1. Introduction
Pachychoroid spectrum of diseases was first taken
into account in 2013 when pachychoroid pigment epitheliopathy (PPE)
was described in a study by Warrow et al (1). The group of diseases analyzed in the
study is characterized by retinal pigment epithelial abnormalities,
accompanied by choroidal thickening.
The term ‘pachy’ is a Greek word ‘παχύ’, which means
thick. The pachychoroid is a relatively new concept referring to a
group of ophthalmological disorders which has as major
characteristics, not only a thickened choroid, but also dilated
choroidal vessels and attenuation of choriochapillaris. These
alterations of the choroidal structure have as a consequence the
dysfunction of the retinal pigment epithelium, a layer of great
importance in maintaining the normal retinal metabolism and
functions and subsequent development of choroidal
neovascularization (2).
The etiology of pachychoroid diseases is
controversial. It is assumed that autosomal dominant heredity is
implicated, together with endogenous and exogenous factors that
trigger the onset of the clinical manifestations.
The pachychoroid spectrum comprises: polypoidal
choroidal vasculopathy/aneurysmal type 1 neovascularization,
pachychoroid neovasculopathy (PNV), PPE, focal choroidal excavation
(FCE), peripapillary pachychoroid syndrome (PPS) and central serous
chorioretinopathy (CSC). All these disorders have one, common
pathogenic process that enables their subsequent evolution.
This review takes into account both the clinical
experience of the authors and the current literature regarding the
pachychoroid spectrum of diseases, emphasizing the comprehension of
the pathogenic process, clinical characteristics and current
therapy trends, in the context of new imaging techniques available
in ophthalmology.
2. Anatomy of the choroid
The choroid represents the vascular layer of the eye
and it is part of the uvea together with the iris and ciliary body.
It extends from the anterior ora serrata to the posterior optic
nerve. The choroid provides vascular supply for the outer third of
the retina. Alterations of the choroidal structure have
repercussions on retinal function and can lead to the death of
retinal pigment epithelial cells and the photoreceptors.
The choroid is comprised of 5 layers: Bruch
Membrane, choriocapillaris, Sattler Layer - medium diameter blood
vessels, Haller's Layer - large diameter blood vessels,
suprachoroid lamina - a transitional zone between choroid and
sclera. The choriochapillaris is important in the rapid transport
of large molecules because in its structure are included large
diameter capillaries and fenestrations of 700-800 nm diameter. The
blood flow of the choroid is higher than that of any other tissue
in the organism, 20 times higher than the retinal flow, providing
oxygen and nutritive substances to the outer retina, retinal
pigment epithelium, avascular fovea and the prelaminar part of the
optic nerve (3).
The choroid is best studied by enhanced depth
imaging-optical coherence tomography (EDI-OCT) and swept
source-optical coherence tomography (SS-OCT), both techniques being
able to display the deep layers of this tissue and correlate its
structural and functional analysis. Optical coherence angiography
(OCTA) is a new, non-invasive, 3D imaging technique, which can
reconstruct the blood flow in all the vascular layers of the
choroid without using contrast substances.
As viewed on OCT, choroid thickness is the greatest
in the subfoveal region and it is thinner in the nasal and temporal
areas. Choroidal thickness varies with age, gender, ethnical group,
refractive error and axial length, but the mean thickness is
considered to be between 260-300 microns (4). Some variations in the thickness of
choroid are possible: it decreases with age and with a longer axial
length of the eye and it temporary increases in the acute stages of
severe posterior uveitis (multifocal choroiditis, multiple white
dot syndrome, Vogt-Koyanagi-Harada syndrome) (5,6).
3. Common characteristics of the
pachychoroid disease spectrum
Advances in OCT imaging technology allowed a better
understanding of the pathological processes implicated in the
manifestation of various ophthalmological diseases. Using EDI-OCT
or SS-OCT it is possible to quantify the choroidal thickness. Thus,
a new group of diseases was described, named generally
‘pachychoroid diseases’ which have a sustained, focal or diffuse
increase in choroidal thickness over 300 microns, as common
characteristic. The thick choroid is due to the dilatation of
Haller's layer vessels, accompanied by subsequent
hyperpermeability. Both the vessels in Satler's layer and the
choriocapilaris have reduced thickness.
In most cases the subfoveal choroidal thickness is
normal, but it is increased in the extrafoveal area by more than 50
microns. Choroidal thickness, although frequently encountered on
OCT, is not the major diagnostic criteria. Each of the diseases
included in the pachychoroid spectrum has specific morphological
alteration important for establishing the diagnostic. Furthermore,
according to Dansingani et al (7), cases that show increased choroidal
thickness on OCT, without any changes in the retina or pigmentary
epithelium are considered as uncomplicated pachychoroid.
The dilated vessels in the Haller layer were named
pachyvessels and can be observed on OCT images as large and
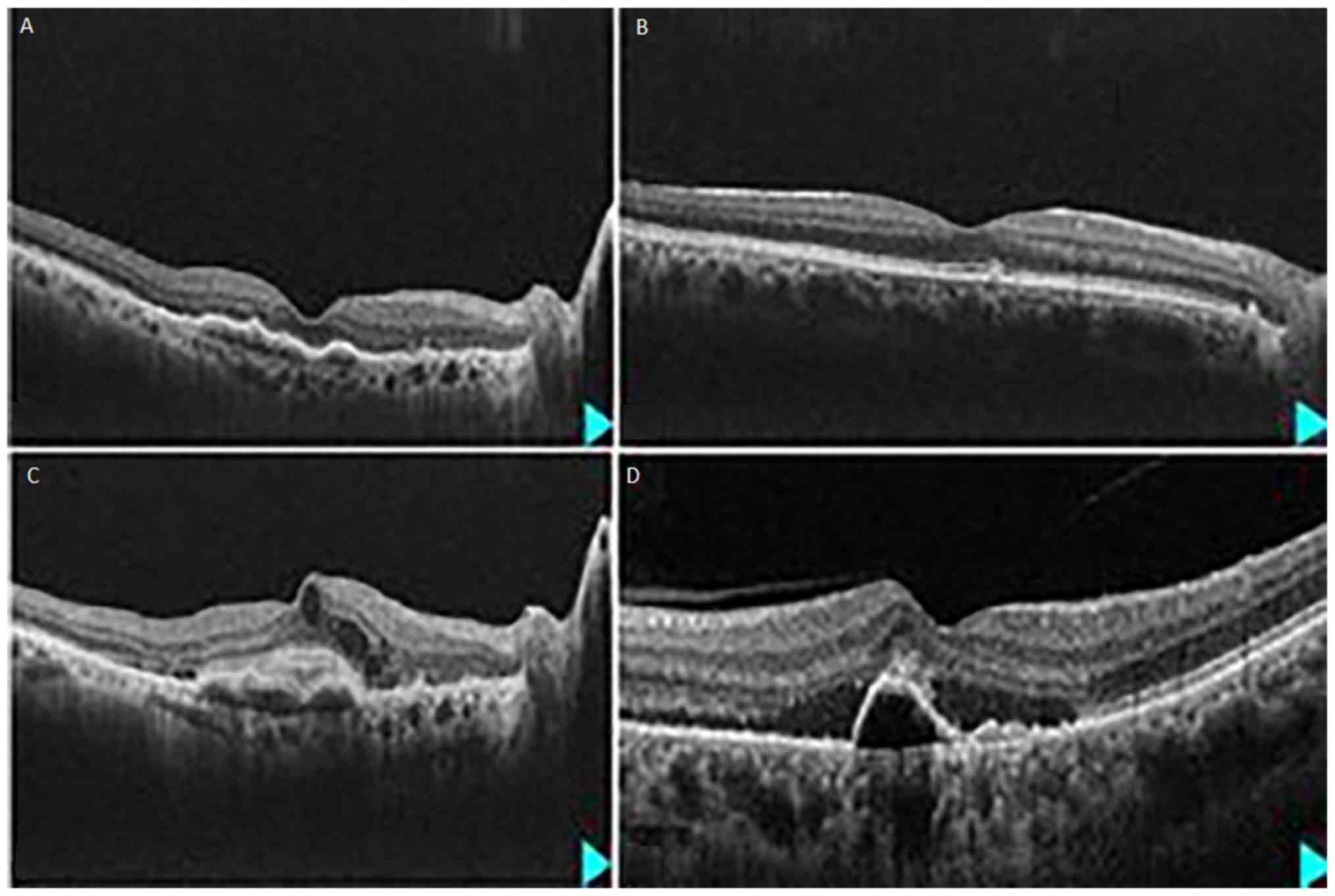
hyporeflective lumens (Fig. 1). Such
vessels were reported to be present, by Yang et al (8), in central serous corioretinopathy,
pachychoroid vasculopathy, PPS and FCE. These vessels do not reduce
their calipers towards the macula, but terminate abruptly. The
layout of pachy vessels may be diffuse or focal and they are
situated in the areas of maximum choroidal thickness and
choriocapilaris thinning, and are accompanied by retinal
morphofunctional changes.
An important feature for pachychoroid disease
spectrum is the attenuation of the choriocapillaris and the
intermediate caliper vessels in the Satler layer. Due to the
thinning of these two vascular layers, some eyes may have a normal
choroidal thickness. This attenuation of small and intermediate
diameter vessels may signify choroidal ischemia and it is
associated, in some cases with retinal outer nuclear layer atrophy,
as seen on OCT images and possibly with photoreceptors
degeneration.
In order to assess the role of choroidal vasculature
features in the development of the disease, automated software that
segments the luminal choroidal area from the stromal area on OCT
images, was developed by Agarwal et al (9). An increased vascular area was
associated with pachychoroid disorders. In a recent study, Saito
et al (10) demonstrated that
choroidal hyperperfusion has an important role in the development
of central serous chorioretinopathy (CSC) and pachychoroid pigment
epitheliopathy (PPE), by measuring the choroidal blood flow
velocity.
4. Clinical entities included in the
pachychoroid disease spectrum
Pachychoroid pigment
epitheliopathy
PPE is considered to be a precursor of CSC. In most
cases the patients are asymptomatic, presenting changes in the
structure of the retinal pigment epithelium, but without the
clinical manifestations specific to CSC.
Some patients diagnosed with unilateral CSC have
alterations specific to PPE in the contralateral eye, which might
be proof that PPE represents the first stage of CSC. Some studies
report that PPE may evolve to another entity from the pachychoroid
spectrum as choroidal neovascular membranes appear, with or without
anevrismal lesions (11).
On spectral OCT these patients do not present
neurosensory retinal detachment, but it is possible to encounter
small pigment epithelium detachments, mottling of the retinal
pigment epithelium in all overlying areas of increased choroidal
thickness (Fig. 1). Often, the PPE
is confused with the onset of age-related macular degeneration
(AMD), but soft drusens are absent in these cases and the OCT
anomalies are present at a younger age than those diagnosed in
AMD.
OCT angiography is useful in documenting the early
changes as it is possible to analyze the increased choroidal
thickness, the presence of pachyveins in Haller's layer and the
thinning in Sattler's layer and choriocapillaris. A recent study by
Sakurada et al demonstrated that there is a direct
relationship between choroidal hyperpermeability, reduced
choriocapilaris flow density and increased choroidal thickness in
PPE patients (12).
Central serous chorioretinopathy
CSC is a disorder that is commonly affecting young
or middle-aged males that present one or more of the following risk
factors: stress, autoimmune diseases, hypertension, alcohol or
tobacco consumers, gastroesophageal reflux disease, users of
corticosteroids, antacids, antihistamines or amphetamines. The
characteristic symptoms are blurred vision, metamorphopsias,
central scotoma, hyperopic shift. Most cases are auto-limited and
resolve within 6 months, but the disease may be recurrent (13).
OCT examination reveals hyporeflective subretinal
fluid, one or more retinal pigment epithelial detachments within or
outside the subretinal fluid area, thick choroid. The exudation
under the retina is the result of the abnormalities that affect the
RPE and Bruch's membrane and allow an increased permeability at the
level of the choroid. A pigment epithelium detachment (PED) is
visible on OCT in many cases. Hwang et al (14) showed that a flat, irregular PED that
accompanies CSC is a risk factor for longer evolution of the
symptoms and the development of a subfoveal neovascular membrane,
thus including these features in the pachychoroid neovasculopathy
(PNV) phenotype.
In some cases the disease becomes chronic and
typical OCT findings in these cases includes persistent subretinal
fluid, RPE degeneration, cystoid edema, elongation of outer
segments of photoreceptors. As the disorder progresses, atrophy
affects the outer retina.
OCT angiography has replaced fluorescein angiography
in the assessment of CSC. Fluorescein angiography shows leakage,
corresponding to the RPE detachments and is classically described
as ‘smokestack’ pattern. OCT angiography can reveal the areas of
choroid dysfunction, hypoperfusion of choriocapillaris and the
hyperperfusion present in the surrounding area.
CSC is considered the second stage of the
pachychoroid disease spectrum. Some studies revealed that the
choroid changes are the main starting point for the
physiopathological process. Due to recent advances in technology,
it is possible to observe the dilated vessels in the choroid and
compare the pathological findings with the asymptomatic fellow eye
(8,15). A new analysis by Demirel et al
(16) of the choroidal features
specific to pachychoroid spectrum diseases revealed that the
choriocapillaris vessel density is much lower in CSC compared with
uncomplicated pachychoroid and PPE.
Pachychoroid neovasculopathy
PNV may be considered a late complication of the
pigment epitheliopathy and CSC. This entity of the pachychoroid
spectrum is characterized by the development of type 1 neovascular
membrane, located under the RPE and overlying areas of thick
choroid and dilated choroidal vessels.
The correspondence between the neovascular membrane
location and the abnormalities inside the choroid is important for
differentiating the neovasculopathy from the neovascular form of
AMD. Usually, the AMD patients are older and have been diagnosed
before with drusens, but the patients with PNV may have presented
previously with a macular aspect consistent with PPE. Sometimes
these patients are diagnosed with a neovascular membrane of unknown
cause. In a recent comparative study, Arf et al (17) concluded that the neovascular
membranes visualized by OCT angiography in PNV patients are similar
to those of type I AMD patients, but appear in younger patients and
are associated with thicker choroids.
The differential diagnosis between these
abnormalities is difficult both by clinical examination and with
the aid of fluorescein angiography, which shows leakage in either
of the situations. OCT and OCT angiography may help in establishing
the correct diagnosis, as the morphology presented by these imaging
techniques are pathognomonic. The hyperpermeability exerted by the
thick choroid determines choriochapilaris and Sattler's layer
ischemia which results in proliferation and ingrowth of the
Haller's layer vessels. In 2019, Matsumo et al (18) demonstrated that choroidal dilated
veins are a characteristic of PNV and this feature has as a
consequence an alteration of the venous drainage routes due to the
appearance of new anastomosis between the superior and inferior
vortex veins.
On OCT it is possible to observe an irregular
detachment of the RPE, with an almost flat or shallow profile and
an inhomogeneous aspect beneath it, which is relevant for the
neovascularization located above an area of pachychoroid (Fig. 1). A ‘double layer sign’ may be
present, representing the visibility of both RPE and Bruch's
membrane. OCT angiography shows a network of vessels, typical for
an occult neovascular membrane, between the RPE and Bruch membrane
(19).
The association between the irregular PED, CSC and
the further development of PNV seems to have a genetic determinism.
Hosoda et al (20) recently
identified two susceptibility loci for pachychoroid related CSC,
both expressed mostly in the choroidal tissue. One of these genes
is also related to the development of AMD, showing that a common
pathway is possible for both diseases.
Polypoidal choroidal vasculopathy
Polypoidal choroidal vasculopathy, also known as
aneurysmal type I neovascularization, was described in a study by
Imamura et al (21) as an
area of choroidal capillaries proliferation under the RPE, which
develops aneurysms, similar to polyps, at its tip. The
proliferation than evolves to serous or hemorrhagic detachment of
the RPE.
This form of the disease, together with the PNV,
represents the most advanced stages of the pachychoroid disease
spectrum. It is considered that 1 in 10 patients with neovascular
AMD is falsely diagnosed and it actually represents a pachychoroid
patient (22).
A recent analysis of the characteristics of the
disease revealed that the aneurysms and the neovascular network are
located between the inner Bruch membrane and the RPE and have the
potential to bleed in this tight space, thus the ‘polyp’
designation is not accurate (23).
The development of the aneurysmal dilations is the
starting point for the exudative process, which evolves in a
cyclical manner. The exudation is auto-limited due to the
thrombosis of the aneurysm, but the vascular network continues to
grow for many years, generating new dilations at the edge of the
original lesion and thus the exudative process is recurrent and
leads to chronic changes. The relapsing manner in which the
pathological process evolves is a stimulus for the growth of the
neovascular membrane, contributing to an additional exudation
(24).
As opposed to the AMD patients, in the polypoidal
choroidal vasculopathy cases, EDI-OCT and SS-OCT show a thickened
choroid, choroidal hyperpermeability, subretinal fluid and narrow,
irregular RPE detachments with polypoid aspect (Fig. 1). A large study on polypoidal
choroidal vasculopathy eyes, conducted by Lee et al
(25), demonstrated that although
the choroidal thickness may have large variability in these cases,
the majority of the patients have pachyvessels as feeding sources
of the aneurysms and attenuation of the inner choroid. A new
comparative study revealed that the fellow eyes of the PCV affected
ones had a Haller layer to subfoveal choroidal thickness ratio
comprised between the ratio of the abnormal and the normal eyes.
Furthermore, during an observation period of 5 years, the choroidal
vascularity index, defined as luminal to total choroidal area
ratio, changed in these eyes and 16% developed PCV specific lesions
(26).
OCT angiography is able to show the network of
filamentous neovessels occupying the outer retina to
choriocapillaris layer (ORCC). A retrospective case series, by
Fujita et al (27), which
included OCT angiography data from 54 eyes, confirmed that OCTA can
replace indocyanine green angiography in detecting the polypoid
lesions.
Peripapillary pachychoroid
syndrome
This syndrome was recently described as an entity of
the pachychoroid disease spectrum in which the thickened choroid is
located in the proximity of the optic disc (28).
As a consequence, the subretinal and intraretinal
fluid is distributed in the nasal macular and peripapillary area.
Some cases may associate pigment epitheliopathy, pigment epithelial
detachment or optic disc edema. Most of the eyes have hyperopic
refraction and short axial lengths. Caution is needed in order to
establish a correct differential diagnosis between the PPS and a
series of inflammatory diseases such as posterior uveitis or
neuro-ophthalmologic disorders.
Although described as a sporadic association,
Govetto et al (29) published
a case study in which the PPS was related to pulmonary arterial
hypertension. In this case, choroidal ischemia is thought to have
been caused by hemodynamic disturbances due to increased central
venous pressure (30).
Focal choroidal excavation
The FCE may be encountered as a single manifestation
of the pachychoroid disease in an eye, or it may be associated with
one of the other entities included in this phenotype. There are
reports of the association of the FCE with CSC, PNV and polypoidal
choroidal vasculopathy, either in the presenting eye or in the
contralateral eye (31,32).
Most of these patients are asymptomatic or only have
minor visual symptoms, blurring and metamorphopsias and present
myopic refraction. The disease manifests in patients younger than
those diagnosed with AMD and without a history of scleral
staphyloma.
On OCT it is possible to observe areas of choroidal
excavation which can manifest under two patterns: with or without
contact between the photoreceptors and the retinal pigment
epithelium. Subretinal fluid may be present at this level. The
choroidal excavation is associated with pachychoroid features. A
study by Rajabian et al (33)
analyzed the OCT angiography features of 28 eyes with FCE and
concluded that choroidal stroma was reduced in areas close to FCE
and both the choriocapillaris and the deep plexus had a lower
vessel density compared with normal eyes. The authors suggested
that the occurrence of FCE is possible due to the weakening of the
choroidal architecture.
Another possible pathogenic theory may be that the
abnormalities represent areas of scarring of the connective tissue
in the choroid, probably of inflammatory nature, that leads to
compression on the choriocapillaris, subsequent ischemia and
predisposition to neovascular membrane development (31).
Banaee et al (34) described an FCE case that developed in
the vicinity of a pachyvessel, in an eye with pachychoroid
characteristics, leading to the disappearance of the vessel and
proposed as a pathogenic mechanism the thrombosis of the
pachyvessel.
5. Discussion
As most studies show, the main pathogenic mechanism
leading to all the abnormalities associated with pachychoroid
disease manifestation is the choroidal dysfunction. The increased
choriocapillaris permeability generates, in time, the attenuation
of this structure with pigmentary changes in the RPE and VEGF
expression. As a result, the disease progresses to neovascular
membranes and exudation (23). The
occurrence of the neovascular membranes and their evolution is
different from those associated with AMD, as choriocapillaris
chronic inflammation and attenuation are the triggers of the
disease (35).
The pathogenesis of the pachychoroid disease remains
controversial, but there are some suppositions that an altered
steroid metabolism is involved. It is known that the choroid
expresses receptors for mineralocorticoid and the continuous
stimulation of these receptors leads to an increase in its
thickness, as Zhao et al (36) demonstrated in their study. Ersoz
et al (37) reported that
prolonged administration of corticosteroids may have as a result
occurrence of PPE features, followed by CSC development, confirming
the supposition that dexamethasone intravitreal implants alter
choroidal functionality.
Another theory states that the increased hydrostatic
pressure at the level of the Bruch membrane, RPE complex is the
main factor to blame in the development of the pigment epithelium
and neurosensory retina detachments. As a result of the
choriocapillaris attenuation and the subsequent ischemia, there is
an overexpression of pro-angiogenic factors that stimulates the
development of a choroidal neovascular membrane. At the same time,
the increase in volume of the Haller's layer vessels and the
mechanical pressure on the RPE causes atrophy, pigmentary changes
and ruptures of the Bruch membrane, facilitating the occurrence of
CNV.
There are some suppositions about the genetic
phenotype that may be common to all the entities included in the
pachychoroid spectrum. Also, some risk genes have been found to be
common with those of AMD, but although these genes have a role in
the occurrence of the neovascular membranes, they do not influence
the pachychoroid features (38). In
some patients presenting the characteristic genetic features of the
pachychoroid, the RPE can eliminate the excess fluid and the
disease is not manifest. These cases are considered to be an
uncomplicated pachychoroid phenotype. In the rest of the cases, the
function of the RPE is altered and the disease manifests as either
minor changes at this level (PPE) or evolves to exudation (CSC),
loss of the vascular layer (choriocapillaris), ischemia and
neovascularization (PCV, PNV). Thus the different entities of the
pachychoroid spectrum appear to be stages of the same disease, but
the evolution from one stage to another is not mandatory.
The therapeutic arsenal for pachychoroid disease
does not include any novelties. Asymptomatic cases can be observed.
In this category are included most CSC, FCE and PPE cases.
The subretinal fluid is spontaneously resorbed in
the majority of acute CSC cases. Early treatment is necessary if
the visual acuity is very low, or the affected eye is the only
functional eye. Subthreshold micropulse laser photocoagulation is
one of the current options of treatment, able to seal the RPE
defect and thus prevent the accumulation of subretinal fluid.
Photodynamic therapy using verteporfirin (PDT) may
be used for chronic CSC, but some adverse effects have been
reported, such as CNV formation and alterations of the
electroretinography aspect. Some studies suggest that half-dose PDT
is safer, but it is effective in reducing choroidal thickness,
especially at the level of Haller layer (39).
The use of anti-VEGF agents is controversial in CSC.
It is postulated that choroidal ischemia accompanies CSC, with a
subsequent rise in the level of VEGF, thus the administration of
anti-VEGF agents is beneficial. Chhablani et al (40) showed that in most cases of FCE, PNV
and polypoid choroidal vasculopathy, the administration of
intravitreal anti-VEGF agents resulted in improved visual acuity
and resolution of subretinal and intraretinal fluid. However, the
effect of anti-VEGF agents on persistent subretinal fluid and
macular edema secondary to CSC was not clearly demonstrated in two
large trials (41,42). Authors such as Schworm et al
(43) and Sacconi et al
(44) concluded that intravitreal
anti-VEGF therapy is useful both in fluid resorption and visual
acuity gain, but the vessel density in the neovascular membrane
does not change after treatment, thus supporting the theory that
arteriogenesis is the main pathogenic mechanism in pachychoroid
related CNV.
The activity of PNV is not only modulated by the
development of the neovascular membrane and the implicit rise in
VEGF levels, but also by the choroidal structural alterations. This
is the reason for a weaker response and a modest response to
intravitreal therapy compared with non-pachychoroid related CNV
(45). PDT is safe and has a better
visual outcome when used in PNV eyes refractory to anti-VEGF. Lee
and Lee (46) showed that adjunctive
photodynamic therapy in eyes non-responsive to anti-VEGF
monotherapy resulted in an almost complete subretinal fluid
resorption and a better visual outcome (46).
As PCV tends to have a high risk of severe visual
acuity loss due to either persistent serous detachment of the
macula that leads to RPE and sensory retina atrophy or acute
rupture and hemorrhage of the polyp, it is justified to commence
the anti-VEGF therapy early in the evolution of the disease. The
EVEREST study showed that, although PDT is more efficient in
inducing regression of the neovascular membrane than anti-VEGF
therapy, more visual acuity was gained after the latter (47). Nevertheless, PCV eyes with
pachychoroid have a weaker response to intravitreal therapy than
those with normal choroidal thickness (48). Baek et al (49) considered that an explanation might be
the fact that the VEGF level of PCV eyes with pachychoroid is lower
than that of normal choroid eyes.
In terms of the most efficient anti-VEGF agent,
there does not seem to be much difference between bevacizumab,
ranibizumab and aflibercept regarding the improvement in visual
acuity, the number of injections and diminishing of the retinal
thickness. Some studies reported that aflibercept has a higher
closure rate of the polyp and is efficient in those cases that do
not respond to ranibizumab (50,51).
Koizumi et al (52) showed
that aflibercept can penetrate the choroid and to reduce its
thickness. Aflibercept has a vasoconstrictor effect on choroidal
vessels and reduces choroidal hyperpermeability, but may lead to
outer retinal atrophy (53).
Combined therapy (PDT and anti-VEGF agents) is more
efficient in controlling the neovascular membranes than
monotherapy. This effect is due to both the diminishing of
exudation by the intravitreal anti-VEGF agent and the thrombosis
induced by PDT in the neovessels (54-58).
Almost one-third of the PCV eyes suffer from
submacular hemorrhage during the first 10 years after diagnosis,
rendering an unfavorable visual outcome in these cases. The
combination of pneumatic displacement of the hemorrhage associated
with intravitreal anti-VEGF seems to be the best therapy for
improving the functional outcome (59).
6. Conclusions
Since the inclusion of the pachychoroid in the
ophthalmologic nomenclature in 2013, significant progress was made
in understanding the pathogenesis of the diseases in this group,
improving the diagnosis tools available to the clinician and
expanding the currently accepted classification. Additional
research is needed in order to better understand the mechanisms
that determine the development of a certain manifestation of the
disease and the progression from one form to another, to improve
the therapeutic management and target the pathogenic mechanism of
each clinical form.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ADM and DCB contributed to the design of the study,
participated in the entire review process and prepared the
manuscript. DC and RLM contributed to the literature research and
the analysis and critical interpretation of the data. ADM, DCB and
MC conceived the review and revised the manuscript. DC gave the
final approval for the publication of the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Warrow DJ, Hoang QV and Freund KB:
Pachychoroid pigment epitheliopathy. Retina. 33:1659–1672.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maranduca MA, Branisteanu D, Serban DN,
Branisteanu DC, Stoleriu G, Manolache N and Serban IL: Synthesis
and physiological implications of melanic pigments. Oncol Lett.
17:4183–4187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mrejen S and Spaide RF: Optical coherence
tomography: Imaging of the choroid and beyond. Surv Ophthalmol.
58:387–429. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lehmann M, Bousquet E, Beydoun T and
Behar-Cohen F: Pachychoroid: An inherited condition? Retina.
35:10–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goldenberg D, Moisseiev E, Goldstein M,
Loewenstein A and Barak A: Enhanced depth imaging optical coherence
tomography: Choroidal thickness and correlations with age,
refractive error, and axial length. Ophthalmic Surg Lasers Imaging.
43:296–301. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Margolis R and Spaide RF: A pilot study of
enhanced depth imaging optical coherence tomography of the choroid
in normal eyes. Am J Ophthalmol. 147:811–815. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dansingani KK, Balaratnasingam C, Naysan J
and Freund KB: En face imaging of pachychoroid spectrum disorders
with sweptsource optical coherence tomography. Retina. 36:499–516.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang L, Jonas JB and Wei W: Choroidal
vessel diameter in central serous chorioretinopathy. Acta
Ophthalmol. 91:e358–e362. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agrawal R, Gupta P, Tan KA, Cheung CM,
Wong TY and Cheng CY: Choroidal vascularity index as a measure of
vascular status of the choroid: Measurements in healthy eyes from a
population-based study. Sci Rep. 6(21090)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saito W, Hashimoto Y, Hirooka K and Ishida
S: Changes in choroidal blood flow velocity in patients diagnosed
with central serous chorioretinopathy during follow-up for
pachychoroid pigment epitheliopathy. Am J Ophthalmol Case Rep.
18(100651)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pang CE and Freund KB: Pachychoroid
neovasculopathy. Retina. 35:1–9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sakurada Y, Fragiotta S, Leong BCS, Parikh
R, Hussnain SA and Freund KB: Relationship between choroidal
vascular hyperpermeability, choriocapillaris flow density and
choroidal thickness in eyes with pachychoroid pigment
epitheliopathy. Retina. 40:657–662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brănişteanu DE, Pintilie A, Andreş LE,
Dimitriu A, Oanţă A, Stoleriu G and Brănişteanu DC: Ethiopathogenic
hypotheses in lichen planus. Rev Med Chir Soc Med Nat Iasi.
120:760–767. 2016.PubMed/NCBI
|
|
14
|
Hwang H, Kim JY, Kim KT, Chae JB and Kim
DY: Flat irregular pigment epithelium detachment in central serous
chorioretinopathy: A form of pachychoroid neovasculopathy? Retina
Oct 1, 2019 (Epub ahead of print). doi:
10.1097/IAE.0000000000002662.
|
|
15
|
Imamura Y, Fujiwara T, Margolis R and
Spaide RF: Enhanced depth imaging optical coherence tomography of
the choroid in central serous chorioretinopathy. Retina.
29:1469–1473. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Demirel S, Değirmenci MFK, Batıoğlu F and
Özmert E: Evaluation of the choroidal features in pachychoroid
spectrum diseases by optical coherence tomography and optical
coherence tomography angiography. Eur J Ophthalmol.
4(1120672119887095)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arf S, Sayman Muslubas I, Hocaoglu M,
Ersoz MG and Karacorlu M: Features of neovascularization in
pachychoroid neovasculopathy compared with type 1 neovascular
age-related macular degeneration on optical coherence tomography
angiography. Jpn J Ophthalmol. 64:257–264. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsumoto H, Kishi S, Mukai R and Akiyama
H: Remodeling of macular vortex veins in pachychoroid
neovasculopathy. Sci Rep. 9(14689)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bousquet E, Bonnin S, Mrejen S, Krivosic
V, Tadayoni R and Gaudric A: Tadayoni R and Gaudric A: Optical
coherence tomography angiography of flat irregular pigment
epithelium detachment in chronic central serous chorioretinopathy.
Retina. 38:629–638. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hosoda Y, Miyake M, Schellevis RL, Boon
CJF, Hoyng CB, Miki A, Meguro A, Sakurada Y, Yoneyama S, Takasago
Y, et al: Genome-wide association analyses identify two
susceptibility loci for pachychoroid disease central serous
chorioretinopathy. Commun Biol. 2(468)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imamura Y, Engelbert M, Iida T, Freund KB
and Yannuzzi LA: Polypoidal choroidal vasculopathy: A review. Surv
Ophthalmol. 55:501–515. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Freund KB, Zweifel SA and Engelbert M: Do
we need a new classification for choroidal neovascularization in
age-related macular degeneration? Retina. 30:1333–1349.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dansingani KK, Gal-Or O, Sadda SR,
Yannuzzi LA and Freund KB: Understanding aneurysmal type 1
neovascularization (polypoidal choroidal vasculopathy): A lesson in
the taxonomy of 'expanded spectra' - a review. Clin Exp Ophthalmol.
46:189–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Uyama M, Wada M, Nagai Y, Matsubara T,
Matsunaga H, Fukushima I, Takahashi K and Matsumura M: Polypoidal
choroidal vasculopathy: Natural history. Am J Ophthalmol.
133:639–648. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee WK, Baek J, Dansingani KK, Lee JH and
Freund KB: Choroidal morphology in eyes with polypoidal choroidal
vasculopathy and normal or subnormal subfoveal choroidal thickness.
Retina. 36 (Suppl 1):S73–S82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee K, Park JH, Park YG and Park YH:
Analysis of choroidal thickness and vascularity in patients with
unilateral polypoidal choroidal vasculopathy. Graefes Arch Clin Exp
Ophthalmol. 258:1157–1164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fujita A, Kataoka K, Takeuchi J, Nakano Y,
Horiguchi E, Kaneko H, Ito Y and Terasaki H: Diagnostic
characteristics of polypoidal choroidal vasculopathy based on
B-scan swept-source optical coherence tomography angiography and
its interrater agreement compared with indocyanine green
angiography. Retina: Jan 20, 2020 (Epub ahead of print). doi:
10.1097/IAE.0000000000002760.
|
|
28
|
Phasukkijwatana N, Freund KB, Dolz-Marco
R, Al-Sheikh M, Keane PA, Egan CA, Randhawa S, Stewart JM, Liu Q,
Hunyor AP, et al: Peripapillary pachychoroid syndrome. Retina.
38:1652–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Govetto A, Sarraf D and Scialdone A: ‘Hide
and seek’ neurosensory retinal detachments in peripapillary
pachychoroid syndrome associated with pulmonary arterial
hypertension. Retin Cases Brief Rep: Nov 21, 2019 (Epub ahead of
print). doi: 10.1097/ICB.0000000000000942.
|
|
30
|
Branisteanu DE, Nichifor M, Dorobat CM,
Brănişteanu DC, Petrariu FD, Molodoi AD, Radu DC and Boda D: Use of
textile biomaterials for the topic treatment of chronic venous
disease. Rom Biotechnol Lett. 20:10618–10625. 2015.
|
|
31
|
Ellabban AA, Tsujikawa A, Ooto S,
Yamashiro K, Oishi A, Nakata I, Miyake M, Akagi-Kurashige Y,
Ueda-Arakawa N, Arichika S, et al: Focal choroidal excavation in
eyes with central serous chorioretinopathy. Am J Ophthalmol.
156:673–683. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lim FP, Wong CW, Loh BK, Chan CM, Yeo I,
Lee SY, Mathur R, Wong D, Wong TY and Cheung CM: Prevalence and
clinical correlates of focal choroidal excavation in eyes with
age-related macular degeneration, polypoidal choroidal vasculopathy
and central serous chorioretinopathy. Br J Ophthalmol. 100:918–923.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rajabian F, Arrigo A, Jampol LM, Mercuri
S, Introini U, Bandello F and Battaglia Parodi M: Optical coherence
tomography angiography features of focal choroidal excavation and
the choroidal stroma variations with occurrence of excavation.
Retina: 2020 Jan 23, 2020 (Epub ahead of print). doi:
10.1097/IAE.0000000000002765.
|
|
34
|
Banaee T, Lyons LJ and El-Annan J:
Development of focal choroidal excavation in non-neovascular age
related macular degeneration with pachy-choroid features. J Curr
Ophthalmol. 31:454–457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kitaya N, Nagaoka T, Hikichi T, Sugawara
R, Fukui K, Ishiko S and Yoshida A: Features of abnormal choroidal
circulation in central serous chorioretinopathy. Br J Ophthalmol.
87:709–712. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao M, Célérier I, Bousquet E, Jeanny JC,
Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, et
al: Mineralocorticoid receptor is involved in rat and human ocular
chorioretinopathy. J Clin Invest. 122:2672–2679. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ersoz MG, Hocaoglu M, Sayman Muslubas I,
Arf S and Karacorlu M: Development of pachychoroid pigment
epitheliopathy and transformation to central serous
chorioretinopathy after intravitreal dexamethasone implantation.
Retin Cases Brief: Sep 26, 2018 (Epub ahead of print). doi:
10.1097/ICB.0000000000000820.
|
|
38
|
Dansingani KK, Perlee LT, Hamon S, Lee M,
Shah VP, Spaide RF, Sorenson J, Klancnik JM Jr, Yannuzzi LA,
Barbazetto IA, et al: Risk alleles associated with
neovascularization in a pachychoroid phenotype. Ophthalmology.
123:2628–2630. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Izumi T, Koizumi H, Maruko I, Takahashi Y,
Sonoda S, Sakamoto T and Iida T: Structural analyses of choroid
after half-dose verteporfin photodynamic therapy for central serous
chorioretinopathy. Br J Ophthalmol. 101:433–437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chhablani J, Kozak I, Pichi F, Chenworth
M, Berrocal MH, Bedi R, Singh RP, Wu L, Meyerle C, Casella AM, et
al: King Khaled Eye Specialist Hospital International Collaborative
Retina Study Group: Outcomes of treatment of choroidal
neovascularization associated with central serous chorioretinopathy
with intravitreal antiangiogenic agents. Retina. 35:2489–2497.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Staurenghi G, Lai TYY, Mitchell P, Wolf S,
Wenzel A, Li J, Bhaumik A and Hykin PG: PROMETHEUS Study Group.
Efficacy and Safety of Ranibizumab 0.5 mg for the treatment of
macular edema resulting from uncommon causes. Twelve-month findings
from PROMETHEUS. Ophthalmology. 125:850–862. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chung YR, Seo EJ, Lew HM and Lee KH: Lack
of positive effect of intravitreal bevacizumab in central serous
chorioretinopathy: Meta-analysis and review. Eye (Lond).
27:1339–1346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Schworm B, Luft N, Keidel LF, Hagenau F,
Kern C, Herold T, Kortuem KU, Priglinger SG and Siedlecki J:
Response of neovascular central serous chorioretinopathy to an
extended upload of anti-VEGF agents. Graefes Arch Clin Exp
Ophthalmol. 258:1013–1021. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sacconi R, Tomasso L, Corbelli E,
Carnevali A, Querques L, Casati S, Bandello F and Querques G: Early
response to the treatment of choroidal neovascularization
complicating central serous chorioretinopathy: A OCT-angiography
study. Eye (Lond). 33:1809–1817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Azuma K, Tan X, Asano S, Shimizu K, Ogawa
A, Inoue T, Murata H, Asaoka R and Obata R: The association of
choroidal structure and its response to anti-VEGF treatment with
the short-time outcome in pachychoroid neovasculopathy. PLoS One.
14(e0212055)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee JH and Lee WK: One-year results of
adjunctive photodynamic therapy for type 1 neovascularization
associated with thickened choroid. Retina. 36:889–895.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y,
Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, et al: EVEREST
study: Efficacy and safety of verteporfin photodynamic therapy in
combination with ranibizumab or alone versus ranibizumab
monotherapy in patients with symptomatic macular polypoidal
choroidal vasculopathy. Retina. 32:1453–1464. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shin JY, Kwon KY and Byeon SH: Association
between choroidal thickness and the response to intravitreal
ranibizumab injection in age-related macular degeneration. Acta
Ophthalmol. 93:524–532. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Baek J, Lee JH and Lee WK: Clinical
relevance of aqueous vascular endothelial growth factor levels in
polypoidal choroidal vasculopathy. Retina. 37:943–950.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Saito M, Kano M, Itagaki K, Ise S,
Imaizumi K and Sekiryu T: Subfoveal choroidal thickness in
polypoidal choroidal vasculopathy after switching to intravitreal
aflibercept injection. Jpn J Ophthalmol. 60:35–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cho HJ, Kim KM, Kim HS, Han JI, Kim CG,
Lee TG and Kim JW: Intravitreal aflibercept and ranibizumab
injections for polypoidal choroidal vasculopathy. Am J Ophthalmol.
165:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Koizumi H, Kano M, Yamamoto A, Saito M,
Maruko I, Kawasaki R, Sekiryu T, Okada AA and Iida T: Short-term
changes in choroidal thickness after aflibercept therapy for
neovascular age-related macular degeneration. Am J Ophthalmol.
159:627–633. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ferrara N: Vascular endothelial growth
factor: Basic science and clinical progress. Endocr Rev.
25:581–611. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yong M, Zhou M and Deng G: Photodynamic
therapy versus anti-vascular endothelial growth factor agents for
polypoidal choroidal vasculopathy: A meta-analysis. BMC Ophthalmol.
15(82)2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Stanca HT, Petrović Z and Munteanu M:
Transluminal Nd:YAG laser embolysis - a reasonable method to
reperfuse occluded branch retinal arteries. Vojnosanit Pregl.
71:1072–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Munteanu M, Rosca C and Stanca H:
Sub-inner limiting membrane hemorrhage in a patient with Terson
syndrome. Int Ophthalmol. 39:461–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Stanca HT, Stanca S, Tabacaru B, Boruga M
and Balta F: Bevacizumab in Wet AMD treatment: A tribute to the
thirteen years of experience from the beginning of the anti-VEGF
era in Romania. Exp Ther Med. 18:4993–5000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Danielescu C, Stanca HT and Balta F: The
management of lamellar macular holes: A review. J Ophthalmol.
2020(3526316)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shin JY, Lee JM and Byeon SH:
Anti-vascular endothelial growth factor with or without pneumatic
displacement for submacular hemorrhage. Am J Ophthalmol.
159:904–14.e1. 2015.PubMed/NCBI View Article : Google Scholar
|