1. Introduction
Sarcopenia is a condition characterized by a
progressive reduction in skeletal muscle mass and strength, which
affects balance, mobility, overall physical performance and quality
of life (1). Risk factors for
sarcopenia include increased age, being of the male sex,
malnutrition and a sedentary lifestyle (2). Primary sarcopenia is often age-related
without apparent underlying causes, whereas secondary sarcopenia is
associated with one or more causes (1). Major co-morbidities associated with
sarcopenia are obesity, osteoporosis and type 2 diabetes mellitus
(3). A meta-analysis of 35 studies
showed that the global prevalence of sarcopenia is 10% in men and
women, and the prevalence is higher in non-Asian countries than in
Asian countries (4). It is estimated
that the cost of hospitalisation associated with sarcopenia was
approximately $40.4 billion USD in the United States, with an
average cost of $260 USD per individual (5). Sarcopenia has also become a topic of
interest in recent years, as an increasing proportion of the global
population being of advanced age is projected to triple between
2017 and 2050(6). The development of
prophylactic and therapeutic strategies for sarcopenia may
therefore become imperative to ensure healthy ageing. It is also
noteworthy that there are no US Food and Drug Administration
(FDA)-approved drugs for the treatment of sarcopenia (7). Understanding the pathogenesis of
sarcopenia will be important in the design of prophylactic and
therapeutic strategies for the disease. Oxidative stress,
inflammation, impairment of mitochondrial function, increased
protein turnover and capillary regression can result in the loss of
skeletal muscle mass and ultimately sarcopenia (8-11).
Previous studies have demonstrated that the administration of
antioxidants reduced the level of oxidative stress during exercise
(12) and the level of muscle
atrophy (13). These findings
suggest a potential role for antioxidants in reversing
sarcopenia.
Astaxanthin is a fat-soluble, naturally occurring
xanthophyll carotenoid identified in numerous organisms, such as
microalgae, crustaceans and fish (such as salmon and trout)
(14). Astaxanthin is a powerful
antioxidant that effectively scavenges free radicals, quenches
singlet oxygen, enhances antioxidant activities and reduces
oxidative stress (15). The
nutraceutical applications of astaxanthin previously reported
include anti-inflammatory (16),
anti-cancer (17) and anti-diabetic
(18) and it has also been reported
to have gastro- (19), hepato-
(20), neuro- (21), cardio- (22), ocular- (23) and skin-protective (24) properties.
A recent review summarised the potential application
of astaxanthin as a dietary supplement in exercising humans. The
author concluded that there was an improvement of exercise
metabolism, performance and recovery following astaxanthin
supplementation (25). In addition,
an in vitro study by Yu et al (26) demonstrated that incubation of the
mouse myoblast C2C12 cell line with astaxanthin (5 µM) during heat
stress (43˚C) prevented adverse changes to the tubular
mitochondrial structure and mitochondrial membrane potential, as
well as reactive oxygen species (ROS) production. Thus, astaxanthin
may have a potential application in preventing muscle injury and
degeneration. In the present review, the health-promoting effects
of astaxanthin on the skeletal muscle in animal models and humans
are presented. The molecular mechanisms underlying the health
benefits of astaxanthin in reversing adverse muscle changes are
also highlighted.
2. Literature search
Evidence acquisition was conducted between February
1st and February 29th 2020 using PubMed and Medline electronic
databases. The key words used to perform the search were
‘astaxanthin AND (sarcopenia OR muscle)’. All in vitro,
in vivo and human studies detailing the effects of
astaxanthin and its underlying mechanisms on muscle health were
extracted. A total of 20 related studies are included in the
present review.
3. Effects of astaxanthin on skeletal
muscle: Evidence from in vivo studies
The effects of astaxanthin on skeletal muscle have
been explored in vivo (Table
I). Recently, Aoi et al (27) compared the effects of three different
forms of astaxanthin on endurance performance in 8-week-old ICR
mice. Astaxanthin derived from Haematococcus pluvialis
(esterified form), synthetic astaxanthin (non-esterified form) or
astaxanthin derived from Phaffia rhodozyma (non-esterified
form) was provided to the animals in their diet at a dose of 0.02%
(w/w) for five weeks. The animals were subjected to treadmill
exercise with a running speed of 25 m/min for the assessment of
endurance and their running time to exhaustion was measured. The
study indicated that animals fed with astaxanthin from H.
pluvialis had the longest running time to exhaustion among the
experimental groups (27). Long-term
effects of astaxanthin supplementation were also evaluated using an
exercised animal model. Adult male Wistar rats were administered
mineral oil (vehicle) or astaxanthin (1 mg/kg) five days per week
for 45 days. The animals treated with astaxanthin had a higher
elapsed time until exhaustion in a forced-swimming activity when
compared with exercised animals without treatment (28).
 | Table ISummary of the findings of studies
exploring the effects of astaxanthin on skeletal muscle in
animals. |
Table I
Summary of the findings of studies
exploring the effects of astaxanthin on skeletal muscle in
animals.
| Animal
species/strain | Experimental
groups | Treatment
period | Summary of
findings | Author (Refs.) |
|---|
| ICR mice (n=40; 8
weeks old) | i) control | 5 weeks | Among all the
experiment groups: | Aoi et al
(27), 2018 |
| | ii) astaxanthin
(0.02% w/w) from Haematococcus pluvialis | | Animals fed with
astaxanthin from Haematococcus pluvialis had the longer | |
| | iii) synthetic
astaxanthin (0.02% w/w) | | running time to
exhaustion and higher | |
| | iv) astaxanthin
(0.02% w/w) from Phaffia rhodozyma | | 5'-AMPK content in
skeletal muscle. | |
| Male Wistar rats
(n=24; age not mentioned) | i) rested
control | | Compared to the
rested control group: | Polotow et
al (28), 2014 |
| | ii) rested control
+ astaxanthin (1 mg/kg; 5 days/week; oral) | 45 days | Astaxanthin
increased elapsed time until | |
| | iii) exercise | | exhaustion in a
forced-swimming activity, | |
| | iv) exercise +
astaxanthin (1 mg/kg; 5 days/week; oral) | | TEAC and FRAP
capacity. Compared to the exercised group: Astaxanthin increased
TEAC and FRAP capacity and lowered TBARS and protein carbonyl
content. | |
| ICR mice with
muscle atrophy (n=44; 7 weeks old) | i) normal | 2 weeks | Compared to the
normal group: | Kawamura et
al (29), 2019 |
| | ii) astaxanthin
(0.06% w/w) | | Astaxanthin
increased relative soleus weight. | |
| | iii) β-carotene
(0.06% w/w) | | A mixture of
antioxidants increased relative | |
| | iv) resveratrol
(0.06% w/w) | | soleus weight, mTOR
phosphorylation level, | |
| | v) mixture of
antioxidants (astaxanthin, β-carotene, resveratrol; 0.02% w/w
each) | | P70S6K
phosphorylation level and reduced carbonylated protein levels. | |
| Male Wistar rats
(n=24; age | i) control | 7 days | Compared to the
hindlimb unloaded group: | Kanazashi et
al (30), 2013 |
| not mentioned) | ii) control +
astaxanthin (50 mg/kg; twice per day; oral) | | Astaxanthin
increased C/F ratio, CAF, capillary | |
| | iii) hindlimb
unloading | | volume, capillary
diameter andHIF-1α, VEGF, | |
| | iv) hindlimb
unloading + astaxanthin (50 mg/kg; twice per day; oral) | | Flt-1, KDR, ANG-1
and Tie-2 levels but reduced ROS production and SOD-1 protein. | |
| Male Sprague-Dawley
rats (n=35; 10 weeks old) | i) control | 2 weeks | Compared to the
hindlimb unloaded group: | Kanazashi et
al (31), 2014 |
| | ii) hindlimb
unloading | | Astaxanthin
increased capillary volume, | |
| | iii) hindlimb
unloading + astaxanthin (50 mg/kg; twice per day; oral) | | capillary diameter,
C/F ratio and eNOS, PGC-1α and VEGF levels and SDH activity | |
| | iv) hindlimb
unloading + intermittent loading | | but decreased ROS
production and SOD-1 | |
| | v) hindlimb
unloading + astaxanthin (50 mg/kg; twice per day; oral) +
intermittent loading | | protein levels.
Astaxanthin + intermittent loading increased soleus mass, FCSA,
capillary volume, capillary diameter, C/F ratio and CAF, eNOS,
PGC-1α and VEGF levels and SDH activity but decreased ROS
production and SOD-1 protein in a greater extent. | |
| Male Sprague-Dawley
rats (n=30; 10 weeks old) | i) control | 1 week | Compared to the
hindlimb unloaded group: | Kanazashi et
al (32), 2019 |
| | ii) hindlimb
unloading | | Astaxanthin
increased SDH activity, C/F ratio | |
| | iii) hindlimb
unloading + astaxanthin (50 mg/kg; twice per day; oral) | | and PGC-1α level
and reduced ubiquitination of proteins, ROS production and SOD-1
protein. | |
| | iv) hindlimb
unloading + electrical stimulation | | Astaxanthin and
electrical stimulation | |
| | v) hindlimb
unloading + astaxanthin (50 mg/kg; twice per day; oral) +
electrical stimulation | | synergistically
increased FCSA, absolute and relative soleus muscle mass,
phosphorylation of FoxO3a, SDH activity, C/F ratio, PGC-1α as well
as reducing ubiquitination of proteins, ROS production and SOD-1
protein. | |
| Male Wistar rats
(n=27; 8 weeks old) | i) control | 3 weeks | Compared to the
hindlimb unloaded group: | Yoshihara et
al (33), 2017 |
| | ii) hindlimb
unloading | | Astaxanthin
increased relative soleus muscle | |
| | iii) hindlimb
unloading + astaxanthin (0.04% w/w) | | mass and FCSA, and
reduced apoptotic nuclei and of protein ubiquitination levels. | |
| Male Wistar rats
(n=49; 8 weeks old) | i) control | 3 weeks | Compared to the
hindlimb unweighting group: | Yoshihara et
al (34), 2018 |
| | ii) hindlimb
unweighting | | A combination of
intermittent reloading, | |
| | iii) hindlimb
unweighting + intermittent reloading | | astaxanthin
supplementation and heat stress | |
| | iv) hindlimb
unweighting + intermittent reloading + astaxanthin (0.04% w/w) | | increased soleus
muscle mass, soleus cross-sectional area and satellite cell
numbers. | |
| | v) hindlimb
unweighting + intermittent reloading + heat stress | | | |
| | vi) hindlimb
unweighting + intermittent reloading + astaxanthin (0.04% w/w) +
heat stress | | | |
| Male Wistar rats
(n=23; 14 weeks old) | i) placebo
diet | 24 days | Compared to the
immobilization + placebo diet | Shibaguchi et
al (38), 2016 |
| | ii) astaxanthin
(0.04%) | | group: Astaxanthin
reduced the percentage of | |
| | iii) astaxanthin
(0.2%) | | immobilized muscle,
SOD level and calpain and | |
| | iv) immobilization
+ placebo diet | | ubiquitin
expression in the atrophied plantaris | |
| | v) immobilization +
astaxanthin (0.04%) | | muscle. | |
| | vi) immobilization
+ astaxanthin (0.2%) | | | |
| Male Wistar
rats | i) control | 3 weeks | Compared to the
joint immobilization group: | Maezawa et
al (39), 2017 |
| (n=28; 7 weeks
old) | ii) control +
astaxanthin (100 mg/kg, daily; oral) | | Astaxanthin lowered
the collagen fibre area, | |
| | iii) joint
immobilization | | transforming growth
factor-β1, α-smooth muscle | |
| | iv) joint
immobilization + astaxanthin (100 mg/kg, daily; oral) | | actin, ROS
production and SOD-1. | |
| Male C57BL/6J mice
(6-week-old) | i) normal chow | 24 weeks | Compared to
high-fat diet group: | Nishida et
al (40), 2020 |
| | ii) normal chow +
astaxanthin | | Astaxanthin
enhanced exercise tolerance and | |
| | iii) high-fat diet
(60%) | | exercise-induced
glucose metabolism Astaxanthin | |
| | iv) high-fat diet
(60%) + astaxanthin (0.02%) | | prevented metabolic
syndrome. | |
| Broiler chicks
(n=32; 15 days old) | i) thermo-neutral
temperature + basal diet | 28 days | Compared to the
basal diet group: | Inoue et al
(41), 2019 |
| | ii) thermo-neutral
temperature + basal diet supplemented with 0.15% Panaferd-P (30 ppm
astaxanthin) | | Astaxanthin
increased breast and leg muscle redness and yellowness; ameliorated
high ambient | |
| | iii) high
temperature + basal diet | | temperature-induced
decrease in muscle redness. | |
| | iv) high
temperature + basal diet supplemented with 0.15% | | Astaxanthin
decreased breast muscle MDA | |
| | v) Panaferd-P (30
ppm astaxanthin) | | concentration under
both thermo-neutral and high ambient temperature conditions. | |
| Female C57BL/6 mice
(n=27; 7 weeks old) | i) rested
control | 3 weeks | Compared to the
intense exercise group: | Aoi et al
(52), 2003 |
| | ii) intense
exercise | | Astaxanthin
decreased the expression of | |
| | iii) intense
exercise + astaxanthin (0.02% w/w) | | 4-HNE-modified
protein, production of 8-OHdG, | |
| | | | MPO activity and CK
activity. | |
| Male C57BL/6 mice
(n=40; 7 weeks old) | i) sedentary
contro | l4 weeks | Compared to the
swimming control group: | Zhou et al
(53), 2019 |
| | ii) swimming
control | | Astaxanthin
decreased GPx, CAT, MDA and CK | |
| | iii) swimming +
astaxanthin (5 mg/kg, 5 days/week; oral) | | levels but
increased SOD level | |
| | iv) swimming +
astaxanthin (15 mg/kg; 5 days/week; oral) | | Astaxanthin
downregulated Nrf2 and Nrf2-dependent | |
| | v) swimming +
astaxanthin (30 mg/kg; 5 days/week; oral) | | enzymes [Keap1,
glutamate-cysteine ligase modifier subunit, glutamate-cysteine
ligase catalytic subunit, NAD(P)H quinone dehydrogenase and heme
oxygenase (decycling) 1]. | |
| ICR mice (n=32; 7
weeks old) | i) rested
control | 2 weeks | Compared to the
exercised group: | Liu et al
(63), 2014 |
| | ii) rested control
+ astaxanthin (0.02% w/w) | | Astaxanthin
decreased plasma non-esterified fatty | |
| | iii) exercise | | acids, increased
intermuscular pH, PGC-1α, | |
| | iv) exercise +
astaxanthin (0.02% w/w) | | cytochrome c and
fibronectin type III domain-containing protein 5. | |
In another study, Kawamura et al (29) investigated the effects of astaxanthin
alone, or in combination with other antioxidants (β-carotene and
resveratrol), on muscle atrophy in 7-week-old male ICR mice. The
knee and ankle joints of one hindlimb were fixed with a cast to
induce muscle atrophy and removed after three weeks. After cast
removal, the animals were fed a basal diet enriched with
astaxanthin, β-carotene, resveratrol or a mixture of the three
antioxidants for two weeks. The animals given a basal diet with
astaxanthin alone or a mixture of the three antioxidants had
significantly higher soleus muscle weight when compared to the
normal animals (29).
An animal model of hindlimb unloading was also used
to assess the effects of astaxanthin on atrophied soleus muscle.
Kanazashi et al (30)
performed hindlimb unloading on adult male Wistar rats for 7 days
by suspending the tail, to prevent weight bearing of the hindlimb
on the floor or contact with the sides of the cage. Astaxanthin was
administered orally at 50 mg/kg twice per day for 7 days.
Astaxanthin was demonstrated to prevent the changes caused by
hindlimb unloading, indicated by the preserved capillary-to-fibre
(C/F) ratio, capillary number per fibre (CAF), capillary volume and
capillary diameter of the treated group. As astaxanthin
supplementation alone was beneficial in preventing capillary
regression, while exerting minimal impact on muscle mass, the same
group of researchers hypothesized that a combination of astaxanthin
and intermittent loading would work synergistically on the
prevention of muscle atrophy and capillary regression during
hindlimb unloading. In the subsequent study, the animals were
subjected to hindlimb unloading followed by the release of the
suspension device to allow for normal cage activity for one hour
daily in darkness. The study duration was extended to two weeks. As
expected, the results indicated that intermittent unloading
combined with astaxanthin ameliorated both soleus muscle atrophy
and capillary regression in the hindlimb unloaded animals (31). A recent study was conducted to
evaluate the effects of a combined therapy of astaxanthin and
electrical stimulation on muscle atrophy using hindlimb unloaded
rats. For electrical stimulation, calf muscles of the rats were
electrically stimulated using a surface electrode (diameter: 1 cm,
frequency: 100 Hz) for 240 sec/day. Treatment with astaxanthin
alone increased the C/F ratio. The combined therapy was more
efficient than astaxanthin alone in reversing the adverse changes
due to hindlimb unloading. The combination of astaxanthin and
electrical stimulation increased absolute soleus muscle mass and
fibre cross-sectional area (FCSA) (32). Another group of researchers reported
that dietary astaxanthin supplementation prior to and during
hindlimb unloading suppressed soleus muscle atrophy. Compared to
the animals subjected to hindlimb unloading without treatment,
astaxanthin supplementation caused higher muscle weight and FCSA in
the soleus muscle (33). A
comprehensive study done by Yoshihara et al (34) illustrated the effects of a
combination of astaxanthin supplementation, heat stress and
intermittent reloading on the hindlimb unweighted rats. Hindlimb
unloading was conducted as aforementioned, whereby the tail was
immobilized in a cast, allowing the animals to move only using
their front feet. The animals were placed in a heat chamber at
41.0-41.5˚C for 30 min. Intermittent reloading was performed during
the heating phase for one hour every other day to allow daily
activities. Astaxanthin was mixed into their basal diet at 0.04%
w/w. The combination of dietary astaxanthin, heat treatment and
intermittent reloading resulted in higher soleus muscle weight and
cross-sectional area in the hindlimb unloaded animals (34).
Hindlimb immobilization is another method to induce
muscle atrophy. Immobilization refers to holding a joint or bone in
place with a cast to prevent its movement, thus inducing muscle
contracture and atrophy (35,36). In
contrast, the rodents are in a head-down position to simulate
weightlessness for hindlimb unloading (37). In an in vivo study, three
groups of male Wistar rats were given either a placebo diet, or a
0.04 or 0.2% astaxanthin diet for 24 days. At day 14, hindlimb
muscle immobilization was introduced to the rats in the maximum
plantar flexion position with a plaster cast. It was demonstrated
that the degree of muscle atrophy was lessened in the rats fed with
a diet supplemented with astaxanthin (38). Similarly, Maezawa et al
(39) introduced joint
immobilization to 7-week-old male Wistar rats using the same
approach. Astaxanthin (100 mg/kg) was administered orally each day
for three weeks (one week before and two weeks during ankle joint
immobilization). The treatment of astaxanthin reduced FCSA in the
rats with joint immobilization (39).
In high-fat diet fed male C57BL/6J mice, astaxanthin
was shown to increase exercise endurance. The astaxanthin-treated
mice were able to run for a longer distance than the untreated mice
when subjected to daily exercise using a treadmill and wheel.
Astaxanthin also increased glucose tolerance after regular daily
training, along with other metabolic syndrome parameters [fasting
blood glucose, insulin, homeostatic model assessment of insulin
resistance (HOMA-IR), glycated haemoglobin (HbA1c), systolic blood
pressure, triglyceride and total cholesterol were reduced]
(40).
The impact of astaxanthin supplementation on meat
colouration in chickens has been evaluated. Using 15-day-old
Broiler chicks as an animal model, Inoue et al (41) randomly assigned the chicks to one of
the four groups using a 2x2 factorial design. The two main
variables in this study were diet [basal diet or basal diet
enriched with 0.15% Panaferd-P (containing 30 ppm astaxanthin)] and
ambient temperature [thermo-neutral temperature (25±1˚C) or high
temperature (35±1˚C)]. It was revealed that a diet containing
Panaferd-P increased muscle carotenoid content, redness and
yellowness of the skeletal muscle (meat) in the broiler chicks
under the condition of thermo-neutral and high ambient temperature
(41). Meat colour determines meat
quality (42). A decrease in muscle
redness might be a consequence of an alteration in muscle myoglobin
concentration (the main protein responsible for meat colour), heat
stress and feed restriction (42,43).
Meanwhile, a reduction in muscle yellowness is an indicator for
decreasing carotenoid (astaxanthin, adonixanthin, canthaxanthin,
adonirubin, lutein and zeaxanthin) accumulations in muscle. Hence,
the increases in muscle redness and yellowness indicated quality
improvement of the meat (41).
4. Effects of astaxanthin on skeletal
muscle: Evidence from human studies
Limited studies have been conducted in humans to
test the effects of astaxanthin on muscle, particularly in the
aspects of muscle injury/damage and muscle strength (Table II). The effects of astaxanthin on
muscle injury were studied among resistance-trained men (n=20, aged
25.1±1.6 years). The subjects were equally divided into the placebo
(administered 1,732 mg safflower oil) or astaxanthin (administered
4 mg astaxanthin and 480 mg lutein) groups. After three weeks of
assigned treatments, the participants were subjected to eccentric
exercise (10 sets of 10 repetitions at 85% of one repetition
maximum) and followed through 96 h post-exercise. The parameters
measured in this study include muscle soreness, creatine kinase
(CK) activity and muscle performance. A similar response in these
variables was noted for both groups, reiterating that astaxanthin
supplementation exerted negligible effects on skeletal muscle
injury following eccentric loading (44). Another human study demonstrated the
effects of astaxanthin supplementation (4 mg) for 90 days on muscle
damage, oxidative stress and antioxidant capacity during soccer
training in elite young soccer players. Treatment with astaxanthin
did not change the levels of thiobarbituric acid-reactive
substances (TBARS) and advanced oxidation protein products (AOPP)
throughout this study. The CK and aspartate aminotransferase (AST)
activities in serum were significantly increased with soccer
training without treatment but were lowered with astaxanthin
administration (45). In a
randomized, double-blind, placebo-controlled study, Liu et
al (46) examined a test
formulation consisting of astaxanthin (12 mg), tocotrienol (10 mg)
and zinc (6 mg) on building strength, endurance and mobility in
exercise training among the elderly. A total of 42 elderly subjects
(aged 65-85 years) were recruited, fed with test formulation or
placebo for 4 months and trained with increasing intervals of
incline walking for three months (three times weekly for 40-60
min). In this study, muscle strength was presented as maximal
voluntary contraction (MVC) in an ankle dorsiflexion exercise, and
the tibialis anterior muscle size was measured as cross-sectional
area (CSA) using magnetic resonance imaging. The authors identified
a greater endurance in a 6-min walk upon exercise training in both
experimental groups. The subjects administered astaxanthin
formulation had higher MVC and CSA, indicating improved muscle
strength and size as compared to the placebo-treated exercised
subjects (46).
 | Table IISummary of the findings of studies
exploring the effects of astaxanthin on skeletal muscle in
humans. |
Table II
Summary of the findings of studies
exploring the effects of astaxanthin on skeletal muscle in
humans.
| Subjects | Groups | Treatment
period | Findings | Author (Refs) |
|---|
| Resistance trained
men (n=20; mean age, 25.1±1.6 years) | i) placebo (1,732
mg safflower oil) | 3 weeks | No significant
difference was observed in | Bloomer et
al (44), 2005 |
| | ii) astaxanthin
[1,732 mg safflower oil + haemotococcus | | muscle soreness,
CK, one repetition maximum | |
| | iii) algae extract
(contains 4 mg astaxanthin and 480 mg lutein)] | | concentric
strength, mean isometric force and mean dynamic force (MDF) between
the two groups. | |
| Male elite soccer
players (n=32, age not mentioned) | i) placebo | 90 days | No change in TBARS
and AOPP levels between | Djordjevic et
al (45), 2012 |
| | ii) astaxanthin (4
mg) | | the two groups. In
comparison with the placebo: Astaxanthin increased SOD activity
after 2 h of soccer exercise. Astaxanthin lowered post-exercise CK
and AST levels. | |
| Elderly men and
women (n=42; aged 65-82 years) | i) exercise
training + placebo (mean age: 72.2±5.2 years) | 4 months | Exercise training
increased endurance (exercise | Liu et al
(46), 2018 |
| | ii) exercise
training + astaxanthin (12 mg) + tocotrienol (10 mg) + zinc (6 mg)
(mean age: 69.1±3.4 years) | | time) and distance
in 6 min walk in both groups. In comparison with a placebo:
Astaxanthin treatment increased MVC and CSA. | |
| Healthy young men
(n=20) | i) exercise
training (mean age: 20.8±0.3 years) | 4 weeks | Maximum workload
and duration of exercise | Takami et al
(47), 2019 |
| | ii) exercise
training + antioxidants (catechin, astaxanthin, quercetin,
glutathione and anthocyanin) (mean age: 21.4±0.4 years) | | were increased in
both groups In the antioxidant group: Oxygen consumption and
carbohydrate oxidation post-training were increased. A positive
correlation was observed between maximum work-load and fat
oxidation Serum insulin was decreased | |
A recent study by Takami et al (47) assessed whether foods containing
antioxidants (such as catechin, astaxanthin, quercetin, glutathione
and anthocyanin) could boost aerobic metabolism during exercise
training. All participants were divided into two groups subjected
to supervised cycling training for 30 min (three days per week) for
four weeks with or without taking antioxidant-rich foods. Several
observations were made in this study. The values of oxygen
consumption and carbohydrate oxidation after training during rest
and exercise conditions were significantly increased in the
antioxidant group. A positive correlation was observed between fat
oxidation during exercise and maximum workload after training. The
magnitude of decrease in serum insulin level after training was
higher in the antioxidant group as compared to the control group
(47).
Overall, the evidence derived from in vivo
studies suggested a beneficial effect of astaxanthin in preventing
muscle degeneration. In humans, the effects of astaxanthin alone or
in combination with other antioxidants on muscle health were
heterogenous, as both positive and negligible effects were
reported.
5. The mechanism of action of
astaxanthin
Understanding of the biological mechanisms
underlying the decline in muscle strength and mass is of
substantial importance in the search for potential therapeutic
agents to prevent sarcopenia. The widely accepted mechanisms
involved in muscle atrophy leading to pathogenesis of sarcopenia
include induction of oxidative stress, impaired mitochondrial
dynamics and functions, negative protein turnover (defined as a
disproportionate decrease in muscle protein synthesis and/or an
increase in muscle protein breakdown) as well as regression of the
capillary network in skeletal muscle (39).
Oxidative stress exerts dual actions on skeletal
muscle, whereby a low level of oxidative stress is beneficial while
excessive oxidative stress is detrimental (48). Oxidative stress is closely associated
with sarcopenia, which is largely attributed to the excessive yield
of reactive oxygen and nitrogen species (RONS) during ageing,
high-intensity exercise and disuse atrophy (49). An increase in ROS level inflicts
direct alteration or damage on important macromolecules, such as
lipids, proteins and nucleic acids, contributing to the loss of
muscle mass and strength (50). The
anti-oxidative properties of astaxanthin have been widely
demonstrated by researchers, evidenced by reduction in various
lipid peroxidation by-products, oxidative stress biomarkers and
markers of muscle damage (51). An
earlier animal study demonstrated that astaxanthin attenuated
exercise-induced skeletal and cardiac muscle damage in 7-week-old
female C57BL/6 mice. The animals were randomly assigned to three
groups: Rested controls, intense exercise and intense exercise
supplemented with dietary astaxanthin (0.02% w/w). Exercise
acclimation (running on a motor-driven treadmill with running
intensity increased from 5 to 28 m/min) performed for 10 min/day
three times per week for three weeks. At the end of the study, the
exercise groups ran on a treadmill at 28 m/min until exhaustion.
The data from this study showed that increases in
4-hydroxy-2-nonenal (4-HNE)-modified protein,
8-hydroxy-2'-deoxyguanosine (8-OHdG), plasma CK activity and
myeloperoxidase (MPO) activity in the gastrocnemius and heart
caused by exercise were attenuated by astaxanthin (52). Astaxanthin treatment was effective in
lowering the concentrations of malondialdehyde (MDA) or TBARS, ROS
and carbonylated protein in various animal models (28-32,38,39,41,53).
The complex endogenous antioxidant defence system,
consisting of key antioxidant enzymes such as glutathione
peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT),
plays a crucial role in neutralizing damaging free radical species
(54). High doses of astaxanthin (15
or 30 mg/kg) were shown to be effective in suppressing the levels
of GPx, CAT and CK and raising SOD activity in plasma and muscle of
mice after moderate-intensity swimming training (53). Another group of researchers
pinpointed the reduction in SOD-1 expression in animals with muscle
atrophy induced by hindlimb unloading treated with astaxanthin
alone (30) or in combination with
other interventions such as intermittent loading (31) or electrical stimulation (32). In line with these studies, a lowered
SOD-1 level was also detected in two other studies using animals
subjected to hindlimb immobilization-induced muscle atrophy
(38,39). Astaxanthin supplementation also
increased antioxidant capacity in the plasma, indicated by higher
Trolox-equivalent antioxidant capacity (TEAC) levels and
ferric-reducing activity of plasma (FRAP) capacity relative to the
control animals (28).
At the molecular level, the signalling pathway
involved in normalizing the disrupted balance between pro-oxidant
and antioxidant levels is that of the nuclear factor erythroid
2-related factor 2 (Nrf2) (55). In
the resting condition, Nrf2 assumes an inactive state by binding to
Kelch-like ECH-associated protein 1 (Keap1) to cause its
ubiquitination and degradation (56). Oxidative stress causes a
conformational change in Keap1 by interfering in the interaction
between Nrf2 and Keap1. Free Nrf2 is subsequently released,
translocated into the nucleus and bound to antioxidant responsive
elements (ARE) to allow transcription of genes that encode for
detoxifying or antioxidant enzymes (56). The transcription of Nrf2 and
Nrf2-dependent enzymes in the mouse heart during moderate-intensity
swimming training were downregulated in astaxanthin-treated animals
(53). In this context, it appears
that either the lack of or excess of ROS and antioxidants elicited
important pathological implications in skeletal muscle. An optimal
amount of ROS and antioxidants may serve as an important factor in
maximizing skeletal muscle performance.
The mitochondrial electron transport chain is the
major site of ROS production in skeletal muscle, thus mitochondrial
DNA is susceptible to oxidative damage by overwhelming ROS
production, affecting mitochondrial homeostasis and function
(57). Changes in mitochondrial
membrane potential, reduction in mitochondrial energy production
capacity, inhibition of mitochondrial oxygen consumption and
reduction in mitochondrial biogenesis are common characteristics of
mitochondrial dysfunction (58),
which are (59). During physical
activity, endothelial nitric associated with the development of
sarcopenia oxide synthase (eNOS) is upregulated to increase nitric
oxide production, which subsequently induces mitochondrial
biogenesis and cell glucose uptake in skeletal muscle. Peroxisome
proliferator-activated receptor gamma coactivator 1-α (PGC-1α) is a
master regulator of mitochondrial biogenesis that regulates the
genes involved in cellular energy metabolism (60,61).
High levels of PGC-1α are an indicator of improved aerobic
metabolism and function of mitochondria (62). Using an exercised mouse model, Liu
et al (63) suggested that
astaxanthin accelerated lipid utilization in skeletal muscle and
reduced intermuscular pH during aerobic exercise through elevation
of PGC-1α. Studies conducted by Kanazashi et al (31,32)
identified a similar pattern to astaxanthin alone, astaxanthin with
intermittent loading and astaxanthin with electrical stimulation
retained mitochondrial biogenesis by raising PGC-1α and eNOS
expression in the soleus muscle of hindlimb unloaded mice. The
total 5'-adenosine monophosphate-activated protein kinase (AMPK)
content in skeletal muscle was also significantly augmented in the
exercised animals fed with astaxanthin from H. pluvialis
than the control group. These findings suggested that astaxanthin
enhanced energy production leading to a longer running time during
treadmill exercise (27).
Under physiological conditions, the maintenance of
skeletal muscle mass depends on the balance between muscle protein
synthesis and muscle protein degradation (64). Muscle atrophy occurs when the rate of
protein degradation outweighs the rate of protein synthesis
(65). The suggested signal
transduction involved in muscle protein synthesis and degradation
includes the phosphatidylinositol-3-kinase (PI3K)/protein kinase B
(Akt)/mammalian target of ripamycin (mTOR) signalling and Forkhead
Box O (FoxO) transcription factor. The activation of PI3K/Akt/mTOR
pathway is modulated by the interaction of insulin growth factor-1
(IGF-1) and insulin with their respective tyrosine kinase
receptors. The activated PI3K/Akt eventually phosphorylates mTOR
and its downstream factor, p70 ribosomal protein S6 kinase (P70S6K)
to promote protein synthesis (66,67). The
mixture of three antioxidants (astaxanthin, β-carotene and
resveratrol) was shown to have greater efficacy than each
antioxidant respectively in increasing relative soleus weight. This
outcome was mediated through the increased phosphorylation of mTOR
and its downstream factor (P70S6K) in male mice with muscle atrophy
(29). FoxO transcription factors
play a role in the catabolic pathway in skeletal muscle. FoxO is
phosphorylated (inhibited) by Akt, thus the genes responsible for
muscle atrophy cannot be transcribed (68). In the study performed by Kanazashi
et al (32), it was noted
that hindlimb unloading induced muscle atrophy in the rats by
activating the ubiquitin-proteasome pathway through reduced
phosphorylation (activation) of Forkhead box class O 3a (FoxO3a).
It is also evident that protein degradation during muscle disuse is
associated with activation of the ubiquitin-proteasome proteolytic
pathway, resulting in increased ubiquitinated protein expression.
Muscle atrophy induced by hindlimb unloading was reversed by the
combined intervention of astaxanthin and electrical stimulation via
increased phosphorylation (inhibition) of FoxO3a (32). In addition, it has been reported that
the induction of oxidative stress stimulated protein degradation by
upregulating calpain (a proteolytic enzyme act upstream of the
ubiquitin-proteasome proteolytic pathway) (69). It was revealed that dietary
astaxanthin intake protected against disuse muscle atrophy in rats,
which was partly due to the reduction of oxidative stress, calpain
and ubiquitin expression (38).
Another mechanism of action that explains the
positive effects of astaxanthin in suppressing disuse skeletal
muscle atrophy involves the inhibition of myonuclear apoptosis.
Apoptosis of myonuclei contributes to the loss of muscle mass.
Previous work by Yoshihara et al (33) indicated that dietary astaxanthin
supplementation prevented the increase of apoptotic nuclei in
soleus muscle [indicated by decreased number of terminal
deoxynucleotidyl transferase dUTP nick end labelling
(TUNEL)-positive nuclei]. Satellite cells (also known as skeletal
muscle stem cells) are the precursors of skeletal muscle cells
required for muscle mass maintenance and muscle regeneration
following muscle atrophy (70).
Previous studies have demonstrated the alterations of satellite
cell activity and density by muscle catabolic conditions, such as
disuse and ageing (71). Indeed,
Yoshihara and co-authors (34) also
revealed that the protection against disuse muscle atrophy exerted
by astaxanthin might be due to the increase in satellite cell
numbers.
The capillary number in skeletal muscle is
proportionate with muscle loading and activity levels. For
instance, exercise and functional overload promote capillary growth
(72) whereas unloading and
immobilization result in capillary regression (73). The regression of the capillary
network during low level muscle loading and activity is often
attributed to an increase in oxidative injury (11). Kanazashi et al (30,31)
performed two studies to assess the effects of astaxanthin on
skeletal muscle capillaries. In these studies, they reported that
hindlimb unloading induced an overproduction of ROS, resulting in
capillary regression and muscle atrophy in the animals. Upon
astaxanthin intervention, the decreases in angiogenic factors [such
as vascular endothelial growth factor (VEGF), hypoxia inducible
factor-1 alpha (HIF-1α), FMS-like tyrosine kinase 1 (Flt-1), kinase
insert domain-containing receptor (KDR), angiopoietin 1 (ANG-1) and
tyrosine kinase with Ig and EGF homology domains 2 (Tie-2)] caused
by hindlimb unloading were counteracted (30). The subsequent study revealed that
hindlimb unloading decreased PGC-1α, VEGF and succinate
dehydrogenase (SDH) activity, which contributed to the detrimental
effects on morphology and number of capillary networks in rat
soleus muscle. Oral astaxanthin administration maintained the
capillary network by increasing PGC-1α, VEGF and SDH activity near
values of animals with sarcopenia (31).
6. Perspectives and conclusion
In the present review, the role of astaxanthin on
skeletal muscle was examined in two major conditions: Physical
exercise and muscle atrophy. Though the direct beneficial effects
of astaxanthin on skeletal muscle were marginal in certain studies,
astaxanthin was shown to be a potentially effective agent to
enhance skeletal muscle performance and counteract the detrimental
effects of skeletal muscle disuse. The mechanisms of action of
astaxanthin may be attributed to its potential to prevent oxidative
stress, increase energy production in mitochondria, regulate the
anabolic (regeneration) and catabolic (proteolysis) processes of
skeletal muscle, suppress programmed cell death of the myonucleus
and activate associated angiogenic pathways to maximize capillary
network (Fig. 1). Among these
molecular mechanisms, oxidative stress appears to be the common
factor that ultimately causes stepwise escalation to the onset and
progression of sarcopenia.
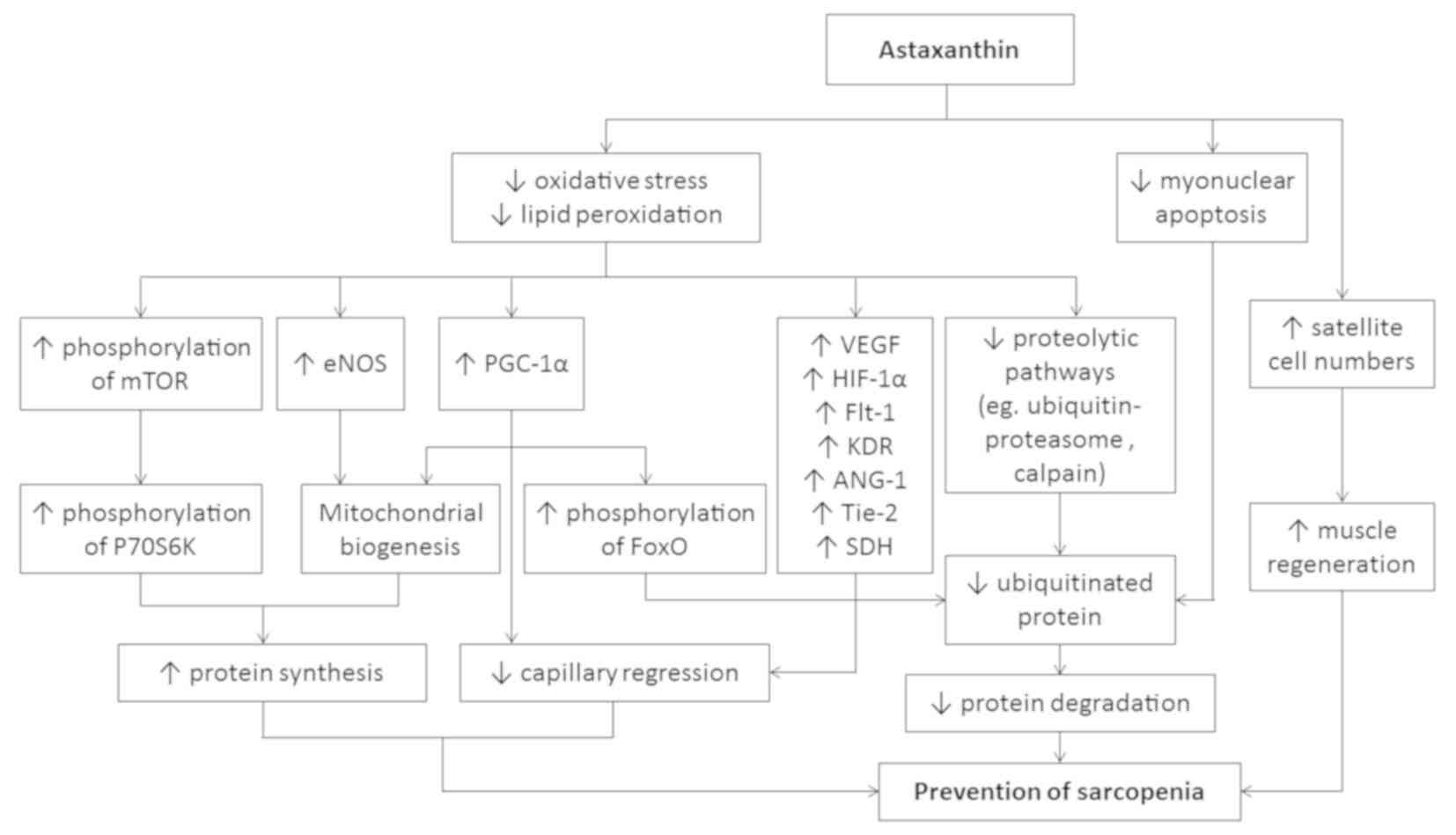 | Figure 1The potential mechanism of action of
astaxanthin in the prevention of sarcopenia. ANG-1, angiopoietin 1;
eNOS, endothelial nitric oxide synthase; Flt-1, FMS-like tyrosine
kinase 1; FoxO, Forkhead Box O; HIF-1α, hypoxia inducible factor-1
alpha; KDR, kinase insert domain-containing receptor; mTOR,
mammalian target of ripamycin; P70S6K, p70 ribosomal protein S6
kinase; PGC-1α, peroxisome proliferator-activated receptor gamma
coactivator 1-alpha; SDH, succinate dehydrogenase; Tie-2, tyrosine
kinase with Ig and EGF homology domains 2; VEGF, vascular
endothelial growth factor. |
Several limitations of the currently available
studies need to be addressed. Firstly, the evidence is largely
preliminary and suggestive of the potential of astaxanthin in the
management of sarcopenia. Much effort should be paid on further
investigations to validate the clinical use of astaxanthin.
Secondly, the induction of oxidative stress in skeletal muscle has
a direct mechanistic link with chronic state of low-grade
inflammation during disuse muscle atrophy (50). Despite exhibiting anti-oxidative
properties, astaxanthin has been reported to be useful for
improving chronic inflammation (24,74).
Therefore, investigation of the anti-inflammatory properties of
astaxanthin during exercise or skeletal muscle atrophy may be a
beneficial area of research. Thirdly, the test formulation provided
in certain studies was a mixture of astaxanthin with other
antioxidants. The positive health outcomes of astaxanthin alone
could not be concluded as the effects might be derived from other
antioxidative agents. As a combination of astaxanthin with other
interventions may show greater efficacy than astaxanthin alone in
promoting skeletal muscle health and performance astaxanthin may be
beneficial when used clinically in conjunction with other
interventions, such as exercise, hormonal and nutritional
intervention to improve muscle health.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the
Universiti Kebangsaan Malaysia and Ministry of Education, Malaysia
(grant nos. MI-2019-006 and FRGS/1/2018/SKK10/UKM/03/1).
Availability of data and materials
Not applicable.
Authors' contributions
SKW performed the literature search and drafted the
manuscript. SIN and KYC provided critical review for the
manuscript. KYC gave final approval for the publication of this
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Santilli V, Bernetti A, Mangone M and
Paoloni M: Clinical definition of sarcopenia. Clin Cases Miner Bone
Metab. 11:177–180. 2014.PubMed/NCBI
|
|
2
|
Trajanoska K, Schoufour JD, Darweesh SK,
Benz E, Medina-Gomez C, Alferink LJ, Lahousse L, Brusselle G,
Stricker B, Darwish Murad S, et al: Sarcopenia and its clinical
correlates in the general population: The rotterdam study. J Bone
Miner Res. 33:1209–1218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Beaudart C, Rizzoli R, Bruyère O,
Reginster JY and Biver E: Sarcopenia: Burden and challenges for
public health. Arch Public Health. 72:45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shafiee G, Keshtkar A, Soltani A, Ahadi Z,
Larijani B and Heshmat R: Prevalence of sarcopenia in the world: A
systematic review and meta-analysis of general population studies.
J Diabetes Metab Disord. 16:21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goates S, Du K, Arensberg MB, Gaillard T,
Guralnik J and Pereira SL: Economic impact of hospitalizations in
US adults with sarcopenia. J Frailty Aging. 8:93–99.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
World Population Ageing: World Population
Ageing 2017. United Nations New York, 2017.
|
|
7
|
Kwak JY and Kwon KS: Pharmacological
interventions for treatment of sarcopenia: Current status of drug
development for sarcopenia. Ann Geriatr Med Res. 23:98–104.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aoi W and Sakuma K: Oxidative stress and
skeletal muscle dysfunction with aging. Curr Aging Sci. 4:101–109.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Londhe P and Guttridge DC: Inflammation
induced loss of skeletal muscle. Bone. 80:131–142. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andreux PA, van Diemen MPJ, Heezen MR,
Auwerx J, Rinsch C, Groeneveld GJ and Singh A: Mitochondrial
function is impaired in the skeletal muscle of pre-frail elderly.
Sci Rep. 8(8548)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fujino H, Kondo H, Nagatomo F and Ishihara
A: Capillary growth and regression in skeletal muscle. J Phys Fit
Sports Med. 3:483–491. 2014.
|
|
12
|
Lee SP, Mar GY and Ng LT: Effects of
tocotrienol-rich fraction on exercise endurance capacity and
oxidative stress in forced swimming rats. Eur J Appl Physiol.
107:587–595. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Servais S, Letexier D, Favier R, Duchamp C
and Desplanches D: Prevention of unloading-induced atrophy by
vitamin E supplementation: Links between oxidative stress and
soleus muscle proteolysis? Free Radic Biol Med. 42:627–635.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Higuera-Ciapara I, Felix-Valenzuela L and
Goycoolea FM: Astaxanthin: A review of its chemistry and
applications. Crit Rev Food Sci Nutr. 46:185–196. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dose J, Matsugo S, Yokokawa H, Koshida Y,
Okazaki S, Seidel U, Eggersdorfer M, Rimbach G and Esatbeyoglu T:
Free radical scavenging and cellular antioxidant properties of
astaxanthin. Int J Mol Sci. 17(103)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park JS, Chyun JH, Kim YK, Line LL and
Chew BP: Astaxanthin decreased oxidative stress and inflammation
and enhanced immune response in humans. Nutr Metab. 7:18.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McCall B, McPartland CK, Moore R,
Frank-Kamenetskii A and Booth BW: Effects of astaxanthin on the
proliferation and migration of breast cancer cells in vitro.
Antioxidants. 7(135)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sila A, Ghlissi Z, Kamoun Z, Makni M,
Nasri M, Bougatef A and Sahnoun Z: Astaxanthin from shrimp
by-products ameliorates nephropathy in diabetic rats. Eur J Nutr.
54:301–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang H and Kim H: Astaxanthin and
β-carotene in Helicobacter pylori-induced Gastric Inflammation: A
mini-review on action mechanisms. J Cancer Prev. 22:57–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rao AR, Sarada R, Shylaja MD and
Ravishankar GA: Evaluation of hepatoprotective and antioxidant
activity of astaxanthin and astaxanthin esters from
microalga-Haematococcus pluvialis. J Food Sci Tech. 52:6703–6710.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Feng Y, Chu A, Luo Q, Wu M, Shi X and Chen
Y: The protective effect of astaxanthin on cognitive function via
inhibition of oxidative stress and inflammation in the brains of
chronic T2DM Rats. Front Pharmacol. 9(748)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fassett RG and Coombes JS: Astaxanthin: A
potential therapeutic agent in cardiovascular disease. Mar Drugs.
9:447–465. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashimoto H, Arai K, Hayashi S, Okamoto H,
Takahashi J, Chikuda M and Obara Y: Effects of astaxanthin on
antioxidation in human aqueous humor. J Clin Biochem Nutr. 53:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Davinelli S, Nielsen ME and Scapagnini G:
Astaxanthin in skin health, repair, and disease: A comprehensive
review. Nutrients. 10(522)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brown DR, Gough LA, Deb SK, Sparks SA and
McNaughton LR: Astaxanthin in exercise metabolism, performance and
recovery: A review. Front Nutr. 4(76)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu T, Dohl J, Chen Y, Gasier HG and
Deuster PA: Astaxanthin but not quercetin preserves mitochondrial
integrity and function, ameliorates oxidative stress, and reduces
heat-induced skeletal muscle injury. J Cell Physiol.
234:13292–13302. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aoi W, Maoka T, Abe R, Fujishita M and
Tominaga K: Comparison of the effect of non-esterified and
esterified astaxanthins on endurance performance in mice. J Clin
Biochem Nutr. 62:161–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Polotow TG, Vardaris CV, Mihaliuc AR,
Gonçalves MS, Pereira B, Ganini D and Barros MP: Astaxanthin
supplementation delays physical exhaustion and prevents redox
imbalances in plasma and soleus muscles of Wistar rats. Nutrients.
6:5819–5838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kawamura A, Aoi W, Abe R, Kobayashi Y,
Wada S, Kuwahata M and Higashi A: Combined intake of astaxanthin,
β-carotene, and resveratrol elevates protein synthesis during
muscle hypertrophy in mice. Nutrition. 69(110561)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kanazashi M, Okumura Y, Al-Nassan S,
Murakami S, Kondo H, Nagatomo F, Fujita N, Ishihara A, Roy RR and
Fujino H: Protective effects of astaxanthin on capillary regression
in atrophied soleus muscle of rats. Acta Physiol. 207:405–415.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kanazashi M, Tanaka M, Murakami S, Kondo
H, Nagatomo F, Ishihara A, Roy RR and Fujino H: Amelioration of
capillary regression and atrophy of the soleus muscle in
hindlimb-unloaded rats by astaxanthin supplementation and
intermittent loading. Exp Physiol. 99:1065–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kanazashi M, Tanaka M, Nakanishi R,
Maeshige N and Fujino H: Effects of astaxanthin supplementation and
electrical stimulation on muscle atrophy and decreased oxidative
capacity in soleus muscle during hindlimb unloading in rats. J
Physiol Sci. 69:757–767. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yoshihara T, Yamamoto Y, Shibaguchi T,
Miyaji N, Kakigi R, Naito H, Goto K, Ohmori D, Yoshioka T and
Sugiura T: Dietary astaxanthin supplementation attenuates
disuse-induced muscle atrophy and myonuclear apoptosis in the rat
soleus muscle. J Physiol Sci. 67:181–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoshihara T, Sugiura T, Miyaji N, Yamamoto
Y, Shibaguchi T, Kakigi R, Naito H, Goto K, Ohmori D and Yoshioka
T: Effect of a combination of astaxanthin supplementation, heat
stress, and intermittent reloading on satellite cells during disuse
muscle atrophy. J Zhejiang Univ Sci B. 19:844–852. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang F, Zhang QB, Zhou Y, Chen S, Huang
PP, Liu Y and Xu YH: The mechanisms and treatments of muscular
pathological changes in immobilization-induced joint contracture: A
literature review. Chin J Traumatol. 22:93–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Frimel TN, Kapadia F, Gaidosh GS, Li Y,
Walter GA and Vandenborne K: A model of muscle atrophy using cast
immobilization in mice. Muscle Nerve. 32:672–674. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Morey-Holton ER and Globus RK: Hindlimb
unloading rodent model: Technical aspects. J Appl Physiol (1985).
92:1367–1377. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shibaguchi T, Yamaguchi Y, Miyaji N,
Yoshihara T, Naito H, Goto K, Ohmori D, Yoshioka T and Sugiura T:
Astaxanthin intake attenuates muscle atrophy caused by
immobilization in rats. Physiol Rep. 4(e12885)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Maezawa T, Tanaka M, Kanazashi M, Maeshige
N, Kondo H, Ishihara A and Fujino H: Astaxanthin supplementation
attenuates immobilization-induced skeletal muscle fibrosis via
suppression of oxidative stress. J Physiol Sci. 67:603–611.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nishida Y, Nawaz A, Kado T, Takikawa A,
Igarashi Y, Onogi Y, Wada T, Sasaoka T, Yamamoto S, Sasahara M, et
al: Astaxanthin stimulates mitochondrial biogenesis in insulin
resistant muscle via activation of AMPK pathway. J Cachexia
Sarcopenia Muscle. 11:241–258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Inoue H, Shimamoto S, Takahashi H,
Kawashima Y, Wataru S, Ijiri D and Ohtsuka A: Effects of
astaxanthin-rich dried cell powder from Paracoccus carotinifaciens
on carotenoid composition and lipid peroxidation in skeletal muscle
of broiler chickens under thermo-neutral or realistic high
temperature conditions. Anim Sci J. 90:229–236. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mancini RA and Hunt MC: Current research
in meat color. Meat Sci. 71:100–121. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zeferino CP, Komiyama CM, Pelícia VC,
Fascina VB, Aoyagi MM, Coutinho LL, Sartori JR and Moura AS:
Carcass and meat quality traits of chickens fed diets concurrently
supplemented with vitamins C and E under constant heat stress.
Animal. 10:163–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bloomer RJ, Fry A, Schilling B, Chiu L,
Hori N and Weiss L: Astaxanthin supplementation does not attenuate
muscle injury following eccentric exercise in resistance-trained
men. Int J Sport Nutr Exerc Metab. 15:401–412. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Djordjevic B, Baralic I, Kotur-Stevuljevic
J, Stefanovic A, Ivanisevic J, Radivojevic N, Andjelkovic M and
Dikic N: Effect of astaxanthin supplementation on muscle damage and
oxidative stress markers in elite young soccer players. J Sports
Med Phys Fitness. 52:382–392. 2012.PubMed/NCBI
|
|
46
|
Liu SZ, Ali AS, Campbell MD, Kilroy K,
Shankland EG, Roshanravan B, Marcinek DJ and Conley KE: Building
strength, endurance, and mobility using an astaxanthin formulation
with functional training in elderly. J Cachexia Sarcopenia Muscle.
9:826–833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Takami M, Aoi W, Terajima H, Tanimura Y,
Wada S and Higashi A: Effect of dietary antioxidant-rich foods
combined with aerobic training on energy metabolism in healthy
young men. J Clin Biochem Nutr. 64:79–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Scicchitano BM, Pelosi L, Sica G and
Musarò A: The physiopathologic role of oxidative stress in skeletal
muscle. Mech Ageing Dev. 170:37–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Brioche T and Lemoine-Morel S: Oxidative
stress, sarcopenia, antioxidant strategies and exercise: Molecular
aspects. Curr Pharm Des. 22:2664–2678. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Meng SJ and Yu LJ: Oxidative stress,
molecular inflammation and sarcopenia. Int J Mol Sci. 11:1509–1526.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sztretye M, Dienes B, Gönczi M, Czirják T,
Csernoch L, Dux L, Szentesi P and Keller-Pintér A: Astaxanthin: A
potential mitochondrial-targeted antioxidant treatment in diseases
and with aging. Oxid Med Cell Longev. 3849692:2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Aoi W, Naito Y, Sakuma K, Kuchide M,
Tokuda H, Maoka T, Toyokuni S, Oka S, Yasuhara M and Yoshikawa T:
Astaxanthin limits exercise-induced skeletal and cardiac muscle
damage in mice. Antioxid Redox Signal. 5:139–144. 2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhou Y, Baker JS, Chen X, Wang Y, Chen H,
Davison GW and Yan X: High-dose astaxanthin supplementation
suppresses antioxidant enzyme activity during moderate-intensity
swimming training in mice. Nutrients. 11(1244)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kurutas EB: The importance of antioxidants
which play the role in cellular response against
oxidative/nitrosative stress: Current state. Nutr J. 15:71.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Vomund S, Schäfer A, Parnham MJ, Brüne B
and von Knethen A: Nrf2, the master regulator of anti-oxidative
responses. Int J Mol Sci. 18(2772)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Guo C, Sun L, Chen X and Zhang D:
Oxidative stress, mitochondrial damage and neurodegenerative
diseases. Neural Regen Res. 8:2003–2014. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ren J, Pulakat L, Whaley-Connell A and
Sowers JR: Mitochondrial biogenesis in the metabolic syndrome and
cardiovascular disease. J Mol Med. 88:993–1001. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Coen PM, Musci RV, Hinkley JM and Miller
BF: Mitochondria as a target for mitigating Sarcopenia. Front
Physiol. 9(1883)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liang H and Ward WF: PGC-1alpha: A key
regulator of energy metabolism. Adv Physiol Educ. 30:145–151.
2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Litvinova L, Atochin DN, Fattakhov N,
Vasilenko M, Zatolokin P and Kirienkova E: Nitric oxide and
mitochondria in metabolic syndrome. Front Physiol.
6(20)2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Austin S and St-Pierre J: PGC1 alpha and
mitochondrial metabolism-emerging concepts and relevance in ageing
and neurodegenerative disorders. J Cell Sci. 125:4963–4971.
2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu PH, Aoi W, Takami M, Terajima H,
Tanimura Y, Naito Y, Itoh Y and Yoshikawa T: The
astaxanthin-induced improvement in lipid metabolism during exercise
is mediated by a PGC-1α increase in skeletal muscle. J Clin Biochem
Nutr. 54:86–89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gordon BS, Kelleher AR and Kimball SR:
Regulation of muscle protein synthesis and the effects of catabolic
states. Int J Biochem Cell Biol. 45:2147–2157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sandri M: Protein breakdown in muscle
wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J
Biochem Cell Biol. 45:2121–2129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Latres E, Amini AR, Amini AA, Griffiths J,
Martin FJ, Wei Y, Lin HC, Yancopoulos GD and Glass DJ: Insulin-like
growth factor-1 (IGF-1) inversely regulates atrophy-induced genes
via the phosphatidylinositol 3-kinase/Akt/mammalian target of
rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 280:2737–2744.
2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ziaaldini MM, Marzetti E, Picca A and
Murlasits Z: Biochemical pathways of sarcopenia and their
modulation by physical exercise: A narrative review. Front Med.
4(167)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sanchez AM, Candau RB and Bernardi H: FoxO
transcription factors: Their roles in the maintenance of skeletal
muscle homeostasis. Cell Mol Life Sci. 71:1657–1671.
2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Smuder AJ, Kavazis AN, Hudson MB, Nelson
WB and Powers SK: Oxidation enhances myofibrillar protein
degradation via calpain and caspase-3. Free Radic Biol Med.
49:1152–1160. 2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dumont NA, Bentzinger CF, Sincennes MC and
Rudnicki MA: Satellite cells and skeletal muscle regeneration.
Compr Physiol. 5:1027–1059. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
McKenna CF and Fry CS: Altered satellite
cell dynamics accompany skeletal muscle atrophy during chronic
illness, disuse, and aging. Curr Opin Clin Nutr Metab Care.
20:447–452. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hoier B and Hellsten Y: Exercise-induced
capillary growth in human skeletal muscle and the dynamics of VEGF.
Microcirculation. 21:301–314. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Fujino H, Kohzuki H, Takeda I, Kiyooka T,
Miyasaka T, Mohri S, Shimizu J and Kajiya F: Regression of
capillary network in atrophied soleus muscle induced by hindlimb
unweighting. J Appl Physiol. 98:1407–1413. 2005.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Miyachi M, Matsuno T, Asano K and Mataga
I: Anti-inflammatory effects of astaxanthin in the human gingival
keratinocyte line NDUSD-1. J Clin Biochem Nutr. 56:171–178.
2015.PubMed/NCBI View Article : Google Scholar
|















