Introduction
Cluster of differentiation 47 (CD47), also known as
integrin-associated protein, is a membrane protein of the
immunoglobulin superfamily (1). CD47
negatively regulates phagocytosis by participating in integrin and
cadherin signaling through interaction with thrombospondin-1
(TSP-1) and signal regulatory protein α (SIRPα) (1). CD47 is ubiquitously expressed in human
cells and has been found to be overexpressed in numerous cancer
types (2-5).
CD47 overexpression on cancer cells predicts adverse prognosis of
breast, gastric, ovarian and esophagus cancer (2-5).
Emerging evidence has demonstrated the critical roles of
phagocytosis and antigen presentation in anti-tumor immunity
(4,5). In preclinical cancer models, CD47
monoclonal antibody treatment has exhibited promising antitumor
effects in various types of cancer by enhancing the activity of
phagocytic cells and innate antitumor immunity (4-7).
However, the effects of CD47 expression on regulating antigen
presentation and T-cell priming in cancer currently remain
unclear.
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most lethal diseases, with an estimated 53,000 new cases in the
United States in 2016(8). Although
comprehensive treatment strategies are available, the 5-year
survival rate of PDAC patients remains dismal (9). Therefore, developing new anti-PDAC
targets for novel treatments represents an urgent and unmet medical
need. Immune checkpoint blockades target immune checkpoints, key
regulators of the immune system, to enhance the immune response,
including the anti-tumor activity (10). Anti-cytotoxic T-lymphocyte associated
protein 4 (anti-CTLA4), an example of an immune checkpoint
blockades, displayed promising anti-tumor effects in various
cancers, including melanoma and colon and lung cancer (11-13);
however, to the best of our knowledge, their effect has not been
investigated in PDAC to date (14).
The effects of anti-CTLA4 treatment largely depend on the
antigen-presenting cell (APC) function and T-cell priming, which is
under the regulation of APCs (15).
Notably, APCs express a high level of SIRPα and are, thus,
influenced by tumor cell CD47 expression.
Given these previous observations, the present study
aimed to investigate whether targeting CD47 is able to enhance the
function of APCs and improve the efficacy of anti-CTLA4 as a
treatment for PDAC in preclinical models.
Materials and methods
Cell culture and transfection
The mouse pancreatic cancer cell line Panc02 was
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Panc02 cells were cultured using RPMI
medium supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), 100 U/ml of penicillin and 100 µg/ml of streptomycin
at 37˚C with 5% CO2. When the cells reached 85%
confluency, subculturing was performed.
To manipulate CD47 expression, wild-type Panc02
cells (CD47-WT) were transfected with CD47-short hairpin RNA
(shRNA) for expression knockdown (CD47-KD group), CD47
overexpression vector (CD47-OE group) or control nonsense vector
(CD47-Ctrl group). All vectors used were lentiviral vectors
purchased from OriGene Technologies, Inc. On day 1, a total of
1.2x107 293 cells (cat. no. 85120602; Sigma-Aldrich;
Merck KGaA) were seeded in a 10-cm dish and cultured with EMEM
medium (cat. no. 30-2003; American Type Tissue Collection) at 37˚C
with 5% CO2. When 70% confluency was reached, the 293
cells were transfected using Lipofectamine 3000 (cat. no. L3000008;
Thermo Fisher Scientific, Inc.) with aCD47 overexpression vector
(cat. no. MR204706L1; OriGene Technologies, Inc.), CD47 shRNA (cat.
no. TL501123; OriGene Technologies, Inc.) or control vector (cat.
no. PS100064; OriGene Technologies, Inc.; 20 µg each) together with
the packaging vector (Lenti-vpak packaging kit; cat. no. TR30037;
OriGene Technologies, Inc.) in order to produce lentiviruses to
infect Panc02 cells. After 48 h of transfection, the lentivirus was
harvested from the supernatant (by centrifugation at 1,500 x g at
4˚C for 5 min to remove cell debris) of the 293 cell culture and
used to transduce the Panc02 cells. Panc02 cells
(1.0x105) were seeded in 24-well plates, and then 0.5 ml
supernatant with virus (107 particles) was added to the
plates to incubate for 24 h at 37˚C. Puromycin (10 µg/ml; cat. no.
A1113802; Thermo Fisher Scientific, Inc.) was added to the culture
medium to select the transduced Panc02 cells on day 3 after
lentiviral transduction. Following 4 days of selection, the
expression of CD47 on the cell membrane was measured by flow
cytometry in each group in order to validate the gene expression
manipulation. Briefly, 1.0x105 cells were collected and
washed with PBS once at room temperature and resuspended in PBS
with 5% BSA. Cells were then stained with CD47 antibody (1:100;
cat. no. 127507; BioLegend, Inc.) for 20 min at room temperature.
Then cells were then washed with PBS and analyzed using a FACSCanto
II flow cytometer (Becton Dickinson & Company) and FlowJo
software (version 9; FlowJo LLC).
Patient samples
Fresh PDAC tissues and the paired adjacent normal
pancreatic tissues were collected from 20 PDAC patients diagnosed
at Xi'an Gaoxin Hospital between June and December 2015. These
patients met the following inclusion criteria: i) The diagnosis of
PDAC was confirmed by hematoxylin-eosin staining by two
certificated pathologists; ii) the patient had not accepted any
chemotherapy or radiotherapy previously; iii) adequate quantities
of tumor tissue and paired tumor adjacent pancreatic tissues were
collected; iv) the patient did not have any immune deficient or
autoimmune disease; v) the patient did not have any other severe
disease; vi) the patient provided informed consent to participation
in the study and agreed to the collection of follow-up information.
Patients were followed-up until death or for up-to 24 months. The
fresh tissues were collected during surgery and immediately used
for flow cytometry analysis (as described) to measure protein
expression levels. The expression of CD47 was measured in tumor and
tumor-adjacent normal tissues, while the expression levels of CD80
and CD86 were measured in tumor-infiltrating APCs. The present
study was approved by the Ethics Committee of Xi'an Gaoxin
Hospital, Ganzhou, Jiangxi, China. Informed consent was obtained
from all patients or their legal representatives through visits or
phone calls and written or oral consents respectively were obtained
in the presence of a public notary.
Animal model
C57BL/6 mice and the Panc02 cell line were used to
establish subcutaneous mouse models of PDAC. Briefly, 5-week old
male mice (Beijing HFK Bioscience, Changping, Beijing, China;
weight, 18-20 g) were obtained and kept in a specific-pathogen-free
environment (27˚C, 45% humidity and 12 h light/dark cycle) with
free access to clean water and standard food. In order to evaluate
the role of CD47 in PDAC development, mice were divided into four
experimental groups (n=10 per group), and 5x105 Panc02
cells with different CD47 expression levels (CD47-OE, CD47-WT,
CD47-Ctrl or CD47-KD) were respectively inoculated into the flanks
of the mice.
Subsequently, the effects of anti-CD47 treatment
were evaluated in the CD47-WT mouse model. Briefly, treatment with
anti-CD47 (5 mg/kg/week; diluted in PBS, cat. no. BE0270; Bio X
Cell, West Lebanon, NH, USA) and/or anti-CTLA4 (5 mg/kg/week;
diluted in PBS, cat. no. BE0131; Bio X Cell) was initiated at 1
week after tumor inoculation via intraperitoneal injection with
Panc02 cells. The tumor volume was measured and calculated every
week. The tumor volume was calculated based on the following
formula: Volume = length x width2 x π/6. A tumor volume
that was >1,500 m3 was considered as the endpoint
(euthanasia took place after 2-8 weeks depending on the rate of
tumor growth and the treatment used).
Phagocytic assay
Panc02 cells were digested into single cell
suspensions and then labeled with carboxyfluorescein succinimidyl
ester (CFSE; eBioscience; Thermo Fisher Scientific, Inc.) following
the manufacturer's protocol. Macrophages were purified from the
peritoneal cell suspension of C57BL/6 mice (the same source and
breeding condition as stated in the animal odel paragraph, pooled
cells from 3 mice). Briefly, 10 ml saline was injected to the
peritoneal cavity and incubated for 10 min. The saline was then
collected for macrophage isolation using the Macrophage Isolation
kit (Peritoneum), mouse, cat. no. 130-110-434, Miltenyi Biotec
GmbH) according to the manufacturer's instructions. Dendritic cells
(DCs) were also isolated from a cell suspension of spleens of
C57BL/6 mice (pooled samples from 3 mice) using a DC isolation kit
(CD8+ Dendritic Cell Isolation kit; cat. no.
130-091-169, Miltenyi Biotec GmbH) following the manufacturer's
protocol. Subsequently, CFSE-labeled Panc02 cells (105)
were co-cultured with the C57BL/6 mouse macrophages
(0.2x105) or DCs (0.2x105) for 2 h. The
phagocytic index was calculated by dividing the number of
CFSE-positive macrophages or DCs by the total number of macrophages
or DCs. To study the effects of CD47 on phagocytosis, tumor cells
were treated with anti-CD47 antibodies. For anti-CD47 treatment,
Panc02 cells were exposed to 20 µg/ml purified anti-mouse CD47
antibody (cat. no. BE0270; Bio X Cell) for 30 min at room
temperature. The control group cells were exposed to the same dose
of isotype IgG (cat. no. BE0089; Bio X Cell).
Fluorescence-activated cell sorting
(FACS) analysis
Single cell suspensions were generated by
dissociating the fresh human and mouse tissue samples (including
normal pancreas and pancreatic cancers) using collagenase IV (5
mg/ml; cat. no. 17104019; Thermo Fisher Scientific, Inc.) and
deoxyribonuclease (DNase I; 50 units/ml; cat. no. EN0525; Thermo
Fisher Scientific, Inc.) at 37˚C for 1 h. Cultured cell lines were
collected by trypsinization (0.05% for 5 min at 37˚C). Next, cells
were incubated with fluorochrome-conjugated primary antibodies on
ice for 30 min, followed by thoroughly washing with PBS. The cells
were then analyzed with the FACSCanto II device (BD Biosciences,
Franklin Lakes, NJ, USA), and the results were read on the FlowJo
software (FlowJo LLC, Ashland, OR, USA). All the primary antibodies
used in the flow cytometry analyses were purchased from BioLegend,
Inc. (San Diego, CA, USA), and are listed in Table I.
 | Table IAntibodies used in the flow cytometry
experiments of the present study. |
Table I
Antibodies used in the flow cytometry
experiments of the present study.
| A, Antibodies used
for flow cytometry in murine samples |
|---|
| Protein antibody
raised against | Catalog number |
|---|
| CD11c | 117307 |
| F4/80 | 123133 |
| MHC-II | 107651 |
| CD11b | 101220 |
| CD68 | 137017 |
| CD80 | 104715 |
| CD86 | 105035 |
| CD8 | 100747 |
| CD3 | 100225 |
| CD4 | 116011 |
| B, Antibodies used
for flow cytometry in human samples |
| Protein antibody
raised against | Catalog number |
| CD11c | 301634 |
| MHC-II | 307625 |
| CD11b | 301323 |
| CD68 | 333819 |
| CD80 | 305213 |
| CD86 | 341407 |
| CD47 | 323108 |
In mouse samples, the
CD11c+MHC-II+ and
CD11b+F4/80+ markers were used for gating DCs
and macrophages, respectively. gp70 protein is known to be widely
expressed by mouse tumor cell lines, but not by normal tumor tissue
(16). Thus, gp70 tetramers
(peptide, VGRVPIGPNP; QuickSwitch™ Custom Tetramer kit; cat. no.
TB-7400-K2; MBL International Corporation, Woburn, MA, USA) were
used to detect gp70+ CD8 T-cell, and these were
considered as tumor-specific T-cells. In human samples, the markers
CD11c+MHC-II+ and
CD11b+CD68+ were used for gating of DCs and
macrophages, respectively. Data were visualized by FlowJo software
(version 9), and the mean fluorescence intensity was used for
statistical analysis.
Statistical analysis
All statistical analyses were performed with SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism
software (version 7; GraphPad Software, Inc.). The differences
between the mean values were accordingly analyzed using t-test or
one-way analysis of variance followed by Tukey's post hoc test.
Survival analysis was performed by the Kaplan-Meier method. The
difference between survival curves was assessed by the log-rank
test. Quantified data presented in the bar plots are shown as the
mean ± standard deviation. A two-tailed P-value of <0.05 was
considered to denote a statistically significant difference.
Results
CD47 is overexpressed in PDAC samples
and is associated with decreased APC activation
To reveal the role of CD47 in PDAC development, the
CD47 expression levels in PDAC tissues were first evaluated. The
clinicopathological features of the patients included in the
present study are summarized in Table
II. The average age of these patients was 61.5±5.8 years. In
total, 12 patients were male and 8 were female. As shown in
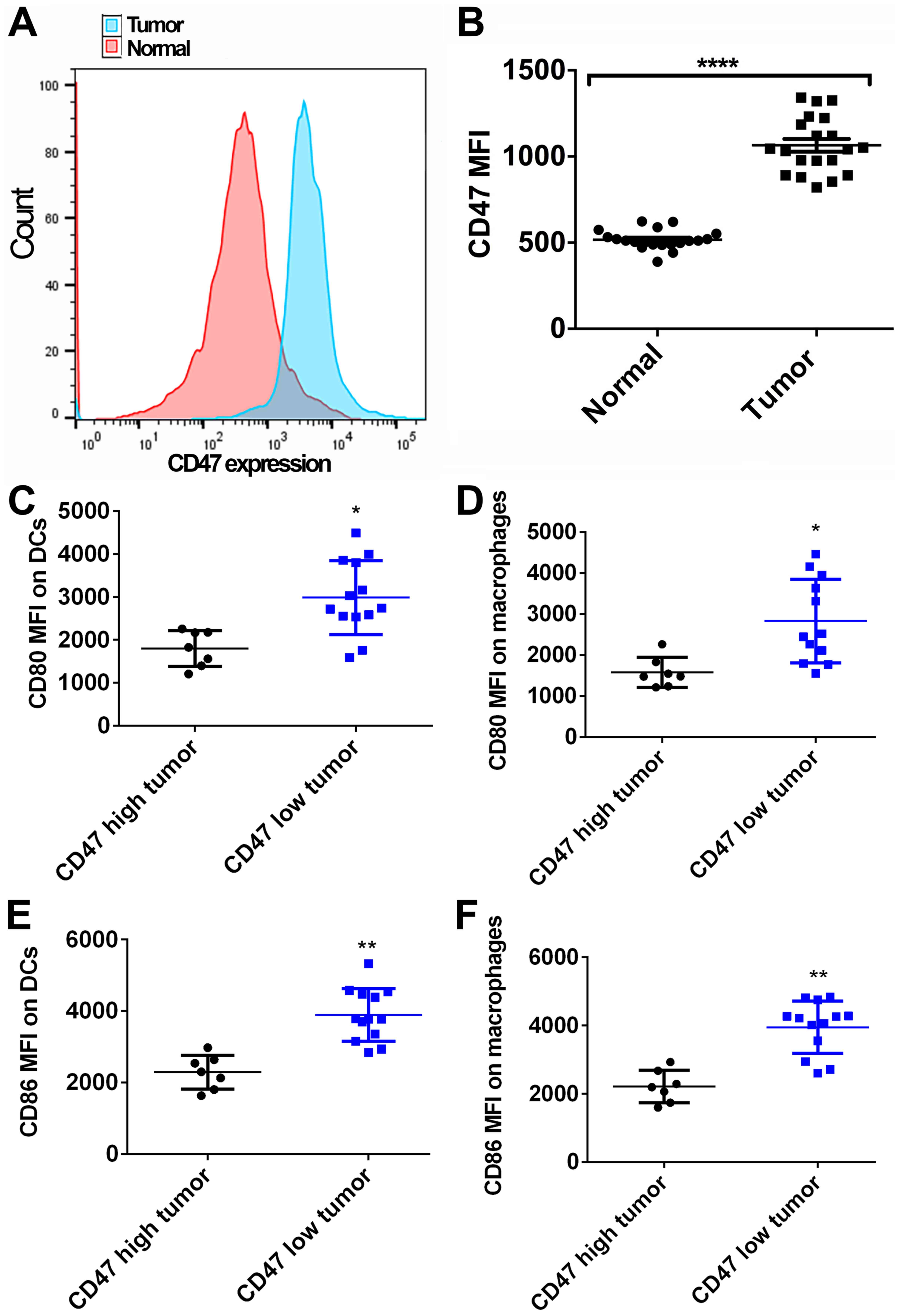
Fig. 1A and B, 20 normal fresh pancreatic tissue samples
and the paired PDAC tissue samples were collected and analyzed by
FACS. The CD47 expression on tumor cells was found to be higher in
the PDAC tissue as compared with that on ductal cells in the
adjacent normal pancreatic tissue (P<0.0001). Furthermore, the
effects of CD47 expression on DC and macrophages' activation were
evaluated. Tumor samples exhibiting higher CD47 expression level
than the mean value were considered as the CD47 high expression
tumors. A total of 7 out of 20 cases were classified as high CD47
expression tumors, while the remaining 13 cases were considered as
CD47 low expression tumors. Significantly higher levels of CD80 and
CD86 were detected in DCs and macrophages identified in CD47 low
expression tumors by FACS, in comparison to the CD47 high
expression tumors (MFI: Mean fluorescent intensity; unpaired t-test
was used to compare the CD80/CD86 MFI between indicated groups;
Fig. 1C-F). Therefore, it is
suggested that CD47 may serve an important role in promoting PDAC
development via suppressing the function of APCs.
 | Table IIClinicopathological features of the
pancreatic ductal adenocarcinoma patients. |
Table II
Clinicopathological features of the
pancreatic ductal adenocarcinoma patients.
| Feature | Value |
|---|
| Age, years |
61.5±5.8a |
| Gender
(male/female) | 12/8 |
| Tumor stage
(T2/T3) | 9/11 |
| Lymph node
metastasis (no/yes) | 14/6 |
| Distant metastasis
(no/yes) | 17/3 |
| Survival,
months |
37.6±9.3a |
| Follow-up outcome
(alive/dead) | 4/16 |
CD47 overexpression in PDAC cells
inhibits the phagocytosis of APCs in vitro
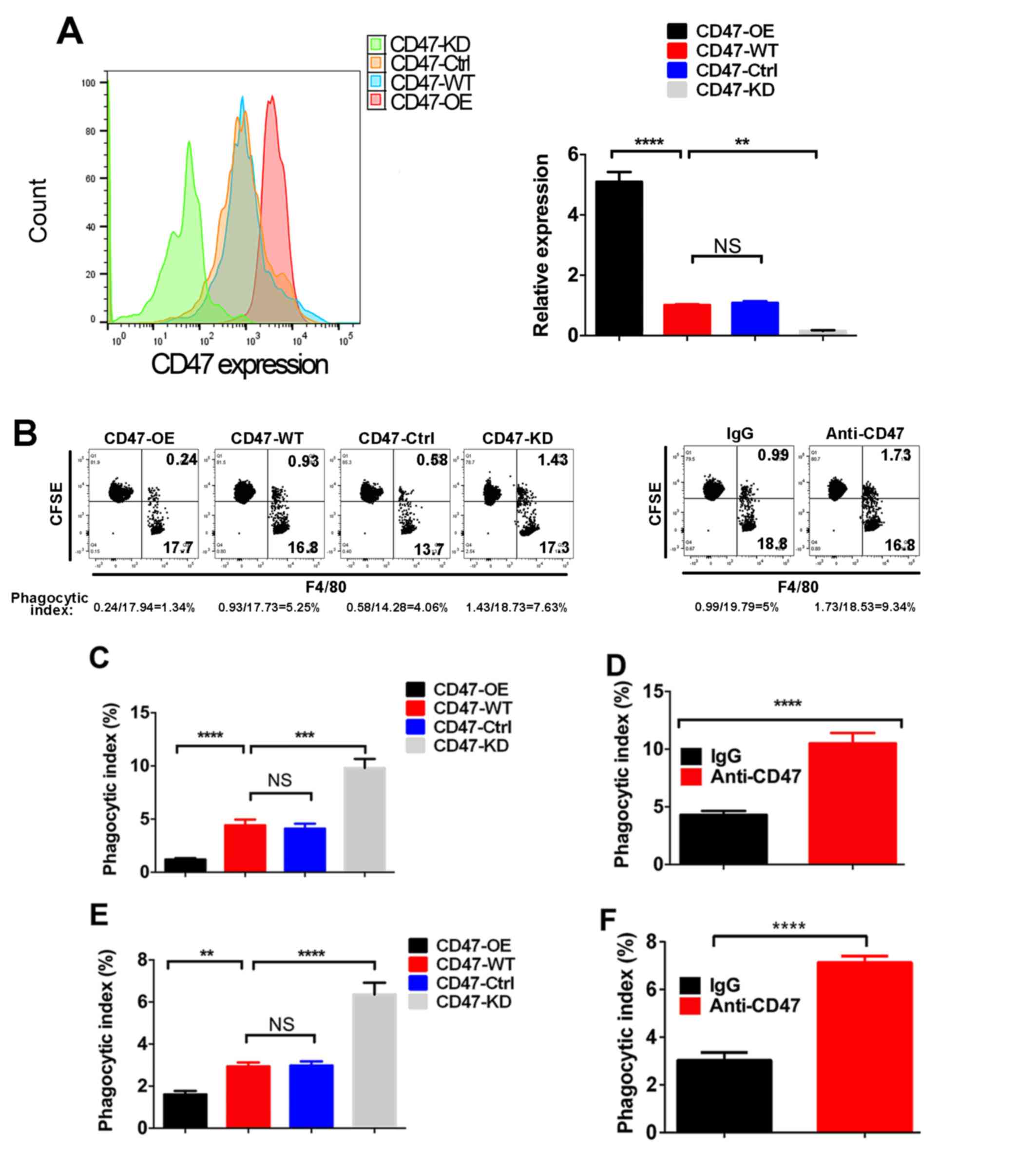
To further explore the role of CD47 in PDAC,
different CD47 expression levels were induced in the PDAC cell line
Panc02, resulting in CD47-OE, CD47-KD, CD47-WT and CD47-Ctrl cells
(Fig. 2A). Next, the phagocytosis
index of these cells was measured in the macrophage-Panc02
co-culture system via FACS method (Fig.
2B). As shown in Fig. 2C, the
phagocytosis index of CD47-OE Panc02 cells was decreased compared
with that in the CD47-WT Panc02 cells and CD47-Ctrl Panc02 cells,
while the phagocytosis index of the CD47-KD Panc02 cells was highly
increased. When CD47 was blocked by the anti-CD47 antibody, the
phagocytic function of macrophages was rescued (Fig. 2D). Similar results were observed in
DCs (Fig. 2E and F).
CD47 overexpression inhibits APC
infiltration and functions in the PDAC mouse model
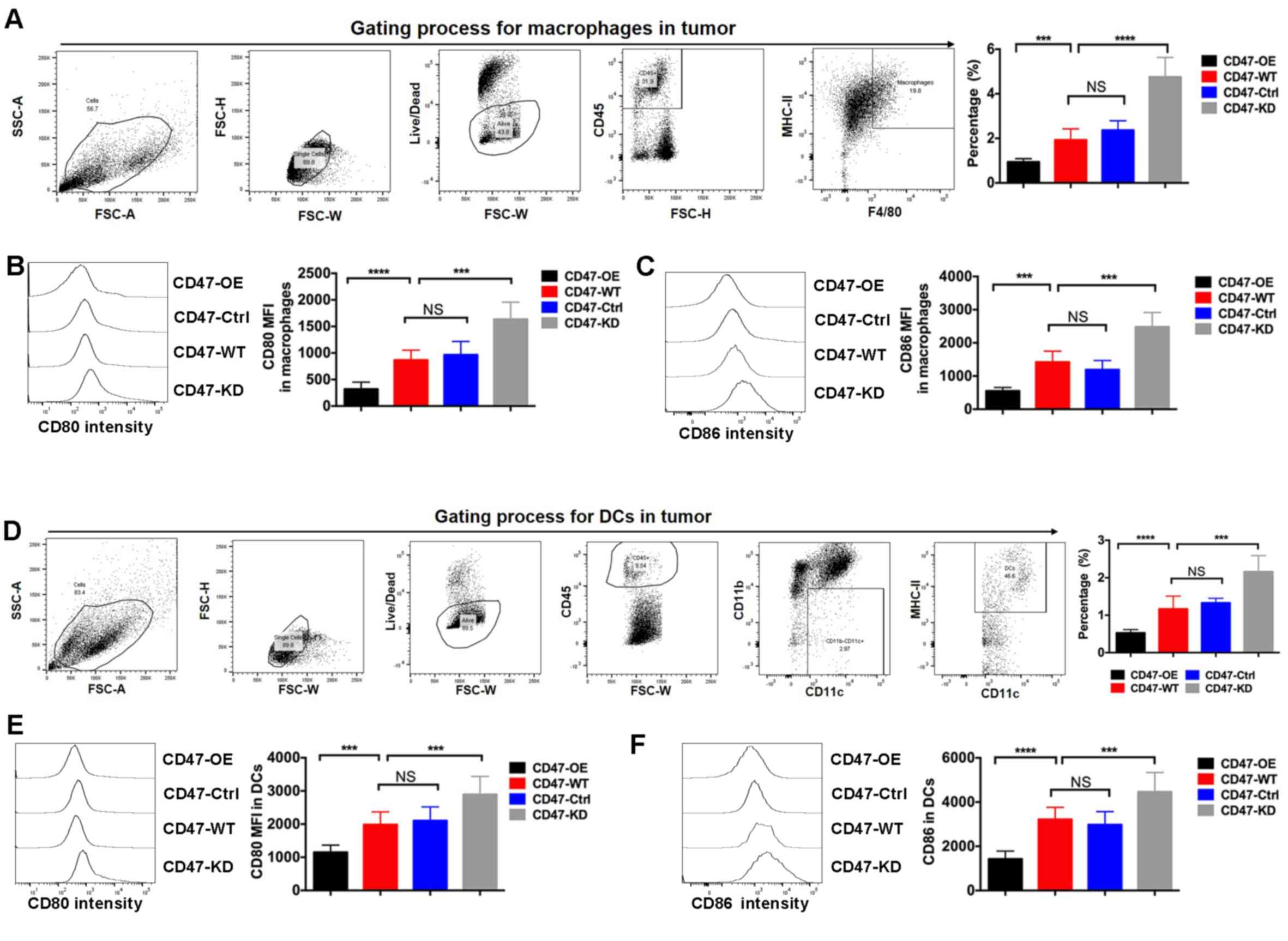
A PDAC syngeneic mouse model was further established
using Panc02 cell lines with various CD47 expression levels.
Through the FACS method, the ratio of two key APCs, namely
macrophages and DCs, was measured in tumor tissues. The results
indicated that the percentages of macrophages and DCs in CD47-OE
tumors were significantly decreased, while these were markedly
increased in the CD47-KD tumors (Fig.
3A and D). CD80 and CD86 are the
two key ligands of CD28 and CTLA4, respectively, which are critical
in modulating T-cell priming. It was further observed that the
expression levels of CD80 and CD86 were reduced in macrophages of
CD47-OE tumors (Fig. 3B and C). Similarly, the expression levels of CD80
and CD86 in DCs isolated from the CD47-OE tumors were decreased
(Fig. 3E and F).
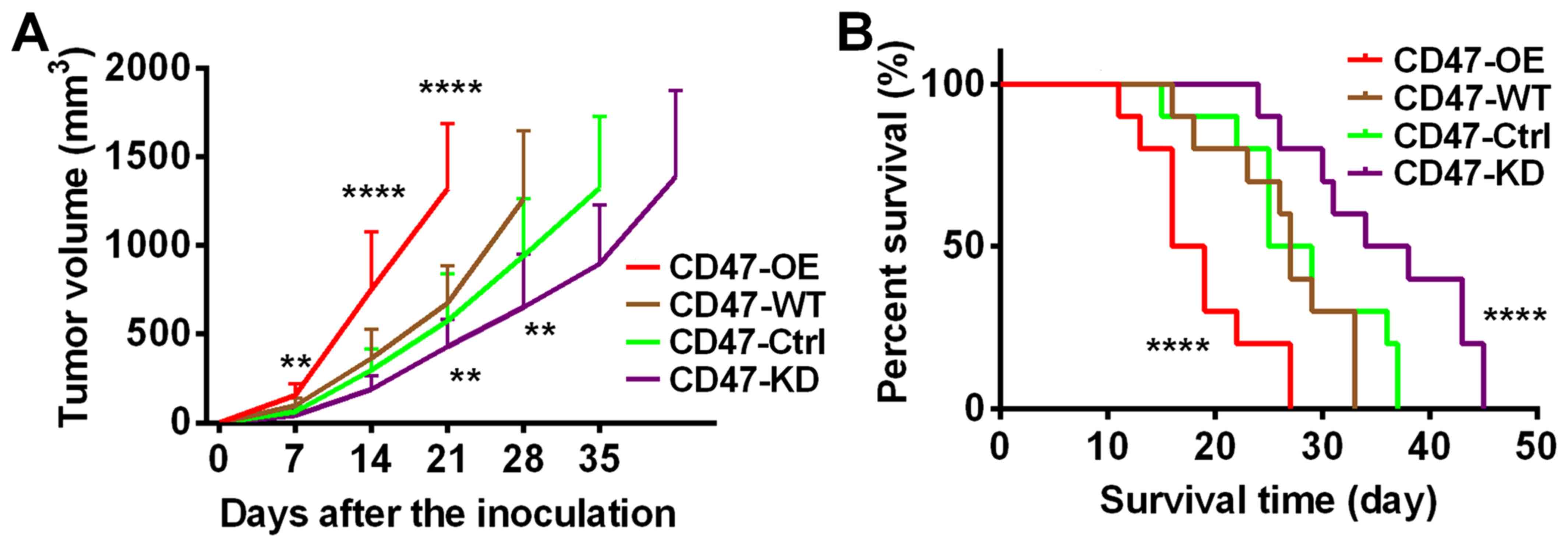
The study also investigated the effects of CD47
expression on tumor development. As shown in Fig. 4A, it was observed that PDAC tumors
with CD47 overexpression grew at a much faster rate compared with
tumors with lower CD47 expression. Consistently, the survival time
of the mice with CD47-OE tumors was the shortest among all groups
(Fig. 4B). Taken together, this
evidence indicated that CD47 suppressed the infiltration and
functions of the macrophages and DCs in PDAC tumors. These data
were in line with our findings in human samples.
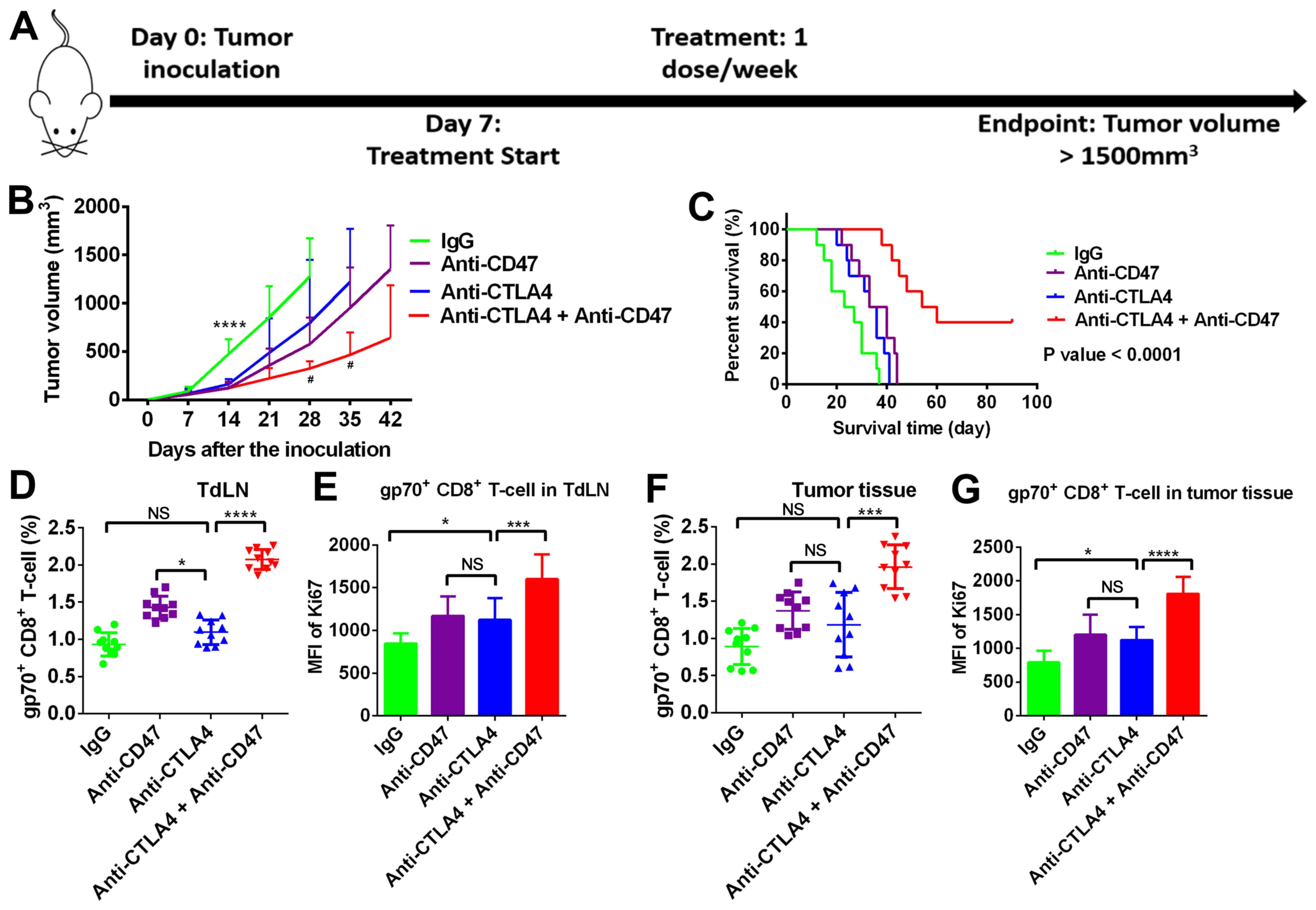
Anti-CD47 treatment enhances the
efficacy of anti-CTLA4 treatment in suppressing PDAC
development
Since anti-CD47 increased phagocytic function and B7
molecule (CD80 and CD86) expression in APCs, the current study
further investigated whether anti-CD47 is able to enhance
anti-CTLA4 efficacy in T-cell priming and suppress PDAC development
(Fig. 5A). In the PDAC subcutaneous
mouse model, the tumors co-treated with anti-CD47 and anti-CTLA4
antibodies grew much slower when compared with the untreated and
monotherapy-treated tumors (Fig.
5B). The survival time of the mice co-treated with anti-CD47
and anti-CTLA4 antibodies was evidently extended (log-rank test
P-value of <0.01; Fig. 5C). The
present study further investigated T-cell priming in the
tumor-draining lymph node (TdLN) and T-cell infiltrating in tumor
tissues. As shown in Fig. 5D and
E, the numbers and proliferation
rate of tumor-specific CD8 T-cells (gp70+) in TdLN were
significantly increased by the anti-CD47 and anti-CTLA4 combination
treatment. Similar results were observed in tumor tissues (Fig. 5F and G).
Discussion
CD47 is a membrane protein of the immunoglobulin
superfamily that is widely expressed on human cells and
overexpressed in certain types of cancer (1). CD47 negatively regulates phagocytosis
of macrophages and DCs by interacting with TSP-1 and SIRPα
(1). Previous studies have
demonstrated that CD47 overexpression promoted tumor development
via suppressing the phagocytic function of macrophages and innate
antitumor immunity. However, the role of CD47 in regulating antigen
presentation in PDAC and the value of targeting CD47 in treating
PDAC have yet to be determined.
The expression and prognostic value of CD47 have
been studied in multiple types of cancer, including breast,
gastric, ovarian and esophagus cancer (2-5).
In line with these previous studies, the results of the present
study revealed that CD47 expression was upregulated in PDAC tumors
by comparing PDAC tissues with tumor-adjacent normal tissues. In
addition, the B7 molecule expression on APCs was compared in human
tumors exhibiting high and low CD47 expression. Notably, the B7
molecules showed higher expression in APCs isolated from CD47 low
expression tumors. These data suggested that CD47 exerted tumor
promoting effects in PDAC and provided the rationale for
investigating its roles in regulating antigen presentation.
Phagocytic cells, such as DCs and macrophages, have
profound antitumor activity via killing tumor cells by secreting
cytokines and functioning as APCs to stimulate adaptive immunity
(17,18). Phagocytosis mainly depends on
macrophage/DC recognition of pro-phagocytic molecules on target
cells, however it can be inhibited by simultaneous expression of
anti-phagocytic molecules, such as CD47(19). In line with previous studies, the
current study revealed that overexpression of CD47 in PDAC cells
significantly reduced the phagocytic activity of macrophages and
DCs in vitro. Furthermore, in the syngeneic PDAC mouse
model, overexpression of CD47 facilitated tumor growth, but reduced
tumor tissue infiltration of macrophages and DCs. The activation of
tumor infiltrating macrophages and DCs was further examined, which
was also found to be suppressed by CD47-overexpressing PDAC
cells.
In the PDAC mouse model of the current study,
blocking CD47 reduced tumor development and prolonged animal
survival. In a previous esophagus cancer model, blocking CD47
significantly restored T-cell activation and delayed tumor
development (5). Similar antitumor
effects of anti-CD47 treatment were also observed in ovarian cancer
and glioma (4,20). More notably, the present study
results indicated that blocking CD47 in tumor cells has
demonstrated positive effects in sensitizing PDAC to the currently
used immune checkpoint blockade agent anti-CTLA4 through the
mechanisms of enhancing antigen presentation. These observations
were in line with the findings of a recent study in an esophageal
cancer model (5). It is known that
impaired antigen presentation, which relies on the function of
phagocytic cells, is one major reason for resistance to immune
checkpoint blockades (21-23).
PDAC is considered as a poor immune infiltrated cancer that is
highly resistant to immunotherapies (24,25).
Thus, the data of the present study implied the potential of
combining anti-CD47 treatment with the existing immune checkpoint
blockades in future clinical trials. The effect of anti-CD47
treatment in PDAC has also been highlighted in the study by Cioffi
and colleagues (26). It was
reported that anti-CD47 treatment alone did not result in direct
inhibition of tumor growth. However, when combined with
chemotherapy, anti-CD47 treatment achieved sustained tumor
regression in patient-derived xenograft models (26). Another study reported that anti-CD47
treatment increased the clearance of PDAC cells by macrophages
(27). In line with these previous
findings, the present study highlighted the therapeutic value of
anti-CD47 on PDAC when combined with immune checkpoint
inhibitors.
In conclusion, the present study revealed that CD47
is overexpressed in PDAC tissues, while overexpression of CD47
accelerated PDAC development by inhibiting the function of APCs.
Blocking the CD47 function using monoclonal antibody treatment
ehanced the efficacy of anti-CLTA4 treatment in suppressing
preclinical PDAC development. Therefore, combining anti-CD47
treatment with current standard immune checkpoint therapies may be
of great value against PDAC in future studies.
Acknowledgements
Not applicable.
Funding
The study was funded by Xi'an Gaoxin Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS and ZL analyzed and interpreted the patient data
and performed the in vitro and in vivo experiments.
JX analyzed the experimental data, and was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xi'an Gaoxin Hospital, Ganzhou, Jiangxi, China.
Informed consent was obtained from all patients or their legal
representatives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matozaki T, Murata Y, Okazawa H and
Ohnishi H: Functions and molecular mechanisms of the CD47-SIRPalpha
signalling pathway. Trends Cell Biol. 19:72–80. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baccelli I, Stenzinger A, Vogel V,
Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M,
Holland-Letz T, Sinn HP, et al: Co-expression of MET and CD47 is a
novel prognosticator for survival of luminal breast cancer
patients. Oncotarget. 5:8147–8160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoshida K, Tsujimoto H, Matsumura K,
Kinoshita M, Takahata R, Matsumoto Y, Hiraki S, Ono S, Seki S,
Yamamoto J and Hase K: CD47 is an adverse prognostic factor and a
therapeutic target in gastric cancer. Cancer Med. 4:1322–1333.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Liu R, Wei H, Gao P, Yu H, Wang K, Fu Z,
Ju B, Zhao M, Dong S, Li Z, et al: CD47 promotes ovarian cancer
progression by inhibiting macrophage phagocytosis. Oncotarget.
8:39021–39032. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tao H, Qian P, Wang F, Yu H and Guo Y:
Targeting CD47 enhances the efficacy of anti-PD-1 and CTLA-4 in an
esophageal squamous cell cancer preclinical model. Oncol Res.
25:1579–1587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu L, Zhang L, Yang L, Li H, Li R, Yu J,
Yang L, Wei F, Yan C, Sun Q, et al: Anti-CD47 antibody as a
targeted therapeutic agent for human lung cancer and cancer stem
cells. Front Immunol. 8(404)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang X, Fan J, Wang S, Li Y, Wang Y, Li
S, Luan J, Wang Z, Song P, Chen Q, et al: Targeting CD47 and
autophagy elicited enhanced antitumor effects in non-small cell
lung cancer. Cancer Immunol Res. 5:363–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paulson AS, Cao HS, Tempero MA and Lowy
AM: Therapeutic advances in pancreatic cancer. Gastroenterology.
144:1316–1326. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Weber J, Mandala M, Vecchio MD, Gogas HJ,
Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: Adjuvant nivolumab versus ipilimumab in
resected stage III or IV melanoma. N Engl J Med. 377:1824–1835.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Reck M, Schenker M, Lee KH, Provencio M,
Nishio M, Lesniewski-Kmak K, Sangha R, Ahmed S, Raimbourg J, Feeney
K, et al: Nivolumab plus ipilimumab versus chemotherapy as
first-line treatment in advanced non-small-cell lung cancer with
high tumour mutational burden: Patient-reported outcomes results
from the randomised, open-label, phase III CheckMate 227 trial. Eur
J Cancer. 116:137–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Winer A, Ghatalia P, Bubes N, Anari F,
Varshavsky A, Kasireddy V, Liu Y and El-Deiry WS: Dual checkpoint
inhibition with ipilimumab plus nivolumab after progression on
sequential PD-1/PDL-1 inhibitors pembrolizumab and atezolizumab in
a patient with lynch syndrome, metastatic colon, and localized
urothelial cancer. Oncologist. 24:1416–1419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Symeonides SN, Anderton SM and Serrels A:
FAK-inhibition opens the door to checkpoint immunotherapy in
pancreatic cancer. J Immunother Cancer. 5(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu FJ and Komarovskaya M: CTLA4 blockade
maximizes antitumor T-cell activation by dendritic cells presenting
idiotype protein or opsonized anti-CD20 antibody-coated lymphoma
cells. J Immunother. 25:455–468. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Scrimieri F, Askew D, Corn DJ, Eid S,
Bobanga ID, Bjelac JA, Tsao ML, Allen F, Othman YS, Wang SC and
Huang AY: Murine leukemia virus envelope gp70 is a shared biomarker
for the high-sensitivity quantification of murine tumor burden.
Oncoimmunology. 2(e26889)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Klimp AH, de Vries EGE, Scherphof GL and
Daemen T: A potential role of macrophage activation in the
treatment of cancer. Crit Rev Oncol Hematol. 44:143–161.
2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Gordon S: Phagocytosis: An immunobiologic
process. Immunity. 44:463–475. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gholamin S, Mitra SS, Feroze AH, Liu J,
Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, et
al: Disrupting the CD47-SIRPalpha anti-phagocytic axis by a
humanized anti-CD47 antibody is an efficacious treatment for
malignant pediatric brain tumors. Sci Transl Med.
9(eaaf2968)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pitt JM, Vétizou M, Daillère R, Roberti
MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M,
Kroemer G and Zitvogel L: Resistance mechanisms to
immune-checkpoint blockade in cancer: Tumor-intrinsic and
-extrinsic factors. Immunity. 44:1255–1269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sindoni A, Minutoli F, Ascenti G and
Pergolizzi S: Combination of immune checkpoint inhibitors and
radiotherapy: Review of the literature. Crit Rev Oncol Hematol.
113:63–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mace TA, Shakya R, Pitarresi JR, Swanson
B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L,
Young G, et al: IL-6 and PD-L1 antibody blockade combination
therapy reduces tumour progression in murine models of pancreatic
cancer. Gut. 67:320–332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cioffi M, Trabulo S, Hidalgo M, Costello
E, Greenhalf W, Erkan M, Kleeff J, Sainz B Jr and Heeschen C:
Inhibition of CD47 effectively targets pancreatic cancer stem cells
via dual mechanisms. Clin Cancer Res. 21:2325–2337. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Michaels AD, Newhook TE, Adair SJ, Morioka
S, Goudreau BJ, Nagdas S, Mullen MG, Persily JB, Bullock TNJ,
Slingluff CL Jr, et al: CD47 blockade as an adjuvant immunotherapy
for resectable pancreatic cancer. Clin Cancer Res. 24:1415–1425.
2018.PubMed/NCBI View Article : Google Scholar
|