. Embolization using the combination of coils,
gelatin and/or polyvinyl alcohol has been successfully used for
RPH. Microcoil embolization has also been used to stop bleeding for
RPH patients (18,25). Additionally, González et al
(5) reported three cases of RPH that
were successfully treated with fluid transfusion and reversal of
anticoagulation. Blood transfusion has also been successfully used
as RPH treatment in a study by Kwok et al (26). In the present study, most patients
received conservative medical treatment and blood transfusion, some
of which have also undergone emergency surgery. If the hemodynamics
and HB concentration of patients remained stable after fluid
infusion or transfusion, conservative treatment could be performed,
otherwise surgical occlusion or balloon occlusion was required. The
current findings provide more evidence that conservative
management, such as blood transfusion, intensive care unit
monitoring, vigorous fluid resuscitation and reversal of
anticoagulation, were effective for most patients with RPH
(9,16,22).
Stopping bleeding in time can also prevent the mortality caused by
RPH, although optimal approaches need to be further explored.
The present study reported several important
findings. Firstly, a 12-year review among Chinese population with
RPH could evaluate practice and long-term outcomes. Secondly,
systematic evidence for RPH after various invasive interventions
provides new insights for the occurrence time of complications
arising from RPH. Thirdly, the current study revealed that any
femoral artery puncture may cause the occurrence of RPH, even if
only one of the invasive interventions was performed. Fourthly, a
higher incidence of RPH was observed when the puncture site was
below the inguinal ligament compared with puncture sites above the
inguinal ligament. Finally, a reduction in HB concentration may be
an important sign of RPH. Patients with low HB concentration should
be subjected to an abdominal CT scan immediately to prevent RPH
occurrence.
There were also several limitations in the present
study. Firstly, the current study is a cross-sectional
observational and a retrospective study. The quality of data mainly
depended on the accuracy of medical records. Secondly, the
epidemiological characteristics of RPH cannot accurately infer
causality, due to an undefined prevalence of RPH caused by PCI, PAI
or OM. To reduce the above limitations, a long-term follow-up was
conducted from 2007 to 2018 in the current study.
In conclusion, RPH is an infrequent complication of
invasive intervention with non-specific clinical manifestations. A
puncture in femoral arterial access under the inguinal ligament may
also result in RPH. RPH mostly occurred within 24 h after
interventions, especially in the patients with hypertension. The
most common symptom was pain, and a reduction in HB concentration
was an important RPH manifestation. Conservative medical treatment
and blood transfusion were suitable for the majority of RPH
patients, and emergency surgery was also required in selected
cases. Future studies are needed to determine the optimal strategy
for managing patients with a high risk of RPH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YGS, YJW and SYT conceived and designed the study.
JQ, YW and KFD measured patient hemoglobin concentrations. YDT and
SBQ performed data acquisition. SYT and JQ performed data analysis
and interpretation. YGS wrote the first draft of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Fuwai Hospital, National Center for Cardiovascular
Diseases, Chinese Academy of Medical Sciences and Peking Union
Medical College (Beijing, China). All written consents from
patients were waived due to the retrospective nature of the current
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frank JJ, Kamalakannan D, Kodenchery M,
Savoy-Moore RT and Rosman H: Retroperitoneal hematoma in patients
undergoing cardiac catheterization. J Interv Cardiol. 23:569–574.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Livraghi T, Solbiati L, Meloni MF, Gazelle
GS, Halpern EF and Goldberg SN: Treatment of focal liver tumors
with percutaneous radio-frequency ablation: Complications
encountered in a multicenter study. Radiology. 226:441–451.
2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Serruys PW, Morice MC, Kappetein AP,
Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand
M, Bass EJ, et al: Percutaneous coronary intervention versus
coronary-artery bypass grafting for severe coronary artery disease.
N Engl J Med. 360:961–972. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kornowski R, Mehran R, Dangas G, Nikolsky
E, Assali A, Claessen BE, Gersh BJ, Wong SC, Witzenbichler B,
Guagliumi G, et al: Prognostic impact of staged versus ‘One-Time’
multivessel percutaneous intervention in acute myocardial
infarction: Analysis from the HORIZONS-AMI (harmonizing outcomes
with revascularization and stents in acute myocardial infarction)
trial. J Am Coll Cardiol. 58:704–711. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
González C, Penado S, Llata L, Valero C
and Riancho JA: The clinical spectrum of retroperitoneal hematoma
in anticoagulated patients. Medicine (Baltimore). 82:257–262.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moscucci M, Mansour KA, Kent KC, Kuntz RE,
Senerchia C, Baim DS and Carrozza JP Jr: Peripheral vascular
complications of directional coronary atherectomy and stenting:
Predictors, management, and outcome. Am J Cardiol. 74:448–453.
1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Akkus NI, Beedupalli J and Varma J:
Retroperitoneal hematoma: An unexpected complication during
intervention on an occluded superficial femoral artery via a
retrograde popliteal artery approach. Rev Port Cardiol. 32:623–627.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kent KC, Moscucci M, Mansour KA, DiMattia
S, Gallagher S, Kuntz R and Skillman JJ: Retroperitoneal hematoma
after cardiac catheterization: Prevalence, risk factors, and
optimal management. J Vasc Surg. 20:905–913. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Testerman GM, Shilad S and George KJ:
Rapid warfarin reversal with factor VIIa in an elderly trauma
patient with retroperitoneal hematoma. Tenn Med. 102:37–39.
2009.PubMed/NCBI
|
|
10
|
Levine MN, Raskob G, Landefeld S and
Kearon C: Hemorrhagic complications of anticoagulant treatment.
Chest. 119 (1 Suppl):108S–121S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ulrich MR, Brock DM and Ziskind AA:
Analysis of trends in coronary artery bypass grafting and
percutaneous coronary intervention rates in Washington state from
1987 to 2001. Am J Cardiol. 92:836–839. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Farouque HM, Tremmel JA, Raissi Shabari F,
Aggarwal M, Fearon WF, Ng MK, Rezaee M, Yeung AC and Lee DP: Risk
factors for the development of retroperitoneal hematoma after
percutaneous coronary intervention in the era of glycoprotein
IIb/IIIa inhibitors and vascular closure devices. J Am Coll
Cardiol. 45:363–368. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waksman R, King SB III, Douglas JS, Shen
Y, Ewing H, Mueller L, Ghazzal ZM and Weintraub WS: Predictors of
groin complications after balloon and new-device coronary
intervention. Am J Cardiol. 75:886–889. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tremmel JA, Tibayan YD, O'Loughlin AJ,
Chan T, Fearon WF, Yeung AC and Lee DP: Most accurate definition of
a high femoral artery puncture: Aiming to better predict
retroperitoneal hematoma in percutaneous coronary intervention.
Catheter Cardiovasc Interv. 80:37–42. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baylis SM, Lansing EH and Glas WW:
Traumatic retroperitoneal hematoma. Am J Surg. 103:477–480.
1962.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sunga KL, Bellolio MF, Gilmore RM and
Cabrera D: Spontaneous retroperitoneal hematoma: Etiology,
characteristics, management, and outcome. J Emerg Med.
43:e157–e161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trimarchi S, Smith DE, Share D, Jani SM,
O'Donnell M, McNamara R, Riba A, Kline-Rogers E, Gurm HS and
Moscucci M: BMC2 Registry. Retroperitoneal hematoma after
percutaneous coronary intervention: Prevalence, risk factors,
management, outcomes, and predictors of mortality: A report from
the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular
Consortium) registry. JACC Cardiovasc Interv. 3:845–850.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Campbell NR, Hull RD, Brant R, Hogan DB,
Pineo GF and Raskob GE: Aging and heparin-related bleeding. Arch
Intern Med. 156:857–860. 1996.PubMed/NCBI
|
|
19
|
French JT, Goins B, Saenz M, Li S,
Garcia-Rojas X, Phillips WT, Otto RA and Bao A: Interventional
therapy of head and neck cancer with lipid nanoparticle-carried
rhenium 186 radionuclide. J Vasc Interv Radiol. 21:1271–1279.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jacques T and Lee R: Improvement of renal
function after relief of raised intra-abdominal pressure due to
traumatic retroperitoneal haematoma. Anaesth Intensive Care.
16:478–482. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Selivanov V, Chi HS, Alverdy JC, Morris JA
Jr and Sheldon GF: Mortality in retroperitoneal hematoma. J Trauma.
24:1022–1027. 1984.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sajnani N and Bogart DB: Retroperitoneal
hemorrhage as a complication of percutaneous intervention: Report
of 2 cases and review of the literature. Open Cardiovasc Med J.
7:16–22. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chan-Tack KM: Fatal spontaneous
retroperitoneal hematoma secondary to enoxaparin. South Med J.
96:58–60. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ahmed M, Keshava SN, Moses V and Valson
AT: Endovascular management of a large retroperitoneal haemorrhage
resulting from dual testicular and intra-renal arterial injury
after renal biopsy. Indian J Radiol Imaging. 28:362–365.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sreeram S, Lumsden AB, Miller JS, Salam
AA, Dodson TF and Smith RB: Retroperitoneal hematoma following
femoral arterial catheterization: A serious and often fatal
complication. Am Surg. 59:94–98. 1993.PubMed/NCBI
|
|
26
|
Kwok CS, Kontopantelis E, Kinnaird T,
Potts J, Rashid M, Shoaib A, Nolan J, Bagur R, de Belder MA, Ludman
P, et al: Retroperitoneal hemorrhage after percutaneous coronary
intervention: Incidence, determinants, and outcomes as recorded by
the british cardiovascular intervention society. Circ Cardiovasc
Interv. 11(e005866)2018.PubMed/NCBI View Article : Google Scholar
|