Introduction
Gastric cancer (GC) is the fourth leading cause of
cancer-associated mortality among the most common malignancies,
with nearly 760,000 deaths resulting from GC each year (1,2). GC has
no specific manifestation at the early stage and >80% of
patients with GC present with advanced or late-stage GC upon
diagnosis (3). Thus, early diagnosis
of GC is particularly important and more effective non-invasive
diagnostic markers for early GC are urgently required.
Peripheral lymphocyte subsets are important cells in
the immune system (4,5). Peripheral blood lymphocyte subsets
mainly include T lymphocytes, B lymphocytes (CD19+) and
natural killer (NK) cells
(CD3-CDl6+CD56+). According to the
presence of different cell surface markers, T lymphocytes may be
divided into various phenotypic and functional groups, including
CD3+, CD4+ and CD8+ T cells, and
regulatory T cells (Tregs;
CD4+CD25+CD127-). T cells and NK
cells are mainly involved in cellular immunity, while B cells are
involved in humoral immunity (5-8).
Changes in the peripheral lymphocyte subsets of
tumor patients not only reflect the state of immune functions but
are also closely linked to the clinical phenotype of tumors
(9). Low levels of CD3+ T
cells in peripheral blood are associated with the poor prognosis of
patients with colon, endometrial or lung cancer (10-12),
while low levels of CD4+ and CD8+ T cells are
associated with the poor prognosis of patients with breast
(13), colorectal (14) and cervical cancer (15). Certain studies have indicated that
the proportion of CD4+ and CD8+ T cells tends
to be stable, fluctuating between 1.3 and 2.0% in healthy
individuals and may also be a good prognostic marker for lung and
cervical cancer (16,17). CD19 is able to specifically reflect
changes in B lymphocytes and CD19+ B cells were proven
to be a prognostic marker for nasopharyngeal carcinoma (18). Tregs are a group of lymphocytes that
negatively regulate the immune response and have an important role
in maintaining self-tolerance and avoiding excessive immune damage
to the body. Studies have suggested that high levels of Tregs lead
to poor prognosis in patients with colorectal or esophageal cancer
(19,20). NK cells are a group of lymphocytes
with innate immune function. Low levels of NK cells have been
indicated to be associated with a favorable prognosis in patients
with esophageal or pancreatic cancer (21,22). In
spite of the increase of clinical research on lymphocyte subsets in
peripheral blood, only a few systematic studies have reported on
the distribution of lymphocyte subsets in the peripheral blood of
patients with GC (23).
In the present study, the distribution of peripheral
lymphocyte subsets in healthy donors (HDs), patients with gastric
ulcer (GU) and patients with GC was explored and the value of NK
cells as a diagnostic and prognostic marker for GC was
revealed.
Materials and methods
Patients
To determine the minimum sample size in the HD, GU
and GC groups for inclusion in the present study, the following
formula was used to calculate the sample capacity:
n1=Zα2Sen(1-Sen)/Δ2,
n2=Zβ2Spe(1-Spe)/Δ2,
where n1 is the case sample capacity, n2 the
control sample capacity, Sen is the sensitivity, Spe is the
specificity, Zα=Zβ=95% CI=1.96 and Δ=0.1. The
minimum sample capacity was determined to be 72 for GC and 68 for
GU and HD. Peripheral blood samples were collected from 122
patients with GC prior to radical D2 surgery for GC at the
Affiliated Hospital of Nantong University (Nantong, China) from
January 2013 to December 2013. Informed consent was obtained from
all included participants. A total of 122 patients with primary GC
scheduled for radical D2 surgery for GC were selected according to
the following criteria: i) Patients were not older than 80 years;
ii) patients did not receive any chemotherapy drugs prior to or
after surgery; iii) patients had no known infectious diseases
within 1 month prior to surgery; iv) patients had no obvious
symptoms of fever; v) no blood transfusion was performed during
surgery and within 1 month after surgery or 3 months prior to
surgery; vi no infection or other complications occurred after
surgery; and vii) tumors were TNM stage I, II or III. A uniform
follow-up procedure was performed based on the clinical guidelines
of the Japanese GC Association Classification of Gastric Carcinoma,
13th edition (24). The present
study was approved by the Ethics Committee of the Affiliated
Hospital of Nantong University (Nantong, China) in accordance with
the tenets of the Declaration of Helsinki.
Blood sample collection and flow
cytometry
Fasting venous blood samples were collected in
heparin anticoagulant tubes and the samples were incubated with
four-color monoclonal antibodies at room temperature for 15 min as
follows: A CD3-FITC/CD8-PE/CD45-peridinin chlorophyll
(PerCP)/CD4-allophycocyanin (APC) four-color kit (Mindray Medical
International Co., Ltd.; cat. no. 340443; 1:1) and a
CD3-FITC/CD16+56-phycoerythrin (PE)/CD45-PerCP/CD19-APC four-color
kit (Mindray Medical International Co., Ltd.; cat. no. 652834, 1:1)
were used to identify CD3+, CD4+,
CD8+ and CD19+ T cells and NK cells,
respectively, while a CD45-PerCP/CD4-APC/CD25-PE/CD127-FITC
four-color kit (BD Biosciences; cat. no. 6029623, 1:1) was used to
identify Tregs. After 15 min, flow cytolytic lysing agent (Mindray
Medical International Co., Ltd.; cat. no. 2018110601) was added.
The aforementioned kits were used according the manufacturers'
protocols and all of the prepared samples were assayed using a
BriCyte E6 flow cytometer (Mindray) and a minimum of
3x103 cells were recorded. A particular uniform protocol
was followed that was published previously (9).
Immunohistochemical (IHC)
analysis
In brief, GC or GU tissues were paraffin-embedded
and cut into sections (4 µm thick). The sections were dewaxed in
xylene and then rehydrated using a graded ethanol series. After
three washes with PBS, the sections were incubated overnight at 4˚C
with the anti-CD56 antibody (Fuzhou Maixin Biotech Co., Ltd.; cat.
no. Kit-0028, 1:100), an NK cell marker. After three washes with
PBS, the sections were incubated with a horseradish
peroxidase-conjugated secondary antibody (Dako, Agilent
Technologies, Inc.; cat. no. S0809; 1:150) for 2 h at room
temperature. Finally, hematoxylin and xylene were used to
counterstain the sections. The staining intensity was scored
manually in the microscope by two independent experienced
pathologists. Brown-stained lymphocytes were defined as positively
stained cells. The final IHC score was calculated using the average
proportion of positively stained cells among lymphocyte cells in
five random fields.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 statistical software (SPSS Inc.). The results are
expressed as mean ± standard deviation (SD). One-way analysis of
variance followed by the Student-Neumann-Keuls post hoc test was
used for comparison among the GC, GU and HD groups. The Student's
t-test was used for comparing the NK cell ratio between cancer
associated death and those who were still alive after 5 years. The
correlation of NK cell levels between serum and paired tumor tissue
samples was analyzed by Spearman correlation analysis. The
sensitivity and specificity to identify GC and HD based on the
peripheral NK cell level were analyzed and the best cutoff was
determined by receiver-operating characteristic (ROC) curve
analysis. The χ2 test was used to determine the
association between the level of peripheral NK cells and
clinicopathological features. Clinical and pathological data at the
time of resection were analyzed to identify factors that influenced
the prognosis via the Cox proportional hazards model. Multivariate
analysis was performed by the Cox proportional hazards regression
model. Kaplan-Meier analysis with a log-rank test was performed to
determine the overall survival (OS) of patients with GC based on
the level of peripheral NK cells. P<0.05 was considered to
indicate statistical significance.
Results
Distribution of lymphocyte subsets in
patients with gastric diseases
Flow cytometry was used to detect the distribution
of peripheral blood lymphocyte subsets in 80 HDs (age, 59.77±12.06
years; 50 male, 30 female), 80 cases of GU (age, 60.26±11.23 years;
42 male, 38 female) and 122 cases of GC (age, 56.41±11.92 years;37
male, 85 female) (Fig. 1).
CD3+, CD4+ and CD8+ T cells, as
well as CD19+ B cells, were lower in patients with GC
than in HDs and GU cases but with no statistical significance
(P>0.05; Fig. 1A-D). Tregs levels
were higher in patients with GC than in HDs or cases of GU, but
there was no significant difference (P>0.05; Fig. 1E). Of note, the level of NK cells in
patients with GC was significantly higher than that in HDs
(P<0.001; 95% CI=7.51-12.51) and patients with GU (P<0.001;
95% CI=8.31-13.67; Fig. 1E).
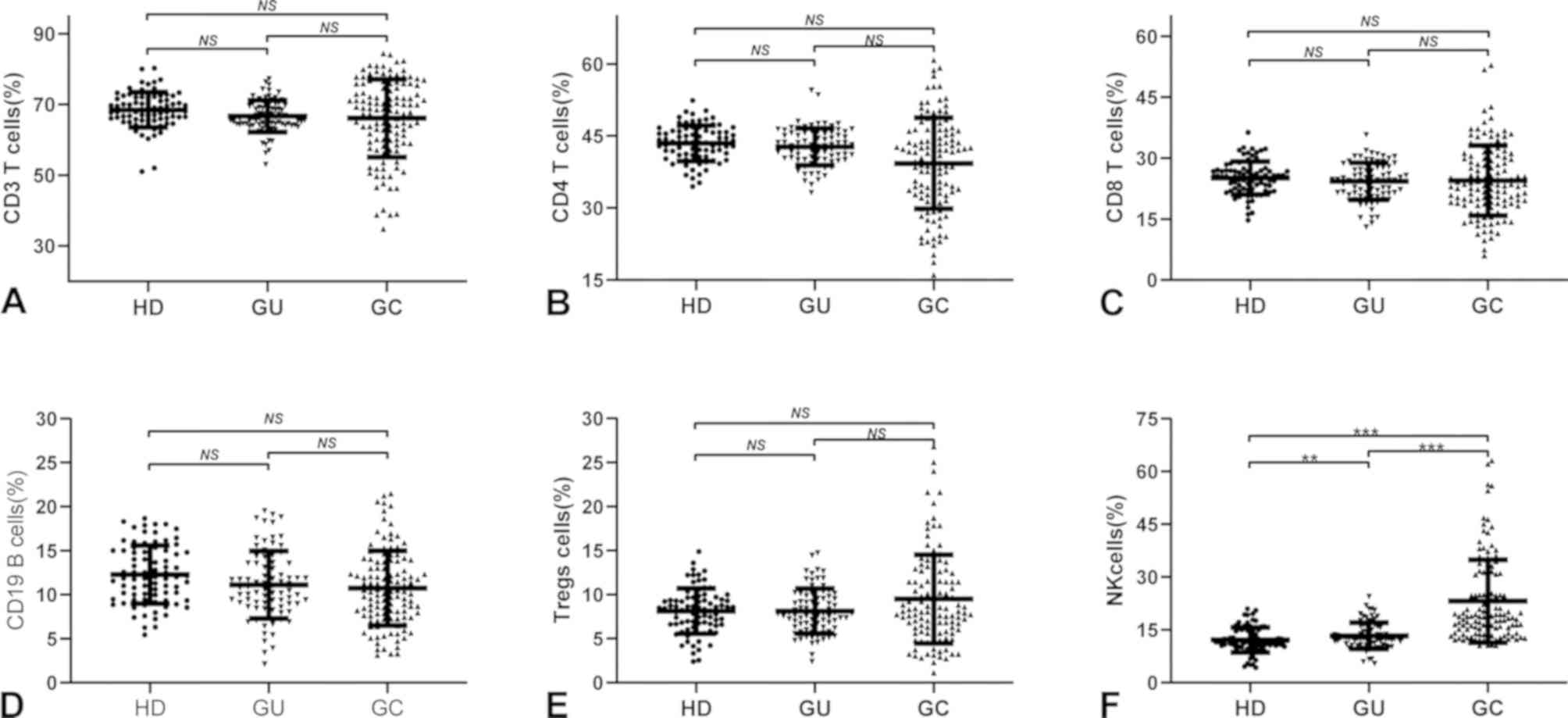 | Figure 1Distribution of peripheral lymphocyte
subsets in different gastric diseases. Distribution of peripheral
(A) CD3+ T cells, (B) CD4+ T cells, (C)
CD8+ T cells, (D) CD19+ T cells, (E) Tregs
cells and (F) NK cells in HDs, GU cases and GC cases, respectively.
One-way analysis of variance was used for comparisons between
groups. **P<0.01, ***P<0.001. NS, no
significance (P>0.05); HD, healthy donor; GU, gastric ulcer; GC,
gastric cancer; Treg, T-regulatory; NK, natural killer. |
NK cell levels are correlated between
paired serum and tumor samples from patients with GC
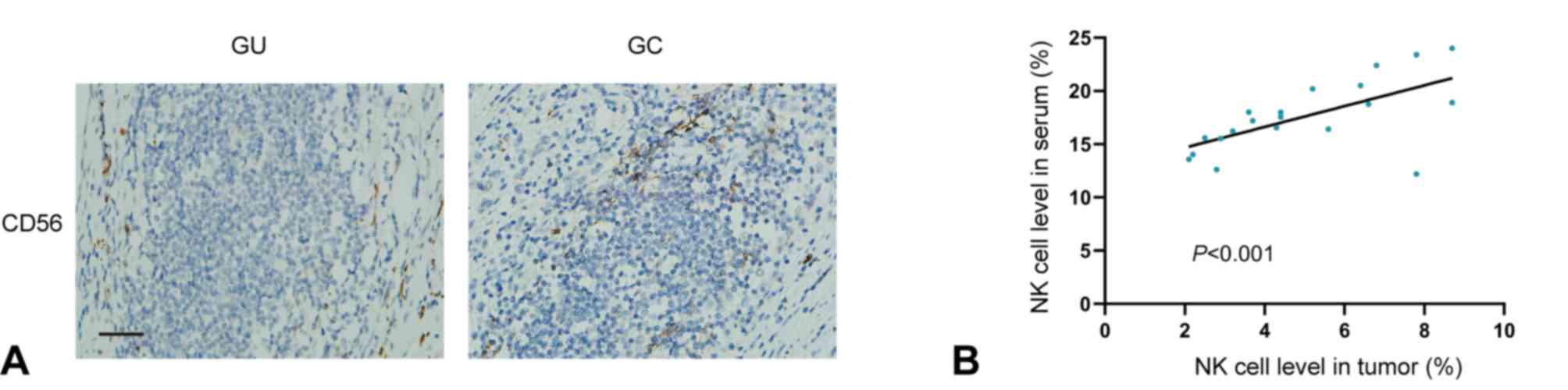
Next, IHC analysis of NK cells (CD56) was performed
in samples from patients with GC (n=20) and ulcer tissue samples
from patients with GU (n=3), revealing that the NK cell level in GC
tissues was notably higher compared with in GU tissues (Fig. 2A). Further comparison revealed a
strong positive correlation between the NK cell levels of paired
tumor serum and tissue samples from patients with GC (Spearman
R=0.898; P<0.001, Fig. 2B).
Peripheral NK cell levels are able to
effectively distinguish patients with GC from HDs
The median proportion of NK cells among lymphocytes
in patients with GC (n=122) was 18.77% and the interquartile range
(IQR) was 13.54-31.18%. However, in HDs (n=80) and patients with GU
(n=80), the median proportion of NK cells was 12.19% (IQR,
10.32-14.77%) and 12.74% (IQR, 11.42-16.01%), respectively. In
addition, the one-way analysis of variance revealed that the level
of peripheral NK cells was increased in the GU group as compared
with that in the HD group, and in the GC group as compared with
that in the GU group (P=0.026 and P<0.001, respectively),
indicating that the level of NK cells increased with the
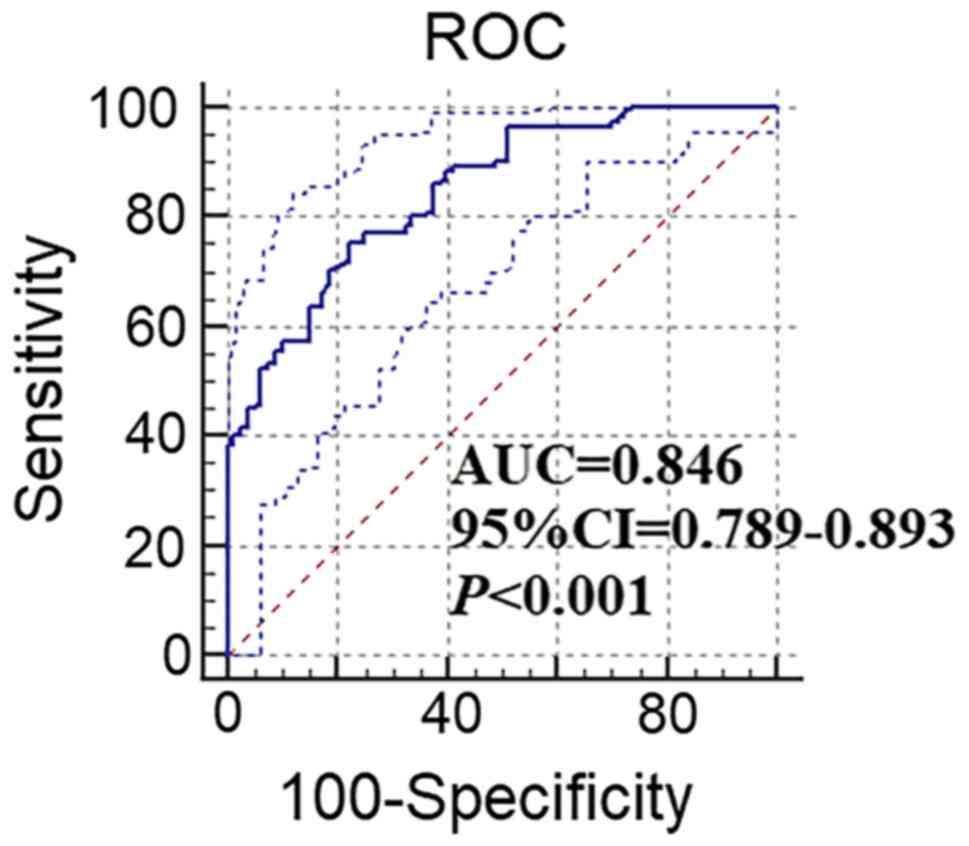
progression of the disease. An ROC curve was generated and
demonstrated that the peripheral NK cell levels were able to
effectively identify patients with GC and HD (Fig. 3). The area under the curve was 0.846
(95% CI: 0.789-0.893; P<0.001), while the best cut-off
diagnostic threshold of NK cells for GC was 15.16%. For this
critical value, a diagnostic sensitivity of 75.41% and a
specificity of 77.45% were determined. The false-positive rate was
24.59% and the false-negative rate was 22.55%. At the critical
value of 15.16%, the proportion of patients with high levels of NK
cells in the GC, HD and GU groups were 75.41% (92/122), 22.55%
(18/80) and 26.25% (21/80), respectively.
Peripheral NK cell levels in patients
with GC are associated with clinicopathological features
To determine the clinical importance of peripheral
NK cell levels in patients with GC, the association of the
peripheral levels of NK cells with various clinicopathological
features in 122 patients with GC was determined. High NK cell
levels were defined as >15.16% and low NK cell levels as ≤15.16%
based on the previously determined NK cell level cutoff in patients
with GC. χ2 test indicated that high levels of
peripheral NK cells were associated with the N stage, T stage and
TNM stage (Table I). However,
multivariate analysis suggested that high levels of peripheral NK
cells were only independently associated with the T stage [P=0.031;
hazard ratio (HR)=3.278; 95% CI: 1.113-9.656].
 | Table IAssociation between the
characteristics of patients with gastric cancer and NK cell ratios
in peripheral blood. |
Table I
Association between the
characteristics of patients with gastric cancer and NK cell ratios
in peripheral blood.
| | NK cell level | | | |
|---|
| Clinicopathological
feature | Low (n=30) (%) | High (n=92)
(%) | Total (n) (%) |
χ2-value | P-value |
|---|
| Sex | | | | 0.756 | 0.384 |
|
Male | 11 (9.0) | 26 (21.3) | 37 (30.3) | | |
|
Female | 19 (15.6) | 66 (54.1) | 85 (69.7) | | |
| Age (years) | | | | 0.005 | 0.941 |
|
≤60 | 20 (16.4) | 62 (50.8) | 82 (67.2) | | |
|
>60 | 10 (8.2) | 30 (24.6) | 40 (32.8) | | |
| Tumor diameter
(cm) | | | | 3.138 | 0.076 |
|
≤4 | 7 (5.7) | 38 (31.1) | 45 (36.8) | | |
|
>4 | 23 (18.9) | 54 (44.3) | 77 (63.2) | | |
| Tumor location | | | | 1.508 | 0.470 |
|
Upper | 5 (4.1) | 10 (8.2) | 15 (12.3) | | |
|
Middle | 9 (7.3) | 38 (31.1) | 47 (38.5) | | |
|
Lower | 16 (13.1) | 44 (36.1) | 60 (49.2) | | |
| Tumor
differentiation | | | | 0.394 | 0.530 |
|
High | 18 (14.8) | 61 (50.0) | 79 (64.8) | | |
|
Low | 12 (9.8) | 31 (25.4) | 43 (35.2) | | |
| CA19-9 (U/ml) | | | | 0.689 | 0.406 |
|
≤37 | 25 (20.5) | 70 (57.4) | 95 (77.9) | | |
|
>37 | 5 (4.1) | 22 (18.0) | 27 (22.1) | | |
| CEA level
(ng/ml) | | | | 1.710 | 0.191 |
|
≤5 | 21 (17.2) | 52 (42.6) | 73 (59.8) | | |
|
>5 | 9 (7.4) | 40 (32.8) | 49 (40.2) | | |
| HP infection | | | | 0.265 | 0.607 |
|
- | 17 (13.9) | 57 (46.7) | 74 (60.6) | | |
|
+ | 13 (10.7) | 35 (28.7) | 48 (39.4) | | |
| TNM stage | | | | 14.407 | 0.001 |
|
I | 18 (14.8) | 21 (17.2) | 39 (32.0) | | |
|
II | 7 (5.7) | 39 (32.0) | 46 (37.7) | | |
|
III | 5 (4.1) | 32 (26.2) | 37 (30.3) | | |
| T stage | | | | 16.505 | 0.001 |
|
1 | 20 (16.4) | 24 (20.0) | 44 (36.1) | | |
|
2 | 5 (4.1) | 26 (21.3) | 31 (25.4) | | |
|
3 | 3 (2.4) | 28 (23.0) | 31 (25.4) | | |
|
4 | 2 (1.6) | 14 (11.5) | 16 (13.1) | | |
| N stage | | | | 9.749 | 0.021 |
|
0 | 18 (14.8) | 28 (23.0) | 46 (37.8) | | |
|
1 | 6 (4.9) | 22 (18.0) | 28 (22.9) | | |
|
2 | 2 (1.6) | 23 (18.9) | 25 (20.5) | | |
|
3 | 4 (3.2) | 19 (15.6) | 23 (18.8) | | |
High levels of NK cells in patients
with GC are linked to poor survival
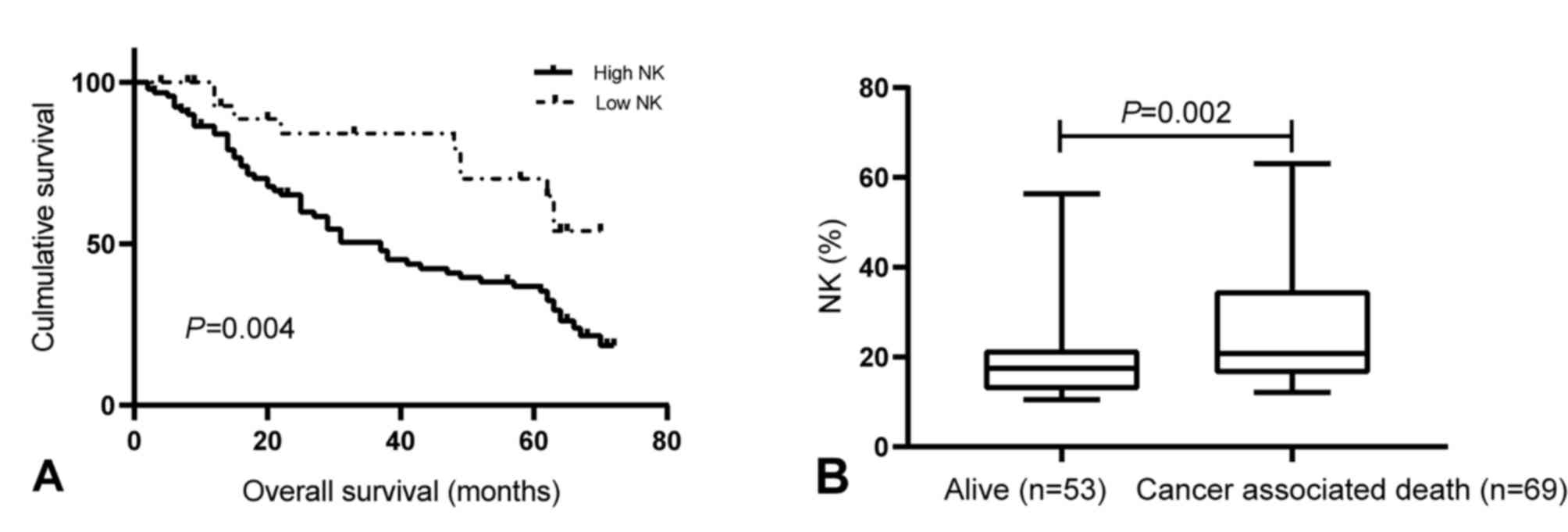
Kaplan-Meier survival curves were drawn to determine
the association of the levels of peripheral NK cells with OS. The
results indicated that patients with high peripheral NK cell levels
had a shorter OS than those with low levels of NK cells (P=0.004;
Fig. 4A). In addition, at the 5-year
follow-up, 69 patients had died, while 53 patients remained alive,
with a 5-year mortality rate of 56.6%. The median proportion of NK
cells was significantly higher in patients who had deceased
(20.77%; IQR, 16.38-34.38%) than in those who were still alive
after 5 years (17.50%; IQR, 12.80-20.82%; P=0.002; Fig. 4B). Next, multivariate analysis
suggested that the level of peripheral NK cells were independent
predictors of OS in patients with GC (P=0.018; HR=2.637; 95% CI:
1.305-5.328; Table II).
 | Table IIUnivariate and multivariate analyses
of prognostic factors for overall survival in patients with gastric
cancer. |
Table II
Univariate and multivariate analyses
of prognostic factors for overall survival in patients with gastric
cancer.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | P-value | HR | 95% CI | P-value |
|---|
| Sex [male (n=85)
vs. female (n=37)] | 0.328 | 1.303 | 0.767-2.214 | NS |
| Age [years; ≤60
(n=82) vs. >60 (n=40)] | 0.574 | 0.864 | 0.519-1.438 | NS |
| Tumor diameter [cm;
≤4 (n=45) vs. >4 (n=77)] | 0.155 | 0.702 | 0.431-1.144 | NS |
| Tumor location
[upper/middle (n=39) vs. lower (n=73)] | 0.597 | 1.136 | 0.708-1.823 | NS |
| Tumor
differentiation [high (n=79) vs. low (n=43)] | 0.209 | 0.828 | 0.616-1.112 | NS |
| CA19-9 level [U/ml;
≤37 (n=95) vs. >37 (n=27)] | 0.362 | 1.316 | 0.729-2.375 | NS |
| CEA level [ng/ml;
≤5 (n=73) vs. >5 (n=49)] | 0.483 | 1.199 | 0.722-1.993 | NS |
| HP infection
[-(n=74) vs. + (n=48)] | 0.096 | 1.505 | 0.930-2.435 | NS |
| T stage [I, II
(n=75) vs. III, IV (n=47)] | 0.037 | 1.671 | 1.033-2.704 | NS |
| N stage [0, 1
(n=74) vs. 2, 3 (n=48)] | 0.015 | 1.820 | 1.124-2.949 | 0.064 |
| NK cells [high
(n=92) vs. low (n=30)] | 0.007 | 2.637 | 1.305-5.328 | 0.018 |
Discussion
The present study aimed to investigate the
diagnostic and prognostic value of peripheral lymphocyte subsets in
GC. The levels of peripheral CD3+, CD4+ and
CD8+ T cells, CD19+ B cells and Tregs in
patients with GC were not significantly different from those in
HDs, but the proportion of peripheral NK cells in patients with GC
(18.77%) was significantly higher than that in HDs (10.85%,
P<0.001) and cases of GU (11.89%, P<0.001). ROC curve
analysis suggested that the peripheral NK cell levels may be used
as a diagnostic marker for patients with GC. In addition to CEA,
CA19-9 and CA72-4 serum markers are widely used for the early
diagnosis of GC (25-27).
Recent studies have indicated that non-coding RNAs, including
Homo sapiens circular RNA 0000467, as well as epigenetic
changes such as RAS association domain family member 10 (RASSF10)
hypermethylation, Helicobacter pylori infection, T stage and
other molecular markers (28), may
also act as diagnostic markers for GC (29-32).
In a previous study, the sensitivity and specificity of CA19-9 were
determined to be 0.605 and 0.559, respectively, while those for CEA
were 0.686 and 0.593, respectively, and those for CA72-4 were 0.667
and 0.592, respectively (29). In
the present study, an ROC curve was generated and the sensitivity
and specificity of NK cells were determined to be 75.41 and 77.45%,
respectively, which were significantly higher than those for CEA,
CA19-9 and CA72-4. These results suggested that the peripheral NK
cell levels may be used as a marker for the early diagnosis of
GC.
The peripheral NK cell level is associated with
various clinicopathological factors. It has been indicated that the
level of peripheral NK cells is associated with lymph node
metastasis in esophageal cancer (21). The present study determined that the
level of peripheral NK cells in patients with GC was associated
with the T stage, suggesting that it may be used to assess tumor
progression in patients with GC. However, this requires further
validation in future studies with larger sample sizes.
Numerous factors affect the prognosis of GC, with
the most widely used factor being the TNM stage (33,34).
However, according to our clinical experience, it appears that
patients with GC and the same TNM stage have different prognoses,
and patients with the same TNM stage may have different molecular
phenotypes. Molecular typing of GC also has an important role in
the prognosis of patients (35). In
recent years, an increasing number of studies have reported that
certain factors, including high co-overexpression of epidermal
growth factor receptor and receptor tyrosine kinase 3, low levels
of microRNA-214 and high methylation of RASSF10, are associated
with poor prognosis in patients with GC (30,36,37). In
the present study, the peripheral NK cell levels were determined to
be an independent prognostic factor in patients with GC and
patients who died within 5 years had a higher level of peripheral
NK cells. Furthermore, the detection of NK cells only requires
peripheral blood extraction, which is convenient to test and
associated with low cost for patients. In the present study, the
specificity and sensitivity of the NK cell ratio were slightly
better than those for established prognostic markers for GC,
including CEA and CA19-9. The present study determined that the NK
cell ratio in the serum exhibited a positive correlation with that
in matched GC tumor tissue samples. In the future, the NK cell
ratio may be implemented in routine diagnostic/prognostic
procedures for GC. However, the present study was limited by the
sample size of patients with GC and HDs. In addition, only common
peripheral blood lymphocytes were examined and the role of the
proportion of NK cells in the prognosis and diagnosis of GC
patients was demonstrated. However, there may be other lymphocyte
clusters that warrant exploration.
In conclusion, the present study indicated that the
peripheral NK cell levels may be used to identify patients with GC
and are associated with certain clinicopathological factors of
malignancy. Of note, high levels of peripheral NK cells were
associated with poor prognosis. The present study demonstrated that
the peripheral NK cell levels may be a good diagnostic and
prognostic marker for patients with GC.
Acknowledgements
The authors thank Dr Nicole Okoh (Liwen Bianji,
Edanz Editing China; www.liwenbianji.cn/ac) for editing the English text of
a draft of this manuscript.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81672409), Jiangsu
Provincial Medical Youth Talent (grant no. QNRC2016700), ‘333
Talent’ Cultivating Project of Jiangsu Province (grant no.
BRA2018394), Postgraduate Research & Practice Innovation
Program of Jiangsu Province (grant no. SJCX20_1153; SJCX20_1159;
KYCX20_2674) and the Scientific and Technological Innovation and
Demonstration Project of Nantong City (grant no. MS12018061).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WJX and YLH conceived and designed the experiments.
YYC and YF performed the experiments. QSM, JZL, XC and PM analyzed
the data. YYC and YFL wrote the manuscript. WL and YFL searched the
literature and performed statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nantong University (Nantong, China). Written informed
consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y,
Zheng L and Li L: Association of intra-tumoral infiltrating
macrophages and regulatory T cells is an independent prognostic
factor in gastric cancer after radical resection. Ann Surg Oncol.
18:2585–2593. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chow MT, Möller A and Smyth MJ:
Inflammation and immune surveillance in cancer. Semin Cancer Biol.
22:23–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zarin P, Wong GW, Mohtashami M, Wiest DL
and Zúñiga-Pflücker JC: Enforcement of γδ-lineage commitment by the
pre-T-cell receptor in precursors with weak γδ-TCR signals. Proc
Natl Acad Sci USA. 111:5658–5663. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Park K, He X, Lee HO, Hua X, Li Y, Wiest D
and Kappes DJ: TCR-mediated ThPOK induction promotes development of
mature (CD24-) gammadelta thymocytes. EMBO J. 29:2329–2341.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Pillars article: Activation of NK cells
and T cells by NKG2D, a receptor for stress-inducible MICA. Science
1999. 285:727–729, J Immunol 200: 2231-2233. 2018.PubMed/NCBI
|
|
8
|
Sardinha LR, Elias RM, Mosca T, Bastos KR,
Marinho CR, D'Império Lima MR and Alvarez JM: Contribution of NK,
NK T, gamma delta T, and alpha beta T cells to the gamma interferon
response required for liver protection against trypanosoma cruzi.
Infect Immun. 74:2031–2042. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J,
Liu L, Liu C and Yu X: Abnormal distribution of peripheral
lymphocyte subsets induced by PDAC modulates overall survival.
Pancreatology. 14:295–301. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu A, Mansure JJ, Solanki S, Siemens DR,
Koti M, Dias ABT, Burnier MM, Brimo F and Kassouf W: Presence of
lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and
immunoscore as prognostic marker in patients after radical
cystectomy. PLoS One. 13(e0205746)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Soo RA, Chen Z, Yan Teng RS, Tan HL,
Iacopetta B, Tai BC and Soong R: Prognostic significance of immune
cells in non-small cell lung cancer: meta-analysis. Oncotarget.
9:24801–24820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zinovkin DA, Pranjol MZI, Bilsky IA and
Zmushko VA: Tumor-associated T-lymphocytes and macrophages are
decreased in endometrioid endometrial carcinoma with MELF-pattern
stromal changes. Cancer Microenviron. 11:107–114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hagland HR, Lea D, Watson MM and Søreide
K: Correlation of blood T-cells to intratumoural density and
location of CD3+ and CD8+ T-cells in colorectal cancer. Anticancer
Res. 37:675–683. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang K, Shen T, Siegal GP and Wei S: The
CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host
interface has prognostic value in triple-negative breast cancer.
Hum Pathol. 69:110–117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shah W, Yan X, Jing L, Zhou Y, Chen H and
Wang Y: A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes
and a high percentage of CD4(+)FOXP3(+) regulatory T cells are
significantly associated with clinical outcome in squamous cell
carcinoma of the cervix. Cell Mol Immunol. 8:59–66. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yasutomo K: The cellular and molecular
mechanism of CD4/CD8 lineage commitment. J Med Invest. 49:1–6.
2002.PubMed/NCBI
|
|
17
|
Hald SM, Bremnes RM, Al-Shibli K, Al-Saad
S, Andersen S, Stenvold H, Busund LT and Donnem T: CD4/CD8
co-expression shows independent prognostic impact in resected
non-small cell lung cancer patients treated with adjuvant
radiotherapy. Lung Cancer. 80:209–215. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu T, Huang Z, Su B, Wang S, Wang D, Wang
C, Wei W, Jiang J, Zhang G, Yang H and Hu W: Prognostic
significance of circulating CD19+ B lymphocytes in EBV-associated
nasopharyngeal carcinoma. Med Oncol. 31(198)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nabeki B, Ishigami S, Uchikado Y, Sasaki
K, Kita Y, Okumura H, Arigami T, Kijima Y, Kurahara H, Maemura K
and Natsugoe S: Interleukin-32 expression and Treg infiltration in
esophageal squamous cell carcinoma. Anticancer Res. 35:2941–2947.
2015.PubMed/NCBI
|
|
20
|
Salama P, Phillips M, Grieu F, Morris M,
Zeps N, Joseph D, Platell C and Iacopetta B: Tumor-infiltrating
FOXP3+ T regulatory cells show strong prognostic significance in
colorectal cancer. J Clin Oncol. 27:186–192. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu B, Chen L, Li J, Zheng X, Shi L, Wu C
and Jiang J: Prognostic value of tumor infiltrating NK cells and
macrophages in stage II+III esophageal cancer patients. Oncotarget.
7:74904–74916. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Karakhanova S, Ryschich E, Mosl B, Harig
S, Jäger D, Schmidt J, Hartwig W, Werner J and Bazhin AV:
Prognostic and predictive value of immunological parameters for
chemoradioimmunotherapy in patients with pancreatic adenocarcinoma.
Br J Cancer. 112:1027–1036. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Maruyama T, Kono K, Mizukami Y, Kawaguchi
Y, Mimura K, Watanabe M, Izawa S and Fujii H: Distribution of Th17
cells and FoxP3(+) regulatory T cells in tumor-infiltrating
lymphocytes, tumor-draining lymph nodes and peripheral blood
lymphocytes in patients with gastric cancer. Cancer Sci.
101:1947–1954. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 Annual report
of the JGCA nationwide registry. Gastric Cancer. 14:301–316.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ning S, Wei W, Li J, Hou B, Zhong J, Xie
Y, Liu H, Mo X, Chen J and Zhang L: Clinical significance and
diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels
in gastric and colorectal cancer patients. J Cancer. 9:494–501.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng
G, Guo M, Lian X, Fan D and Zhang H: Diagnostic and prognostic
value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC
Cancer. 17(737)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shen M, Wang H, Wei K, Zhang J and You C:
Five common tumor biomarkers and CEA for diagnosing early gastric
cancer: A protocol for a network meta-analysis of diagnostic test
accuracy. Medicine (Baltimore). 97(e0577)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yoon JH, Park YG, Nam SW and Park WS: The
diagnostic value of serum gastrokine 1 (GKN1) protein in gastric
cancer. Cancer Med. 8:5507–5514. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu J, Zhang PY, Xie JW, Wang JB, Lin JX,
Chen QY, Cao LL, Huang CM, Li P and Zheng CH: Hsa_circ_0000467
promotes cancer progression and serves as a diagnostic and
prognostic biomarker for gastric cancer. J Clin Lab Anal.
33(e22726)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xue WJ, Feng Y, Wang F, Li P, Liu YF, Guo
YB, Wang ZW and Mao QS: The value of serum RASSF10 hypermethylation
as a diagnostic and prognostic tool for gastric cancer. Tumour
Biol. 37:11249–11257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abbaszadegan MR, Taghehchian N, Aarabi A
and Moghbeli M: MAEL Cancer-testis antigen as a diagnostic marker
in primary stages of gastric cancer with Helicobacter pylori
infection. J Gastrointest Cancer. 51:17–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kouzu K, Tsujimoto H, Hiraki S, Nomura S,
Yamamoto J and Ueno H: Diagnostic accuracy of T stage of gastric
cancer from the view point of application of laparoscopic proximal
gastrectomy. Mol Clin Oncol. 8:773–778. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Deng J, Liu J, Wang W, Sun Z, Wang Z, Zhou
Z, Xu H and Liang H: Validation of clinical significance of
examined lymph node count for accurate prognostic evaluation of
gastric cancer for the eighth edition of the American joint
committee on cancer (AJCC) TNM staging system. Chin J Cancer Res.
30:477–491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei J, Yao T, Wang Y, Li L, Pan C and
Zhang N: Prognostic analysis of stage III gastric cancer after
curative surgery according to the newest TNM classification. Clin
Transl Oncol. 21:232–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu TP, Wang WY, Ma P, Shuai Y, Zhao K,
Wang YF, Li W, Xia R, Chen WM, Zhang EB and Shu YQ: Upregulation of
the long noncoding RNA FOXD2-AS1 promotes carcinogenesis by
epigenetically silencing EphB3 through EZH2 and LSD1, and predicts
poor prognosis in gastric cancer. Oncogene. 37:5020–5036.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moghbeli M, Makhdoumi Y, Soltani Delgosha
M, Aarabi A, Dadkhah E, Memar B, Abdollahi A and Abbaszadegan MR:
ErbB1 and ErbB3 co-over expression as a prognostic factor in
gastric cancer. Biol Res. 52(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ji B, Huang Y, Gu T, Zhang L, Li G and
Zhang C: Potential diagnostic and prognostic value of plasma long
noncoding RNA LINC00086 and miR-214 expression in gastric cancer.
Cancer Biomark. 24:249–255. 2019.PubMed/NCBI View Article : Google Scholar
|