Introduction
Osteoarthritis (OA) is a disease that causes
articular cartilage degeneration, and there is currently no
effective drug to prevent disease progression (1). Medical care is mainly based on
alleviating painful symptoms (2).
The pathological changes in OA involve all tissues that form
joints. During the development of OA, intra-articular tissues
exhibit inflammation of varying severity, and joint inflammation
may cause joint destruction. It is hypothesized that the immune
system is one of the factors involved in the pathogenesis,
occurrence and development of OA (3). During OA development, the production
and function of cytokines vary according to the duration and
severity of the disease (4).
Cytokines can be divided into pro- and anti-inflammatory according
to their roles in OA (3). Cytokines
interfere with the process of catabolism and anabolism,
particularly in tissues that are often subjected to high mechanical
loads, such as the human joints. The imbalance between synthesis
and catabolism causes progressive degeneration of the articular
cartilage, causing gradual development of the disease (5).
Mesenchymal stem cells (MSCs) are adult stem cells
derived from the multi-directional differentiation potential of
tissues and organs and can differentiate into osteogenic (6), cartilage (7) and liver cells (8). Previous studies have demonstrated that
MSCs can be transformed into chondrocytes and induced into
articular cartilage to repair damage, and have therapeutic effects
on experimental OA (9,10). Currently, two-dimensional cultures
cannot maintain the stability of MSCs. This is primarily due to the
senescence of MSCs during the process of a traditional plate
culture, self-differentiation of osteoblasts, decrease in
anti-inflammatory ability and decline in proliferation ability
which severely affect the function of MSCs as a cell therapy
(11). Previous studies have
reported that aggregation of MSCs into 3-dimensional (3D) spheroids
could markedly enhance their trophic and anti-inflammatory
properties (11,12).
Chitosan (CS) is a deacetylated derivative of chitin
and is the second most abundant natural polysaccharide in the world
(13). The biocompatibility of CS
has been attributed to its structural and functional similarity to
glycosaminoglycans, making it a biomaterial candidate for cartilage
engineering (14). In the present
study, MSCs were cultured on CS membranes (CMs) to form 3D
multicellular spheres. The formation of multicellular spheres had a
significant effect on the expression of anti-inflammatory genes in
MSCs. The aim of the present study was to investigate the impact of
a CM cultures on the expression of pro- and anti-inflammatory
cytokines in human umbilical cord MSCs and to establish a
foundation for further study on the role of CM-cultured MSCs in OA
cartilage repair.
Materials and methods
Experimental materials
CS powder (Sinopharm Group Chemical Reagent Co.,
Ltd.), FBS (Gibco; Thermo Fisher Scientific, Inc.), DMEM (Gibco;
Thermo Fisher Scientific, Inc.), a SDS-PAGE gel preparation kit
(cat. no. AS1012; Aspen), RIPA total protein lysis and extraction
buffer (cat. no. AS1004; Aspen), a BCA protein concentration assay
kit (cat. no. AS1086; Aspen), protein markers (Thermo Fisher
Scientific, Inc.), a fluorescence quantitative PCR instrument
(Thermo Fisher Scientific, Inc.), TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), SYBR®
Premix Ex Taq™ (Takara Biomedical Technology, Co., Ltd.),
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Biomedical
Technology, Co., Ltd.), PCR primers (Wuhan Google Biotechnology
Company Co., Ltd.), trypsin EDTA (Gino Biomedical Technology Co.,
Ltd.) and PBS (Gino Biomedical Technology Co., Ltd) were used in
the present study.
Preparation of the CM
CS powder was dissolved in 1% glacial acetic acid
solution(Sinopharm Group Chemical Reagent Co., Ltd.) to obtain a 1%
CS solution, which was spread evenly at the bottom of a 6-well
culture plate at 1.2 ml/well. The liquid was dried in an oven at
65˚C for 24 h to prepare a CM substrate. The CM substrate was
exposed to ultraviolet light overnight, neutralized using a 0.5
mol/l NaOH solution for 10 min at room temperature. Lye residue was
thoroughly washed with sterile water three times. The CM substrate
was subsequently washed with PBS three times.
Cultivation of primary MSCs
The present study was approved by the Ethics
Committee of the Renmin Hospital of Wuhan University (Wuhan, China)
and written informed consent. Umbilical cords were obtained from
three healthy postpartum women (age, Patient 1, 28 years; Patient
2, 28 years; and Patient 3, 30 years) in May 2017. Umbilical cords
were collected and washed thoroughly with PBS containing 100 µg/ml
streptomycin and 100 U/ml penicillin. Wharton's jelly tissue was
separated from the umbilical cords with forceps, cut into smaller
pieces with scissors and digested with 0.1% type I collagenase
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 16 h to
release MSCs. MSCs were obtained following by centrifugation for 5
min at 400 x g at room temperature and cultured in high glucose
MEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (Hyclone; GE Healthcare Life Sciences) and 10 ng/ml
basic fibroblast growth factor (Peprotech EC Ltd.). The medium was
replaced every 3 days with fresh medium. MSCs were identified by
morphological and flow cytometric analyses using an inverted
microscope at a magnification of x40 and x100 (Olympus
Corporation). When their proliferation reached 80%, the P1
generation was used as seed cells.
Experimental grouping and cell
culture
The following groups were used in the present study:
i) The experimental group, in which CM covered the bottom of the
6-well plate; and ii) the control group, in which the 6-well plate
did not undergo treatment. MSCs P1 cells were inoculated into the
groups (seeding density, 3x105/well) in a low-sugar DMEM
medium containing 10% FBS in a CO2 incubator at 37˚C. At
72 h post-inoculation, cell morphologies were observed using an
inverted microscope at a magnification of x40 and x100 (Olympus
Corporation) and cells were collected for subsequent
experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Expression levels of pro- and anti-inflammatory
genes in MSCs cultured in the experimental and control groups were
detected using RT-qPCR. Total RNA was extracted from the two groups
of cells cultured for 72 h and transcribed into cDNA using a
reverse transcriptase kit. A total of 2 µl cDNA was used as a PCR
amplification template. β-actin was used as the internal reference
gene. All qPCR reactions were performed using the following
thermocycling conditions: Initial denaturation at 95˚C for 30 sec,
followed by 40 cycles of 95˚C for 5 sec, 58˚C for 30 sec and 72˚C
for 30 sec. SYBR® Premix Ex Taq™ (Takara Biomedical
Technology, Co., Ltd.)was used for the quantitative analysis using
a PCR instrument. The 2-ΔΔCq method was used to analyze
results (15). Primer sequences are
presented in Table I.
 | Table IPrimer sequences of the genes used
for RT-qPCR. |
Table I
Primer sequences of the genes used
for RT-qPCR.
| | Primer sequence
(5'-3') |
|---|
| Target gene | Forward | Reverse |
|---|
| β-actin |
GTCCACCGCAAATGCTTCTA |
TGCTGTCACCTTCACCGTTC |
| IL-1β |
ACGATGCACCTGTACGATCACT |
GAGAACACCACTTGTTGCTCCA |
| TNF-α |
CTCTTCTCCTTCCTGATCGTGG |
CTTGTCACTCGGGGTTCGAG |
| IL-6 |
TCAGCCCTGAGAAAGGAGACAT |
GCTCTGGCTTGTTCCTCACTACT |
| IL-18 |
TGCATCAACTTTGTGGCAATG |
CTTCAAATAGAGGCCGATTTCC |
| IL-10 |
AACCTGCCTAACATGCTTCG |
GAGTTCACATGCGCCTTGAT |
| LIF |
AGGTCTTGGCGGCAGTACAC |
CCAAGGTACACGACTATGCGG |
| IL6ST |
ACTTGGAGCCAGATTCCTCCT |
CCCACTTGCTTCTTCACTCCA |
| IL-4 |
GCAGTTCTACAGCCACCATGAG |
TCTCTCTCATGATCGTCTTTAGCC |
| IL-13 |
CAACATCACCCAGAACCAGAAG |
GCATCCTCTGGGTCTTCTCG |
| TSG6 |
GATGGATGGCTAAGGGCAGAG |
CGTGTGGGTTGTAGCAATAGGC |
| TGF-β1 |
CAGCAACAATTCCTGGCGATA |
GCTAAGGCGAAAGCCCTCAAT |
Western blotting
Expression levels of pro- and anti-inflammatory
cytokines in MSCs were detected using western blotting. MSCs in the
experimental and control groups were lysed with pre-cooled RIPA
buffer for 30 min and centrifuged at 13,000 x g for 5 min at 4˚C.
The supernatant was collected and total protein amounts were
measured using a BCA kit (Beyotime Institute of Biotechnology). A
total of 20 µg total protein/lane was electrophoresed on SDS-PAGE
(10% gel) and transferred to 0.22-µm PVDF membranes. Membranes were
blocked with 5% skim milk TBST (10 mmol/l Tri-HCl; 150 mmol/l NaCl;
0.25% Tween-20; pH 7.5) for 1 h at room temperature. Membranes were
then incubated with polyclonal rabbit anti-human β-actin (1:10,000;
cat. no. TDY051; BEIJING TDY BIOTECH CO.,LTD.), interleukin (IL)-1β
(1:1000; cat. no. ab2105; Abcam), tumor necrosis factor-α (TNF-α;
1:500; cat. no. ab66579; Abcam), IL-6 (1:1000; cat. no. 21865-1-AP;
Proteintech Group, Inc), IL-18 (1:1000; cat. no. 10663-1-AP;
Proteintech Group, Inc), IL-4 (1:1000; cat. no. ab9622; Abcam),
IL-10 (1:1000; cat. no. DF6894; affbiotech), IL-13 (1:500; cat. no.
ab106732; Abcam), leukemia inhibitory factor (LIF; 1:1000; cat. no.
ab113262; Abcam), glycoprotein 130 (IL6ST; 1:500; cat. no.
ab202850; Abcam), TNF-α-stimulated gene 6 (TSG6; 1:500; cat. no.
ab128266; Abcam), transforming growth factor (TGF) β1 (1:1000; cat.
no. AF1027; affbiotech) overnight at 4˚C. Membranes were washed
with TBST and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibodies (1:10000; cat. no. AS1107;
Wuhan Aspen Biotechnology, Co., Ltd.) for 1 h at room temperature.
Proteins were visualized using ECL Western blotting kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and densitometric
values were analyzed using AlphaEaseFC software (AlphaInnotech,
Inc. version no. 4.0).
Immunofluorescence detection
Sterile coverslips were placed on 6-well plates.
Suspended cell culture solutions were added to the coverslip and
incubated with 5% CO2 for 2 h at 37˚C in an incubator
until cells were fixed. Cell cultures were then fixed with 4%
paraformaldehyde (cat. no. AS1018; Wuhan Aspen Biotechnology, Co.,
Ltd.) for 30 min at room temperature. After washing three times
with phosphate-buffered saline (cat. no. AS1025; Wuhan Aspen
Biotechnology, Co., Ltd.), the cells were incubated for 1 h at room
temperature in PBS containing 5% bovine serum albumin (cat. no.
10735078001; Roche Diagnostics (Shanghai), Co., Ltd.). Cells were
incubated with polyclonal rabbit anti-human COX2 (1:200; cat. no.
ab52237; Abcam), IL-4 (1:100; cat. no. bs-20685R; bioss), IL-10
(1:100; cat. no. ab34843; Abcam), leukemia inhibitory factor (LIF;
1:200; cat. no. ab113262; Abcam) overnight at 4˚C followed by
incubation with corresponding secondary antibodies of FITC-labeled
Goat Anti-Rabbit (cat. no. AS-1110), FITC-labeled Goat Anti-Mouse
(cat. no. AS-1112), CY3-labeled Goat Anti-Rabbit (cat. no.
AS-1109), CY3-labeled Goat Anti-Mouse (cat. no. AS-1111),
CY3-labeled Donkey Anti-Goat (cat. no. AS-1113) for 50 min at room
temperature. All secondary antibodies were purchased from Wuhan
Aspen Biotechnology, Co., Ltd. and were used at a dilution of 1:50.
Cells were then washed three times with PBS for 5 min each time.
DAPI staining solution was added to each well, incubated for 5 min
at room temperature and washed three times with PBS for 5 min each
time. Following this, an anti-fluorescence quencher (cat. no.
AS1089; Wuhan Aspen Biotechnology, Co., Ltd.) was added to the
cells and samples were viewed under a fluorescence microscope at a
magnification of x200.
Statistical analysis
Data were analyzed using SPSS software (version
22.0; IBM Corp.). Data are presented as the mean ± standard
deviation and paired Student's t-test were used for comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphological observation of control
and experimental MSCs
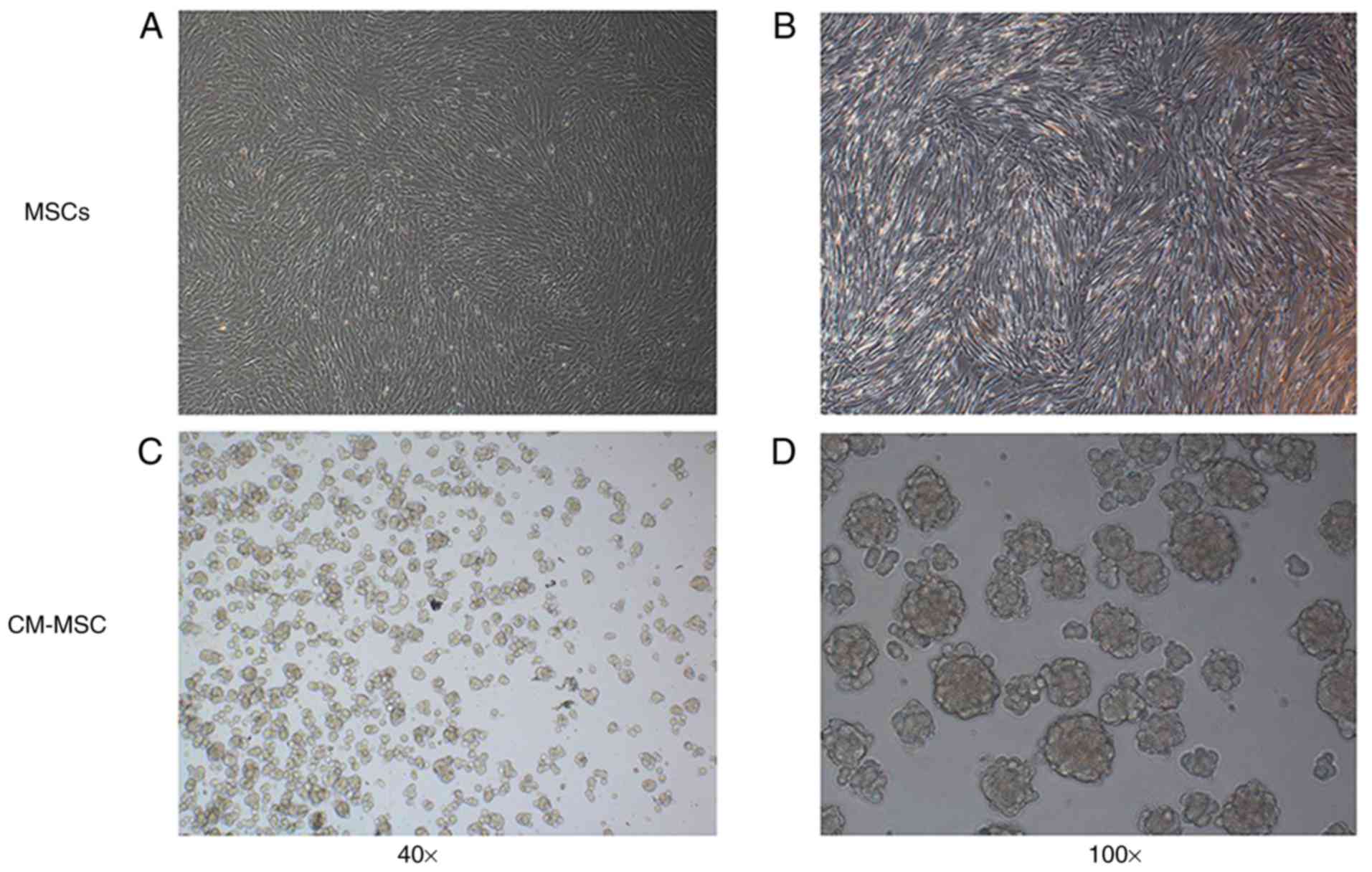
As presented in Fig.
1, MSCs in the control group were adherent, fibroblast-like
cells, which aggregated into clusters following proliferation. MSCs
cultured with CM shown suspended growth. Initially, MSCs were
suspended on the surface of the CM as a single sphere; however,
after ~24 h, cells aggregated spontaneously into multicellular
spheroids on the surface of the CM and increased progressively
(data not shown).
Effects of CM on pro- and
anti-inflammatory cytokine mRNA expression in MSCs
The mRNA expression levels of pro-inflammatory genes
IL-1β, TNF-α, IL-6 and IL-18 were significantly decreased in the
experimental group compared with the control group
(*P<0.05, **P<0.01; Fig. 2A). By contrast, the mRNA expression
levels of anti-inflammatory genes TGF-β1 were significantly
increased in the experimental group compared with the control group
(P<0.01; Fig. 2B). The relative
expression levels of IL-4 and IL-13 in the experimental group were
10-fold higher compared with the control group.
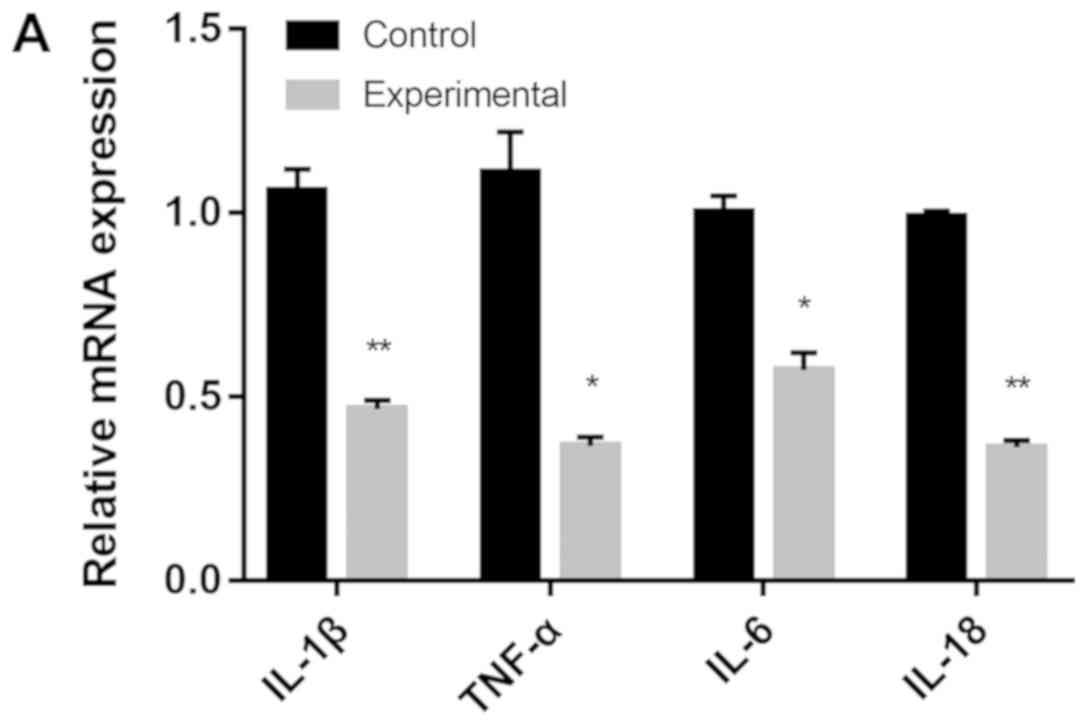 | Figure 2(A) mRNA expression levels of IL-1β,
TNF-α, IL-6 and IL-18 in the control and experimental groups. (B)
The mRNA expression levels of IL-4, IL-10, IL-13, TSG6, LIF, IL6ST
and TGF-β1 in the control and experimental groups. The control
group was cultured using a traditional plate culture, while the
experimental group was cultured in a plate with a CM. IL,
interleukin; TNF-α, tumor necrosis factor α; TSG6, TNF-α-stimulated
gene 6; LIF, leukemia inhibitory factor; IL6ST, glycoprotein 130;
TGF-β1, transforming growth factor β1; CM, chitosan membrane.
*P<0.05, **P<0.01. |
Effects of CM on pro- and
anti-inflammatory cytokine protein expression in MSCs
The protein expression levels of pro-inflammatory
genes IL-1β, TNF-α, IL-6 and IL-18 in the experimental group were
significantly decreased compared with the control group
(*P<0.05, **P<0.01,
***P<0.001; Fig. 3).
Furthermore, the protein expression levels of TGF-β1 were
significantly increased in the experimental group compared with the
control group (P<0.01; Fig.
3).
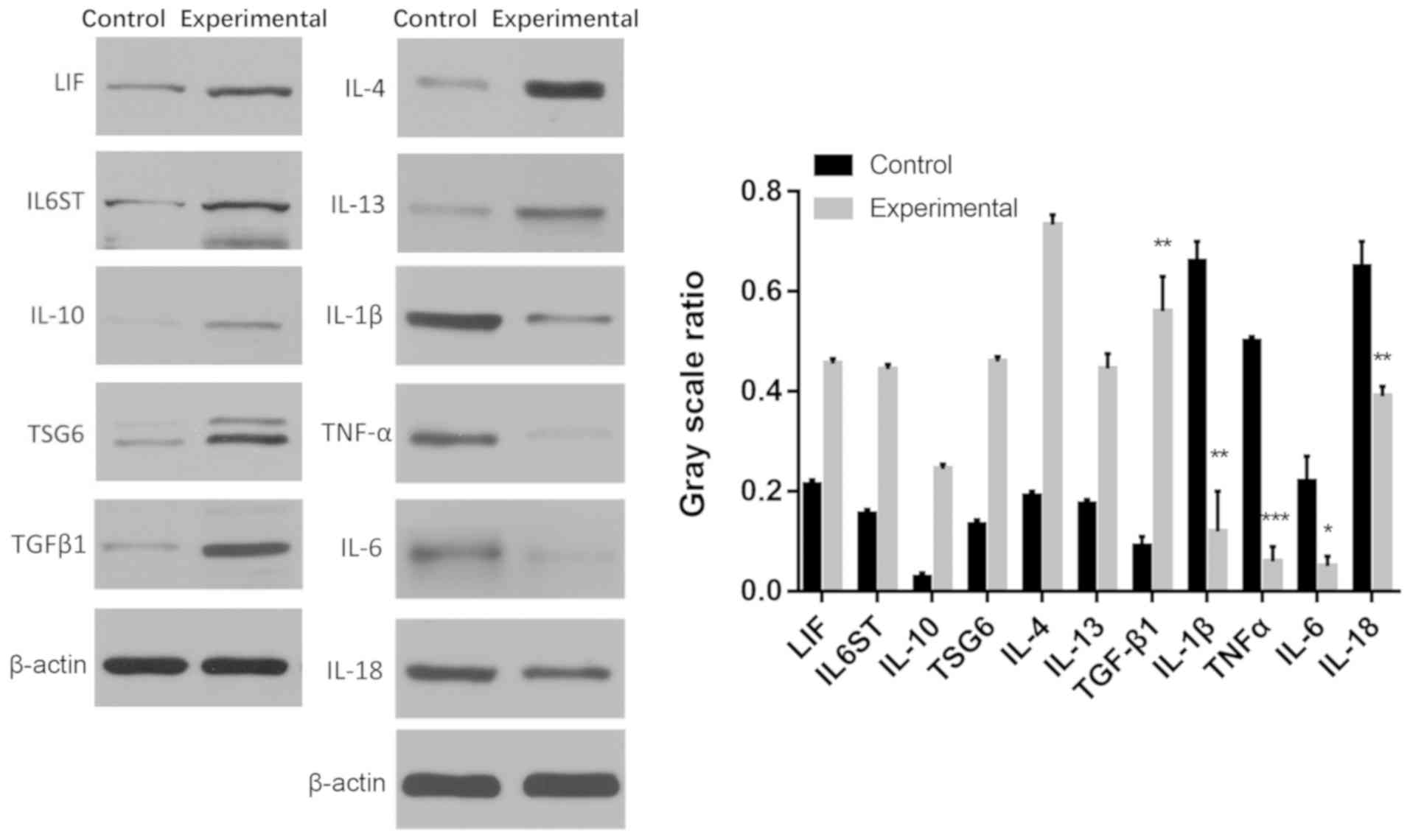 | Figure 3Protein expression of pro- and
anti-inflammatory cytokines LIF, IL6ST, IL-10, TSG6, TGF-β1, IL-4,
IL-13, IL-1β, TNF-α, IL-6 and IL-18 in the control and experimental
groups. The control group was a traditional plate culture, while
the experimental group was cultured in a plate with a CM. LIF,
leukemia inhibitory factor; IL6ST, glycoprotein 130; IL,
interleukin; TSG6, TNF-α-stimulated gene 6; TGF-β1, transforming
growth factor β1; TNF-α, tumor necrosis factor α; CM, chitosan
membrane. *P<0.05, **P<0.01,
***P<0.001. |
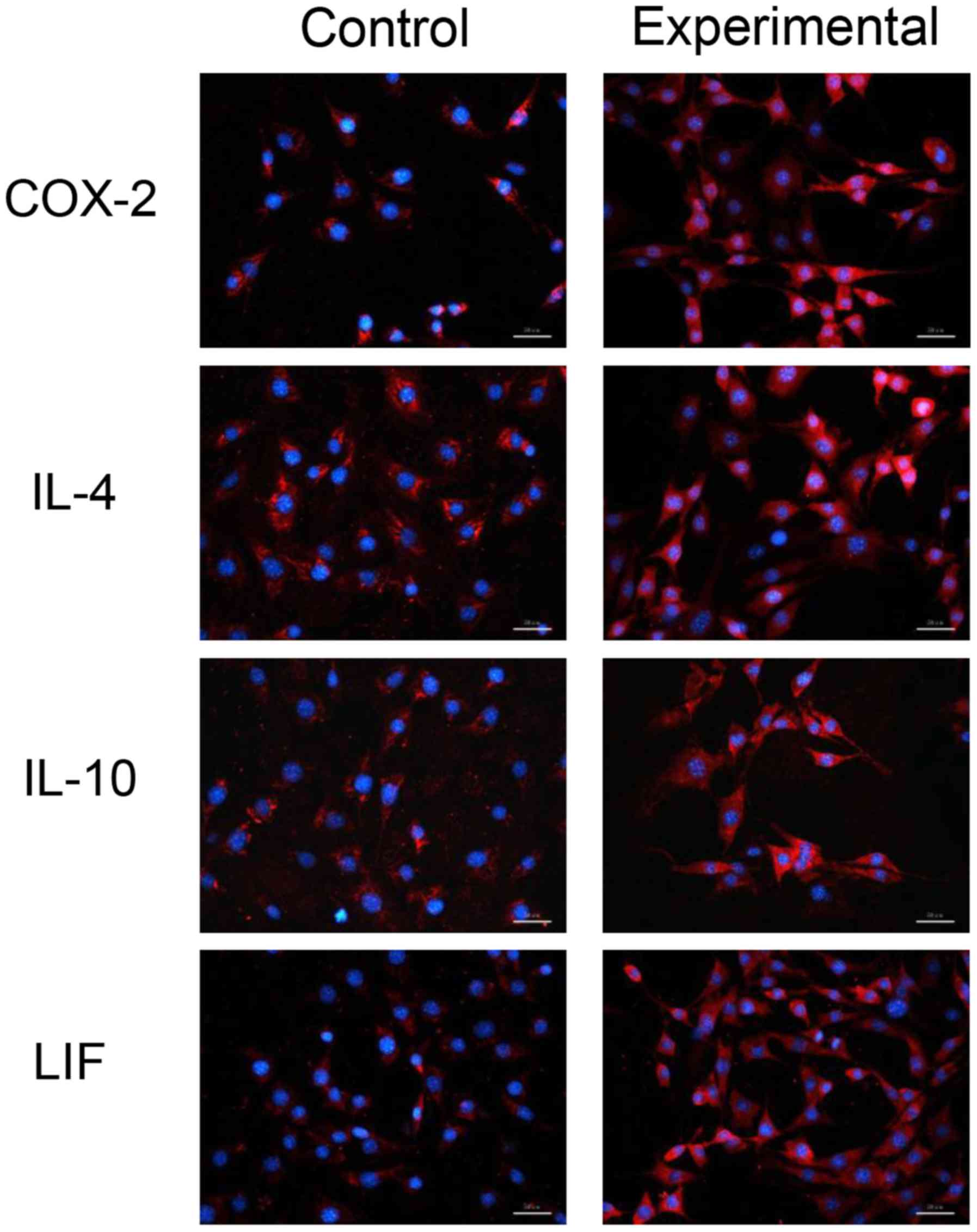
Immunofluorescence observations
Proteins expressed by prostaglandin synthase-2
(COX-2), IL-4, IL-10 and LIF in the experimental and control groups
were located in cells and belonged to endogenous proteins (Fig. 4). The protein content of COX-2, IL-4,
IL-10 and LIF in the experimental group was higher compared to the
control group, indicating that the CM increased the expression of
COX-2, IL-4, IL-10 andLIF in MSCs.
Discussion
The incidence and development of OA is characterized
by the inflammatory catabolism process, which damages tissues, and
the anti-inflammatory anabolism process, which protects tissues
(5). Various pro- and
anti-inflammatory factors mediate these processes. In OA,
inflammatory cytokines interact and restrict each other to maintain
the healthy metabolism of articular cartilage (1). An imbalance of inflammatory cytokines
causes the abnormal metabolism of articular cartilage, which leads
to deformation, loss and degradation of articular cartilage
(3).
In the pathogenesis of OA, pro-inflammatory
cytokines IL-1β and TNF-α induce a release of inflammatory
mediators via the regulation of NF-κB and microtubule-associated
protein kinase (MAPK) signaling pathways, causing articular
cartilage degeneration, destruction and degradation. The
pro-inflammatory effects of IL-1β and TNF-α in the pathogenesis of
OA have been confirmed (16-18).
Previous studies have demonstrated that IL-1β and TNF-α promote the
absorption and degradation of articular cartilage, causing the
occurrence of OA (19,20). In the pathogenesis of OA, IL-1β and
TNF-α initially bind to their respective receptors and regulate the
release of pro-inflammatory cytokines, including IL-1β, TNF-α, IL-6
and IL-18 via the NF-κB and MAPK signaling pathways (21). The expression levels of a disintegrin
and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4),
ADAMTS-5, nitric oxide and prostaglandin E2 are
upregulated and the contents of matrix metallopeptidase (MMP)-1,
MMP-3 and MMP-13 are increased in chondrocytes and synovial
fibroblasts, thereby enhancing the inflammatory reaction, promoting
extracellular matrix (ECM) degradation, inhibiting extracellular
matrix synthesis and, ultimately, leading to articular cartilage
degeneration, destruction and degradation (3).
Furthermore, anti-inflammatory cytokines serve
important roles in the pathogenesis of OA by inhibiting
pro-inflammatory cytokines, downregulating the expression of MMPs,
hindering the inflammatory response, destroying articular
chondrocytes, promoting the synthesis of proteoglycans and collagen
in chondrocytes, and inhibiting the progression of OA.
Anti-inflammatory cytokines in OA primarily include IL-4, IL-10,
IL-13, LIF, IL6ST, TSG6 and TGF-β1(22).
Previous studies have reported that IL-4 inhibits
IL-1 and induces the release of MMP-13, thereby preventing the
degradation of cartilage ECM, protecting cartilage integrity and
delaying the process of OA (23,24).
Furthermore, IL-4 has been revealed to inhibit the expression of
ADAMTS-4 and ADAMTS-5, delay the degradation of articular cartilage
ECM, and promote the synthesis of proteoglycans and collagen in
chondrocytes (23). Reportedly,
IL-10 inhibits the synthesis and secretion of IL-6 and other
related pro-inflammatory cytokines, thus serving a role in the
regulation of inflammatory immunity (25). Additionally, IL-10 inhibits the
release of MMP-1, MMP-3, MMP-13 and nitric oxide in cartilage
tissues, inhibits the inflammatory reaction and destruction of
articular chondrocytes, and promotes the synthesis of proteoglycan
and collagen in chondrocytes (23).
TGF-β is a cytokine secreted by various cells and has a regulatory
effect on cell growth, differentiation and immune function
(26). Previous studies have
demonstrated that TGF-β promoted the expression of metallopeptidase
inhibitor-4 RNA, inhibited the activity of MMPs, reduced the
formation of MMPs and protected cartilage, which delayed the
development of OA (27,28). Furthermore, TGF-β promotes
chondrocyte DNA synthesis, increases the number of chondrocytes and
repairs inflammation-induced cartilage damage (29).
MSCs are multipotent, undifferentiated cells with
extensive differentiation potential and self-replication abilities.
MSCs have become a focus of interest in the treatment of OA as they
can be easily isolated and exhibit in vitro proliferative
abilities, plasticity, immunosuppressive characteristics,
autocrine- and paracrine-mediated effects, migration ability to
injured sites, and phenotypic stability (9). MSCs involved in tissue regeneration and
can migrate to injured sites. In the local microenvironment, the
directional differentiation of MSCs into specific types of
functional cells directly participates in the process of tissue
repair (30). Furthermore, MSCs
secrete various bioactive substances, including cytokines and
growth factors, which can improve microenvironment regeneration of
the injured sites and inhibit local inflammation (31). Previous studies have confirmed that
intra-articular injection of MSCs can be used to treat early
arthritis, protect articular cartilage and increase the expression
of certain anti-inflammatory-related genes and cartilage-protective
factors (32,33).
The survival rate of MSCs amplified by traditional
adherent cultures in clinical and animal experiments is low and
biological activities and therapeutic effects are poor (32). The division and differentiation of
MSCs are closely associated with their microenvironment, and
cytokines and proteins in the microenvironment all influence the
differentiation of MSCs (34).
Previous studies have demonstrated that 3D cell cultures can
produce multicellular spheres and reproduce the microenvironment
and associated physiological activities in vivo (35,36).
Therefore, 3D MSCs exhibit stronger biological functions and
therapeutic effects compared with traditional adherent MSCs
(37). The results of the present
study revealed that a 3D CM culture was more effective in
maintaining the anti-inflammatory properties of MSCs compared with
a 2D traditional culture.
CS has high biocompatibility, no immunogenicity and
low toxicity (38). The present
study demonstrated that CM-cultured MSCs overcame the shortcomings
of plate cultures. Similarly to the 3D culture model of hanging
drop culture, CM-cultured MSCs form spheroids and exhibit enhanced
transformation efficiency (39).
Previous studies have reported that MSCs adhering to the CM can
self-assemble to form 3D spherical cells (14). During this process, MSCs adhere and
diffuse on the CM and reduce the number of pseudopods to form
multicellular spheres. This aggregation process is different from
that of suspensions or suspension cultures (13).
It has been reported that the formation of spheroids
in the CM is associated with the involvement of various genes and
proteins, including cadherin (40,41), the
Rho/Rho-associated protein kinase pathway (12) and Wnt (41). Although certain changes in gene and
protein expression levels have been observed, the exact mechanism
of spheroid formation in the CM remains unclear.
3D suspension cultures of MSCs form multicellular
spheroids, affect the epigenetic status of MSCs and improve the
anti-inflammatory properties of MSCs (12). The formation of multicellular spheres
affects the expression of inflammatory cytokines in MSCs. The
results of the present study demonstrated that, in the CM group,
the expression levels of pro-inflammatory genes IL-1β, TNF-α, IL-6
and IL-18 were significantly lower compared with the control group,
and the expression levels of anti-inflammatory genes TGF-β1 were
significantly higher compared with the control group, indicating
that the formation of multicellular spheres in CM-cultured MSCs was
more conducive to maintaining the anti-inflammatory characteristics
of MSCs.
The present study had certain limitations. Firstly,
the amount of cytokines secreted by CM-cultured MSCs compared with
MSCs was not examined. Secondly, cells were identified as MSCs by
cell morphology. Further cell type identification assays would have
been beneficial.
In conclusion, compared with the control group,
CM-cultured MSCs formed multicellular spheres, significantly
decreased the expression of pro-inflammatory cytokines and
significantly increased the expression of anti-inflammatory-related
genes, indicating that CM cultures could enhance the
anti-inflammatory properties of MSCs. Although the role of pro- and
anti-inflammatory cytokines in the pathogenesis of OA remains
unclear, the present study provided a novel approach that may be
beneficial for the OA research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science
and Technology Support Project of Hubei (grant no. 2015BCA316) and
the Science and Technology Program in Wuhan (grant no.
2016060101010045).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and PY reviewed literature, researched data and
drafted the manuscript. PY was involved in revising the manuscript
and participated in the interpretation of data. HL made
contributions to the acquisition of data. BQ was responsible for
the conception, design of the study and revising the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Renmin Hospital of Wuhan University, Wuhan,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis.
Osteoarthritis Lancet. 386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Charlier E, Deroyer C, Ciregia F, Malaise
O, Neuville S, Plener Z, Malaise M and de Seny D: Chondrocyte
dedifferentiation and osteoarthritis (OA). Biochem Pharmacol.
165:49–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vangsness CT Jr, Burke WS, Narvy SJ,
MacPhee RD and Fedenko AN: Human knee synovial fluid cytokines
correlated with grade of knee osteoarthritis - a pilot study. Bull
NYU Hosp Jt Dis. 69:122–127. 2011.PubMed/NCBI
|
|
5
|
Mueller MB and Tuan RS: Anabolic/Catabolic
balance in pathogenesis of osteoarthritis: Identifying molecular
targets. PM R. 3 (Suppl 1):S3–S11. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aboushady IM, Salem ZA, Sabry D and
Mohamed A: Comparative study of the osteogenic potential of
mesenchymal stem cells derived from different sources. J Clin Exp
Dent. 10:e7–e13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gao W, Lin M, Liang A, Zhang L, Chen C,
Liang G, Xu C, Peng Y, Chen C, Huang D, et al: Melatonin enhances
chondrogenic differentiation of human mesenchymal stem cells. J
Pineal Res. 56:62–70. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Du Z, Wei C, Cheng K, Han B, Yan J, Zhang
M, Peng C and Liu Y: Mesenchymal stem cell-conditioned medium
reduces liver injury and enhances regeneration in reduced-size rat
liver transplantation. J Surg Res. 183:907–915. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Delling U, Brehm W, Ludewig E, Winter K
and Jülke H: Longitudinal evaluation of effects of intra-articular
mesenchymal stromal cell administration for the treatment of
osteoarthritis in an ovine model. Cell Transplant. 24:2391–2407.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Saulnier N, Viguier E, Perrier-Groult E,
Chenu C, Pillet E, Roger T, Maddens S and Boulocher C:
Intra-articular administration of xenogeneic neonatal Mesenchymal
Stromal Cells early after meniscal injury down-regulates
metalloproteinase gene expression in synovium and prevents
cartilage degradation in a rabbit model of osteoarthritis.
Osteoarthritis Cartilage. 23:122–133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Y, Wang Y, Zhou L, Zou Y, Huang G, Gao
G, Ting S, Lei X and Ding X: Spheroid-cultured human umbilical
cord-derived mesenchymal stem cells attenuate hepatic
ischemia-reperfusion injury in rats. Sci Rep.
8(2518)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bartosh TJ, Ylöstalo JH, Mohammadipoor A,
Bazhanov N, Coble K, Claypool K, Lee RH, Choi H and Prockop DJ:
Aggregation of human mesenchymal stromal cells (MSCs) into 3D
spheroids enhances their antiinflammatory properties. Proc Natl
Acad Sci USA. 107:13724–13729. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yeh HY, Liu BH, Sieber M and Hsu SH:
Substrate-dependent gene regulation of self-assembled human MSC
spheroids on chitosan membranes. BMC Genomics.
15(10)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang GS, Dai LG, Yen BL and Hsu SH:
Spheroid formation of mesenchymal stem cells on chitosan and
chitosan-hyaluronan membranes. Biomaterials. 32:6929–6945.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen S, Guo J, Luo Y, Zhang W, Cui Y, Wang
Q, Zhang Z and Wang T: Functional proteomics revealed IL-1β
amplifies TNF downstream protein signals in human synoviocytes in a
TNF-independent manner. Biochem Biophys Res Commun. 450:538–544.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ismail HM, Yamamoto K, Vincent TL, Nagase
h, Troeberg L and Saklatvala J: Interleukin-1 Acts via the JNK-2
Signaling Pathway to Induce Aggrecan Degradation by Human
Chondrocytes. Arthritis Rheumatol. 67:1826–1836. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Osta B, Roux JP, Lavocat F, Pierre M,
Ndongo-Thiam N, Boivin G and Miossec P: Differential Effects of
IL-17A and TNF-α on Osteoblastic Differentiation of Isolated
Synoviocytes and on Bone Explants from Arthritis Patients. Front
Immunol. 6(151)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lawrence JT, Birmingham J and Toth AP:
Emerging ideas: Prevention of posttraumatic arthritis through
interleukin-1 and tumor necrosis factor-alpha inhibition. Clin
Orthop Relat Res. 469:3522–3526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Furman BD, Mangiapani DS, Zeitler E,
Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F and Olson SA:
Targeting pro-inflammatory cytokines following joint injury: Acute
intra-articular inhibition of interleukin-1 following knee injury
prevents post-traumatic arthritis. Arthritis Res Ther.
16(R134)2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Mobasheri A, Henrotin Y, Biesalski HK and
Shakibaei M: Scientific evidence and rationale for the development
of curcumin and resveratrol as nutraceutricals for joint health.
Int J Mol Sci. 13:4202–4232. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang T and He C: Pro-inflammatory
cytokines: The link between obesity and osteoarthritis. Cytokine
Growth Factor Rev. 44:38–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Assirelli E, Pulsatelli L, Dolzani P,
Platano D, Olivotto E, Filardo G, Trisolino G, Facchini A, Borzì RM
and Meliconi R: Human osteoarthritic cartilage shows reduced in
vivo expression of IL-4, a chondroprotective cytokine that
differentially modulates IL-1β-stimulated production of chemokines
and matrix-degrading enzymes in vitro. PLoS One.
9(e96925)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Uchimura T, Foote AT, Smith EL, Matzkin EG
and Zeng L: Insulin-Like Growth Factor II (IGF-II) Inhibits
IL-1β-Induced Cartilage Matrix Loss and Promotes Cartilage
Integrity in Experimental Osteoarthritis. J Cell Biochem.
116:2858–2869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fytili P, Giannatou E, Karachalios T,
Malizos K and Tsezou A: Interleukin-10G and interleukin-10R
microsatellite polymorphisms and osteoarthritis of the knee. Clin
Exp Rheumatol. 23:621–627. 2005.PubMed/NCBI
|
|
26
|
Coricor G and Serra R: TGF-β regulates
phosphorylation and stabilization of Sox9 protein in chondrocytes
through p38 and Smad dependent mechanisms. Sci Rep.
6(38616)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang W, Mabrouk ME, Sylvester J, Dehnade
F and Zafarullah M: Enhanced expression of tissue inhibitor of
metalloproteinases-4 gene in human osteoarthritic synovial
membranes and its differential regulation by cytokines in
chondrocytes. Open Rheumatol J. 5:81–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Dong C, Li N, Ma Q, Yun Z, Cai C,
An M and Ma B: Modulation of TGF β activity by latent TGF β binding
protein 1 in human osteoarthritis fibroblast like synoviocytes. Mol
Med Rep. 17:1893–1900. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mueller MB, Fischer M, Zellner J, Berner
A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS and Angele
P: Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of
TGF-beta isoforms and chondrogenic conditioning. Cells Tissues
Organs. 192:158–166. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Spees JL, Lee RH and Gregory CA:
Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res
Ther. 7(125)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang S, Tong M, Hu S and Chen X: The
Bioactive Substance Secreted by MSC Retards Mouse Aortic Vascular
Smooth Muscle Cells Calcification. BioMed Res Int.
2018(6053567)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ichiseki T, Shimasaki M, Ueda Y, Ueda S,
Tsuchiya M, Souma D, Kaneuji A and Kawahara N:
Intraarticularly-Injected Mesenchymal Stem Cells Stimulate
Anti-Inflammatory Molecules and Inhibit Pain Related Protein and
Chondrolytic Enzymes in a Monoiodoacetate-Induced Rat Arthritis
Model. Int J Mol Sci. 19(203)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Emadedin M, Ghorbani Liastani M, Fazeli R,
Mohseni F, Moghadasali R, Mardpour S, Hosseini SE, Niknejadi M,
Moeininia F, Aghahossein Fanni A, et al: Long-Term Follow-up of
Intra-articular Injection of Autologous Mesenchymal Stem Cells in
Patients with Knee, Ankle, or Hip Osteoarthritis. Arch Iran Med.
18:336–344. 2015.PubMed/NCBI
|
|
34
|
Liu J, Chen B, Yan F and Yang W: The
Influence of Inflammatory Cytokines on the Proliferation and
Osteoblastic Differentiation of MSCs. Curr Stem Cell Res Ther.
12:401–408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huang BW and Gao JQ: Application of 3D
cultured multicellular spheroid tumor models in tumor-targeted drug
delivery system research. J Control Release. 270:246–259.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xu Y, Shi T, Xu A and Zhang L: 3D spheroid
culture enhances survival and therapeutic capacities of MSCs
injected into ischemic kidney. J Cell Mol Med. 20:1203–1213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ahsan SM, Thomas M, Reddy KK, Sooraparaju
SG, Asthana A and Bhatnagar I: Chitosan as biomaterial in drug
delivery and tissue engineering. Int J Biol Macromol. 110:97–109.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cheng NC, Wang S and Young TH: The
influence of spheroid formation of human adipose-derived stem cells
on chitosan films on stemness and differentiation capabilities.
Biomaterials. 33:1748–1758. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hsu SH, Huang GS and Feng F: Isolation of
the multipotent MSC subpopulation from human gingival fibroblasts
by culturing on chitosan membranes. Biomaterials. 33:2642–2655.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yeh HY, Liu BH and Hsu SH: The
calcium-dependent regulation of spheroid formation and
cardiomyogenic differentiation for MSCs on chitosan membranes.
Biomaterials. 33:8943–8954. 2012.PubMed/NCBI View Article : Google Scholar
|