Introduction
Cementocytes are the cellular components of
cementum. The cementum, which is the thin mineral layer lining the
dental root and connecting the periodontal ligaments (PDLs),
functions as the anchor of the teeth to the PDLs and as a regulator
of tooth position (1). Loss of
cementum leads to periodontal disorder and tooth displacement and
loss (2). It has been previously
reported that 48-66% of roots suffer resorption of cementum after
orthodontic treatment, indicating the susceptibility of the
cementum to orthodontic force (3).
Although the root's outermost layer, the cellular cementum, which
is also known as cellular intrinsic fiber cementum, is considered
to exhibit a general protective function against resorption, the
response and function of cementocytes are largely unknown (4).
The limited understanding of the regulatory role of
cementocytes under orthodontic stimulation is attributed to: i)
In vitro difficulties in accessing and characterizing the
cementocytes, which are terminally differentiated cells, and are
embedded with hard dental roots in small quantities; ii) lack of
immortalized cell lines that resemble the phenotypic features of
non-proliferating mature cementocytes (5,6); iii)
technical challenges in establishing an in vitro stress
loading model to replicate the orthodontic force on the cementum,
using regular two-dimensional (2D) monolayer cultured cells
(7-9).
To improve the understanding of cementocytes, the
cementocyte cell line IDG-CM6 has been established, and efforts
have been made to generate an in vitro analysis system
(6). Force types, such as cyclic
tensile and intermittent compression forces, have been used to
analyze cementoblasts and periodontal cells (10,11).
However, the results of these systems are variable, and the
association of these systems with in vivo mechanical loading
requires additional elucidation. The majority of the stress loading
models, which have been developed in vitro to study cellular
mechanotransduction (11-17),
are based on cells cultured in 2D monolayer disks or flasks, which
lack the characteristics of the natural tissue microenvironment
(11,14,16,17).
Previous studies have indicated that three-dimensional (3D)
cultures can induce differentiation of osteoblasts (18), simulate the in vivo
differentiation pathway (19) and
replicate a more realistic tissue microenvironment where cells may
be subjected to mechanical loading (8). 3D cell models are mostly assembled by
gels or scaffolds (13,20-23),
both of which can imitate the in vivo spatial structure, and
demonstrate the features of cells subjected to mechanical forces.
Due to their elasticity, collagen gels are widely used as supports
and as a transfer of direct compressive force (15,24-26).
These 3D culture models are principally used to study
differentiation and mineralization of osteoblasts and osteocytes.
However, 3D culture models for ex vivo investigation of
cementocytes have not been reported to date, to the best of our
best knowledge.
The principal aims of the current study were to
establish an in vitro 3D stress loading model replicating
orthodontic force on cementocytes and investigate the response of
cementocytes to continuous compressive force of varying magnitude,
via evaluating the expression of the biomarkers involved in
cementum remodeling.
Materials and methods
2D cell culture
The murine cementocyte-like cell line IDG-CM6 was
kindly provided by Professor Lynda F. Bonewald (Indiana University,
Indianapolis, USA) and used in the experiments after mycoplasma
testing using a mycoplasma detection kit (Beijing Solarbio Science
& Technology Co., Ltd.) (6).
This murine cell line was derived from the cementum of
Immortomouse/dentin matrix protein 1 (DMP1)-green fluorescent
protein (GFP)+/– mice, and the immortalization was
mediated via interferon-gamma (IFN-γ) to express a thermolabile
large T antigen, as previously described (6). Cells within passages 5-15 were thawed
at 33˚C and resuspended in α-minimum essential medium (α-MEM) with
L-glutamine and nucleosides (HyClone; Cytiva), supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin. Recombinant mouse IFN-γ (50
U/ml; Thermo Fisher Scientific, Inc.) was also added in the
proliferation medium. Cells were then distributed on
collagen-coated dishes (rat tail type I collagen at a concentration
of 0.15 mg/ml in 0.02 M acetic acid; Corning Inc.) and incubated at
33˚C with 5% CO2 for 24 h. At 80-90% confluency, cells
were sub-cultured with 0.05% trypsin/0.53 mM EDTA solution (Gibco;
Thermo Fisher Scientific, Inc.) at 33˚C for 2 min.
3D cell culture system
The 3D culture system was based on a protocol
described previously (27), which
was slightly modified. The cells were resuspended in α-MEM and
added to the hydrogel mixture on ice. The constructs comprised 5%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 10% 5X αMEM (Gibco;
Thermo Fisher Scientific, Inc.), 20% Matrigel (8-12 mg/ml; Corning
Inc.), 40% rat tail type I collagen (3.79-4.10 mg/ml; Corning Inc.)
and 25% cell suspension (1-2x106 cells/ml), and were
neutralized with 1M sodium hydroxide (Merck KGaA). 500 µl of the
mixture were distributed per well in a 24-well plate. The plate was
subsequently incubated at 37˚C for 1 h for gel polymerization. The
proliferation medium was α-MEM supplemented with L-glutamine and
nucleosides, 10% FBS, penicillin (100 U/ml) and streptomycin (100
µg/ml) and recombinant mouse IFN-γ (50 U/ml). The cell constructs
were cultured at 33˚C with 5% CO2 for 24 h.
Cell osteogenic differentiation
The overexpression of the thermolabile large T
antigen, which is a technique widely used in cell line
immortalization, induces cell division in immortalized cells
(28). In a previous study, in the
case of the IDG-CM6 cell line, T antigen was expressed at 33˚C,
which induced cell division, but the level of T antigen was
decreased after culture of the cells for 24 h at 37˚C. In the
absence of T antigen, the cells better resemble the mature
cementocytes in the osteogenic condition (6). To induce cementocyte-like
differentiation, IDG-CM6 cells in 2D and 3D cultures were incubated
at 37˚C with 5% CO2 for 0-35 days. The first day
incubated at 37˚C was called day 0. The growth medium was replaced
by differentiation medium comprising α-MEM containing 10% FBS, 50
mg/ml ascorbic acid (Merck KGaA), 4 mM β-glycerophosphate (Merck
KGaA), penicillin (100 U/ml) and streptomycin (100 µg/ml). The
cells were observed by light microscopy (Zeiss GmbH) at x200
magnification every 2-3 days.
Proliferation assay
The cell proliferation of IDG-CM6 cells under
differentiation conditions was assessed via Cell Counting Kit-8
(CCK-8) assay (Shanghai Yeasen Biotechnology Co., Ltd.) according
to the manufacturer's recommendations. In brief, cells were
cultured in the aforementioned 3D culture system and
differentiation conditions for 0, 3, 7, 10, 14, 21 and 35 days. The
cells at each time point were incubated with CCK-8 solution for 2 h
at 37˚C, and the absorbance at a wavelength of 450 nm was measured.
The aforementioned IDG-CM6 cells differentiated on conventional
monolayer dishes (2D) at each time point were used as the control
group.
Alkaline phosphatase (ALP) staining
and ALP activity assay
5-Bromo-4-chloro-3-indolyl-phosphate/nitro blue
tetrazolium (BCIP/NBT) Color Development kit (Beyotime Institute of
Biotechnology) was used for ALP staining according to the
manufacturer's instructions. The 3D and 2D cultured cells were
seeded in 24-well-plates at a density of 2x105
cells/well in differentiation medium at 37˚C with 5% CO2
for 0, 5, 7, 10, 14 and 21 days. Subsequently, cells were fixed
with 4% paraformaldehyde for 10 min at room temperature. After
washing twice with ice-cold PBS, the cells were stained with
BCIP/NBT for 45 min at room temperature prior to observation under
a scanner (Canon, Inc.).
For the ALP activity assay, 3D and 2D cultured cells
were incubated in 24-well-plates at a density of 2x105
cells/well in differentiation medium at 37˚C with 5%
CO2. After 0, 5, 7, 10, 14 and 21 days of incubation,
cells were washed twice with PBS and were lysed on ice for 20 sec
in 450 µl ALP buffer with 0.2% TritonX-100 (Beijing Solarbio
Science & Technology Co., Ltd.), followed by two freeze-thaw
cycles, and the total protein level was subsequently quantified
using a BCA kit (Thermo Fisher Scientific, Inc.). A total of 100 µl
p-nitrophenyl phosphate (pNPP; Merck KGaA) was added to the same
volume of cell lysate, and incubated at 37˚C for 30 min. The
absorbance at 405 nm was read in triplicate, and ALP activity was
normalized to the total protein level and expressed as µmol pNPP
produced per min/mg protein.
GFP expression
The IDG-CM6 cell line is derived from
Immortomouse/DMP1-GFP+/− mice, which dispose a
DMP1-cis-regulatory system driving DMP1-induced GFP expression
(29). Therefore, using IDG-CM6
cells derived from DMP1-GFP+/− mice allowed the
identification of DMP1-expressing cells via monitoring the
expression of GFP. For the observation of DMP1-GFP, the green
fluorescent signal was examined on days 3, 7, 14, 21 and 35 via
fluorescence microscopy at x40 magnification (Olympus Corporation).
For quantification purposes, 3D and 2D cultured IDG-CM6 cells were
incubated in 24-well plates at a density of 2x105
cells/well in differentiation medium at 37˚C with 5%
CO2. After 0, 7, 14, 21, 28 and 35 days of
differentiation, cells were lysed with RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.) on ice and centrifuged
at 12,000 x g for 10 min at 4˚C. The fluorescence intensity of 100
µl cell lysates was measured with an Infinite® 200
fluorescence plate reader (96-well) at an excitation of 460 nm and
an emission of 508 nm (Tecan Group, Ltd). The relative fluorescence
units were normalized to the total protein concentration quantified
by the BCA assay, as previously described (6).
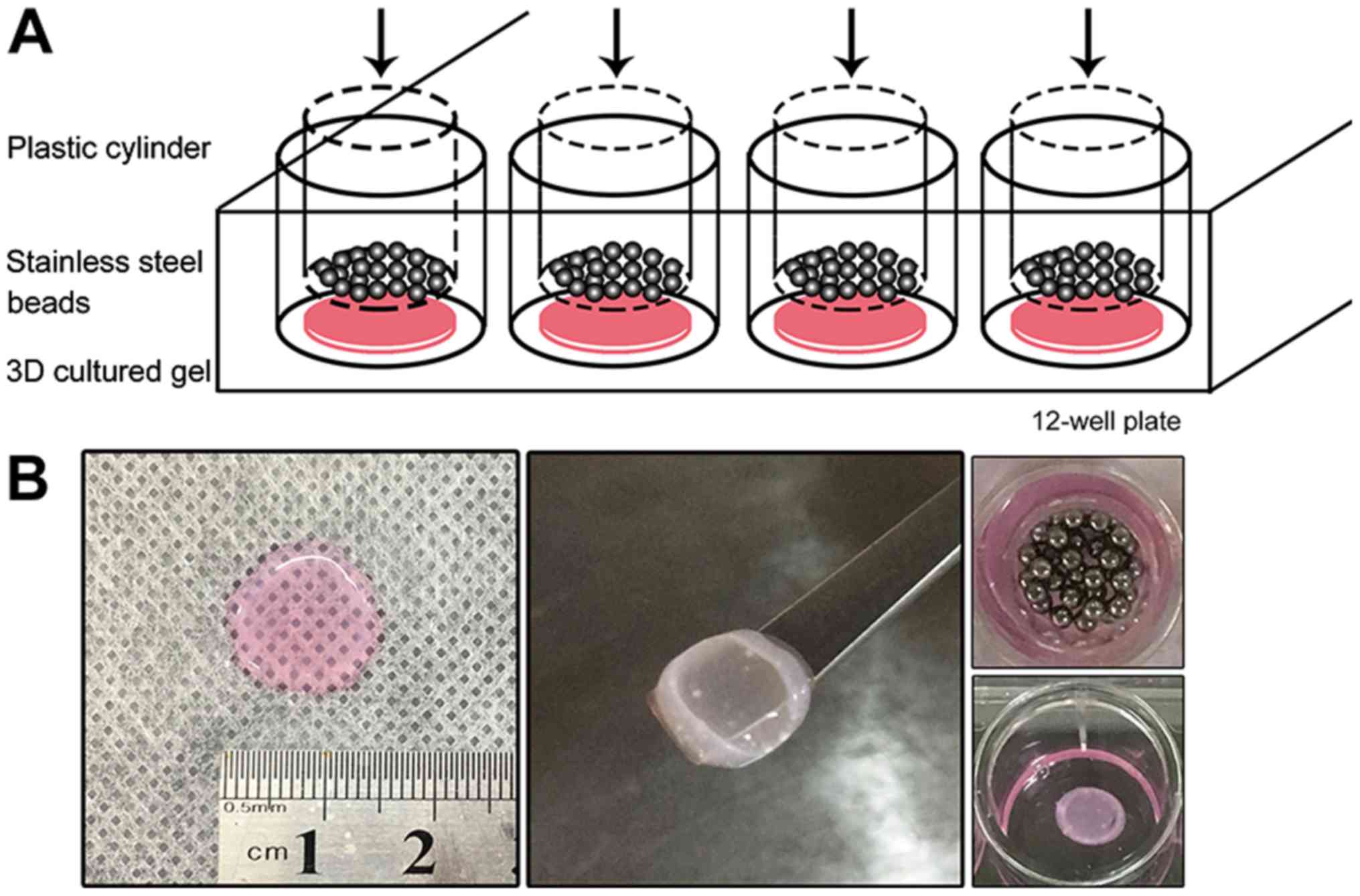
Static compressive loading
The 3D cultured gel with a density of
2x105 cells/gel was transferred to a 12-well plate with
differentiation medium after the size of the gel was measured
(Fig. 1). The gels were 137±17 mm in
diameter and 1.71±0.08 mm in thickness. Sterilized stainless-steel
beads were weighed and placed in a sterilized plastic cylinder. The
beads and cylinder were placed onto the gels, using the gravity to
replicate the static compression applied to the cells. The pressure
loaded on the gel was calculated and adjusted to 2, 4, 6 and 8
g/cm2 using the following formula: P=4m/πd2
(m, total weight of the beads and cylinder; d, diameter). Previous
studies have reported cell response to compressive loading ranging
from 1-24 h (11,19). A total of 8 g/cm2 of force
was applied on the 3D cultured gel for 2-24 h in the preliminary
test. It was found that within 6 h, the cell viability did not
significantly decrease, but increasing cell death was detected
thereafter. Thus, a 6 h duration was used for subsequent tests.
During the compression, the cells were incubated at 37˚C with 5%
CO2.
Cell viability assay
LIVE/DEAD™ Viability/Cytotoxicity kit (Thermo Fisher
Scientific, Inc.) was used for evaluating cell viability according
to the manufacturer's protocol. A solution of 2 µM calcein
acetoxymethyl (calcein AM) and 4 µM ethidium homodimer (EthD-1) was
added to the gel sample, and subsequently incubated for 30 min at
room temperature. Calcein AM is retained in live cells generating
green fluorescence, whereas EthD-1 generates red fluorescence in
dead cells (30). At least five
separate fields at x200 magnification were captured with a
fluorescence microscope (Olympus Corporation). Positive cells were
counted in each field using ImageJ v1.52 software (National
Institutes of Health). Cell viability was calculated as a
percentage of the calcein AM positive cells to the total number of
cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
The 3D and 2D cultured IDG-CM6 cells were incubated
in 24-well plates at a density of 2x105 cells/well in
differentiation medium at 37˚C with 5% CO2. After 0 and
7 days of differentiation, total RNA of the cells was isolated with
a MiniBEST Universal RNA extraction kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. In addition,
after static compressive loading for 6 h, the 3D cultured gel with
a density of 2x105 cells/gel was ground in liquid
nitrogen for 1 min and total RNA was isolated with the same
extraction kit. The 3D cultured gel without compressive loading was
used as a control. The concentration was calculated by measuring
the absorbance at a wavelength of 260 nm using a
NanoDrop™ 1000 spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.). A total of 1 mg RNA was used to
synthesize complementary DNA with PrimeScript™ RT
Reagent kit (Takara Biotechnology Co., Ltd.). The reverse
transcription temperature protocol was as follows: 37˚C for 15 min
and reverse transcriptase inactivation at 85˚C for 5 sec. RT-qPCR
was performed with Applied Biosystems™ 7500 Real-Time
PCR system (Thermo Fisher Scientific, Inc.), using SYBR®
Premix Ex Taq™ (Takara Biotechnology Co., Ltd.) as a
probe. The mRNA levels of mineral metabolic markers, including
sclerostin (SOST), osteoprotegerin (OPG), receptor activator of
NF-κB ligand (RANKL) and osteocalcin (OCN) were quantified. The
thermocycling conditions of the qPCR was as follows: Initial
denaturation at 95˚C for 30 sec; followed by 40 cycles of
denaturation at 95˚C for 5 sec, annealing at 60˚C for 34 sec and
extension at 72˚C for 30 sec. The results were analyzed with the
2-ΔΔCq method to calculate the relative RNA levels,
which were normalized to GAPDH (31). The primers were synthesized by Sangon
Biotech Co., Ltd., and the primer sequences are listed in Table I.
 | Table IList of primers used for reverse
transcription quantitative PCR. |
Table I
List of primers used for reverse
transcription quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| GAPDH |
ATGTGTCCGTCGTGGATCTG |
TGAAGTCGCAGGAGACAACC |
| OPG |
ACCCAGAAACTGGTCATCAGC |
CTGCAATACACACACTCATCACT |
| RANKL |
CAGCATCGCTCTGTTCCTGTA |
CTGCGTTTTCATGGAGTCTCA |
| SOST |
AGCCTTCAGGAATGATGCCAC |
CTTTGGCGTCATAGGGATGGT |
| OCN |
CTGACCTCACAGATCCCAAGC |
TGGTCTGATAGCTCGTCACAAG |
Statistical analysis
All experiments were repeated in triplicate. The
data are presented as the mean ± standard deviation. The
statistical analysis was performed using one-way ANOVA followed by
Tukey's post hoc test and two-way ANOVA followed by Sidak post hoc
test to determine significant differences with SPSS version 19.0
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
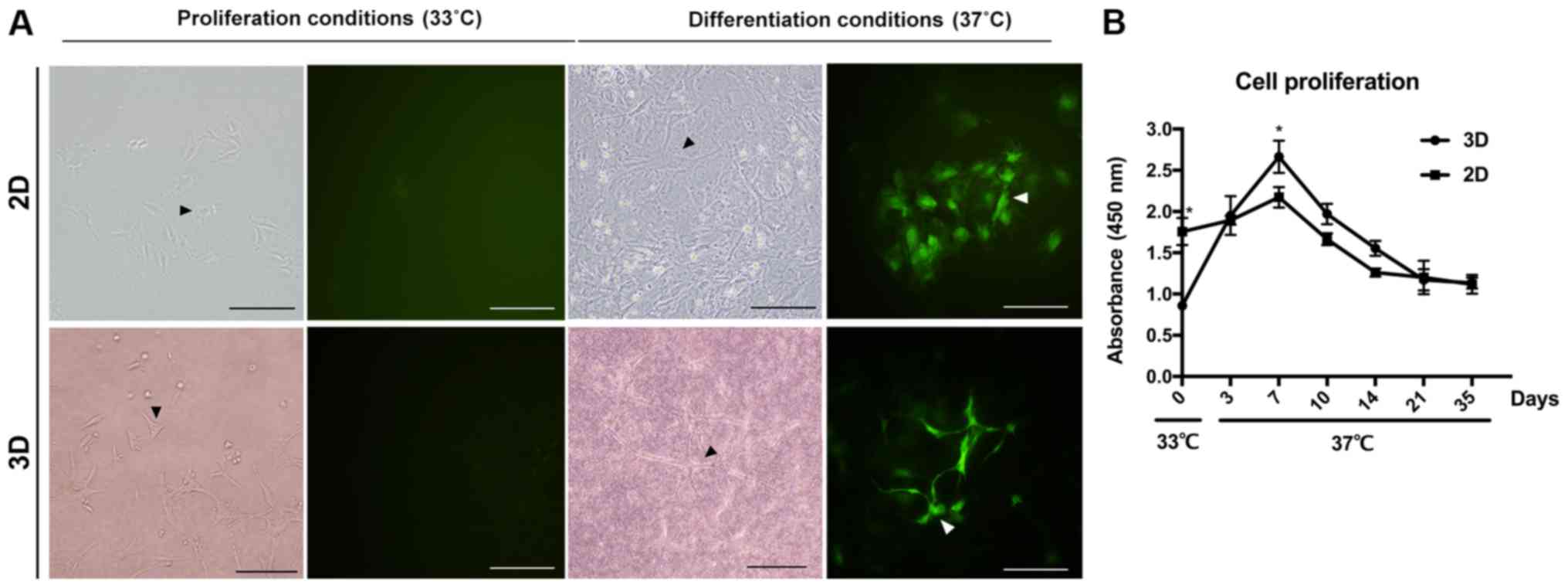
IDG-CM6 cells exhibit cementocyte-like
morphology in both 2D and 3D differentiation conditions
Cementocytes are a type of multi-dendrite cells. The
IDG-CM6 cell line is conditionally immortalized with a thermolabile
large T antigen, which induces IDG-CM6 cell division (6). Following culture of IDG-CM6 cells in
the differentiation conditions, the dendritic processes were
increased and extended both in the 2D and 3D differentiation
system. On day 28 (Fig. 2A), the
canalicular network has been formed by the extending dendrites,
which is consistent with the in vivo cellular network of
cementocytes (5,6). CCK-8 assay was performed to evaluate
the proliferation of cementocytes under differentiation conditions
in both 2D and 3D groups. In the same differentiation medium, the
cells in the 3D system exhibited lower proliferation on day 0
(Fig. 2B; P<0.05), however, at
day 7, the proliferation of the 3D was faster compared with the 2D
group (Fig. 2B; P<0.05). With
additional differentiation, cell proliferation declined from day 7
to 35 within each group, and no significance between 2D and 3D
group was observed (Fig. 2B).
IDG-CM6 cell line expresses DMP1 and
ALP in the 3D scaffold
DMP1 is a marker for cementocytes. The IDG-CM6 cell
line has been demonstrated to express DMP1 under differentiation
conditions but not immortalized conditions (6). The expression of DMP1 was evaluated via
monitoring the expression of GFP, since the IDG-CM6 cell line is
derived from the DMP1-GFP+/− mice. GFP expression was
not observed in cells cultured in immortalized conditions but in
cells in differentiation conditions, starting at day 14 in the 2D
group and day 7 in the 3D group (Fig.
3A). Relative fluorescence units of GFP (Fig. 3D) showed that after differentiation
for 21 to 35 days, the 3D group expressed higher GFP levels
compared with the 2D group (P<0.05). To evaluate the capability
of mineral deposition, which is a marker for mineral cell
differentiation, ALP staining and ALP activity assay were
performed. Decreased expression of ALP was observed in the 3D
compared with the 2D group (Fig.
3C), with a significant difference observed at days 5, 7 and 10
(P<0.05). On days 14 and 21, ALP expression peaked and exhibited
no significant difference between the groups.
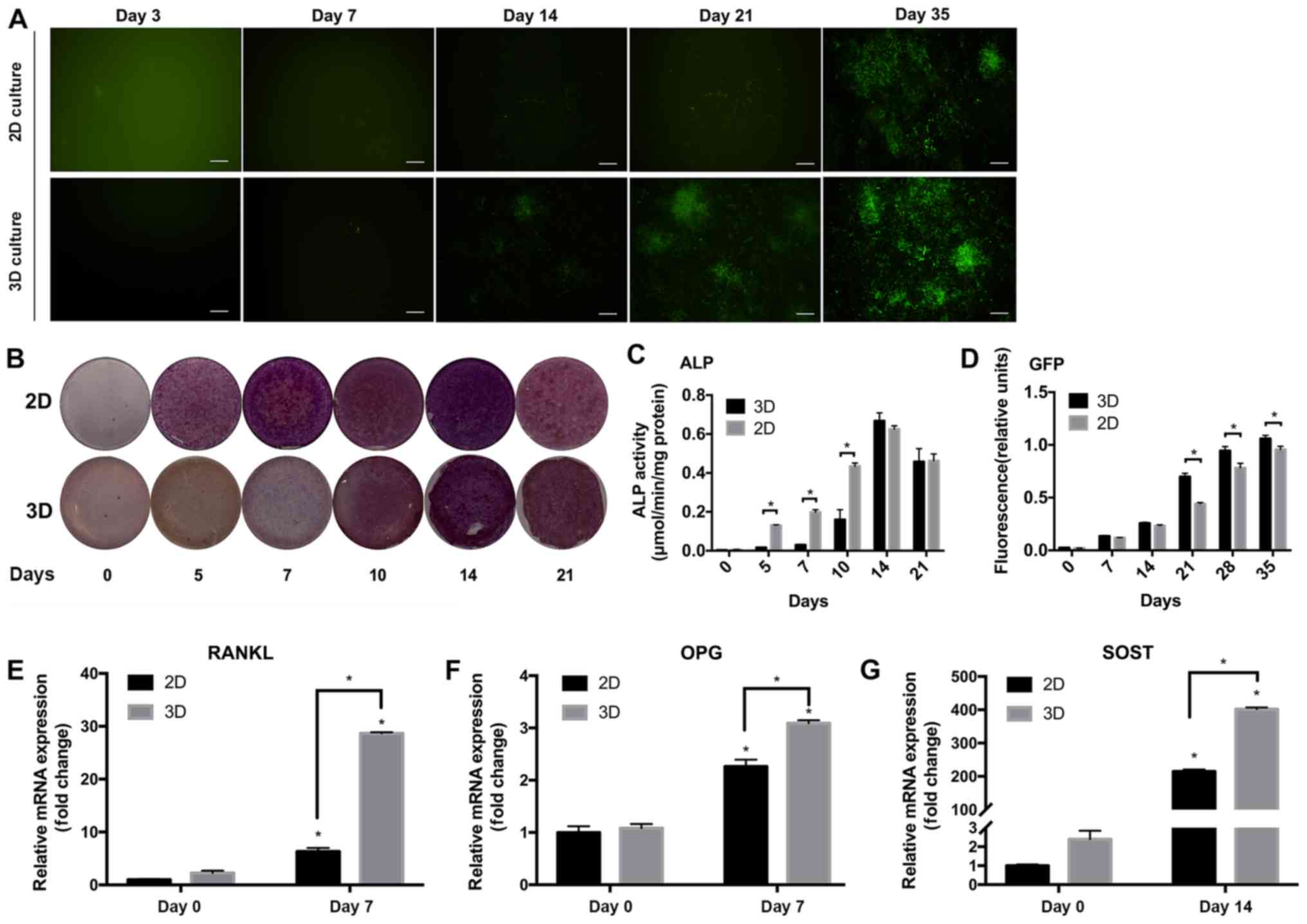 | Figure 3Differentiation of the IDG-CM6 cell
line in the 3D scaffold. (A) DMP1-associated GFP expression was
detected via fluorescence microscopy and (D) quantified. GFP
protein (indicated as green in panel A) was quantified in relative
fluorescence units and normalized to the total protein
concentration. (B) Alkaline phosphatase activity was detected by
ALP staining and (C) quantified in µmol p-nitrophenol phosphate
produced per min per mg protein. Relative mRNA expression of (E)
RANKL, (F) OPG and (G) SOST genes normalized to GAPDH. The
differentiation at 0 and 7 days was compared in the 2D or 3D
groups. Data are presented as the mean ± standard deviation.
*P<0.05. Scale bars, 20 µm. 2D, two-dimensional; 3D,
three-dimensional; GFP, green fluorescent protein; ALP, alkaline
phosphatase; RANKL, receptor activator of NF-κB ligand; OPG,
osteoprotegerin; SOST, sclerostin; DMP1, dentin matrix protein
1. |
IDG-CM6 cells express OPG, RANKL and
SOST mRNA in the 3D differentiation system
OPG, RANKL and SOST are key regulatory cytokines of
mechanotransduction, and have been detected in cementocytes
(6). The RT-qPCR results of the
current study indicated that the cells expressed higher levels of
these markers under 3D differentiation conditions compared with the
2D group or cells without differentiation (P<0.05; Fig. 3E-G).
Static compressive loading indicates
that cell viability is magnitude-dependent
The ratio of living cells (stained in green with
LIVE/DEAD staining) to total cells (stained in green and red) was
calculated to evaluate the cell viability after compression
(Fig. 4A-C). A preliminary
experiment with 8 g/cm2 compression force was applied to
optimize the treatment time. Cell viability was decreased after 12
h (63.74±3.51%; P<0.05) and 24 h (59.50±3.21%; P<0.05) of
compression (Fig. 4B). The
expression of SOST (Fig. 4D) and
RANKL (Fig. 4F) mRNA increased after
6 h of compression, but additional increase was not detected at 24
h. No significant change in OPG expression (Fig. 4E) was detected at 2, 6 and 24 h of
compression. Therefore, 6 h of compression was used for subsequent
studies to compare different force magnitudes. Cell viability
decreased by values of compressive loading (Fig. 4C), being the lowest in the 8
g/cm2 force group (72.60±0.08%). No significant
differences were observed in the 2 or 4 g/cm2 force
groups, while cell viability was significantly decreased in the 6
and 8 g/cm2 pressure groups compared with the control
group (P<0.05, respectively).
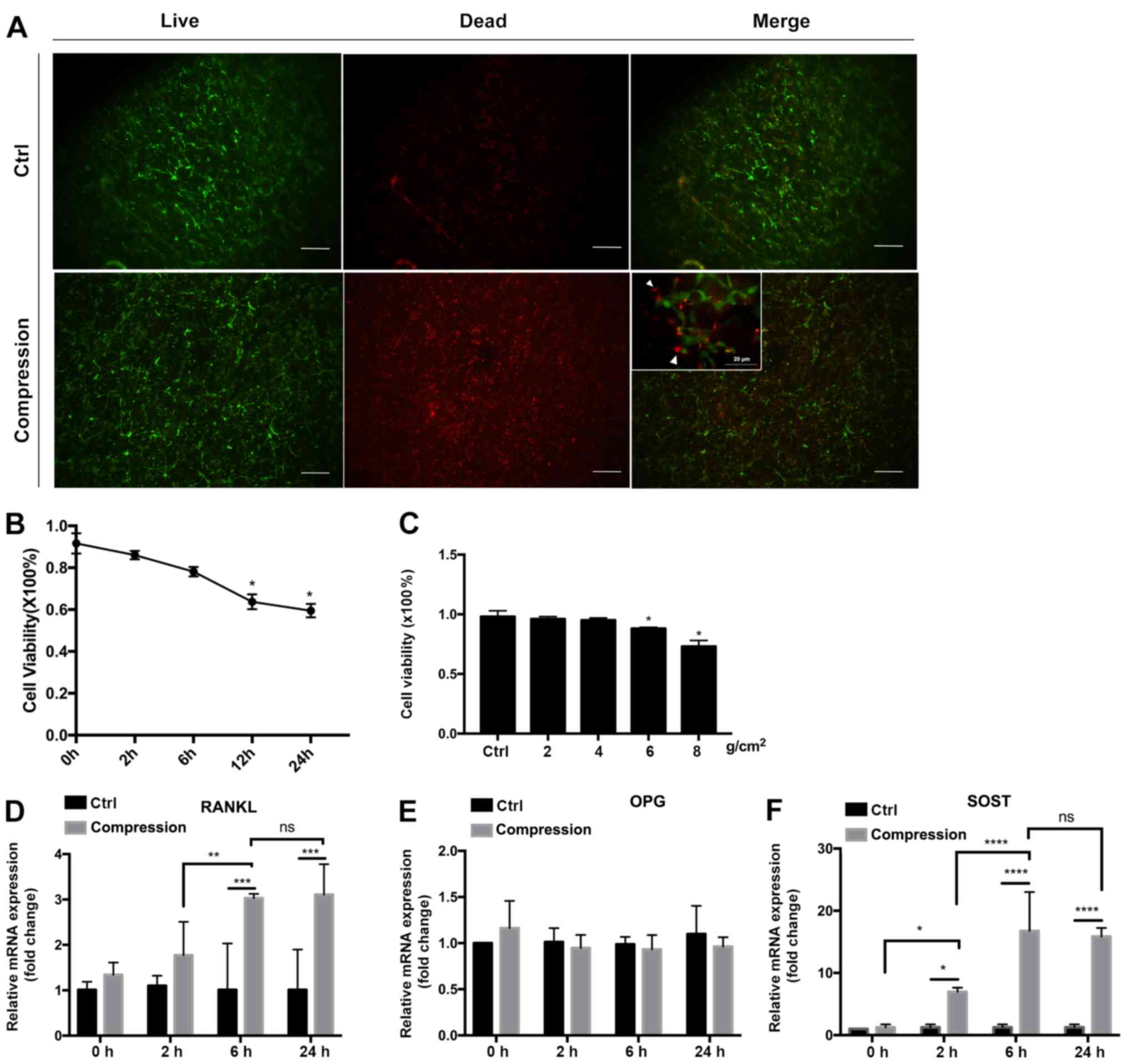 | Figure 4Cell viability and gene expression
under static compressive loading. (A) LIVE/DEAD staining at x40
magnification of IDG-CM6 cells under compression of 8
g/cm2 for 6 h and without compression (Ctrl). Inset,
merged images at x400 magnification. Living cells were stained
green, while dead cells were stained red (white arrows). The number
of red-stained cells was higher in the compression group than in
the control group. The numbers of living and dead cells were
counted, and the ratio of living vs. dead cells was calculated. (B)
Association between the cell viability and force duration when 8
g/cm2 force was applied. *P<0.05 between
each timepoint vs. 0 h. (C) Association of the cell viability with
the force magnitude when force was applied for 6 h.
*P<0.05 between each magnitude vs. ctrl. (D-F)
Relative mRNA expression of (D) RANKL, (E) OPG and (F) SOST
normalized to GAPDH in IDG-CM6 cells in response to 8
g/cm2 force. The mRNA expression was compared between
the control and the loading group, and the loading groups were
compared relatively to the force duration. Data are presented as
the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. Scale bars, 20 µm. Ctrl, control; ns,
not significant; RANKL, receptor activator of NF-κB ligand; OPG,
osteoprotegerin; SOST, sclerostin. |
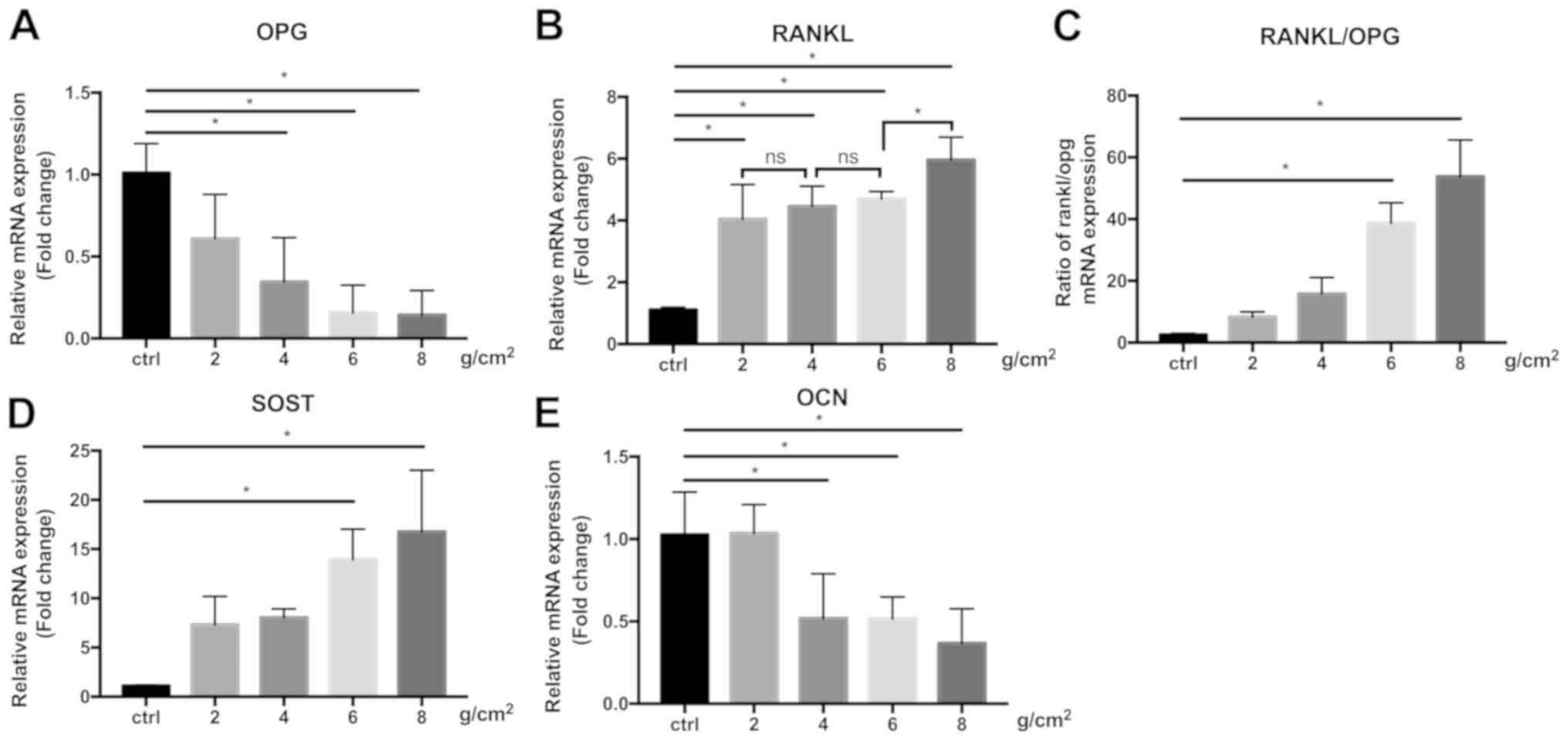
IDG-CM6 upregulates the expression of
SOST mRNA and the RANKL/OPG ratio and downregulates OCN in response
to compression
SOST is a negative marker of bone formation
(32), and the increase of SOST mRNA
expression after compression was observed in association with the
force magnitude (Fig. 5D), with 8
g/cm2 force inducing the highest increase in SOST
expression (16.76±6.26 fold-change vs. GAPDH). RANKL expression was
also significantly increased under 2, 4, 6 and 8 g/cm2
force, however differences between the force magnitudes were only
observed between 6 and 8 g/cm2(P<0.05), but not
between 2, 4 and 6 g/cm2. (Fig. 5B). IDG-CM6 cells exhibited a
decreased OPG expression (Fig. 5A)
in a force magnitude-dependent manner, with the lowest expression
observed in the 8 g/cm2 force group (0.14±0.15
fold-change vs. GAPDH). Therefore, the RANKL/OPG ratio (Fig. 5C), which is a marker of bone
remodeling during orthodontic tooth movement (33), was significantly increased in the 6
and 8 g/cm2 force groups compared with the control group
(P<0.05), and peaked in the 8 g/cm2 group
(53.78±11.86 fold-change vs. GAPDH). OCN is another marker
expressed in the cellular cementum and participates in mineral
metabolism (5,34). When subjected to compression, a
significant decline in OCN expression was observed in the 4, 6, and
8 g/cm2 (P<0.05) force groups compared with the
control group, but not in the 2 g/cm2 group (Fig. 5E).
Discussion
The current study described the establishment of a
3D cementocyte differentiation model, which may be used to
investigate cellular behavior in response to continuous compressive
loading. The results indicated that the 3D differentiation system
induced mineralized differentiation of the IDG-CM6 cell line and
maintained the cementocyte phenotype. The continuous compression on
cementocytes was indicated to induce cell death and the expression
of osteoclastogenic markers, which has also been observed in other
in vitro and clinical studies (16,35). The
results of the current study suggested that the response of
cementocytes to compression loading may be associated with
orthodontic-induced tooth root resorption.
Similar to osteocytes, cementocytes are a group of
terminally mature cells that are embedded in hard tissues. The
terminal differentiation and surrounding minerals represent
challenges for studying the function and biological behavior of
cementocytes. Previous studies have focused on in vivo
methods to explore the impact of external stimulation on the
cementum, and have revealed that orthodontic forces, inappropriate
occlusion and periodontal inflammation lead to osteoclast
differentiation and cementum resorption (3,4,16). IDG-CM6 is an immortalized cell line,
which reproduces the expression profile of cementocytes observed
in vivo (36). Zhao et
al (6) reported that IDG-CM6
presented a different cell behavior in altered culture conditions.
In immortalized conditions, the cells have been indicated to
exhibit certain features of undifferentiated cells, such as high
proliferation and low expression of DMP1, ALP and other
mineralization markers (6). On the
contrary, the absence of thermo-sensitive T antigen has been
demonstrated to induce the cells to express these mineralization
markers in differentiation conditions (28). In the present study, IDG-CM6 cells
did not express GFP (which was directly associated with DMP1
expression) under proliferation conditions. The results also
indicated that when they were transferred in the differentiation
conditions (37̊C without IFN-γ), the cells exhibited decreased
proliferation, increased expression of DMP1, ALP activity and
expression of mineralization regulators, and differentiated into
cementocyte-like cells. These results were consistent with a
previous study (6). The results of
the present study revealed that under the same differentiation
conditions, the cells cultured in 3D system expressed a higher
level of DMP1, SOST, OPG and RANKL compared with cells cultured in
conventional 2D plates. Previous studies have indicated that
collagen-based 3D scaffolds facilitated the formation of dendritic
processes in mineral cells and replicated the cell network in
vivo (22,37,38). It
has also been reported that the addition of Matrigel to collagen I
maintained the properties of osteocytes and inhibited cell
dedifferentiation (26,39). However, to the best of our knowledge,
the impact of 3D culture systems on the differentiation of
cementocytes has not been reported. In the present study, the
lacunar structure of collagen in the 3D culture system may enhance
the liquid transfer and the cell network formation, thereby
maintaining the cementocyte properties.
DMP1 has been indicated to localize in cementoblasts
and cementocytes in human and animal cementum (40,41),
acting as a key regulator of the Wnt signaling pathway in mineral
metabolism and biomineralization (42). During the formation of the cellular
cementum, DMP1 protein has been detected in the pericellular
cementum of cementocytes, including their processes, but not in
cementoblasts (43). Bae et
al (44) have reported that the
upregulation of DMP1 was associated with the thin and
hypomineralized cementum and root dentin of the
OC-Cre:Catnb+/lox(ex3) mutant mice, suggesting that DMP1
was involved in the local modulation of the Wnt/β-catenin signaling
pathway. In the current study, the cementocytes that were cultured
in 3D hydrogel supplemented with differentiation medium, expressed
a high level of GFP, which indicated that the cells also expressed
a high level of DMP1. In addition, this expression in the 3D model
was observed higher than 2D culture at day 21, 28 and 35,
indicating a more differentiated cell type.
Increased ALP activity in the 2D culture group
compared with 3D culture at days 5, 7 and 10 indicated a delay in
the increase of ALP activity in the 3D hydrogel. A marked increase
in ALP activity was detected between days 10 and 14. Therefore, the
3D culture system exhibited an equivalent level of ALP expression
as that of the 2D culture, indicating a delayed but comparative
capacity of mineralization.
Moreover, the 3D system induced the higher
expression of SOST, OPG and RANKL genes compared with the 2D
system. These markers are expressed by mature cementocytes and act
as key cytokines in mechanotransduction and mineral metabolism
(45-47).
As the cementum is the outmost layer of the tooth root, it
withstands the stress from mechanical alterations in the oral
environment, such as orthodontic therapies. Therefore, it is
possible that the 3D culture system in the present study better
represented the physiological conditions of cementocytes and
induced IDG-CM6 cells to express a higher level of metabolic
genes.
The present study also established a static
compression loading system to mimic the orthodontic compression
force, which is applied to the tooth root. Sustained orthodontic
compression has been indicated to initiate sequential events that
cause tooth movement, including the matrix and cell strain, which
result in cell activation and differentiation. However, the
underlying mechanism remains unclear (48). To explore the cellular response to
mechanical loading, efforts have been made to mimic the
microenvironment using in vitro models, with cyclic and
fluid flow shear stress being primarily been used as the loading
force (10,12). The compression system of the present
study, which utilized gravity to create a static compression force,
has been indicated to better mimic the in vivo
microenvironment (23). The
elasticity of the hydrogel has been demonstrated to lead to matrix
deformation and strain (26,49), which resemble the in vivo
matrix strain that is induced by orthodontic forces. The 3D
scaffolds have been indicated to embed the cells, and the sparse
structure has been revealed to allow the cells to spontaneously
form a canalicular network (8,22). In
addition, the compression applied to the scaffold has been
indicated to prevent direct cell damage compared with the direct
forces that are exerted on the monolayer of cells cultured on disks
or flasks (16).
The force magnitude and duration time of the
compression applied in the current study, which are two factors
closely associated with cell and tissue responses, can be adjusted
via varying the number of stainless beads and the application time.
Therefore, the 3D compression system may be an efficient tool for
investigation of the biological processes occurring during
orthodontic tooth movement and other mechanotransduction
pathways.
During orthodontic tooth movement, the forces that
are applied on the teeth are directly and indirectly transferred to
the cells surrounding the tooth root (50). Cells that are sensitive to mechanical
stress, including osteocytes and PDL cells, respond quickly to the
stimulation (51). In the
osteocyte-like cell line MLO-Y4, SOST expression has been indicated
to decrease under 1 h of compression stress (52), while in human PDL cells, SOST and
RANKL expression has been reported to increase in 24 h of
compression force (51). The current
study applied a heavy force of 8 g/cm2 to examine the
response of the cementocytes to compression, and revealed an
increase in SOST and RANKL expression, which was consistent with
the previous results on PDL cells. However, compared to the
previous study regarding with the PDL cells (51) and osteocytes (52), the response of cementocytes was
earlier compared with PDL cells, and comparable to that of the
osteocytes, indicating the potential role of cementocytes in early
mechanotransduction of tooth movement.
The results of the current study indicated that
static compression resulted in increased cell death and expression
of osteoclastic markers in cementocytes, while osteogenic markers
were inhibited. Moreover, cell viability and the downregulation of
OPG and OCN mRNA were indicated to exhibit a force
magnitude-dependent pattern, while SOST expression was also
associated with the force magnitude. The results of the present
study suggested that cementocytes may participate in the modulation
of the force-related osteoclast differentiation via the
Wnt/β-catenin signaling pathway. These results are consistent with
those of previous studies, where animal models have been
demonstrated to exhibit root resorption and bone remodeling under
compression (53,54). Other in vitro studies have
also indicated that mechanoreceptor cells, including PDLs and
osteocytes, detected forces and activated an intercellular
signaling cascade that ultimately results in bone and tooth
resorption (11,55,56).
However, the results of the current study are in contradiction with
a previous study that utilized fluid flow shear stress on
cementocytes, which induced an increased cementogenesis
differentiation (6). These
contradictory results may be attributed to the different type of
loading, higher magnitude or longer duration of force employed in
these previous studies (6,16,35).
Fluid flow shear stress has been considered to increase cell
viability, induce well-organized cytoskeleton formation, increase
filopodia processes and regulate the osteogenic differentiation of
osteocytes (57,58). Different types of force have been
reported to induce variable cell responses. The cementoblast-like
cell line OCCM-30 has been indicated to suppress the expression of
bone sialoprotein and increase that of osteopontin under
2,000-4,000 microstrain of cyclic stress, and to upregulate
microRNA-146b-5p and downregulate SMAD4 under tension (59,60).
Based on previous findings indicating that mild
force caused a continuous and constant tooth movement, while heavy
force resulted in a periodical and declining tooth movement
(50), it can be hypothesized that
cementocytes are the potential regulators of compression-induced
osteoclast activation, bone resorption and quick tooth movement via
regulating the RANKL/OPG ratio, SOST and OCN expression. The
cellular response is likely regulated by the magnitude of the force
that is applied to the tooth.
The limitations of the current study include the
absence of insights into the association between the duration of
the force and the cellular response to mechanical loading, which is
an important factor in orthodontic therapies. Future studies should
explore the cellular crosstalk between cementocytes and adjacent
cells such as cementoclasts. Additional studies are required to
clarify the mechanism of mechanotransduction and the link between
cellular response and tissue remodeling.
In conclusion, the present study established a 3D
collagen-based cementocyte model subjected to continuous
compressive loading, which simulated the microenvironment of
cellular cementum under orthodontic force. The cementocyte-like
cell line IDG-CM6 maintained a cementocyte profile in the model and
was sensitive to pressure loading in association with the magnitude
of force. The present study has revealed that cementocytes possibly
function as stress receptors of the tooth in the
mechanotransduction process during orthodontic tooth movement.
These results expand our knowledge of the biological processes of
orthodontic tooth movement and root resorption. This reproducible
model is a potential tool for additional studies and for in-depth
research on novel therapeutics for tooth movement acceleration.
Acknowledgements
The authors would like to thank Professor Lynda
Bonewald (Indiana University, Indianapolis, USA) for the providing
cells used in the study and valuable discussions, as well as Dr
Xiaolin Wang (Shanghai Jiao Tong University, Shanghai, China) for
assistance during the experiments and valuable discussions.
Funding
The current study was funded by National Natural
Science Foundation of China (grant nos. 81470765 and 81000420) and
the Interdisciplinary Program of Shanghai Jiao Tong University
(grant no. YG2016MS06).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW performed the experiments, analyzed the data and
drafted the manuscript. YX contributed to data acquisition and
interpretation, and critically revised the manuscript. XW
contributed to the statistical analysis and drafted the manuscript.
NZ contributed to the conception of the study and interpretation of
the data, and critically revised the manuscript. GS contributed to
the conception and design of the study, and critically revised the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pitaru S, Pritzki A, Bar-Kana I, Grosskopf
A, Savion N and Narayanan AS: Bone morphogenetic protein 2 induces
the expression of cementum attachment protein in human periodontal
ligament clones. Connect Tissue Res. 43:257–264. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feller L, Khammissa G, Thomadakis G,
Fourie J and Lemmer J: Apical external root resorption and repair
in orthodontic tooth movement: Biological events. Biomed Res Int.
2016(4864195)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walker SL, Tieu LD and Flores-Mir C:
Radiographic comparison of the extent of orthodontically induced
external apical root resorption in vital and root-filled teeth: A
systematic review. Eur J Orthod. 35:796–802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brezniak N and Wasserstein A:
Orthodontically induced inflammatory root resorption Part I: The
basic science aspects. Angle Orthod. 72:175–179. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grzesik WJ, Kuzentsov SA, Uzawa K, Mankani
M, Robey PG and Yamauchi M: Normal human cementum-derived cells:
Isolation, clonal expansion, and in vitro and in vivo
characterization. J Bone Miner Res. 13:1547–1554. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao N, Nociti FH Jr, Duan P, Prideaux M,
Zhao H, Foster BL, Somerman MJ and Bonewald LF: Isolation and
functional analysis of an immortalized murine cementocyte cell
line, IDG-CM6. J Bone Miner Res. 31:430–442. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Redlich M, Roos H, Reichenberg E, Zaks B,
Grosskop A, Bar Kana I, Pitaru S and Palmon A: The effect of
centrifugal force on mRNA levels of collagenase, collagen type-I,
tissue inhibitors of metalloproteinases and beta-actin in cultured
human periodontal ligament fibroblasts. J Periodontal Res.
39:27–32. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Q, Gu Y, Zhang W, Dziopa L, Zilberberg
J and Lee W: Ex vivo 3D osteocyte network construction with primary
murine bone cells. Bone Res. 3(15026)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Verbruggen SW, Vaughan TJ and McNamara LM:
Fluid flow in the osteocyte mechanical environment: A
fluid-structure interaction approach. Biomech Model Mechanobiol.
13:85–97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li S, Li F, Zou S, Zhang L and Bai Y:
PTH1R signalling regulates the mechanotransduction process of
cementoblasts under cyclic tensile stress. Eur J Orthod.
40:537–543. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Manokawinchoke J, Limjeerajarus N,
Limjeerajarus C, Sastravaha P, Everts V and Pavasant P: Mechanical
force-induced TGFB1 increases expression of SOST/POSTN by hPDL
cells. J Dent Res. 94:983–989. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nieponice A, Maul TM, Cumer JM, Soletti L
and Vorp DA: Mechanical stimulation induces morphological and
phenotypic changes in bone marrow-derived progenitor cells within a
three-dimensional fibrin matrix. J Biomed Mater Res A. 81:523–530.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Atkins GJ, Welldon KJ, Holding CA, Haynes
DR, Howie DW and Findlay DM: The induction of a catabolic phenotype
in human primary osteoblasts and osteocytes by polyethylene
particles. Biomaterials. 30:3672–3681. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Diercke K, König A, Kohl A, Lux CJ and
Erber R: Human primary cementoblasts respond to combined IL-1beta
stimulation and compression with an impaired BSP and CEMP-1
expression. Eur J Cell Biol. 91:402–412. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Damaraju S, Matyas JR, Rancourt DE and
Duncan NA: The effect of mechanical stimulation on mineralization
in differentiating osteoblasts in collagen-I scaffolds. Tissue Eng
Part A. 20:3142–3153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Diercke K, Kohl A, Lux CJ and Erber R:
Compression of human primary cementoblasts leads to apoptosis: A
possible cause of dental root. resorption? J Orofac Orthop.
75:430–445. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tripuwabhrut P, Mustafa K, Brudvik P and
Mustafa M: Initial responses of osteoblasts derived from human
alveolar bone to various compressive forces. Eur J Oral Sci.
120:311–318. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Boukhechba F, Balaguer T, Michiels JF,
Ackermann K, Quincey D, Bouler JM, Carle GF and Rochet N: Human
primary osteocyte differentiation in a 3D culture system. J Bone
Miner Res. 24:1927–1935. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vazquez M, Evans BA, Riccardi D, Evans SL,
Ralphs JR, Dillingham CM and Mason DJ: A new method to investigate
how mechanical loading of osteocytes controls osteoblasts. Front
Endocrinol (Lausanne). 5(208)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jagodzinski M, Breitbart A, Wehmeier M,
Hesse E, Haasper C, Krettek C, Zeichen J and Hankemeier S:
Influence of perfusion and cyclic compression on proliferation and
differentiation of bone marrow stromal cells in 3-dimensional
culture. J Biomech. 41:1885–1891. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamamoto M, Kawashima N, Takashino N,
Koizumi Y, Takimoto K, Suzuki N, Saito M and Suda H:
Three-dimensional spheroid culture promotes odonto/osteoblastic
differentiation of dental pulp cells. Arch Oral Biol. 59:310–317.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun Q, Choudhary S, Mannion C, Kissin Y,
Zilberberg J and Lee WY: Ex vivo replication of phenotypic
functions of osteocytes through biomimetic 3D bone tissue
construction. Bone. 106:148–155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Coyac BR, Chicatun F, Hoac B, Nelea V,
Chaussain C, Nazhat SN and McKee MD: Mineralization of dense
collagen hydrogel scaffolds by human pulp cells. J Dent Res.
92:648–654. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ahearne M: Introduction to cell-hydrogel
mechanosensing. Interface Focus. 4(20130038)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Damaraju S, Matyas JR, Rancourt DE and
Duncan NA: The role of gap junctions and mechanical loading on
mineral formation in a collagen-I scaffold seeded with
osteoprogenitor cells. Tissue Eng Part A. 21:1720–1732.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Honma M, Ikebuchi Y, Kariya Y and Suzuki
H: Establishment of optimized in vitro assay methods for evaluating
osteocyte functions. J Bone Miner Metab. 33:73–84. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fusenig NE, Breitkreutz D, Dzarlieva RT,
Boukamp P, Bohnert A and Tilgen W: Growth and differentiation
characteristics of transformed keratinocytes from mouse and human
skin in vitro and in vivo. J Invest Dermatol. 81 (Suppl
1):S168–S175. 1983.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Woo M, Rosser J, Dusevich V, Kalajzic I
and Bonewald F: Cell line IDG-SW3 replicates
osteoblast-to-late-osteocyte differentiation in vitro and
accelerates bone formation in vivo. J Bone Miner Res. 26:2634–2646.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kalajzic I, Braut A, Guo D, Jiang X,
Kronenberg MS, Mina M, Harris MA, Harris SE and Rowe DW: Dentin
matrix protein 1 expression during osteoblastic differentiation,
generation of an osteocyte GFP-transgene. Bone. 35:74–82.
2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tawakoli PN, Al-Ahmad A, Hoth-Hannig W,
Hannig M and Hannig C: Comparison of different live/dead stainings
for detection and quantification of adherent microorganisms in the
initial oral biofilm. Clin Oral Investig. 17:841–850.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR,
Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI and Bellido
T: Sost downregulation and local Wnt signaling are required for the
osteogenic response to mechanical loading. Bone. 50:209–217.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Silva I and Branco JC: Rank/RANKL/OPG:
Literature review. Acta Reumatol Port. 36:209–218. 2011.PubMed/NCBI
|
|
34
|
García-Martín A, Reyes-García R,
Avila-Rubio V and Muñoz-Torres M: Osteocalcin: A link between bone
homeostasis and energy metabolism. Endocrinol Nutr. 60:260–263.
2013.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
35
|
Matsuzawa H, Toriya N, Nakao Y,
Konno-Nagasaka M, Arakawa T, Okayama M and Mizoguchi I: Cementocyte
cell death occurs in rat cellular cementum during orthodontic tooth
movement. Angle Orthod. 87:416–422. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Li T, Wang X, Yin X, Zhao N, Zou
S, Duan P and Bonewald L: The role of sphingosine-1-phosphate
signaling pathway in cementocyte mechanotransduction. Biochem
Biophys Res Commun. 523:595–601. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pedraza CE, Marelli B, Chicatun F, McKee
MD and Nazhat SN: An in vitro assessment of a cell-containing
collagenous extracellular matrix-like scaffold for bone tissue
engineering. Tissue Eng Part A. 16:781–793. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Uchihashi K, Aoki S, Matsunobu A and Toda
S: Osteoblast migration into type I collagen gel and
differentiation to osteocyte-like cells within a self-produced
mineralized matrix: A novel system for analyzing differentiation
from osteoblast to osteocyte. Bone. 52:102–110. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dewitt DD, Kaszuba SN, Thompson DM and
Stegemann JP: Collagen I-matrigel scaffolds for enhanced Schwann
cell survival and control of three-dimensional cell morphology.
Tissue Eng Part A. 15:2785–2793. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bosshardt DD: Are cementoblasts a
subpopulation of osteoblasts or a unique phenotype? J Dent Res.
84:390–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sawada T, Ishikawa T, Shintani S and
Yanagisawa T: Ultrastructural immunolocalization of dentin matrix
protein 1 on Sharpey's fibers in monkey tooth cementum. Biotech
Histochem. 87:360–365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Toyosawa S, Okabayashi K, Komori T and
Ijuhin N: mRNA expression and protein localization of dentin matrix
protein 1 during dental root formation. Bone. 34:124–133.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bae CH, Lee JY, Kim TH, Baek JA, Lee JC,
Yang X, Taketo MM, Jiang R and Cho ES: Excessive Wnt/β-catenin
signaling disturbs tooth-root formation. J Periodont Res.
48:405–410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lehnen SD, Gotz W, Baxmann M and Jager A:
Immunohistochemical evidence for sclerostin during cementogenesis
in mice. Ann Anat. 194:415–421. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jäger A, Götz W, Lossdörfer S and
Rath-Deschner B: Localization of SOST/sclerostin in cementocytes in
vivo and in mineralizing periodontal ligament cells in vitro. J
Periodontal Res. 45:246–254. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li Y, Jacox LA, Little SH and Ko CC:
Orthodontic tooth movement: The biology and clinical implications.
Kaohsiung J Med Sci. 34:207–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Taki M, Yamashita T, Yatabe K and Vogel V:
Mechano-chromic protein-polymer hybrid hydrogel to visualize
mechanical strain. Soft Matter. 15:9388–9393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Krishnan V and Davidovitch Z: Cellular,
molecular, and tissue-level reactions to orthodontic force. Am J
Orthod Dentofacial Orthop. 129:469.e1–e32. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Odagaki N, Ishihara Y, Wang Z, Ei Hsu
Hlaing, Nakamura E, Hoshijima M, Hayano M, Kawanabe S and Kamioka
N: Role of osteocyte-PDL crosstalk in tooth movement via
SOST/Sclerostin. J Dent Res. 97:1374–1382. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shu R, Bai D, Sheu T, He Y, Yang X, Xue C,
He Y, Zhao M and Han X: Sclerostin promotes bone remodeling in the
process of tooth movement. PLoS One. 12(e0167312)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gonzales C, Hotokezaka H, Yoshimatsu M,
Yozgatian JH, Darendeliler MA and Yoshida N: Force magnitude and
duration effects on amount of tooth movement and root resorption in
the rat molar. Angle Orthod. 78:502–509. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen L, Mo S and Hua Y: Compressive
force-induced autophagy in periodontal ligament cells downregulates
osteoclastogenesis during tooth movement. J Periodontol.
90:1170–1181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bumann EE and Frazier-Bowers SA: A new
cyte in orthodontics: Osteocytes in tooth movement. Orthod
Craniofac Res. 20 (Suppl 1):S125–S128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Murshid SA: The role of osteocytes during
experimental orthodontic tooth movement: A review. Arch Oral Biol.
73:25–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yan Z, Wang P, Wu J, Feng X, Cai J, Zhai
M, Li J, Liu X, Jiang M, Luo E and Jing D: Fluid shear stress
improves morphology, cytoskeleton architecture, viability, and
regulates cytokine expression in a time-dependent manner in MLO-Y4
cells. Cell Biol Int. 42:1410–1422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ajubi NE, Klein-Nulend J, Nijweide PJ,
Vrijheid-Lammers T, Alblas MJ and Burger EH: Pulsating fluid flow
increases prostaglandin production by cultured chicken osteocytes-a
cytoskeleton-dependent process. Biochem Biophys Res Commun.
225:62–68. 1996.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Huang L, Meng Y, Ren A, Han X, Bai D and
Bao L: Response of cementoblast-like cells to mechanical tensile or
compressive stress at physiological levels in vitro. Mol Biol Rep.
36:1741–1748. 2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang L, Hu H, Cheng Y, Chen J, Bao C, Zou
S and Wu G: Screening the expression changes in MicroRNAs and their
target genes in mature cementoblasts stimulated with cyclic tensile
stress. Int J Mol Sci. 17(2024)2016.PubMed/NCBI View Article : Google Scholar
|