Introduction
Cisplatin (DDP) is recognized as one of the most
effective antineoplastic drugs and is extensively used in several
types of cancer, such as lung, liver, ovarian, bladder and cervical
cancers (1-5).
However, the clinical application of cisplatin is limited due to
its toxic side effects, particularly acute kidney injury (AKI)
(6). Thus, development of novel
adjuvant therapeutic strategies remains critical to alleviate
nephrotoxicity induced by cisplatin. Puerarin is an isoflavonoid
extracted from the Chinese medical herb Radix puerariae
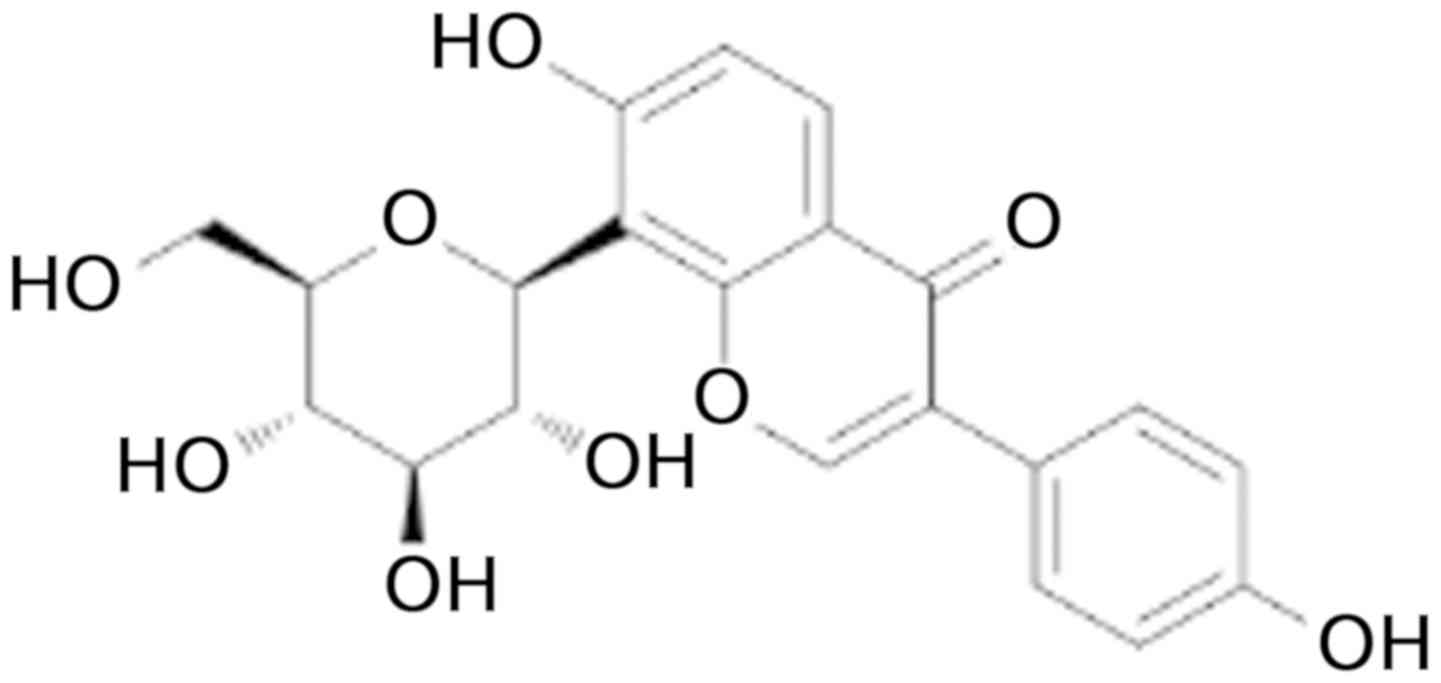
(R. puerariae), with a structure of
7-hydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one-8-β-D-glucopyranoside
(Fig. 1) (7). Puerarin has recently attracted interest
for its extensive pharmacological properties in the treatment of
kidney diseases, including AKI and chronic renal injury (8-11).
Furthermore, it was suggested that puerarin exerted a protective
effect against DDP-induced nephrotoxicity (12). However, the molecular mechanisms
underlying renal protection of puerarin in DDP-induced AKI remain
unclear.
MicroRNAs (miRNAs/miRs) play a regulatory role in
biological processes, including inflammation, by negatively
regulating several coding genes (13). miR-31 was demonstrated to be
associated with the progress of renal cell carcinoma (RCC)
(14). In addition, miR-31-5p was
markedly downregulated in RCC tissues compared with paired adjacent
normal tissues (15). miR-31 was
also found to directly target the 3'-untranslated region (UTR)
sequence of the Numb gene (16).
Furthermore, miR-31-5p was reported to promote the proliferation of
colorectal cancer cells by targeting the Numb protein (17). Numb is considered an intrinsic
determinant in Drosophila, which alters the cell fate by
negatively regulating the Notch signaling pathway (18). The depletion of Numb can promote
dynamin-related protein 1-mediated mitochondrial fission and
exacerbate mitochondrial dysfunction and tubular cell apoptosis in
DDP-induced AKI (19). Numb has also
been demonstrated to alleviate puromycin aminonucleoside-induced
renal proximal tubular cell apoptosis by suppressing the Notch
signaling pathway (20). The present
study aimed to investigate whether puerarin exerts its
renoprotective function via the miR-31-mediated Numb/Notch1
signaling pathway, using a DDP-induced AKI rat model.
Materials and methods
Reagents and chemicals
Puerarin was obtained from Zhejiang Conba
Pharmaceutical Co., Ltd. DDP was purchased from Qilu Pharmaceutical
Co., Ltd. Other chemicals were all purchased from Beijing Solarbio
Science & Technology Co., Ltd.
Cells and animals
The human renal proximal tubular epithelial (HK-2)
cell line was provided by the Central aboratory, the Second
Hospital of Shandong University. HK-2 cells were cultured in DMEM
(4.5 g/l glucose) with 10% FBS and 1% penicillin-streptomycin at
37˚C in 5% CO2. HK-2 cells were treated with 40 µM DDP
at different timepoints (0, 12, 24 and 48 h) or 100 µM
puerarin.
Female Sprague-Dawley rats (age 6-8 weeks; weight
180-220 g) were purchased from the Division of Animals of Peking
University (China) and housed in specific-pathogen free conditions
at the animal center of the Second Hospital of Shandong University,
China. The use of rats was approved by the Institutional Care and
Use Committee of the Second Hospital of Shandong University [permit
no. KYLL-2017 (GJ) A-0028]. The rats were disposed in compliance
with the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health.
Experimental design
A total of 18 rats were randomly divided into three
groups (21,22): i) Sham control group (Sham, n=6),
which received intraperitoneal (i.p.) injection of 2 ml saline and
intravenous (i.v.) injection of 0.1 ml saline every other day over
the course of 14 days; ii) DDP group (n=6), which received i.p.
injection of 20 mg/kg DDP on day 1 and iii) puerarin + DDP (Pue+DDP
group, n=6), which received i.p. injection of 20 mg/kg DDP on day 1
and with i.v. injection of puerarin (50 mg/kg) every other day over
the course of 14 days.
The dosage regimen was implemented according to
previous studies (2,12). The weights of each rat were recorded
every day during the entire experimental period, and the kidneys
were assessed in g per g of body weight. On day 14, the body and
kidney weight of rats were recorded, and blood was collected before
the rats sacrificed by spinal dislocation after intraperitoneal
injection of 4% chloral hydrate (300 mg/kg) for ~10 min. Kidney
samples were removed for western blotting.
Evaluation of renal function
Blood was collected into a centrifuge tube
containing heparin and then centrifuged at 500 x g for 4 min at
room temperature to obtain plasma samples. An automated blood
analyzer (Dimension Xpand Plus; Siemens Healthineers) and standard
diagnostic kits (urea nitrogen, cat. no. EIABUN; Invitrogen; Thermo
Fisher Scientific, Inc. and creatinine, cat. no. C011-1-1; Nanjing
Jiancheng Bioengeneering Institute) was used to determine blood
urea nitrogen (BUN) and serum creatinine (SCR) content.
Hematoxylin and eosin (HE) and TUNEL
examination
Kidneys were fixed in 10% buffered formalin
overnight at 4˚C, embedded in paraffin, sectioned (4-6 µm
thickness) and stained with HE at room temperature for 6 min. A
light microscope (CKX41; Olympus Corporation) was used to detect
histopathological results at x4 magnification. Kidney sections were
also used to determine apoptosis. Apoptotic cells were identified
using a TUNEL assay (In Situ Cell Death Detection kit; cat.
no. 11684817910; Roche; Merck KGaA). TUNEL staining was performed
according to the manufacturer's instructions. Apoptotic cells were
monitored by dark brown nuclear staining and counted with 100 cells
from six random microscopic fields under a fluorescent microscope
(x10 magnification) in each group. The values were presented as the
percentage of the total number of cells.
Assessment of oxidative stress
Tissue samples were homogenized with saline solution
(0.9%) at 4˚C and centrifuged at 200 x g for 30 min at room
temperature. The supernatants were collected for oxidative stress
detection. The activities of superoxide dismutase (SOD; SOD assay
kit; cat. no. A001-3-2; Nanjing Jiancheng Bioengineering Institute)
and malondialdehyde (MDA; MDA assay kit; cat. no. A003-1-1; Nanjing
Jiancheng Bioengineering Institute), and the contents of
glutathione S-transferase (GS; GST Fluorometric Assay kit; cat. no.
KT002; AmyJet Scientific) and catalase (CA; CAT assay kit; cat. no.
A007-1-1; Nanjing Jiancheng Bioengineering Institute) in kidneys
were determined according to the manufacturer's protocols.
Cell transfection
HK-2 cells were plated in 6-well plates at a density
of 1x105 cells/well until 80 90% confluence and were
transfected with miR-31 inhibitor and negative control (NC) (both,
final concentration 100 nM) using Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. HK-2 cells transfected with miR-NC were used as controls.
Briefly, following culture for 24-48 h, the expression of Numb and
Notch-1 genes were detected using reverse
transcription-quantitative PCR (RT-qPCR) analysis. After 6 h of
incubation at 37˚C with 5% CO2, the approximate
transfection efficiency was measured, where expression of miR-31
significantly decreased in miR-31 inhibitor-treated (12.52%) or
both miR-31 inhibitor and DDP (18.12%), compared with sham (97.83%)
or DDP treatment (85.45%), respectively (Fig. S1). The results were calculated using
fluorescence-activated cell sorting (Nikon Eclipse C1; Nikon
Corporation and ImageJ; National Institutes of Health). The cells
were continued to be cultured after the medium was replaced once
the transfection efficiency was calculated. Total RNA was extracted
from the groups at 48 h after transfection.
RT-qPCR analysis
Total miRNA from HK-2 cells and kidneys were
extracted using an miRNeasy mini kit (Qiagen GmbH, 217704)
accordance with the manufacturer's protocol. cDNA was reverse
transcribed from miRNA using a miScript II RT kit according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.) at 72˚C for 10 min, 42˚C for 1 h and 72˚C for 5 min. TaqMan
fluorescent probe (cat. no. 4351379; Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the qPCR. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 2 min and 28 cycles of 95˚C for 1 min,
53˚C for 1 min and 72˚C for 5 min. The expression of levels Numb
and Notch1were quantified using the 2-ΔΔCq method
(23). U6 were used as internal
reference genes to calculate the mRNA miRNA expression of the
target genes, respectively. All experiments were performed three
times. The following primer pairs were used for the qPCR: miRNA-31
forward, 5'-CAGCTATGCCAGCATCTTG CCT-3' and miRNA-31 reverse,
5'-ATATGGAACGCTTCAC GAATT-3'; Numb forward, 5'-TTTCGAAAGTGTTGGGATT
ATATAC-3' and reverse, 5'-AACTACAATAAACCAAAATC GCG-3'; Notch1
forward, 5'-GCGAGGTCAACACAGAC GAG-3' and reverse,
5'-CAGGCACTTGGCACCATTC-3'; GAPDH forward,
5'-TTCACCACCATGGAGAAGGC-3' and reverse, 5'-GGCATGGACTGTGGTCATGA-3'
and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Western blotting
Kidney tissues were homogenized in ice-cold
suspension buffer (BioTeke Corporation) and HK-2 cells were lysed
with RIPA buffer (Thermo Fisher Scientific, Inc.). The samples were
centrifuged at 500 x g for 20 min at 4˚C, and protein concentration
was determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). The proteins (30 µg/lane) were separated
using 10% SDS-PAGE and then transferred onto PVDF membranes (EMD
Millipore).
Milk (5% non-fat) was used to block the non-specific
binding sites at room temperature for 2 h. Subsequently, the
membranes were incubated with the following primary antibodies:
Bcl-2 (cat. no. 3869s; 1:1,000; Cell Signaling Technology, Inc.),
Bax (cat. no. 2772s; 1:1,000; Cell Signaling Technology, Inc.),
caspase-3 (cat. no. 9654s; 1:1,000; Cell Signaling Technology,
Inc.), Numb (cat. no. 2761s; 1:1,000; Cell Signaling Technology,
Inc.), Notch1 (cat. no. 4830s; 1:1,000; Cell Signaling Technology,
Inc.) and β-actin (cat. no. 4970s; 1:1,000; Cell Signaling
Technology, Inc.) overnight at 4˚C. Protein signals were all
normalized to β-actin expression.
The membranes were washed thrice with TBS-10%
Tween-20 at room temperature and incubated with
horseradish-peroxidase conjugated anti-mouse immunoglobulin G
secondary antibody (cat. no. 7076S; 1:1,100; Cell Signaling
Technology, Inc.) for 2 h at room temperature. Protein signals were
detected using ECL reagent (Beijing Solarbio Science &
Technology Co., Ltd.), and analyzed using Gel-Pro Analyzer
densitometry software (version 6.3; Media Cybernetics, Inc.).
Triplicate experiments with triplicate samples were performed.
Statistical analysis
SPSS/Win 15.0 software (SPSS Inc.) was used for data
analysis. Data were expressed as the mean ± standard deviation.
Differences were analyzed using one-way ANOVA and Student's t-test.
Tukey's post hoc test was used for pairwise comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Puerarin suppresses mi-31 expression
in DDP-induced AKI
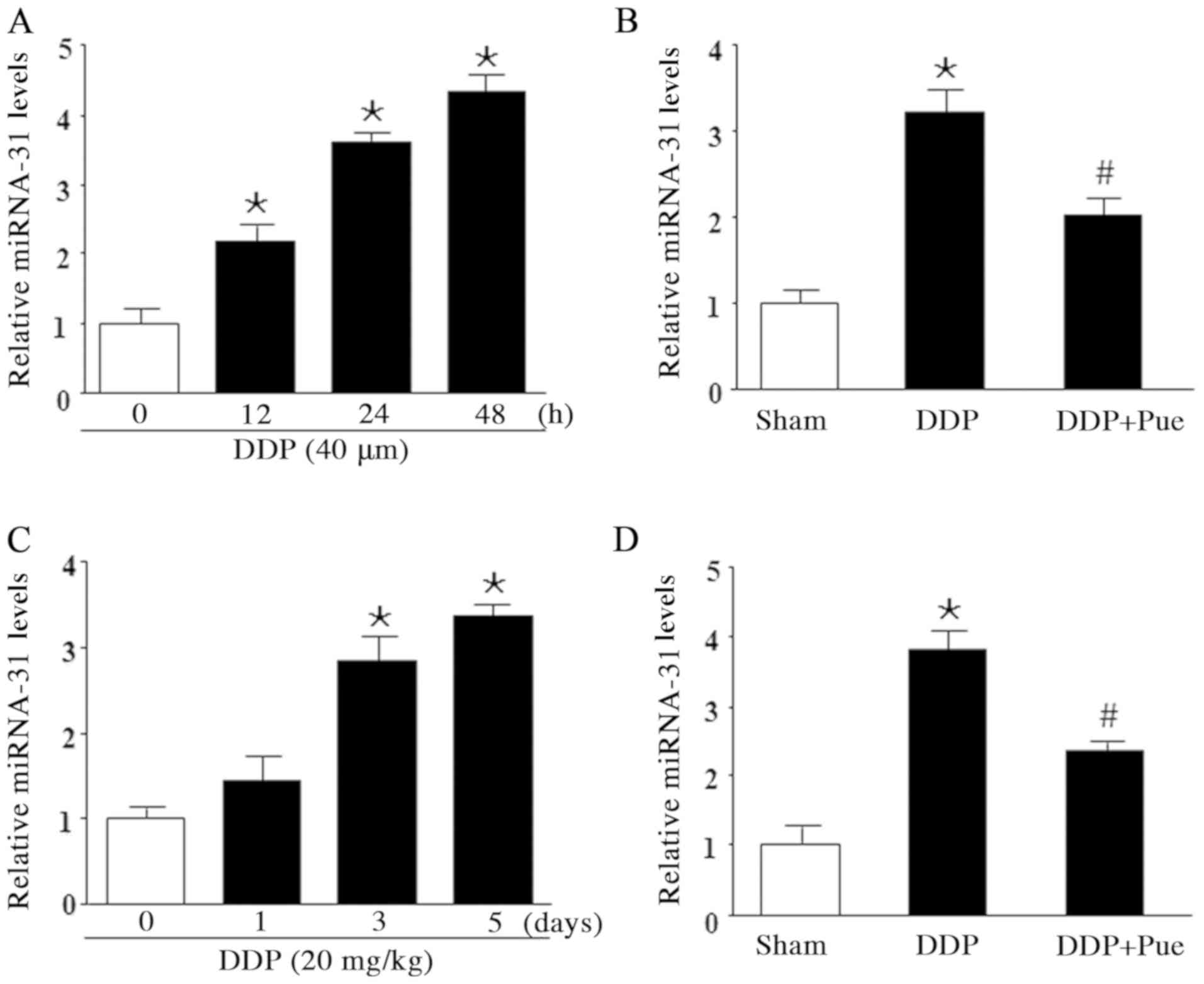
RT-qPCR was performed to determine miR-31 expression
in DDP-treated HK-2 cells and SD rats. The results demonstrated
that miR-31 significantly increased following treatment with DDP
compared with the controls. Furthermore, treatment with 40 µM DDP
for 12, 24 and 48 h increased miR-31 expression in a
concentration-dependent manner (Fig.
2A). To determine the effect of puerarin on miR-31 expression
in DDP-induced AKI, HK-2 cells were treated with 40 µM DDP and/or
100 µM puerarin. miR-31 expression was significantly upregulated
following DDP treatment compared with controls. Furthermore, the
combined treatment of DDP and puerarin significantly counteracted
the increased expression levels of miR-31 expression (Fig. 2B). miR-31 expression levels were
assessed in DDP-treated rats (20 mg/kg) on days 1, 3 and 5. The
results demonstrated that miR-31 levels significantly increased on
day 3 and 5 compared with day 0 (Fig.
2C). Conversely, combination treatment with 20 mg/kg DDP and 50
mg/kg puerarin downregulated miR-31 expression compared with DDP
treatment alone (Fig. 2D).
Puerarin improves DDP-induced renal
injury
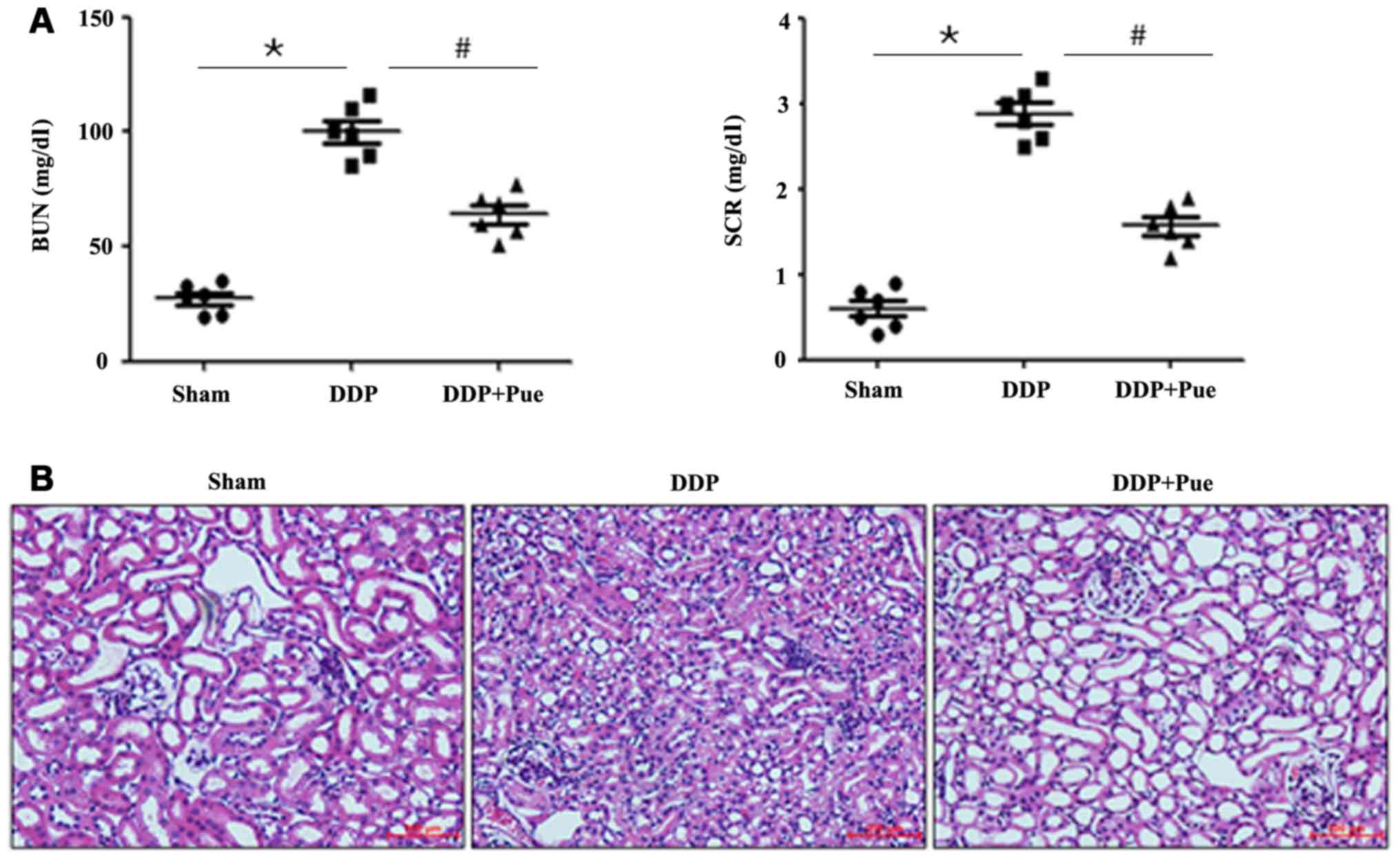
The contents of the kidney injury biomarkers (BUN)
and (SCR) significantly increased in DDP-treated rats compared with
controls (Fig. 3A). Conversely,
combined treatment of puerarin and DDP significantly decreased BUN
and SCR contents. Furthermore, a significant increase in the
absolute and relative kidney weights were observed in the
DDP-treated group compared with controls, whereas puerarin
prevented the alterations compared with the DDP-treated group
(Table I).
 | Table IEffect of DDP treatment (alone and
with puerarin) on the body weight, kidney weight and kidney/body
weight ratio in Sprague-Dawley rats. |
Table I
Effect of DDP treatment (alone and
with puerarin) on the body weight, kidney weight and kidney/body
weight ratio in Sprague-Dawley rats.
| Group | Body weight (g) | Kidney weight
(g) | Kidney/body weight
ratio (%) |
|---|
| Sham | 219.5±6.12 | 1.98±0.09 | 0.91±0.12 |
| DDP |
185.2±5.83a |
2.79±0.11a |
1.51±0.21a |
| DDP+Puerarin | 200.5±3.39 |
2.25±0.13b |
1.13±0.18b |
Consistent with functional assay results, HE-stained
kidney sections from the control rats revealed integrated
histomorphology (Fig. 3B). Treatment
with DDP was associated with severe kidney injury, characterized by
tubular injury, infiltration of inflammatory cells, interstitial
hemorrhage and necrosis. However, combined treatment of puerarin
and DDP relieved the histopathological alterations compared with
DDP treatment alone. The results indicated that DDP treatment alone
(20 mg/kg) was associated with distinct deterioration of renal
function after 48 h.
Puerarin reverses DDP-induced changes
in renal antioxidants
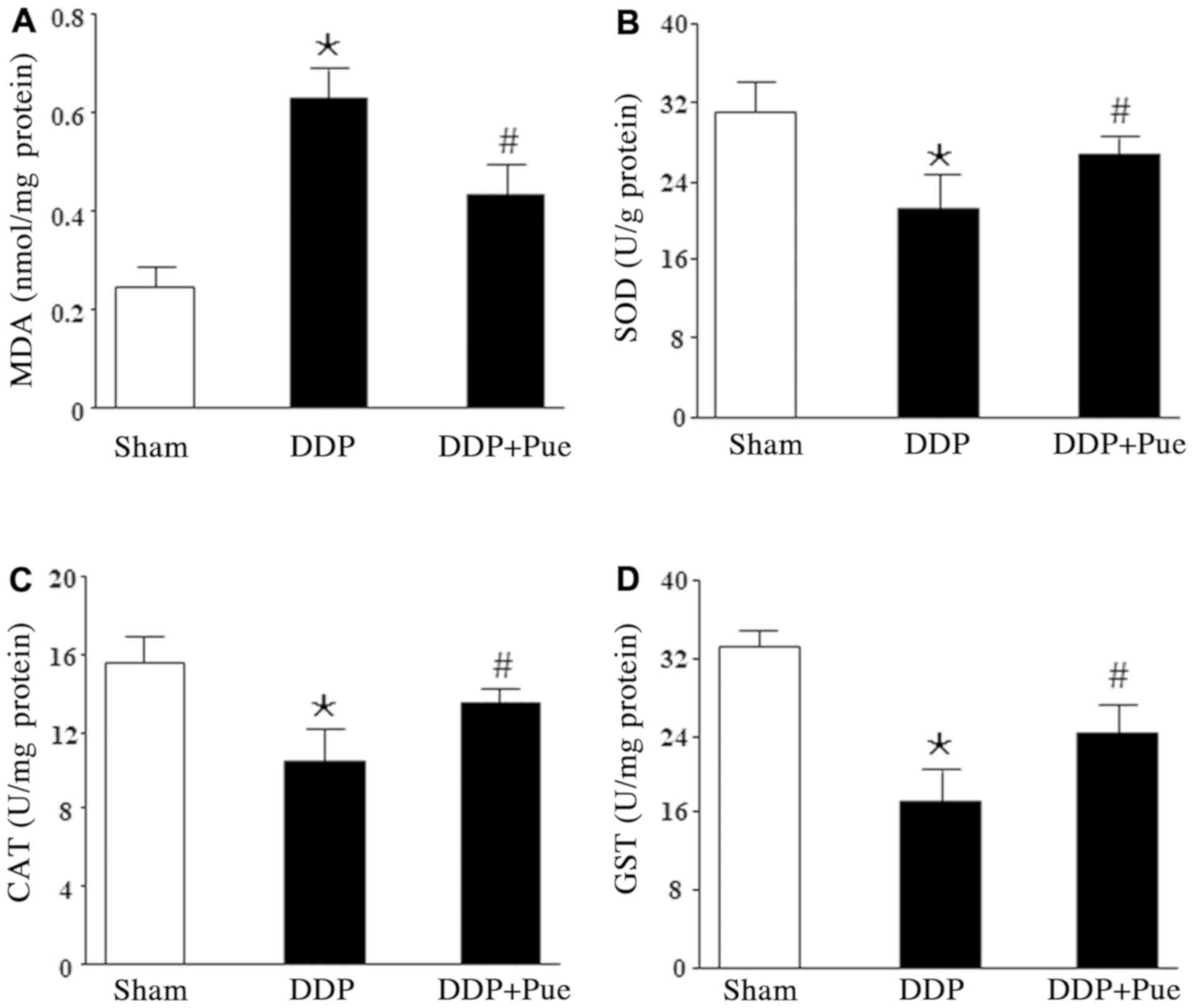
The rats were treated with 20 mg/kg DDP, the dose
which is able to induce AKI (2). As
shown in Fig. 4A-D, treatment with
DDP suppressed renal antioxidant defense, evidenced by
significantly increased MDA content and significantly decreased
expression levels of SOD, CAT and GST compared with the controls.
Furthermore, combined treatment with puerarin significantly
reversed the alterations in renal antioxidant activities compared
with DDP treatment alone.
Puerarin suppresses DDP-induced
apoptosis-associated protein expression
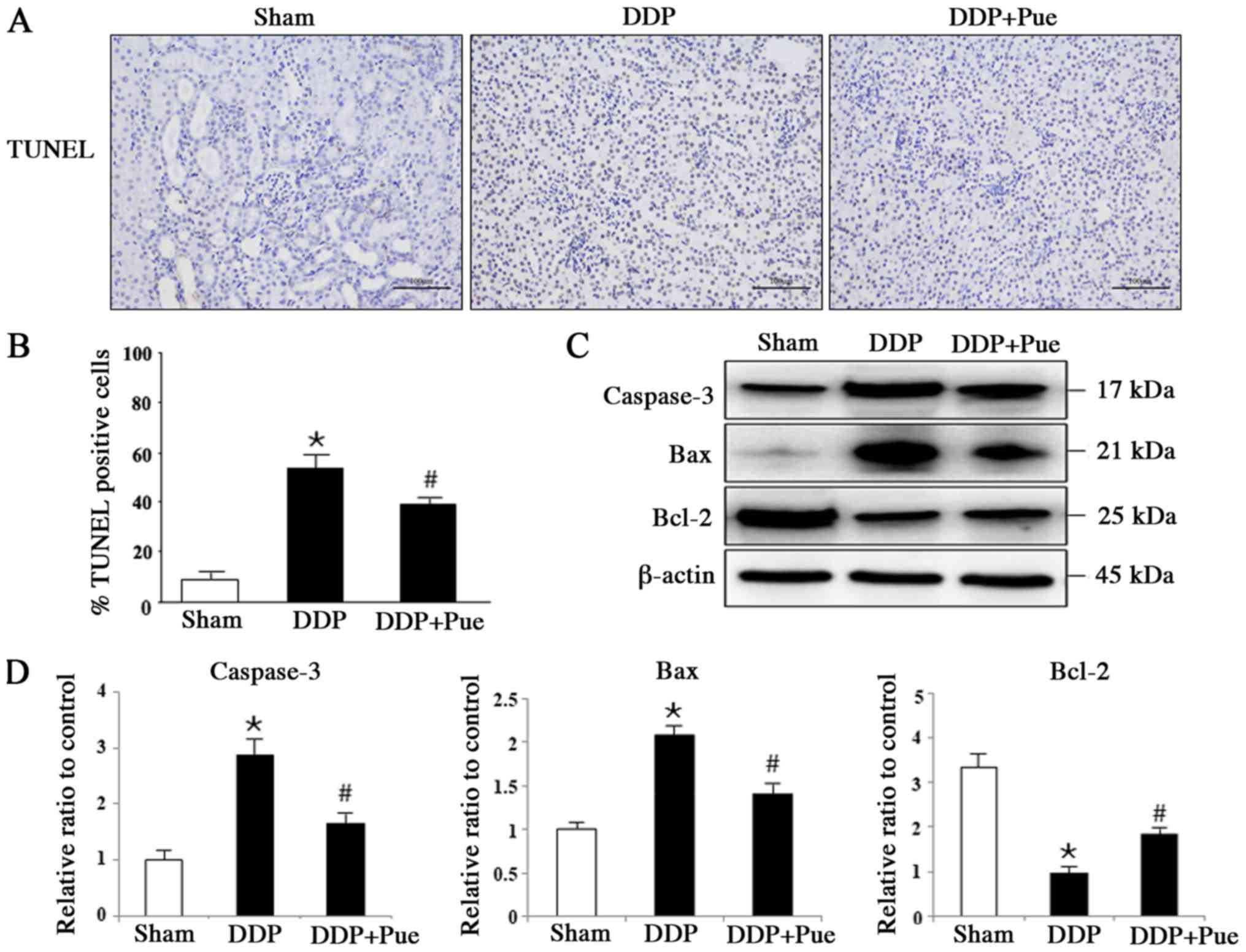
The apoptotic rates exhibited in TUNEL-stained
sections from rats were detected and calculated based on six
randomly selected fields (Fig. 5A
and B). Treatment with DDP induced
apoptosis in 56.98% of kidney cells compared with the Sham group,
while combination treatment with puerarin and DDP induced apoptosis
in 37.33% of cells compared with the DDP group (n=6). The
expression levels of apoptosis-associated proteins in kidneys were
determined via western blot analysis (Fig. 5C and D). The expression levels of cleaved
caspase-3 and Bax significantly increased while Bcl-2 significantly
decreased in DDP-treated rats compared with controls. Furthermore,
combination treatment with puerarin was demonstrated to block
DDP-induced activation of caspase-3 and Bax, and inactivation of
Bcl-2 in kidney tissues.
Puerarin protects against DDP-induced
AKI by regulating the Numb/Notch1 signaling pathway
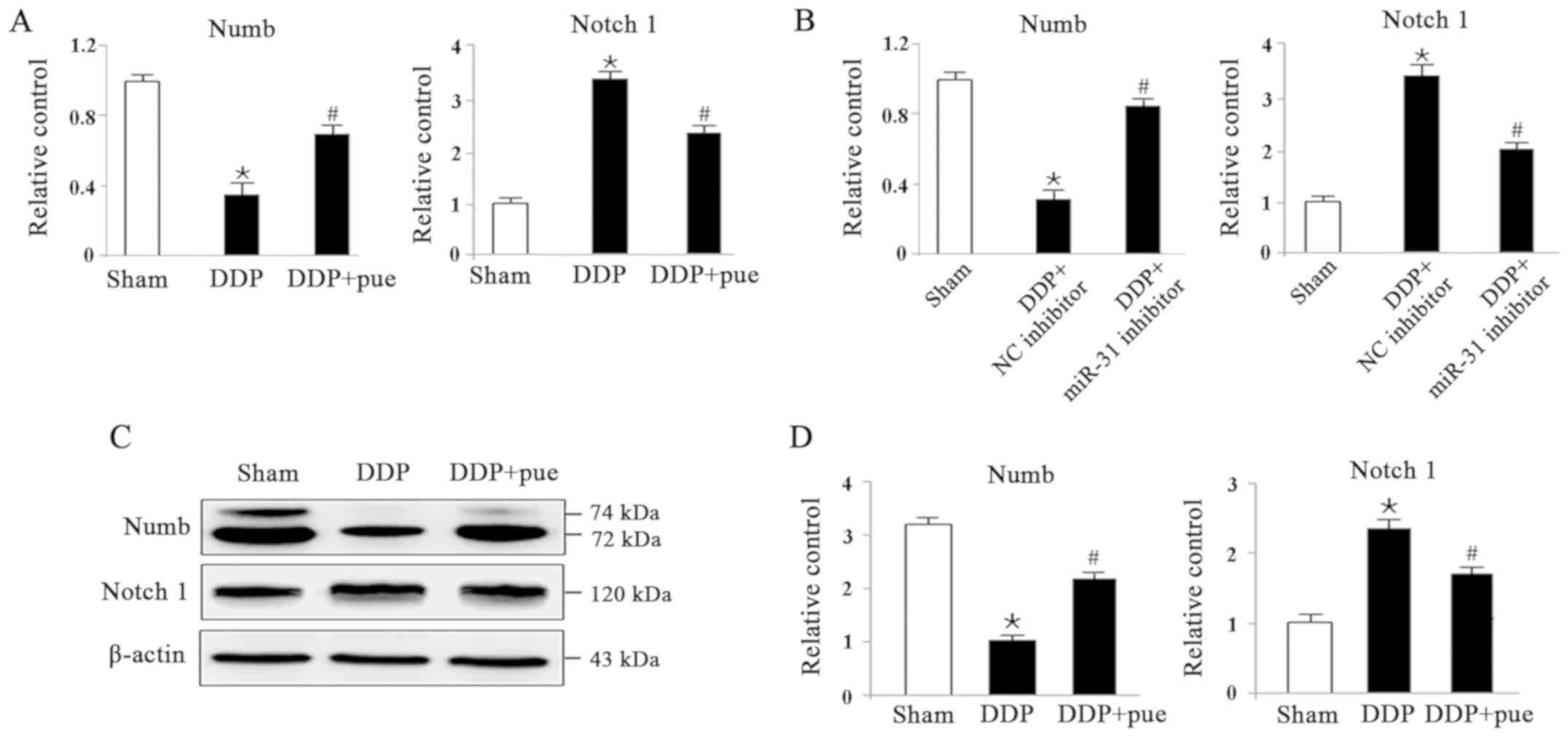
To determine the role of the miRNA-31-related
Numb/Notch1 signaling pathway in DDP-induced AKI, the expression of
Numb and Notch1 both at mRNA and protein levels we detected using
RT-qPCR and western blotting, respectively, in HK-2 cells. As shown
in Fig. 6A, compared with the
controls, RNA levels of Numb and Notch1 significantly decreased and
increased in DDP- treated cells, respectively, while the
combination of DDP and puerarin showed opposite effects. The same
was observed in Sham, DDP combined with NC or miR-31 inhibitor
groups as shown in Fig. 6B. Fig. 6C shows the expression levels of Numb
protein, which significantly decreased, while Notch1 expression
increased in DDP-induced AKI compared with the controls.
Furthermore, combination treatment with DDP and puerarin
significantly suppressed DDP-induced Numb inhibition and Notch1
activation compared with DDP treatment alone (Fig. 6D).
Discussion
Puerarin is an isoflavonoid extracted from the
Chinese medical herb R. puerariae, which regulates several
physiological functions, including anti inflammatory and
antioxidant processes (24).
Recently, puerarin has been extensively used in the therapy of
different types of disease, such as renal fibrosis, liver fibrosis
and myocardial ischemia (25-27).
Furthermore, puerarin is considered to induce beneficial effects on
the treatment of different types of kidney disease, including acute
renal injury and chronic renal injury (8-11).
Gao et al (28) reported that
puerarin provides renoprotection in rat models by altering
apoptosis and autophagy. Furthermore, puerarin was demonstrated to
exhibit a renal protective effect against DDP-induced
nephrotoxicity by regulating the toll-like receptor 4/NF-κB
signaling pathway and attenuating diabetic kidney injury via
suppression of NADPH oxidase 4 expression (29,21).
However, the molecular mechanisms underlying the renal protection
of puerarin in DDP-induced AKI remain unclear. The results of the
present study demonstrated that puerarin exerted a protective
effect on DDP-induced renal injury in both HK-2 cells and rat
kidneys.
In the present study, treatment with DDP notably
increased miR-31 expression in a dose-dependent manner, expression
levels of renal function biomarkers (BUN and SCR), MDA content and
apoptosis-associated proteins (caspase-3 and Bax), while
significantly decreasing the expression levels of GST, SOD and CAT
antioxidants in serum, and antiapoptotic factor, Bcl-2 in kidney
samples. Histopathological analysis, including HE and TUNEL
demonstrated similar results. The effects induced by DDP were
reversed following treatment with puerarin, suggesting that
puerarin may protect DDP-induced renal injury. Furthermore, western
blot analysis demonstrated that treatment with DDP decreased Numb
protein expression, while increasing Notch1 protein expression in
HK-2 cells. Conversely, treatment with puerarin and transfection
with miR-31-mimics altered the effects on the Numb/Notch1 signaling
pathway induced by DDP.
miRNAs play a regulatory role across several
biological processes, such as carcinogenesis, inflammation and
numerous signaling pathways including the Numb/Notch1 signaling
pathway (12,13). miR-31 is associated with the
progression of renal cell carcinoma via targeting cyclin-dependent
kinase 1(15). Numb is confirmed as
the downstream target of miR-31-5p and the overexpression of Numb
inhibited the proliferation, migration, invasion and induced cell
cycle arrest and apoptosis in colorectal cancers (16). In addition, the symmetric division of
mouse Lewis lung carcinoma stem cells was regulated via
miR-31-mediated Numb expression (30). A previous study reported that
puerarin facilitated the proliferation, migration and invasion of
colorectal cancer cells by targeting Numb protein (18). Numb was originally identified as an
intrinsic determinant in Drosophila, affecting the cell fate
by negatively altering the Notch signaling pathway (17). Numb is predominantly expressed in
normal proximal tubules and detected in interstitial cells of
fibrotic kidneys (31). Activation
of Numb facilitated interstitial fibrosis by inducing
G2/M arrest of tubular cells, while inhibition of Numb
promoted dynamin-associated protein 1-mediated mitochondrial
fission and exacerbates mitochondrial fragmentation and dysfunction
in AKI (32). A previous study
reported that Numb alleviates puromycin aminonucleoside-induced
renal proximal tubular cell apoptosis by suppressing Notch
signaling activity (21). For the
vital function of Notch signaling in regulating the balance among
cell proliferation and apoptosis that influence the progress of
various organ injuries, the present study hypothesized that the
negative regulation of Numb affects Notch signaling via miR-31 in
DDP-induced AKI.
The present study indicated that puerarin may
alleviate DDP-induced acute renal damage by suppressing miR-31
expression, resulting in enhancing Numb activation, thereby
inhibiting the Notch signaling pathway. However, the present study
only observed the protective effects of puerarin associated with
miR-31 and Numb/ Notch 1 signaling pathway. Further studies will
confirm the function of puerarin protection in DDP-induced renal
injury via knockdown of Numb to verify that puerarin played its
protection role via regulating miR-31/Numb/Notch pathway. Puerarin
may act as a novel therapeutic candidate in the treatment of
DDP-induced AKI.
Supplementary Material
Transfection efficiency determination.
(A) FACS results and (B) quantitative analysis showing miR-31
expression. (C) miR-31 levels detected in DDP-treated groups. Data
are presented as the mean ± standard deviation. n=3.
*P<0.05. DDP, cisplatin; NS, not significant; miR,
microRNA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW, CL, QL, JL and XL participated in the study
design, performed experiments, collected and analyzed the data and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
requirements of the Institutional Care and Use Committee of the
Second Hospital of Shandong University.
Patient consent for publication
Not applicable.
Competing interests.
The authors declare that they have no competing
interests.
References
|
1
|
Ma G, Cheng W and Ma M: Effect of
Docetaxel Combined with Cisplatin Preoperative Neoadjuvant
Chemotherapy for Stage III NSCLC. J Coll Physicians Surg Pak.
29:1230–1231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sultana S, Verma K and Khan R:
Nephroprotective efficacy of chrysin against cisplatin-induced
toxicity via attenuation of oxidative stress. J Pharm Pharmacol.
64:872–881. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen H, Liu S, Li M, Huang P and Li X:
circ_0003418 Inhibits Tumorigenesis And Cisplatin Chemoresistance
Through Wnt/β-Catenin Pathway In Hepatocellular Carcinoma.
OncoTargets Ther. 12:9539–9549. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu W, Wang W, Wang X, Xu C, Zhang N and
Di W: Cisplatin-stimulated macrophages promote ovarian cancer
migration via the CCL20-CCR6 axis. Cancer Lett. 472:59–69.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li Y, Zu X, Hu X, Wang L and He W:
Forkhead Box R2 Knockdown Decreases Chemoresistance to Cisplatin
via MYC Pathway in Bladder Cancer. Med Sci Monit. 25:8928–8939.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luo X, Wei J, Yang FL, Pang XX, Shi F, Wei
YX, Liao BY and Wang JL: Exosomal lncRNA HNF1A-AS1 affects
cisplatin resistance in cervical cancer cells through regulating
microRNA-34b/TUFT1 axis. Cancer Cell Int. 19(323)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li WQ, Wu JY, Xiang DX, Luo SL, Hu XB,
Tang TT, Sun TL and Liu XY: Micelles Loaded With Puerarin And
Modified With Triphenylphosphonium Cation Possess Mitochondrial
Targeting And Demonstrate Enhanced Protective Effect Against
Isoprenaline-Induced H9c2 Cells Apoptosis. Int J Nanomedicine.
14:8345–8360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ma JQ, Ding J, Xiao ZH and Liu CM:
Puerarin ameliorates carbon tetrachloride-induced oxidative DNA
damage and inflammation in mouse kidney through ERK/Nrf2/ARE
pathway. Food Chem Toxicol. 71:264–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
She S, Liu W, Li T and Hong Y: Effects of
puerarin in STZ-induced diabetic rats by oxidative stress and the
TGF-β1/Smad2 pathway. Food Funct. 5:944–950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhong Y, Zhang X, Cai X, Wang K, Chen Y
and Deng Y: Puerarin attenuated early diabetic kidney injury
through down-regulation of matrix metalloproteinase 9 in
streptozotocin-induced diabetic rats. PLoS One.
9(e85690)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Lin S, Li Z, Yang D and Wang Z:
Protective effects of puerarin on experimental chronic lead
nephrotoxicity in immature female rats. Hum Exp Toxicol.
32:172–185. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma X, Yan L, Zhu Q and Shao F: Puerarin
attenuates cisplatin-induced rat nephrotoxicity: The involvement of
TLR4/NF-κB signaling pathway. PLoS One. 12(e0171612)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang J, Ni J, Beretov J, Thompson J,
Graham P and Li Y: Exosomal microRNAs as liquid biopsy biomarkers
in prostate cancer. Crit Rev Oncol Hematol.
145(102860)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He J, He J, Min L, He Y, Guan H, Wang J
and Peng X: Extracellular vesicles transmitted miR-31-5p promotes
sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int
J Cancer. 146:1052–1063. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Quan J, Chen F, Pan X, Zhuang C,
Xiong T, Zhuang C, Li J, Huang X, Ye J, et al: miR-31-5p acts as a
tumor suppressor in renal cell carcinoma by targeting
cyclin-dependent kinase 1 (CDK1). Biomed Pharmacother. 111:517–526.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chou CH, Tu HF, Kao SY, Chiang CF, Liu CJ,
Chang KW and Lin S: Targeting of miR-31/96/182 to the Numb gene
during head and neck oncogenesis. Head Neck. 40:808–817.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu GF, Chu T, Ruan Z, Zhang M, Zhou M,
Zhang Q, Zhang R and Wu L: Inflammation-Related MicroRNAs Are
Associated with Plaque Stability Calculated by IVUS in Coronary
Heart Disease Patients. J Interv Cardiol.
2019(9723129)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peng H, Wang L, Su Q, Yi K, Du J and Wang
Z: miR-31-5p promotes the cell growth, migration and invasion of
colorectal cancer cells by targeting NUMB. Biomed Pharmacother.
109:208–216. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z, Li H, Su J, Xu S, Zhu F, Ai J, Hu
Z, Zhou M, Tian J, Su Z, et al: Numb Depletion Promotes
Drp1-Mediated Mitochondrial Fission and Exacerbates Mitochondrial
Fragmentation and Dysfunction in Acute Kidney Injury. Antioxid
Redox Signal. 30:1797–1816. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Luo Z, Mu L, Zheng Y, Shen W, Li J, Xu L,
Zhong B, Liu Y and Zhou Y: NUMB enhances Notch signaling by
repressing ubiquitination of NOTCH1 intracellular domain. J Mol
Cell Biol. 12:345–358. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ding X, Zhu F, Li T, Zhou Q, Hou FF and
Nie J: Numb protects renal proximal tubular cells from puromycin
aminonucleoside-induced apoptosis through inhibiting Notch
signaling pathway. Int J Biol Sci. 7:269–278. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Wang H, Yu L and Chen J: The
Puerarin improves renal function in STZ-induced diabetic rats by
attenuating eNOS expression. Ren Fail. 37:699–703. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu J, Xu L, Sun C, Zhang B, Li J, Sun J,
Zhang Y and Sun D: Paeonol alleviates epirubicin-induced renal
injury in mice by regulating Nrf2 and NF-κB pathways. Eur J
Pharmacol. 795:84–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song Q, Zhao Y, Li Q, Han X and Duan J:
Puerarin protects against iron overload-induced retinal injury
through regulation of iron-handling proteins. Biomed Pharmacother.
122(109690)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen
C, Shan G and Yao Q: Puerarin attenuates renal fibrosis by reducing
oxidative stress induced-epithelial cell apoptosis via MAPK signal
pathways in vivo and in vitro. Ren Fail. 39:423–431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Zhang H, Pan L, Zou H, Miao X, Cheng
J and Wu Y: Puerarin alleviates liver fibrosis via inhibition of
the ERK1/2 signaling pathway in thioacetamide-induced hepatic
fibrosis in rats. Exp Ther Med. 18:133–138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gao S, Li L, Li L, Ni J, Guo R, Mao J and
Fan G: Effects of the combination of tanshinone IIA and puerarin on
cardiac function and inflammatory response in myocardial ischemia
mice. J Mol Cell Cardiol. 137:59–70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song X, Li Z, Liu F, Wang Z and Wang L:
Restoration of autophagy by puerarin in lead-exposed primary rat
proximal tubular cells via regulating AMPK-mTOR signaling. J
Biochem Mol Toxicol. 31(e21869)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang J, Zhou T, Sun Z, Ye T, Zhou S, Li J,
Liu Y, Kong L, Tang J, Liu D and Xing HR: Zeb1 Regulates the
Symmetric Division of Mouse Lewis Lung Carcinoma Stem Cells Through
Numb Mediated by miR-31. Int J Biol Sci. 14:1399–1410.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jadhav S, Ajay AK, Trivedi P, Seematti J,
Pellegrini K, Craciun F and Vaidya VS: RNA-binding Protein Musashi
Homologue 1 Regulates Kidney Fibrosis by Translational Inhibition
of p21 and Numb mRNA. J Biol Chem. 291:14085–14094. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu F, Liu W, Li T, Wan J, Tian J, Zhou Z,
Li H, Liu Y, Hou FF and Nie J: Numb contributes to renal fibrosis
by promoting tubular epithelial cell cycle arrest at G2/M.
Oncotarget. 7:25604–25619. 2016.PubMed/NCBI View Article : Google Scholar
|