Introduction
Lung cancer is the most frequently diagnosed cancer
worldwide, comprising 20% of all cancer-related mortalities
(1). Non-small cell lung cancer
(NSCLC) accounts for 80-85% of lung cancer cases (2). Anlotinib hydrochloride is a
multi-target tyrosine kinase inhibitor, which is able to prolong
progression-free survival and overall survival in patients with
advanced NSCLC, who have an advanced stage of the disease despite
receiving >2 types of systemic chemotherapy (3). Anlotinib is able to inhibit cancer
progression and targets factors associated with angiogenesis,
including vascular endothelial growth factor (VEGF) receptors 1-3,
fibroblast growth factor (FGF) receptors 1-4, platelet-derived
growth factor (PDGF) receptor α and β and stem cell factor receptor
(4). Various types of cardiovascular
toxicity have been reported in the ALTER 0302 and 0303 clinical
trials, including hypertension, sinus tachycardia and QTc
prolongations; however, acute myocardial infarction (AMI) has not
been reported (3,5). The present case study, to the best of
our knowledge, was the first to report on a case with advanced
squamous cell carcinoma (SqCC) who developed AMI following
treatment with anlotinib.
Case report
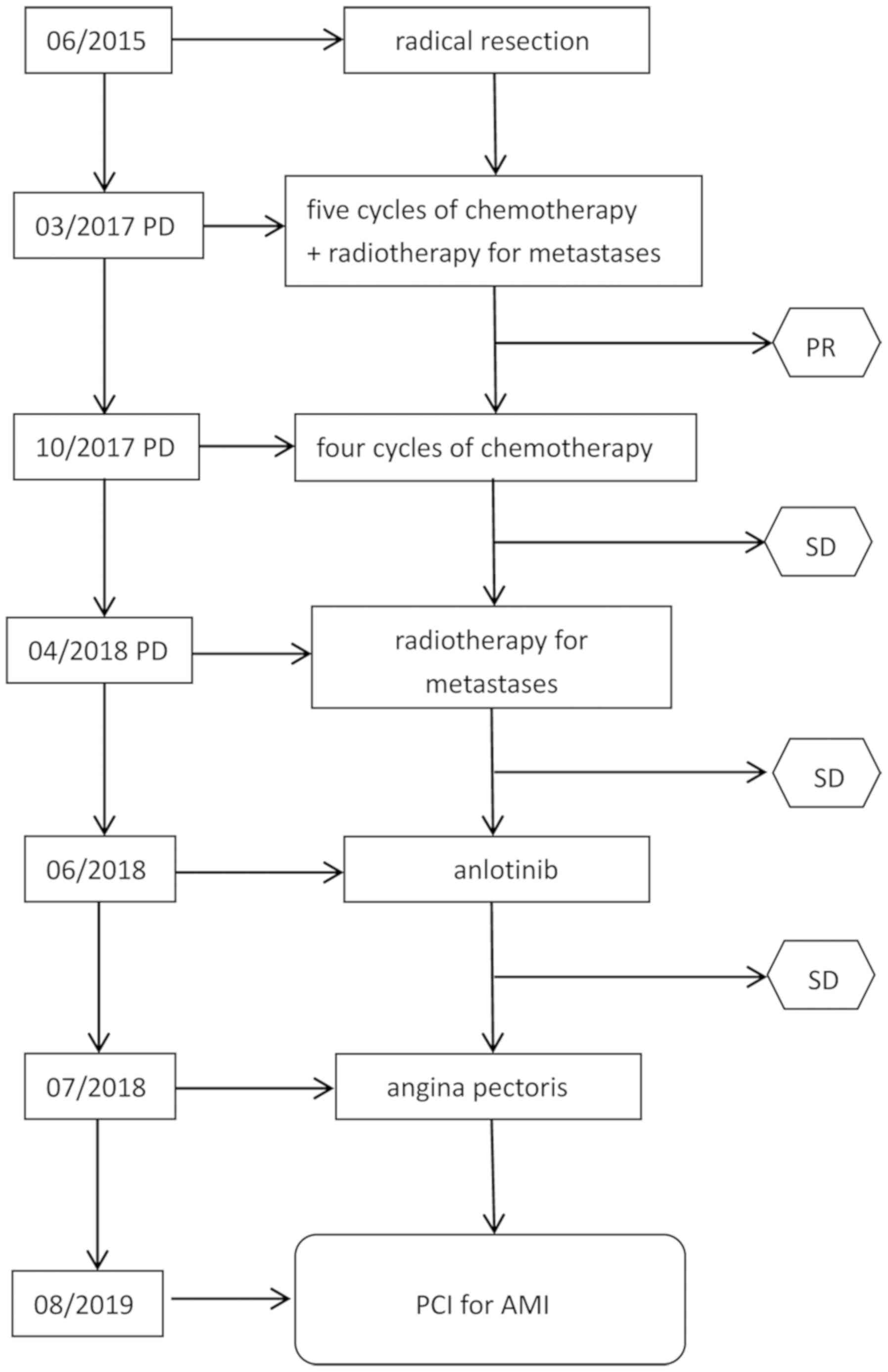
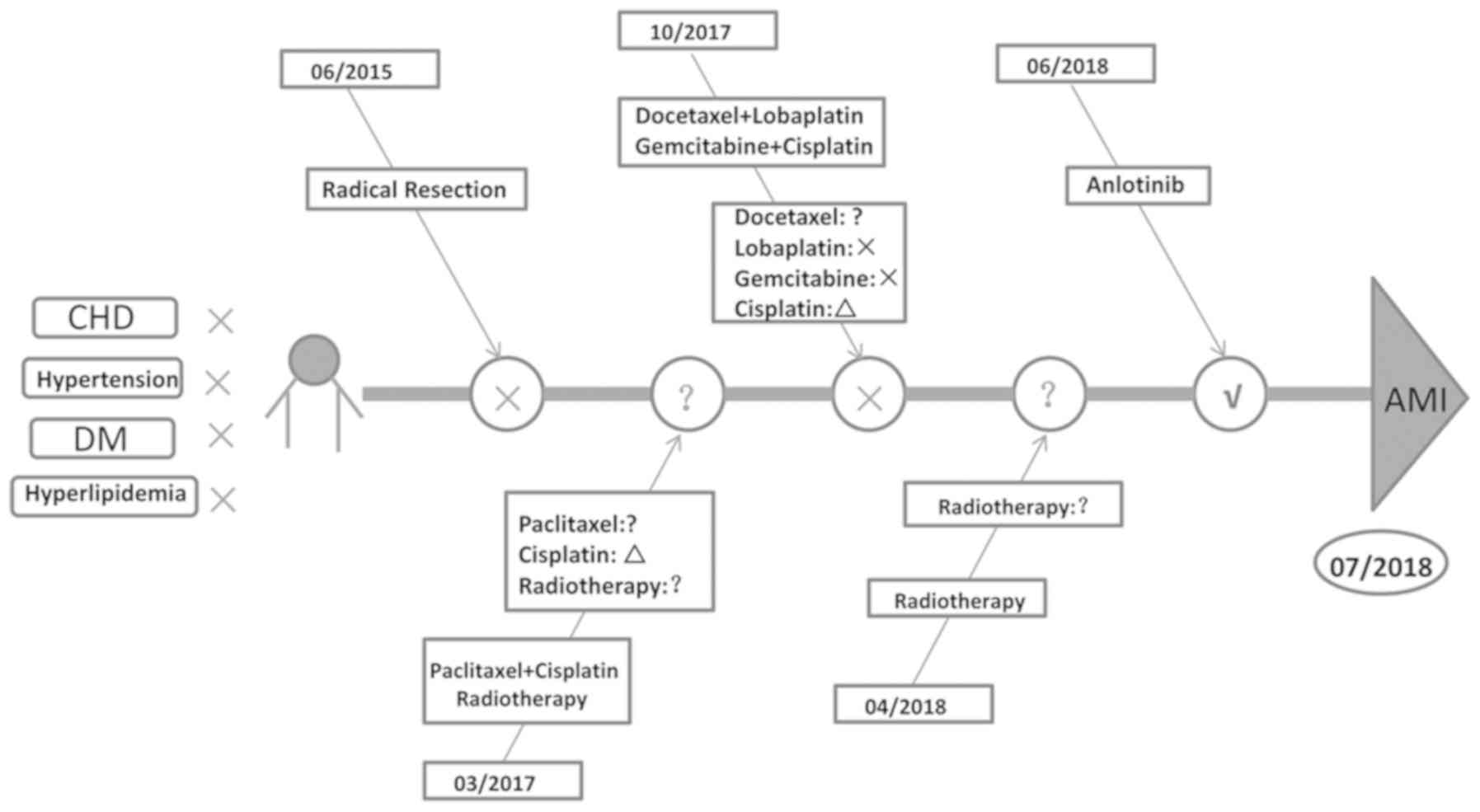
A 49-year-old male with a history of smoking (20
packs per year) and without coronary heart disease, hypertension,
diabetes mellitus or hyperlipidemia presented with a cough and
dyspnea in June 2015 at the West China Hospital (Chengdu, China). A
CT scan revealed a shadow of the left lower lobe. A pathological
diagnosis of T3N0M0 (stage II) SqCC was made following radical
resection according to the eighth edition of the TNM classification
of lung cancer (6). No epidermal
growth factor receptor, ALK or ROS-1 mutations were identified. The
patient refused adjuvant treatment following surgery and
experienced back pain in March 2017. An additional CT scan
indicated the third thoracic vertebral metastasis. The patient was
subsequently prescribed five cycles of paclitaxel and cisplatin
chemotherapy and 44 Gy/22f radiotherapy and achieved partial
response. The CT performed at reexamination in October 2017
revealed that the thoracic spine lesion was larger than before and
was accompanied by left hilar lymph node enlargement. A total of 2
cycles of docetaxel and lobaplatin chemotherapy were subsequently
administered to the patient and stable disease (SD) was achieved.
To improve the efficacy, treatment was changed to 2 cycles of
gemcitabine and cisplatin chemotherapy and SD was maintained. In
April 2018, the CT scan revealed the tumor had invaded the spinal
cord. The patient received 33 Gy/30f radiotherapy for the
metastatic masses prior to systemic treatment. In June 2018, the
blood pressure, glucose and lipid levels of the patient were all
within normal ranges (Table I);
therefore, the patient was prescribed anlotinib (12 mg orally, once
a day), until AMI was diagnosed (Fig.
1).
 | Table ILaboratory parameters of the patient
in June 2018. |
Table I
Laboratory parameters of the patient
in June 2018.
| Parameter | Value | Normal range |
|---|
| Blood pressure
(mmHg) | 128/60 | (90-139)/(60-89) |
| Fasting glucose
(mmol/l) | 5.12 | 3.9-5.9 |
| Cholesterol
(mmol/l) | 4.29 | 2.8-5.7 |
| Triglyceride
(mmol/l) | 0.54 | 0.29-1.83 |
| HDL-C (mmol/l) | 1.35 | >0.9 |
| LDL-C (mmol/l) | 2.83 | <4.0 |
After a month of using anlotinib, the patient
developed occasional chest pain, high blood pressure (140/100
mmHg), hyperlipidemia and hand-foot syndrome. The patient ignored
the thoracalgia which had developed, resulting in more frequent and
serious attacks requiring the use of nitroglycerin to relieve the
pain, one week prior to AMI. On August 7, 2019, the patient
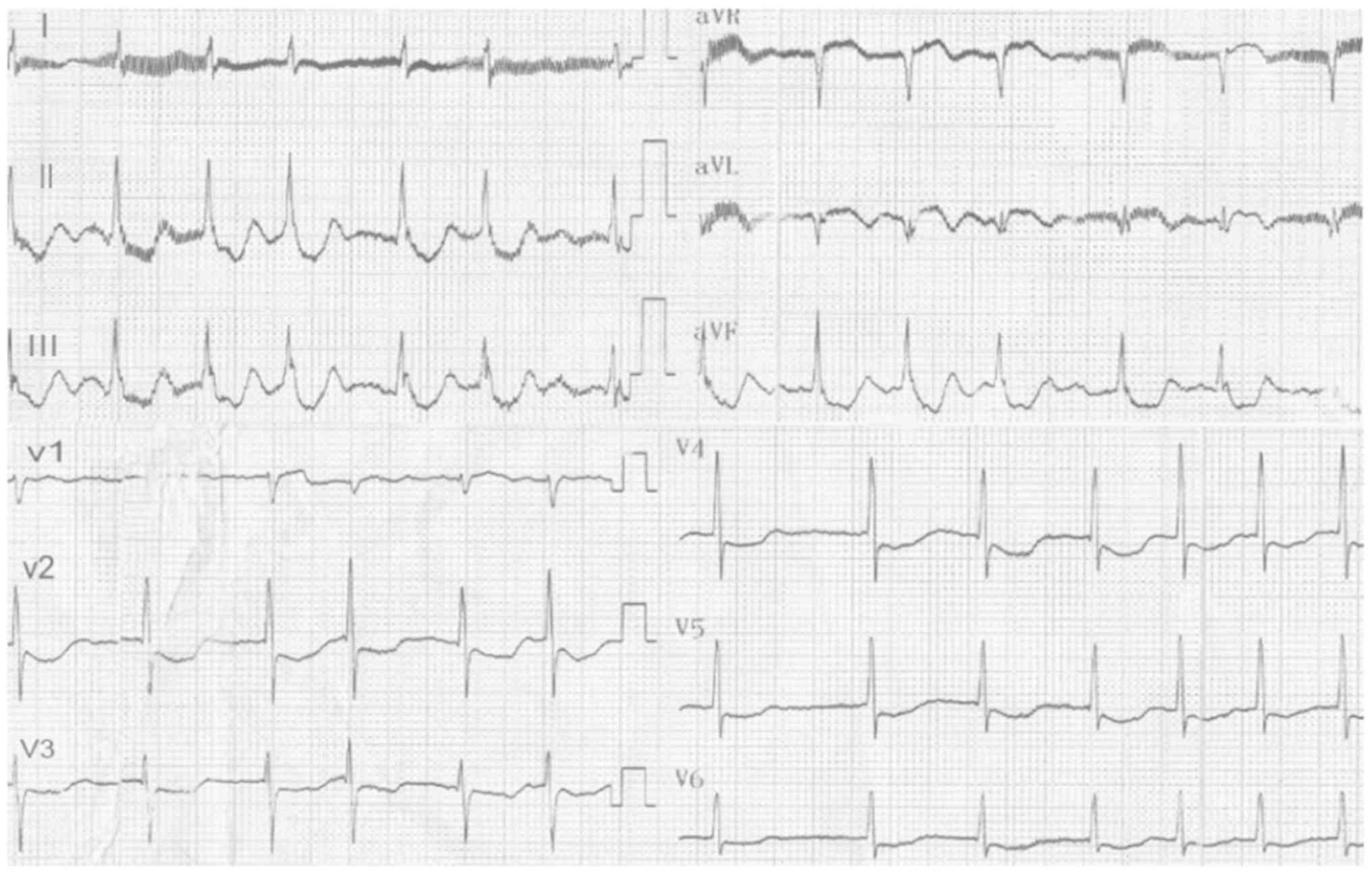
presented with unbearable chest pain, which was not relieved by
nitroglycerin. The electrocardiogram revealed atrial fibrillation,
ST elevation in leads AVR and V1 (AVR>V1) and ST depression in
leads V2 to V6, II, III and AVF (Fig.
2) with the amount of cardiac isoform of troponin T reaching
140.70 pg/ml (normal range, 0-14 pg/ml). The cardiologists
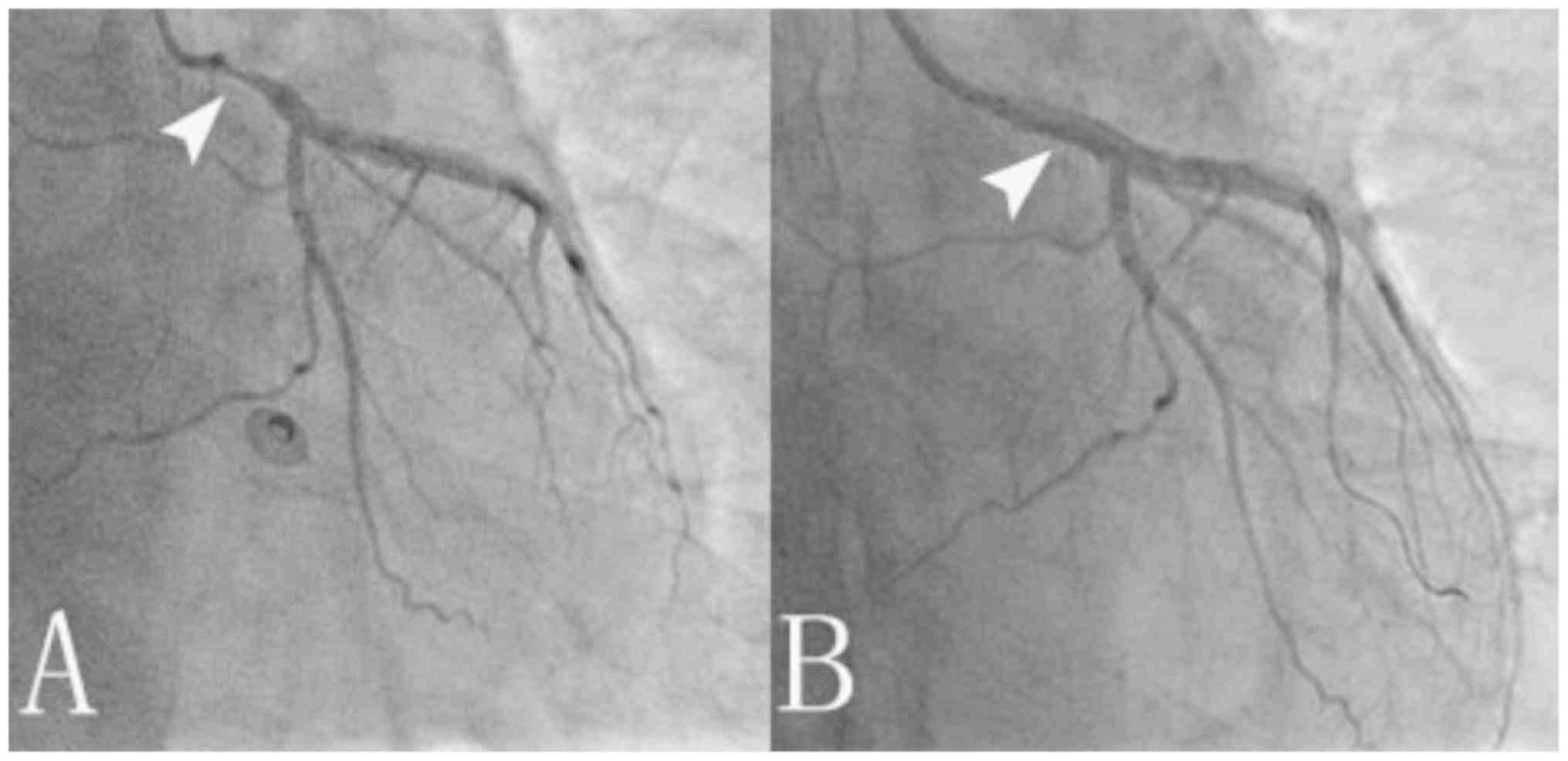
considered that the patient had experienced AMI. Emergency coronary
angiography revealed 85% proximal stenosis of the left major
coronary artery and coronary intervention with a drug-eluting stent
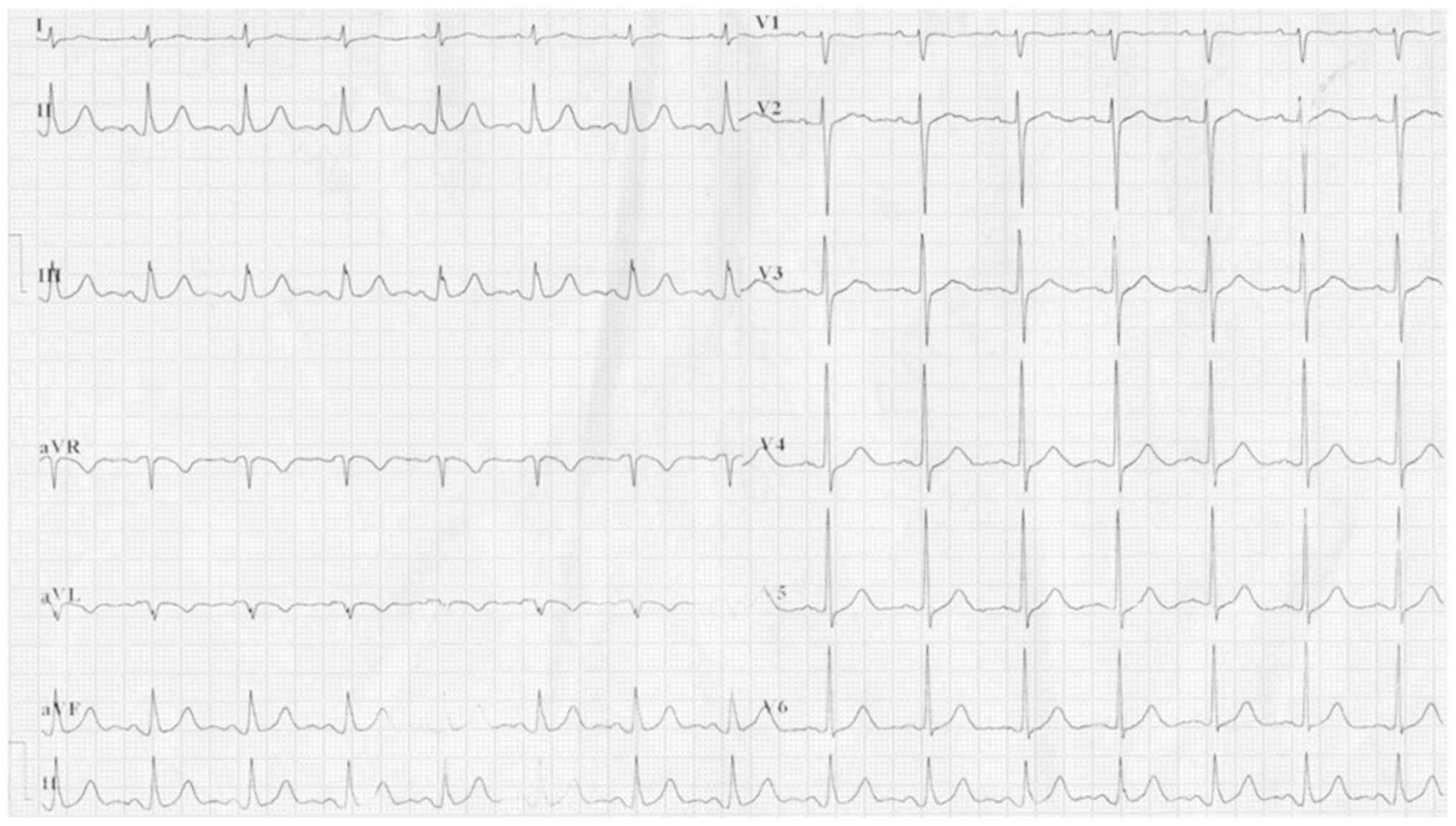
was performed (Fig. 3). The patient
did not experience any further chest pain and the postoperative
electrocardiogram was normal (Fig.
4). Subsequently, the patient received dual antiplatelet
therapy and stopped the use of anlotinib. The patient is still
alive following the administration of regular immunotherapy.
Discussion
Coronary heart disease, hypertension, diabetes
mellitus and hyperlipidemia, which are risk factors for AMI, were
not previously observed in the middle-aged patient of the present
study, who only exhibited a history of smoking prior to treatment
with anlotinib (7). Several types of
chemotherapeutic drugs have been administered following recurrence.
The occurrence of cisplatin-associated AMI between the first dose
and 4 weeks after the last dose has been reported (8). In several cases, the use of taxanes was
reported to induce AMI (9,10), and percutaneous coronary intervention
with a paclitaxel-coated balloon is widely used to remedy this
(11). Gemcitabine was only reported
to cause peripheral ischemia and Raynaud's phenomenon in resistant
arteries (12), while lobaplatin was
not observed to cause any vascular lesions (13). Radiotherapy may cause damage to the
vasculature and the rates of coronary events were demonstrated to
increase linearly with increasing dosages directly exposed to the
heart (14). AMI caused by
radiotherapy is primarily observed in patients with breast cancer
and Hodgkin's lymphoma due to the high dosages exposed to the heart
(15). The incidence of AMI is
relatively lower in patients with lung cancer due to the low
cardiac dose and the improvements in radiation techniques, which
avoid radiation exposure of the heart (16). In the present case, the radiotherapy
site of the patient was located in the third thoracic spine region,
indicating that the anatomic location of the lesion was higher than
that of the heart. In other words, the heart of the patient was not
as exposed as the location of lesion. The patient suffered angina
pectoris following 1 month of treatment with anlotinib, indicating
that pathological changes occurred in the coronary artery.
Therefore, the AMI that had developed in the patient was reasonably
attributed to anlotinib (Fig.
5).
Anlotinib serves an anti-tumor role by inhibiting
several types of tyrosine kinases (4). In 1971, Folkman (17) proposed that tumor growth depends on
angiogenesis and numerous anti-angiogenesis drugs have since
emerged, including anlotinib. Vessels are lined by a single layer
of endothelial cells that rely on VEGF and are surrounded by smooth
muscle cells (18,19). Arteries have a muscular layer that
regulates the vascular tone, whereas capillaries are more sparsely
supported by specialized mesenchymal cells known as pericytes, and
depend on the production of PDGF by endothelial cells (20). Tumor cells secrete FGF to promote
excess growth and dysregulation of the vasculature (21). Anti-angiogenesis drugs exert
effective tumor inhibition effects by suppressing the
aforementioned factors (22).
Notably, toxicities also arise due to their
mechanism. One such type is cardiovascular toxicity, including
hypertension, congestive heart failure, cardiac ischemia and AMI
(23). AMI is a fatal cardiovascular
event, which is the result of cardiac ischemia (7). Coronary atherosclerosis and arterial
thrombosis account for cardiac ischemia, and are formed due to the
following factors: VEGF signaling pathway inhibitors increase the
risk of arterial thrombosis, as they decrease endothelial cell
survival and proliferation (24).
Furthermore, administration of anti-angiogenesis drugs was
hypothesized to decrease nitric oxide synthesis and lead to
hypertension, which may increase endothelial damage (25). Hyperlipidemia is another side effect
of anti-angiogenesis drugs, resulting in an acceleration of the
formation of plaques and causing them to be unstable (7). A meta-analysis of angiogenesis
inhibitors, including bevacizumab, aflibercept, ramucirumab,
sunitinib, sorafenib, pazopanib, vandetanib, cabozantinib,
axitinib, ponatinib and regorafenib, indicated that the risk of
cardiac ischemia almost tripled and fatal cardiovascular events
increased by 1.26-fold (23).
Myocardial infarction has also been reported in association with
the use of bevacizumab, ramucirumab, sunitinib and sorafenib
(25-27).
Cardiovascular toxicity was notable in phase I, II
and III clinical trials of anlotinib (3-5);
however, to the best of our knowledge, the present study was the
first to report on a case of anlotinib-induced AMI. The number of
patients and follow-up time were limited in these clinical trials
and the longest median duration of anlotinib treatment was 24 weeks
in these clinical trials, a longer time period may be required to
observe the occurrence of AMI in a larger population. Furthermore,
anlotinib was only approved for the treatment of patients with
advanced NSCLC who have progressed following treatment with >2
types of prior systemic chemotherapy (28). Thus, the effects of anlotinib are
difficult to observe in a clinical setting, as the health of the
patients is generally poor, with a short life expectancy (3-5).
However, the patient in the present case study received anlotinib
for >1 year, with a good response, which enabled the observation
of the occurrence of AMI.
In conclusion, to the best of our knowledge, the
present study was the first to report on a case of AMI induced by
anlotinib. Fatal cardiovascular events may also occur due to
anlotinib, which is similar to the effects of other
anti-angiogenesis drugs; therefore, patients treated with anlotinib
require further and regular monitoring in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL analyzed and interpreted the data, and was a
major contributor in drafting the manuscript. TC collected the
clinical data and was involved in drafting the manuscript. ZD
supervised the collection and interpretation of data and critically
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chengdu No. 7 People's Hospital, Chengdu Tumor
Hospital, (Chengdu, China) and informed consent was obtained from
the patient.
Patient consent for publication
The patient provided written informed consent
regarding the publication of the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li
L, Wang F, Hao Y, Li C and Chi Y: Safety, pharmacokinetics, and
antitumor properties of anlotinib, an oral multi-target tyrosine
kinase inhibitor, in patients with advanced refractory solid
tumors. J Hematol Oncol. 9(105)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou
J, Lu Y, Shi Y, Wang Z, Jiang L, et al: Anlotinib as a third-line
therapy in patients with refractory advanced non-small-cell lung
cancer: A multicentre, randomised phase II trial (ALTER0302). Br J
Cancer. 118:654–661. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rami-Porta R, Bolejaek V, Giroux DJ,
Chansky K, Crowley J, Asamura H and Goldstraw P: International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee, Advisory Board Members and Participating
Institutions. The IASLC lung cancer staging project: The new
database to inform the eighth edition of the TNM classification of
lung cancer. J Thorac Oncol. 9:1618–1624. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Anderson JL and Morrow DA: Acute
myocardial infarction. N Engl J Med. 376:2053–2064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moore RA, Adel N, Riedel E, Bhutani M,
Feldman DR, Tabbara NE, Soff G, Parameswaran R and Hassoun H: High
incidence of thromboembolic events in patients treated with
cisplatin-based chemotherapy: A large retrospective analysis. J
Clin Oncol. 29:3466–3473. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Rowinsky EK, McGuire WP, Guarnieri T,
Fisherman JS, Christian MC and Donehower RC: Cardiac disturbances
during the administration of taxol. J Clin Oncol. 9:1704–1712.
1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ali RM, Abdul Kader MASK, Wan Ahmad WA,
Ong TK, Liew HB, Omar AF, Zuhdi ASM, Nuruddin AA, Schnorr B and
Scheller B: Treatment of coronary drug-eluting stent restenosis by
a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc Interv.
12:558–566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dasanu CA: Gemcitabine: Vascular toxicity
and prothrombotic potential. Expert Opin Drug Saf. 7:703–716.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gu L, Zhong D, Yu T, Tang P, Meng F and
Qin Q: Retrospective study of the efficacy and toxicity of
lobaplatin-etoposide chemotherapy in small cell lung cancer. Thorac
Cancer. 10:226–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Darby SC, Ewertz M, McGale P, Bennet AM,
Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante
B, et al: Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 368:987–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zamorano JL, Lancellotti P, Rodriguez
Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ,
Lip GYH, Lyon AR, et al: 2016 ESC position paper on cancer
treatments and cardiovascular toxicity developed underthe auspices
of the ESC committee for practice guidelines: The task force for
cancertreatments and cardiovascular toxicity of the European
society of cardiology (ESC). Eur Heart J. 37:2768–2801.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee CC, Zheng H, Soon YY, Foo LL, Koh WY,
Leong CN, Vellayappan B, Tey JCS and Tham IWK: Association between
radiation heart dosimetric parameters, myocardial infarct and
overall survival in stage 3 non-small cell lung cancer treated with
definitive thoracic radiotherapy. Lung Cancer. 120:54–59.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31.
1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Baluk P, Hashizume H and McDonald DM:
Cellular abnormalities of blood vessels as targets in cancer. Curr
Opin Genet Dev. 15:102–111. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Claesson-Welsh L: Blood vessels as targets
in tumor therapy. Ups J Med Sci. 117:178–186. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abdel-Qadir H, Ethier JL, Lee DS,
Thavendiranathan P and Amir E: Cardiovascular toxicity of
angiogenesis inhibitors in treatment of malignancy: A systematic
review and meta-analysis. Cancer Treat Rev. 53:120–127.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ivy SP, Wick JY and Kaufman BM: An
overview of small-molecule inhibitors of VEGFR signaling. Nat Rev
Clin Oncol. 6:569–579. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Choueiri TK, Schutz FA, Je Y, Rosenberg JE
and Bellmunt J: Risk of arterial thromboembolic events with
sunitinib and sorafenib: A systematic review and meta-analysis of
clinical trials. J Clin Oncol. 28:2280–2285. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dahlberg SE, Sandler AB, Brahmer JR,
Schiller JH and Johnson DH: Clinical course of advanced
non-small-cell lung cancer patients experiencing hypertension
during treatment with bevacizumab in combination with carboplatin
and paclitaxel on ECOG 4599. J Clin Oncol. 28:949–954.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Herbst RS, Arkenau HT, Santana-Davila R,
Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG,
Martin-Liberal J, et al: Ramucirumab plus pembrolizumab in patients
with previously treated advanced non-small-cell lung cancer,
gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A
multicohort, non-randomised, open-label, phase 1a/b trial. Lancet
Oncol. 20:1109–1123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou M, Chen X, Zhang H, Xia L, Tong X,
Zou L, Hao R, Pan J, Zhao X, Chen D, et al: China national medical
products administration approval summary: Anlotinib for the
treatment of advanced non-small cell lung cancer after two lines of
chemotherapy. Cancer Commun (Lond). 39(36)2019.PubMed/NCBI View Article : Google Scholar
|