Introduction
Hepatocellular carcinoma (HCC) is one of common
types of gastrointestinal cancer with high rates of recurrence and
metastasis in the liver, and the overall prognosis for patients is
poor (1-3).
The 5-year survival rate of HCC is ~32.5% in China, and only ~59.1%
in patients diagnosed at an early stage (4,5). In
Western countries, the 5-year survival rate has been reported to be
<12% (4,5). In 1869, Armand Trousseau observed that
cancer patients may be complicated by venous thrombosis, and this
is known as Trousseau syndrome (6).
One of the high risk factors for induction of venous thrombosis has
been demonstrated to be cancer, and the incidence rate of venous
thrombosis reached ~12.3% for cancer patients following diagnosis
(7). However, the mechanisms
underlying tumor thrombus induced by malignant tumor remain
unclear.
It has been observed that numerous microRNAs (miRs)
and mRNAs are involved in Trousseau syndrome (8). Different factors, including integrin
β3(9) and nuclear factor-κB
(10), participate in the processes.
Similar to the development of tumor, the induction of tumor
thrombus is closely associated with angiogenesis (11). Recent studies mainly focused on the
roles and signaling transductions associated with vascular
endothelial growth factor (VEGF) in the progression of tumor
thrombus (12-14).
The excessive growth of blood vessels is one of the important
conditions for the formation of tumors and tumor thrombi (15-17),
therefore the EA.hy926 cell line was used in the current study.
In the present study, VEGF expression was detected
at the mRNA and protein levels in blood and tumor thrombus
specimens collected from HCC patients complicated with vein tumor
thrombus. The association between miR-186 and VEGF expression
levels was also validated.
Materials and methods
Sample collection
The current study included 29 cases of HCC patients
with portal vein tumor thrombus, whose tumor thrombus was resected
between January 2013 and September 2015 in the Department of
Hepatobiliary Surgery of Huai'an First People's Hospital (Huai'an,
China), and the peritumoral tissues of the tumor thrombus were also
collected as control tissues. During the same period, blood samples
from 39 HCC patients without tumor thrombus were used as the
control group. There were 18 males and 11 females among the HCC
patients with portal vein tumor thrombus, and the age range was
between 26 and 66 years, with a median age of 45.6 years. In the
control group without tumor thrombus, there were 26 males and 13
females, and their age ranged between 21 and 77 years, with a
median age of 49.5 years. All the included patients presented
first-onset HCC, and had not received hormone therapy, medication,
radiotherapy and chemotherapy prior to inclusion. All the patients
were diagnosed by pathologists at the Huai'an First People's
Hospital (18). The portal vein
tumor thrombus was confirmed by ultrasound or based on the
symptoms, signs and diagnosis history. The resected tumor thrombus
and peritumoral tissues were stored at -80˚C within 2 h. Fasting
peripheral blood samples were collected from all patients upon
diagnosis and stored at -20˚C following addition of EDTA
anticoagulant. Prior written and informed consent was obtained from
every patient, and the study was approved by the Ethics Review
Board of Nanjing Medical University (Huai'an, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from blood and tissue samples was
extracted by TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The RNA quality of samples was checked by
1% gel electrophoresis and the absorbance ratio at 260/280 nm was
detected by a spectrophotometer. The cDNA was obtained with the
TIANScript II cDNA first strand synthesis kit (cat. no. KR107;
Tiangen Biotech Co., Ltd., Beijing, China). The SuperReal PreMix
(SYBR Green) kit (cat. no. FP204; Tiangen Biotech Co., Ltd.) was
used to detect the mRNA expression of VEGF, with β-actin used as
the internal reference. Primers used in qPCR were as follows: VEGF
forward, 5'-TTGCCTTGCTGCTCTACCTC-3', and reverse,
5'-AAATGCTTTCTCCGCTCTGA-3'; β-actin forward,
5'-TGACGTGGACATCCGCAAAG-3', and reverse,
5'-CTGGAAGGTGGACAGCGAGG-3'. The reaction was performed in a 25-µl
system, including 10 µl SuperReal PreMix (SYBR Green), 1 µl forward
primer, 1 µl reverse primer, 2 µl cDNA template and 11 µl
ddH2O. The cycle conditions were the following: 94˚C for
2 min, followed by 35 cycles of 94˚C for 30 sec, 55˚C for 30 sec,
71˚C for 1 min and a further 71˚C for 2 min. Relative expression of
VEGF against β-actin was calculated by the
2-ΔΔCq method (19).
Reverse transcription of miRNA followed the
procedure provided by the manufacturer of the miRcute miRNA cDNA
kit (KR201; Tiangen Biotech Co., Ltd.). qPCR was then used to
detect the expression of miR-186, with U6 used as an internal
reference. The primers were as follows: miR-186 forward,
5'-CCCGATAAAGCTAGATAACC-3', and reverse, 5'-CAGTGCGTGTCGTGGAGT-3';
U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3', and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'. The reaction was conducted in a
25-µl system, including 10 µl SuperReal PreMix (SYBR Green), 1 µl
forward primer, 1 µl reverse primer, 2 µl cDNA template and 11 µl
ddH2O. The thermal cycling conditions were the
following: 95˚C for 5 min, followed by 40 cycles of 95˚C for 10
sec, 60˚C for 20 sec and 72˚C for 20 sec. The relative expression
of miR-186/U6 was calculated by the
2-ΔΔCq method.
ELISA
The peripheral blood was centrifuged at 1,000 x g at
4˚C for 10 min to separate the serum and red blood cells, and the
serum was used for subsequent analysis. ELISA was performed
according to the instructions provided by the human VEGFA ELISA kit
(cat. no. 100663; Abcam, Cambridge, MA, USA). Next, 50-µl standard
were added into different wells on the ELISA plate, with 10 µl
sample and 40 µl dilution buffer added into each well. With the
exception of blank wells, 100 µl horseradish peroxidase
(HRP)-labeled antibody was added to each well, covered with
microplate sealers and incubated for 1 h. Subsequent to five washes
with the washing solution in the kit 50 µl substrate A and 50 µl
substrate B were added into each well. After incubation at 37˚C for
15 min, 50 µl stop solution was added into each well. The optical
density value was measured at a wavelength of 450 nm within 15
min.
Bioinformatics prediction
Bioinformatics prediction is the basis to explore
the functions of miRNAs. The miRanda (http://www.microma.org/rnicroma/home.do), TargetSean
(www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTA (http://pictar.mdc-berlin.de/) databases were used in
the present study to predict the potential miRNAs that can directly
regulate VEGF physiologically and in pathological conditions,
according to previously described methods (20-22).
Based on the results of bioinformatics analysis, miR-186 was
selected as a miRNA regulating VEGF expression. The authors of the
current study investigated miR-186 previously. Additionally,
previous reports have demonstrated that miR-186 could act as tumor
suppressor (23,24); therefore, miR-186 was selected for
analysis in the current study.
Dual-luciferase reporter assay
Based on the bioinformatics prediction, the
wild-type 3'-untranslated region (UTR) and the mutant 3'-UTR of
VEGF were synthesized in vitro, and were cloned into the
downstream of pMIR-REPORT luciferase vector (Ambion; Thermo Fisher
Scientific, Inc.) by Spe-1 and HindIII enzyme. 293T cells
were co-transfected with agomiR-186 (100 nM; Sangon Biotech Co.,
Ltd., Shanghai, China) mimics and with wild-type VEGF 3'-UTR or
mutant 3'-UTR. Following transfection with Lipofectamine 2000
reagent (cat. no. 11668-027; Thermo Fisher Scientific, Inc.) for 24
h, cells were lysed and luciferase intensity was measured by GloMax
20/20 luminometer (Promega Corp., Madison, WI, USA) based on the
standard protocol of the luciferase kit (cat. no. E1910; Promega
Corp.). The intensity of Renilla was used as a control, and
the fluorescence intensity in the NC, wild-type and mutant groups
was analyzed.
Cell transfection
At 24 h prior to transfection, 3x105
logarithm growth EA.hy926 cells (Type Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) were seeded in
24-well plate and cultured into antibiotics-free F12/Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum.
When cells reached ~70% confluence, transfection was performed. A
total of 1 µg/µl plasmid, 30 nM VEGF small interfering RNA (siRNA;
target sequence, 106-AATCATCACGAAGTGGTGAAGTT; forward,
5'-UCAUCACGAAGUGGUGAAGdTdT-3' and reverse
5'-CUUCACCACUUCGUGAUGAdTdT-3') or 30 nM agomiR-186 (forward,
5'-CAAAGAAUUCUCCUUUUGGGCU-3' and reverse
5'-GCCCAAAGGUGAAUUUUUUGGG-3'; all Sangon Biotech Co., Ltd.) and
Lipofectamine 2000 were added into EP tubes containing 50 µl DMEM,
respectively. The two tubes were mixed together after 5-min
incubation at room temperature, and the mixture was added to each
well after incubation for 20 min at room temperature. After
transfection for 48 h, the cells were collected to detect the
expression of VEGF at the mRNA and protein levels.
Western blot analysis
Total proteins were extracted by protein lysis using
RIPA buffer (P0013B; Beyotime Institute of Biotechnology, Haimen,
China) based on a standard protocol, and the protein concentration
was detected by a BCA assay kit (Zhongke Ruitai Biotechnology Co.,
Ltd., Beijing, China). After boiling with loading buffer for 5 min,
20 µg protein was subjected to 10% SDS-PAGE and then transferred to
a polyvinylidene fluoride membrane under ice bath (constant voltage
of 100 V for 2 h). Subsequent to blocking by 5% skim milk, rabbit
anti-human polyclonal VEGF (1:1,000; cat. no. ab46154) and rabbit
anti-human β-actin (1:5,000; cat. no. ab129348) primary antibodies
were added at 4˚C overnight. Next, HRP-conjugated goat anti-rabbit
IgG secondary antibody (1:3,000; cat. no. ab6721) was added at 37˚C
for 1 h. All the antibodies were purchased from Abcam. Finally, the
membrane was developed by the BeyoECL Plus enhanced
chemiluminescence reagent (P0018; Beyotime Institute of
Biotechnology). The developed film was scanned and analyzed by
Image Lab 3.0 software (Bio-Rad Laboratories Inc., Hercules, CA,
USA). β-actin was used as an internal control to calculate the
relative expression of VEGF.
MTT assay
EA.hy926 cells were seeded in 96-well plates with
2x103 cells/well, and each sample had three replicates.
At 24, 48 and 72 h, 20 µl MTT (5 g/l) was added into each well,
followed by addition of 150 µl dimethyl sulfoxide to resolve the
purple crystals. After incubation for 4 h at 37˚C, the absorbance
of cells was measured at 490 nm wavelength and the proliferation
curves were plotted.
Statistical analysis
The SPSS version 18.0 software (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis. All the data are
presented as the mean ± standard deviation, and normality test was
used. One-way analysis of variance was used to compare difference
among multiple groups. When variance was homogenous, the least
significant difference and Student-Newman-Keuls methods were used;
otherwise, Tamhane's T2 or T3 methods were applied. P<0.05 was
considered as an indicator of statistically significant
differences.
Results
Expression of VEGF mRNA in tumor
thrombus and blood samples
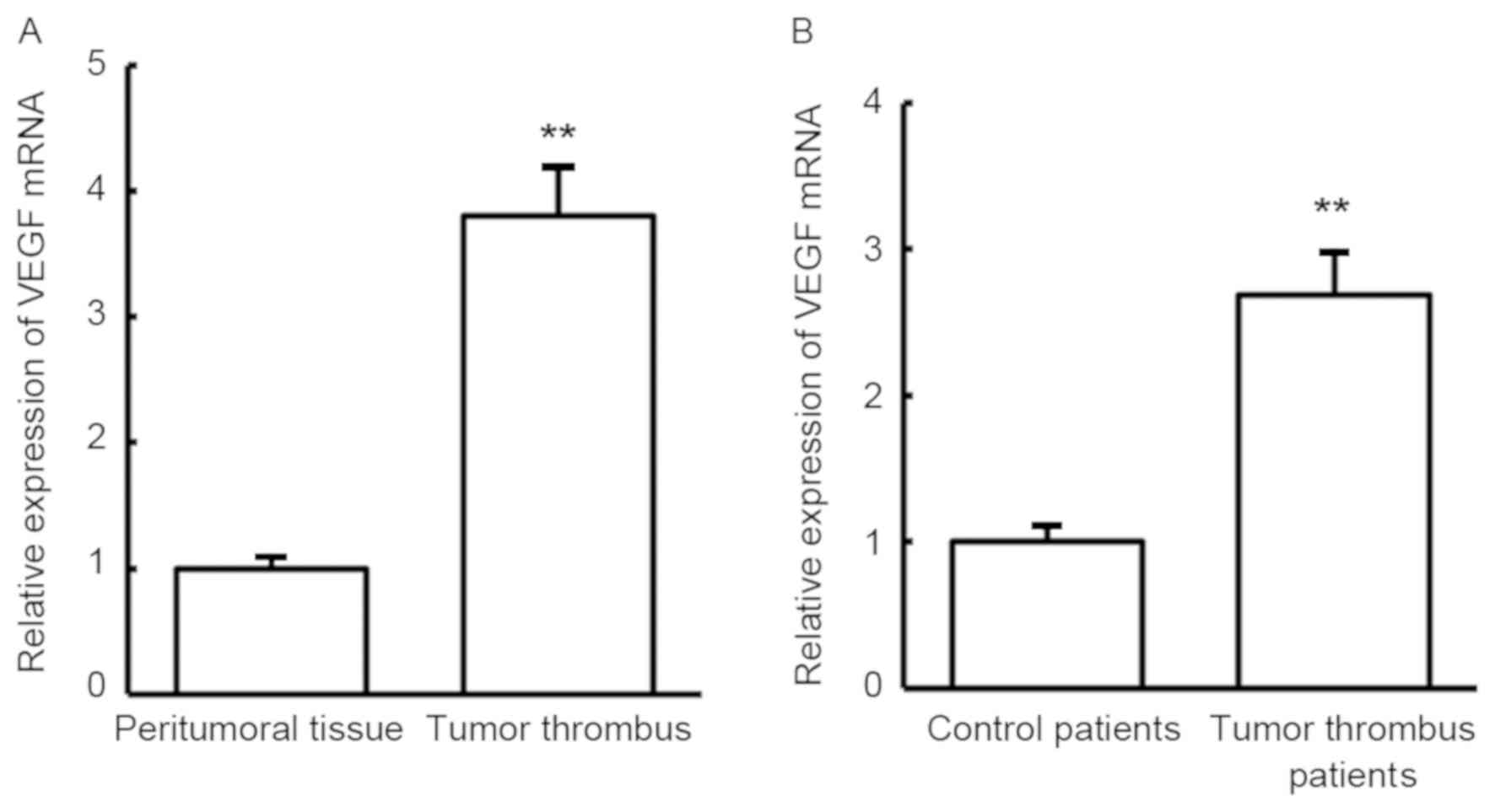
To examine the expression changes between tumor
thrombus and peritumoral tissues in HCC patients with portal vein
tumor thrombus, RT-qPCR was conducted to detect VEGF mRNA. Compared
with peritumoral tissues, VEGF mRNA expression was significantly
increased in the tumor thrombus (P<0.01; Fig. 1A). Similar to the results in tumor
tissues, VEGF mRNA expression was also significantly increased in
the blood of patients with portal vein tumor thrombus when compared
with that in the blood of patients without tumor thrombus
(P<0.01; Fig. 1B). The results
indicate that VEGF may serve regulatory roles in the development of
tumor thrombus in HCC.
Expression of VEGF protein in tumor
thrombus and blood
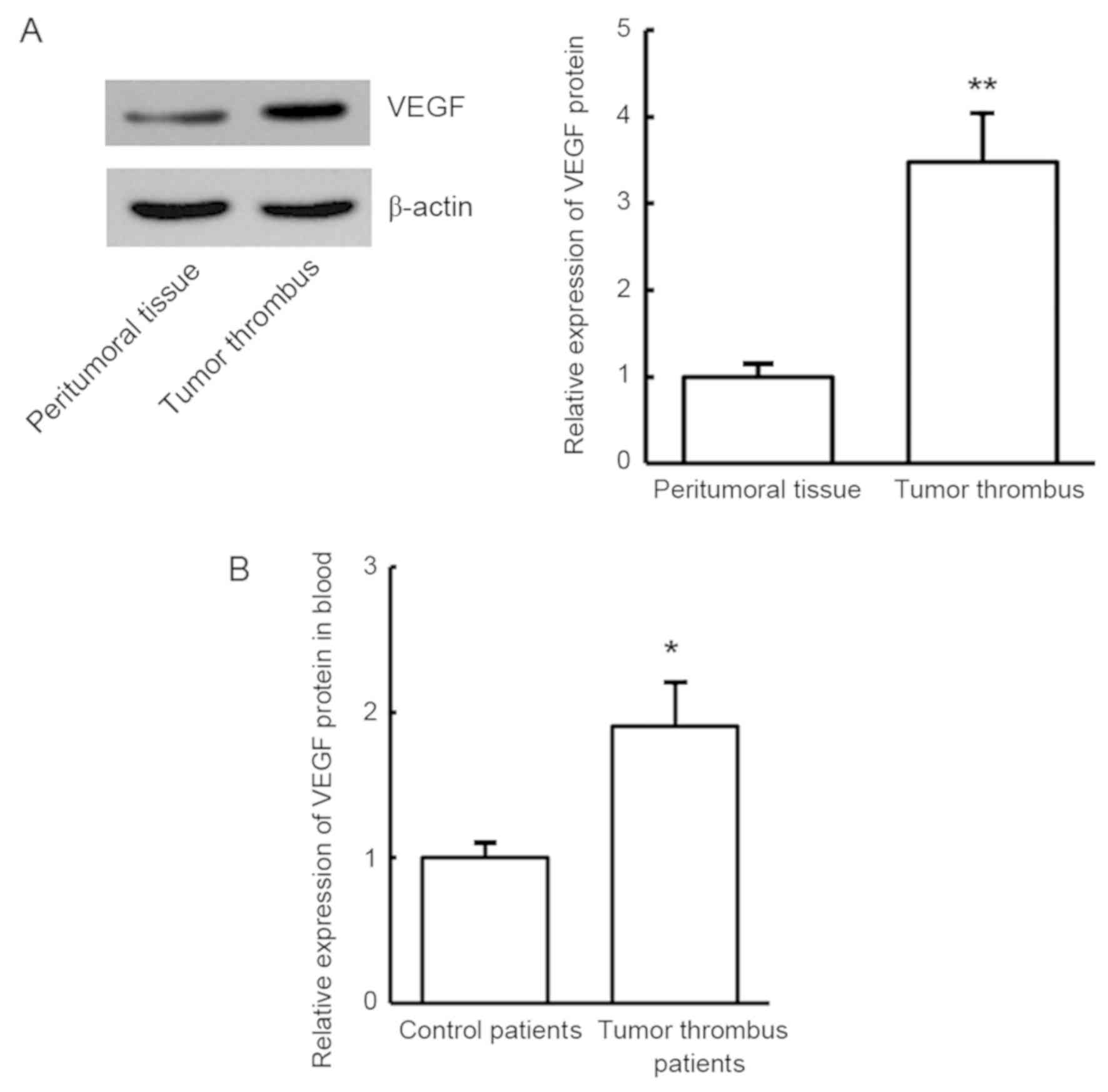
To validate the expression of VEGF protein in the
tumor thrombus tissues and blood samples in HCC patients with tumor
thrombus, western blot analysis and ELISA were used, respectively.
Compared with the control group, VEGF protein was significantly
increased in the tumor thrombus tissues and blood of patients with
portal vein tumor thrombus (P<0.01; Fig. 2). The results revealed that VEGF
protein expression was consistent with VEGF mRNA expression, which
further indicates that VEGF may be involved in the development of
tumor thrombus in HCC patients.
Expression of miR-186 in tumor
thrombus and blood
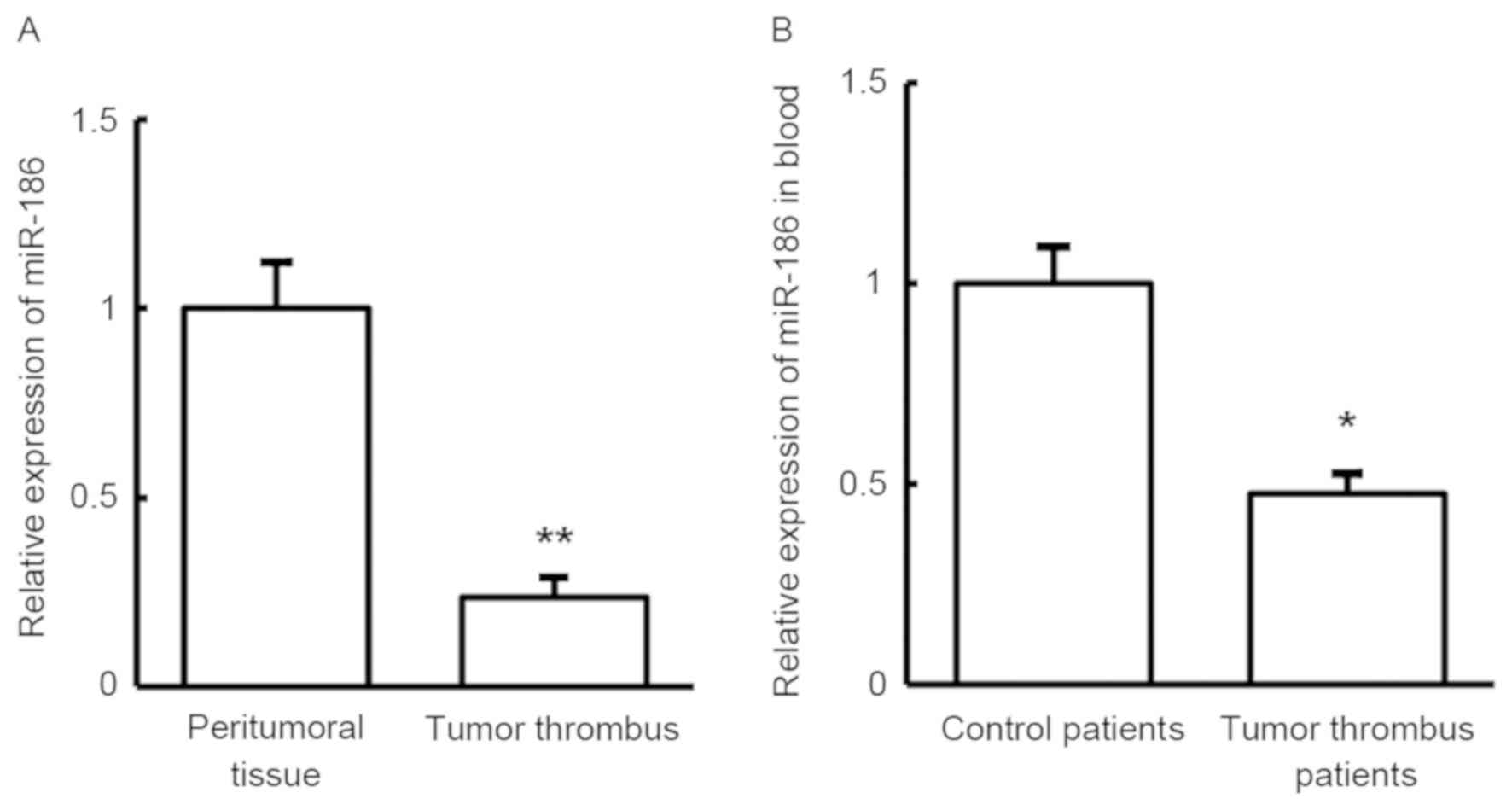
In order to investigate the roles of miR-186 in
tumor thrombus in HCC patients, RT-qPCR was used to detect its
expression in tumor thrombus tissue and blood samples. As shown in
Fig. 3, miR-186 expression was
significantly downregulated both in the tumor thrombus tissues and
in the blood, when compared with the control samples (P<0.05).
This result indicates that miR-186 may serve a role in the
development of tumor thrombus in HCC patients.
VEGF is directly targeted by
miR-186
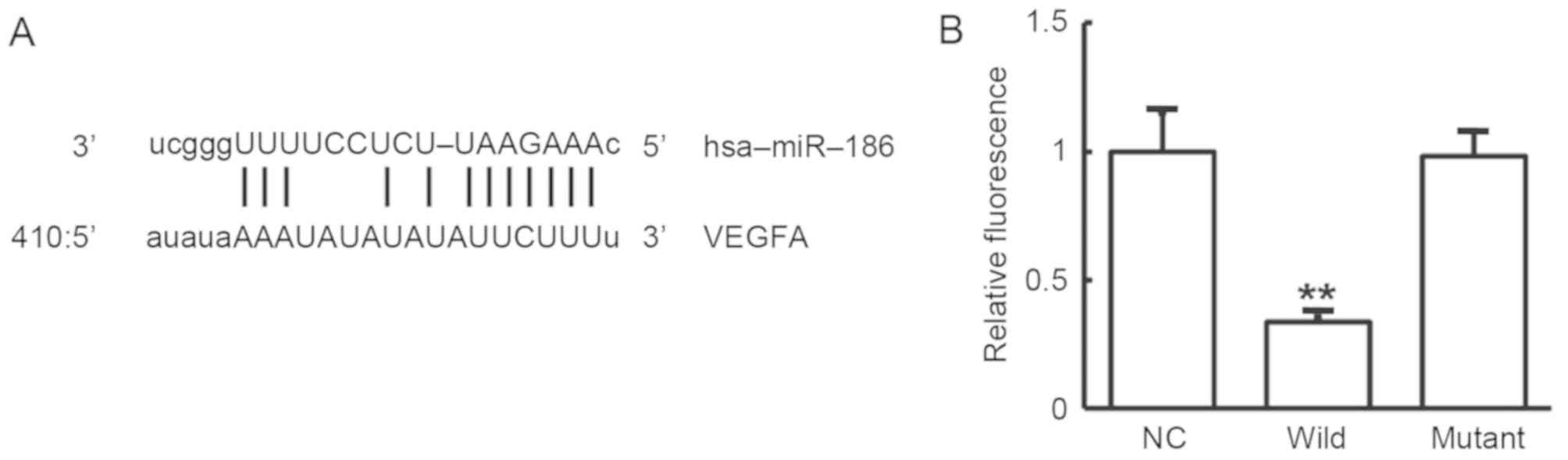
To determine whether VEGF was directly targeted by
miR-186, bioinformatics prediction was performed. The miR-186 was
predicted as one of the miRNAs that regulates VEGF. The
complementary binding site of miR-186 with VEGF is shown in
Fig. 4A. To further verify this
result, the dual-luciferase reporter assay was conducted. As shown
in Fig. 4B, the fluorescence
intensity was significantly downregulated in 293T cells
co-transfected with agomiR-186 and pMIR-REPORT-wild type plasmid in
comparison with the negative control group (P<0.01). By
contrast, there was no significant difference between cells
co-transfected with agomiR-186 and pMIR-REPORT-mutant in comparison
with the negative control group (P>0.05). These results indicate
that miR-186 can regulate VEGF expression through complementary
binding to the 3'-UTR of VEGF mRNA.
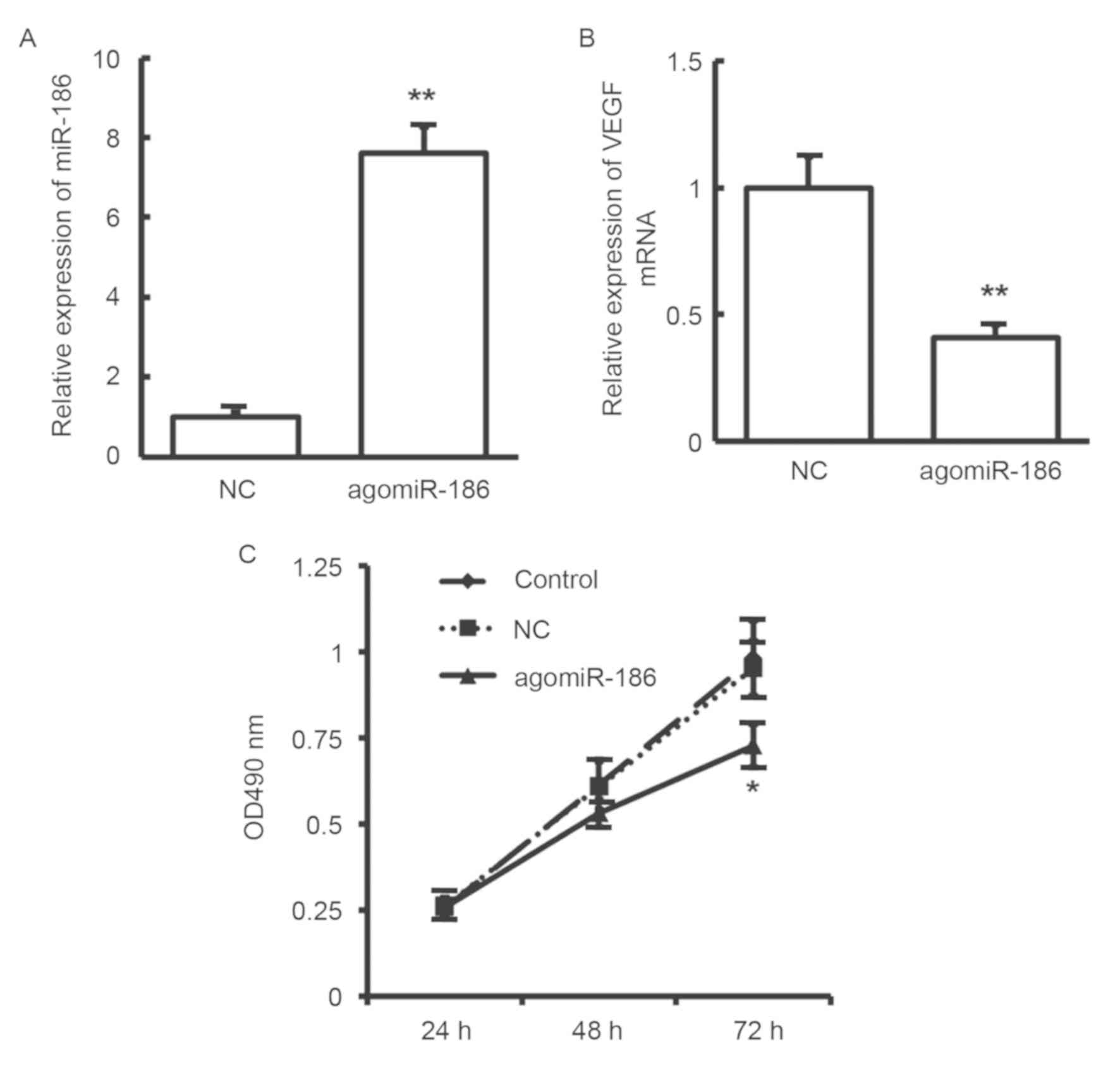
Effects of miR-186 on proliferation of
EA.hy926 cells
To analyze the influence of miR-186 on cell
proliferation, MTT assay was performed. As shown in Fig. 5A, miR-186 expression was
significantly increased in EA.hy926 cells subsequent to
transfection with agomiR-186 (P<0.01). At the same time, as
shown in Fig. 5B, VEGF expression
was significantly decreased in EA.hy926 cells following
transfection with agomiR-186 (P<0.01). Compared with the control
group, EA.hy926 cell proliferation was significantly inhibited in
cells after transfection with agomiR-186 (P<0.05; Fig. 5C). These results suggest that miR-186
may inhibit the proliferation of EA.hy926 cells through regulating
VEGF expression.
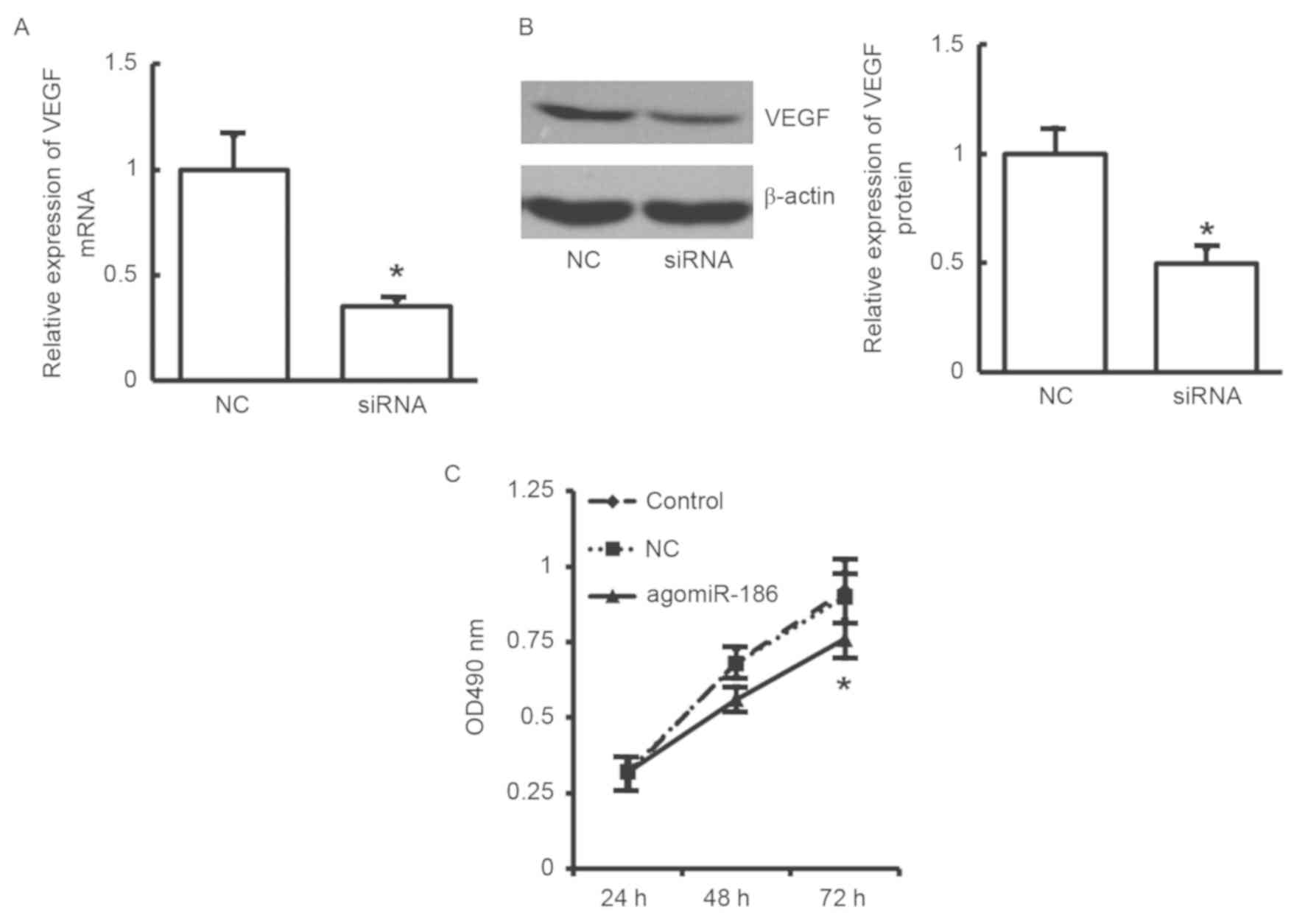
Effects of VEGF siRNA on proliferation
of EA.hy926 cells
To further validate the aforementioned results
indicating that EA.hy926 cell proliferation was inhibited by
miR-186 through downregulation of VEGF, a siRNA was used to
downregulate VEGF expression and then cell proliferation was
detected by MTT assay. As shown in Fig.
6A and B, VEGF expression at the
mRNA and protein levels was significantly decreased following
transfection with VEGF siRNA in EA.hy926 cells (P<0.05). In
addition, the MTT results revealed that cell proliferation was
significantly reduced in the agomiR-186 transfection group
(Fig. 6C). The results further
confirm that downregulated VEGF may inhibit the proliferation of
EA.hy926 cells.
Discussion
In the present study, VEGF expression was detected
in the blood and tumor thrombus tissues of HCC patients with portal
vein tumor thrombus, and the expression of upstream regulator
miR-186 was analyzed. Through cell experiments, the functions of
miR-186 and VEGF were preliminary discussed, and then the
underlying molecular mechanisms were examined.
Vein tumor thrombus is a common complication in
malignant process and the second most common cause of patient
mortality besides cancer itself (25). Approximately 10% of patients with
renal cell cancer will develop venous tumor thrombus (26,27), and
60% of patients with tumor thrombus are associated with metastasis
(28). For patients with tumor
thrombus, complete resection of the tumor is a treatment method
(26). Currently, blocking cell
signaling pathways and angiogenesis in tumors to inhibit tumor
growth is one of the efficient targeted therapies to treat cancer.
Certain studies have reported that targeted therapies are a new
adjuvant treatment for tumor thrombus (29-33).
In normal environment, VEGF is one of the most effective
pro-angiogenic factors and regulators of blood vessel growth. Its
basic function is to promote angiogenesis and increase blood supply
(34). It has also been reported
that VEGF is an important factor in HCC (35). The EA.hy926 cell line was selected
for the current study based on previous studies (15-17).
In line with previous findings, the present study observed that
VEGF was abnormally expressed in the blood and tumor thrombus
tissues of HCC patients with portal vein tumor thrombus, whereas
silencing VEGF slowed down the proliferation of EA.hy926
endothelial cells. All these results indicate that VEGF may serve
an important role in the development of tumor thrombus in HCC
patients.
To further investigate the regulatory mechanisms of
VEGF, the upstream regulators of VEGF were predicted through
bioinformatics methods. miRNAs may inhibit mRNA translation through
degrading mRNA (36). In fact, these
regulatory mechanisms of miRNAs to upregulate or downregulate
several genes serve important roles in the development and
progression of tumors (37,38). According to bioinformatics
prediction, the current study identified that miR-186 may be an
upstream miRNA to regulate VEGF.
Several studies have demonstrated that miR-186 may
be a novel target in tumor prevention, diagnosis and treatment. For
instance, Zhang et al (39)
found that miR-186 can be used as a diagnostic biomarker in
pancreatic cancer, while it also influenced proliferation and
invasion of tumor cells. In addition, Lee et al (40) demonstrated that upregulated miR-186
was associated with recession of fibroblast proliferation. Sun
et al (41) also observed
that miR-186 participated in the formation of fibroblasts in
tumors. Furthermore, a study by Cui et al (42) demonstrated that miR-186 inhibited
cell proliferation and metastasis in non-small cell lung cancer
through regulating Rock1. To further examine the molecular
mechanism underlying the action of miR-186 on VEGF, human
endothelial cell EA.hy926 were cultured in vitro and
transfected with agomiR-186 to analyze the alterations in cell
proliferation by MTT assay. The results indicated that miR-186
reduced the proliferation of EA.hy926 cells, while upregulated
miR-186 induced the downregulation of VEGF. In addition,
dual-luciferase reporter assay confirmed that miR-186 was able to
directly bind to the 3'-UTR of VEGF mRNA, suggesting that miR-186
may regulate VEGF mRNA expression.
In conclusion, the findings of the present study
suggested that miR-186 serves important roles in the development of
portal vein tumor thrombus in HCC through regulating VEGF
expression. miR-186 may be used as a target for diagnosis,
prevention and treatment of HCC patients with tumor thrombus. The
current findings provide novel insight for understanding the
development and progression of tumor thrombus in HCC patients.
Acknowledgements
The authors would like to thank Professor Dianhua Gu
(Department of Hepatobiliary Surgery, Huai'an First People's
Hospital, Nanjing Medical University, Huai'an, China) for his
valuable help during the preparation of this manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WDY designed the study and performed the
experiments. FGL designed the study, performed the statistical
analysis and prepared the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanda M, Sugimoto H and Kodera Y: Genetic
and epigenetic aspects of initiation and progression of
hepatocellular carcinoma. World J Gastroenterol. 21:10584–10597.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kuszyk BS, Beauchamp NJ Jr and Fishman EK:
Neurovascular applications of CT angiography. Semin Ultrasound CT
MR. 19:394–404. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wedd JP, Nordstrom E, Nydam T, Durham J,
Zimmerman M, Johnson T, Thomas Purcell W and Biggins SW:
Hepatocellular carcinoma in patients listed for liver
transplantation: Current and future allocation policy and
management strategies for the individual patient. Liver Transpl.
21:1543–1552. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Yang BH, Xia JL, Huang LW, Tang ZY, Chen
MS, Li JQ, Liang AM, Mo QG, Lu HS, Dai CL, et al: Changes of
clinical aspect of primary liver cancer in China during the past 30
years-control study for 3,250 cases with primary liver cancer.
Zhonghua Yi Xue Za Zhi. 83:1053–1057. 2003.(In Chinese). PubMed/NCBI
|
|
5
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Trousseau A: Lectures on clinical
medicine, delivered at the Hotel-Dieu, Paris: Translated and edited
with notes and appendices, by P. Victor Bazire. Lindsay and
Blakiston, 1869.
|
|
7
|
Blom JW, Vanderschoot JP, Oostindiër MJ,
Osanto S, van der Meer FJ and Rosendaal FR: Incidence of venous
thrombosis in a large cohort of 66,329 cancer patients: Results of
a record linkage study. J Thromb Haemost. 4:529–535.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meyer G: Venous thromboembolism and
cancer. Rev Prat. 65:216–219. 2015.(In French).
|
|
9
|
Bianconi D, Schuler A, Pausz C,
Geroldinger A, Kaider A, Lenz HJ, Kornek G, Scheithauer W,
Zielinski CC, Pabinger I, et al: Integrin beta-3 genetic variants
and risk of venous thromboembolism in colorectal cancer patients.
Thromb Res. 136:865–869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Malaponte G, Signorelli SS, Bevelacqua V,
Polesel J, Taborelli M, Guarneri C, Fenga C, Umezawa K and Libra M:
Increased levels of NF-kB-dependent markers in cancer-associated
deep venous thrombosis. PLoS one. 10(e0132496)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Connolly GC, Phipps RP and Francis CW:
Platelets and cancer-associated thrombosis. Semin Oncol.
41:302–310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chang LH, Pan SL, Lai CY, Tsai AC and Teng
CM: Activated PAR-2 regulates pancreatic cancer progression through
ILK/HIF-α-induced TGF-α expression and MEK/VEGF-A-mediated
angiogenesis. Am J Pathol. 183:566–575. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee S and Goldfinger LE: RLIP76 regulates
HIF-1 activity, VEGF expression and secretion in tumor cells, and
secretome transactivation of endothelial cells. FASEB J.
28:4158–4168. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Posch F, Thaler J, Zlabinger GJ,
Königsbrügge O, Koder S, Zielinski C, Pabinger I and Ay C: Soluble
Vascular Endothelial Growth Factor (sVEGF) and the risk of venous
thromboembolism in patients with cancer: Results from the Vienna
Cancer and Thrombosis Study (CATS). Clin Cancer Res. 22:200–206.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arutyunyan I, Fatkhudinov T, Kananykhina
E, Usman N, Elchaninov A, Makarov A, Bolshakova G, Goldshtein D and
Sukhikh G: Role of VEGF-A in angiogenesis promoted by umbilical
cord-derived mesenchymal stromal/stem cells: In vitro study. Stem
Cell Res Ther. 7(46)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gapizov SS, Petrovskaya LE, Shingarova LN,
Svirschevskaya EV, Dolgikh DA and Kirpichnikov MP: The effect of
TNF and VEGF on the properties of Ea.hy926 endothelial cells in a
model of multi-cellular spheroids. Acta Naturae. 10:34–42.
2018.PubMed/NCBI
|
|
17
|
Wei Y, Yang Q, Zhang Y, Zhao T, Liu X,
Zhong J, Ma J, Chen Y, Zhao C and Li J: Plumbagin restrains
hepatocellular carcinoma angiogenesis by suppressing the migration
and invasion of tumor-derived vascular endothelial cells.
Oncotarget. 8:15230–15241. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ladizinski B and Federman DG: Trousseau
syndrome. CMAJ. 185(1063)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Witkos TM, Koscianska E and Krzyzosiak WJ:
Practical aspects of microRNA target prediction. Curr Mol Med.
11:93–109. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5(23)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li J, Xia L, Zhou Z, Zuo Z, Xu C, Song H
and Cai J: MiR-186-5p upregulation inhibits proliferation,
metastasis and epithelial-to-mesenchymal transition of colorectal
cancer cell by targeting ZEB1. Arch Biochem Biophys. 640:53–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Su BB, Zhou SW, Gan CB and Zhang XN:
MiR-186 inhibits cell proliferation and invasion in human cutaneous
malignant melanoma. J Cancer Res Ther. 14 (Suppl):S60–S64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thodiyil PA and Kakkar AK: Variation in
relative risk of venous thromboembolism in different cancers.
Thromb Haemost. 87:1076–1077. 2002.PubMed/NCBI
|
|
26
|
Lambert EH, Pierorazio PM, Shabsigh A,
Olsson CA, Benson MC and McKiernan JM: Prognostic risk
stratification and clinical outcomes in patients undergoing
surgical treatment for renal cell carcinoma with vascular tumor
thrombus. Urology. 69:1054–1058. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karnes RJ and Blute ML: Surgery insight:
Management of renal cell carcinoma with associated inferior vena
cava thrombus. Nat Clin Pract Urol. 5:329–339. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lam JS, Klatte T, Kim HL, Patard JJ, Breda
A, Zisman A, Pantuck AJ and Figlin RA: Prognostic factors and
selection for clinical studies of patients with kidney cancer. Crit
Rev Oncol Hematol. 65:235–262. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bex A, Van der Veldt AA, Blank C,
Meijerink MR, Boven E and Haanen JB: Progression of a caval vein
thrombus in two patients with primary renal cell carcinoma on
pretreatment with sunitinib. Acta Oncol. 49:520–523.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Harshman LC, Srinivas S, Kamaya A and
Chung BI: Laparoscopic radical nephrectomy after shrinkage of a
caval tumor thrombus with sunitinib. Nat Rev Urol. 6:338–343.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Karakiewicz PI, Suardi N, Jeldres C, Audet
P, Ghosn P, Patard JJ and Perrotte P: Neoadjuvant sutent induction
therapy may effectively down-stage renal cell carcinoma atrial
thrombi. Eur Urol. 53:845–848. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shuch B, Riggs SB, LaRochelle JC,
Kabbinavar FF, Avakian R, Pantuck AJ, Patard JJ and Belldegrun AS:
Neoadjuvant targeted therapy and advanced kidney cancer:
Observations and implications for a new treatment paradigm. BJU
Int. 102:692–696. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hakenberg OW: Comment on Di Silverio et
al: Neodajuvant therapy with sorafenib in advanced renal cell
carcinoma with vena cava extension submitted to radical
nephrectomy. Urol Int 2008. 80:451–453, Urol Int 80: 454;.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Roberts E, Cossigny DA and Quan GM: The
role of vascular endothelial growth factor in metastatic prostate
cancer to the skeleton. Prostate Cancer.
2013(418340)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: MiR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic beta-cells. J Biol Chem. 287:31155–31164.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Zhang ZL, Bai ZH, Wang XB, Bai L, Miao F
and Pei HH: miR-186 and 326 predict the prognosis of pancreatic
ductal adenocarcinoma and affect the proliferation and migration of
cancer cells. PLoS One. 10(e0118814)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee YH, Kim SY and Bae YS: Upregulation of
miR-760 and miR-186 is associated with replicative senescence in
human lung fibroblast cells. Mol Cells. 37:620–627. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun P, Hu JW, Xiong WJ and Mi J: miR-186
regulates glycolysis through Glut1 during the formation of
cancer-associated fibroblasts. Asian Pac J Cancer Prev.
15:4245–4250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cui G, Cui M, Li Y, Liang Y, Li W, Guo H
and Zhao S: MiR-186 targets ROCK1 to suppress the growth and
metastasis of NSCLC cells. Tumour Biol. 35:8933–8937.
2014.PubMed/NCBI View Article : Google Scholar
|