Introduction
Lower back pain is a ubiquitous disorder that is
associated with limitations to daily activities that significantly
reduce the quality of life. Its lifetime prevalence is >80%
worldwide (1-4).
Symptomatic degenerative defects to the lumbar spine are the main
causes of lower back pain (5,6), which
costs over $100 billion each year in the US (7). Since the process of degeneration itself
does not always result in pain, factors and mechanisms that are
available to distinguish symptomatic from asymptomatic degeneration
remain elusive (8). Furthermore,
lower back pain is not caused by a known etiology or mechanism. The
origin of tissue pain may emanate from ≥1 sources, including the
intervertebral disc, facet joints, longitudinal ligaments,
musculature and fascia (9,10).
Animal models have provided valuable insights into
the mechanism of symptomatic degeneration of the spine. A number of
disc degeneration animal models have been established previously,
which are important for mechanistic studies and the development of
treatment strategies (11). However,
the number of animal models of lumbar facet joint degeneration that
have been established remain insufficient. As the only true
synovial joints of the spine, facet joints are highly innervated
and as such are important load-bearing structures in the posterior
aspect of the vertebral column (12). Stress overload may result in
osteoarthritis of the facet joint, which has been determined to be
a possible source of lower back pain (13). Despite its prevalence, few studies
have utilized animal models to study lumbar facet joint
osteoarthritis. Difficulties in creating animal models stem from
the fact that both chemical and mechanical interventions are more
difficult to perform in the facet joint compared with the same
procedures in the disc. Among these models, the majority belong to
the class of chemically induced model of facet joint
osteoarthritis, which induces a rapid and severe autoimmune
reaction by injecting chemical agents into the lumbar facet joints
(14).
In this present study, a novel rat model of
mechanically-induced lumbar facet joint injury was developed. In
contrast to previously described chemically-induced animal models,
the current model was created by physically loading increased
stress, which was closer to the etiology of human facet joint
syndrome (12). This rat model may
provide an ideal experimental model for further investigations into
the etiology, pathogenesis and development of therapies for lumbar
facet joint osteoarthritis.
Materials and methods
Ethics
All experimental procedures were reviewed and
approved by the Sixth Medical Center of the Chinese PLA General
Hospital Animal Ethics Board (Beijing, China).
Design and manufacture of the
compression spring
A modified intraspinal compression spring was
designed according to the anatomy measurements of the male Sprague
Dawley (SD) rat lumbar spine (Fig.
1). The modified intraspinal compression spring can provide
persistent compressive stress to the lumbar facet joints. The
spring was manufactured by the Yongxing Kangli Hardware Factory
(Wenzhou, China) with the following dimensions: i) External
diameter, 2 mm; ii) length, 2 mm; iii) wire diameter, 0.4 mm; and
iv) 2 mm accessory length. The string was made of 316L stainless
steel, which can offer 50 g in force when stretched to 4 mm. The
force of the spring was set according to the biomechanics of the
facet joint in humans. Generally, the bilateral facet joints can
bear ~20% of the body weight (15).
Therefore, for a rat weighing ~250 g, the application of 50 g in
pressure can simulate the facet stress that is typically observed
in humans (15).
Animal model induction
A total of 40 healthy male SD rats (aged 6-8 weeks;
weight, 255-320 g) were provided by the Experimental Animal Center
of the Sixth Medical Center of Chinese PLA General Hospital [Animal
production license number: SCXK (Beijing) 2015-0012]. Animals were
kept in cages with the environmental temperature, maintained at a
temperature of 22-26˚C and a relative humidity at 40-80% under a 12
h light-dark cycle, and allowed free access to food and water.
Routine cleaning and disinfection were performed daily. For each
rat, one of the L4/5 and L5/6 spinal fragments was selected
randomly using a random number Table marked as Compression level
for persistent compressive injury. The other level of the same
animal was indicated as Control level treated with sham operation.
All rats were anesthetized by an intraperitoneal injection of
ketamine (5 mg/100 g), xylazine (0.5 mg/100 g) and acepromazine
(0.1 mg/100 g). Following skin preparation and disinfection, a
posterior midline incision was performed, where the multifidus
muscle at the L4-L6 spinous process was resected to expose the
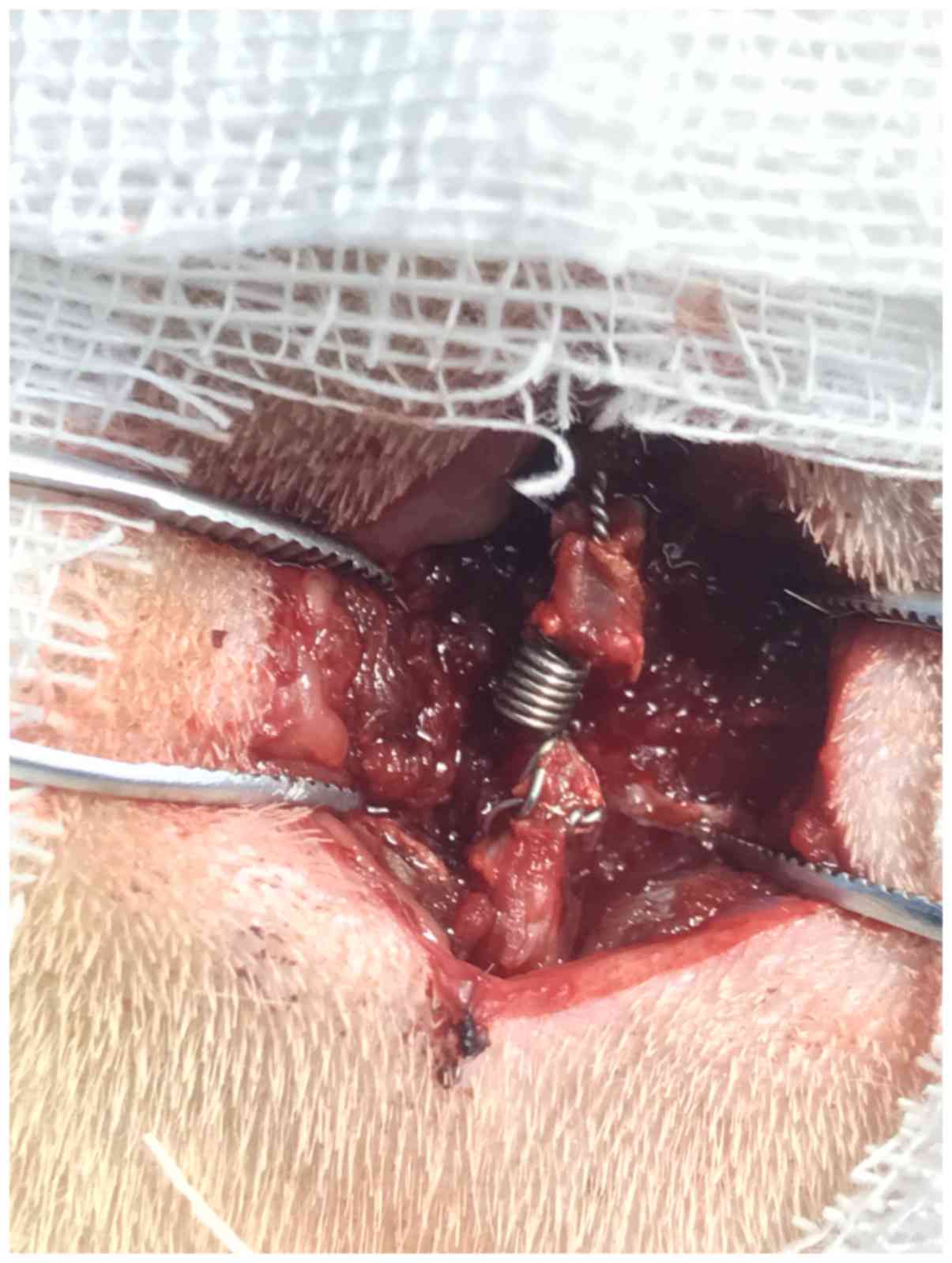
L4-L6 spinal processes. The modified intraspinal compression
springs were implanted into the defined compression level by
attachment to the spinous processes (Fig. 2). The control level in the same
animal underwent the same incision and exposure as that of
compression level without implantation of the compression spring.
Subsequently, the muscle was sutured and the skin was then closed
at both compression and control levels. The animals were treated
with subcutaneous injections of antibiotics (penicillin, 80,000
Units) once per day per rat for 3 days. Following surgery, the rats
were allowed to recover and were subsequently examined on days 7,
14, 28, 42 and 56 as described below.
Macroscopic observation
On days 7, 14, 28, 42 and 56 following surgery, 8
rats were randomly euthanized by an intraperitoneal overdose
injection of pentobarbital sodium (200 mg/kg). Anteroposterior (AP)
and lateral X-ray examinations of the rats were performed to verify
the spring location prior to lumbar vertebral segment collection.
The L4-L6 spinal sample was harvested en bloc after being
sacrificed via cervical dislocation. The pathological defects in
the L4/5 and L5/6 lumbar facet joints were evaluated by macroscopic
observation. A macroscopic tissue observation scoring system
proposed by Pelletier et al (16) was used, with a score of 0-4
corresponding to the following conditions: i) 0, intact articular
surface with normal calamine blue color; ii) 1, rough articular
surface with a gray color; iii) 2, defected superficial cartilage;
iv) 3, defected deep cartilage with ulceration; and v) 4,
exfoliated cartilage with exposed subchondral bone.
Histological examination
Following macroscopic observation, the L4-L6
segments were fixed in 4% formaldehyde for 48 h at room
temperature, followed by decalcification in EDTA solution for 4-8
weeks. The decalcified facet joints were paraffin-embedded and
subsequently sliced into 4-6 µm thick sections in the transverse
plane. Serial facet joint sections of the experimental and control
levels were deparaffinized using xylene followed by a descending
series of ethanol at room temperature. The sections were stained
with toluidine blue at 20˚C for 10 min. The results were detected
using a light microscope (Olympus Corporation) at a magnification
of x100, and three fields of view were scored for articular
cartilage degeneration according to the Osteoarthritis Research
Society International (OARSI) score (ranging from 0 to 24 points)
(17).
Immunohistochemical staining
The aforementioned deparaffinized facet joint
sections were incubated with rabbit-anti rat antigen monoclonal
antibodies targeting interleukin (IL)-1β (1:100; cat. no. ab9722;
Abcam) and tumor necrosis factor (TNF)-α (1:100; cat. no. ab6671;
Abcam) at 4˚C overnight. Subsequently, the sections were further
incubated with a biotin-labeled goat-anti rabbit secondary antibody
(1:200; cat. no. 074-1506; KPL, Inc.) for 60 min at room
temperature. The staining was made visible using
3,3'-diaminobenzidine, where cells exhibiting positive staining are
brown in color. Staining intensity and the percentage of positive
cells were evaluated from three random fields for each section
under a light microscope (Olympus Corporation; magnification,
x100). A computer interface (Image-Pro Plus; version 6.0; Media
Cybernetics Inc.) was used for image analysis. The relative
expression of the targeted molecules was calculated by multiplying
the staining intensity score with the positive cell score. Staining
intensity was scored from 0 to 3, representing no staining, light,
moderate and strong staining, respectively. Positive cells (the
ratio of brown cells to total cells) were scored from 1 to 4 as
follows: i) <10%; ii) 11-50%; iii) 51-75%; and iv) >75%,
respectively.
Statistical analysis
Continuous variables are presented as the mean ± SD.
Comparisons made for non-parametric data, between the control and
treated groups at different time points were performed using
Wilcoxon signed rank sum tests with Bonferroni's adjustments and
parametric data comparisons were performed using the paired
Student's t-test. All statistical analyses were performed using the
SPSS 17.0 software (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Macroscopic observation
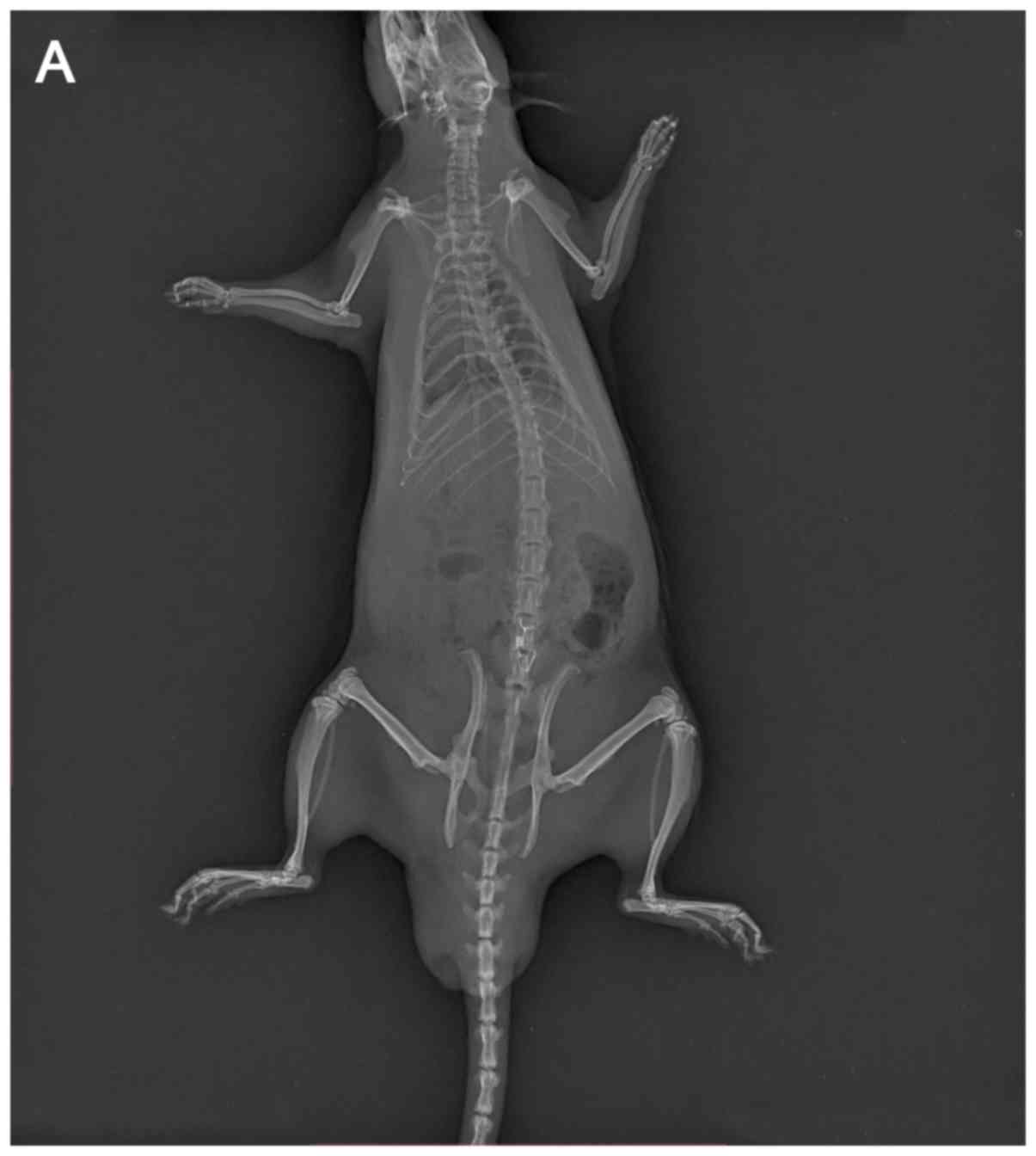
AP and lateral X-ray examinations were performed on
all rats at the indicated days prior to sacrifice. All springs were
found to be in the correct position without any dislocation
(Fig. 3). The facet joint articular
cartilage in the control group exhibited a bright calamine blue
color without cracks or defects at 7, 14, 28, 42 and 56 days
following surgery. Compared with the control group, time-dependent
pathological changes were observed in the experimental group. In
addition, joint synovial membranes were slightly swollen at 7 and
14 days following surgery. Marked joint swelling and a gray, dim
articular cartilage surface was observed at 28 days, whereas at 42
and 56 days following surgery, a rough cartilage surface and minor
cracks were observed, respectively (data not shown).
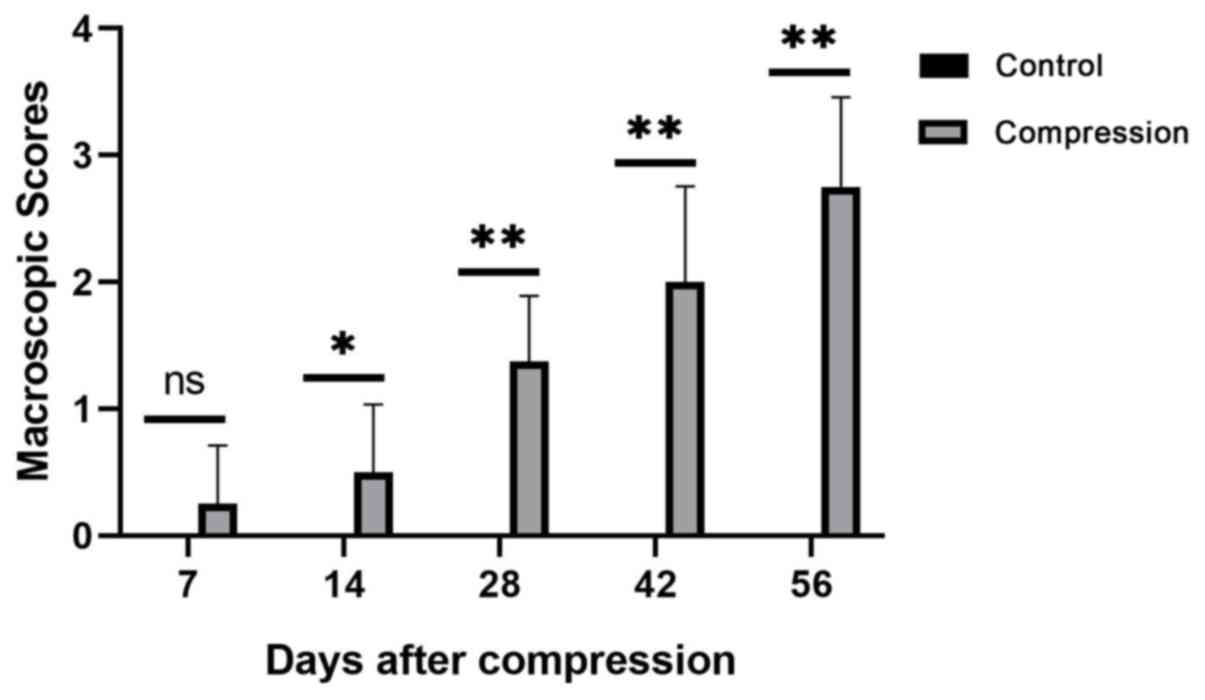
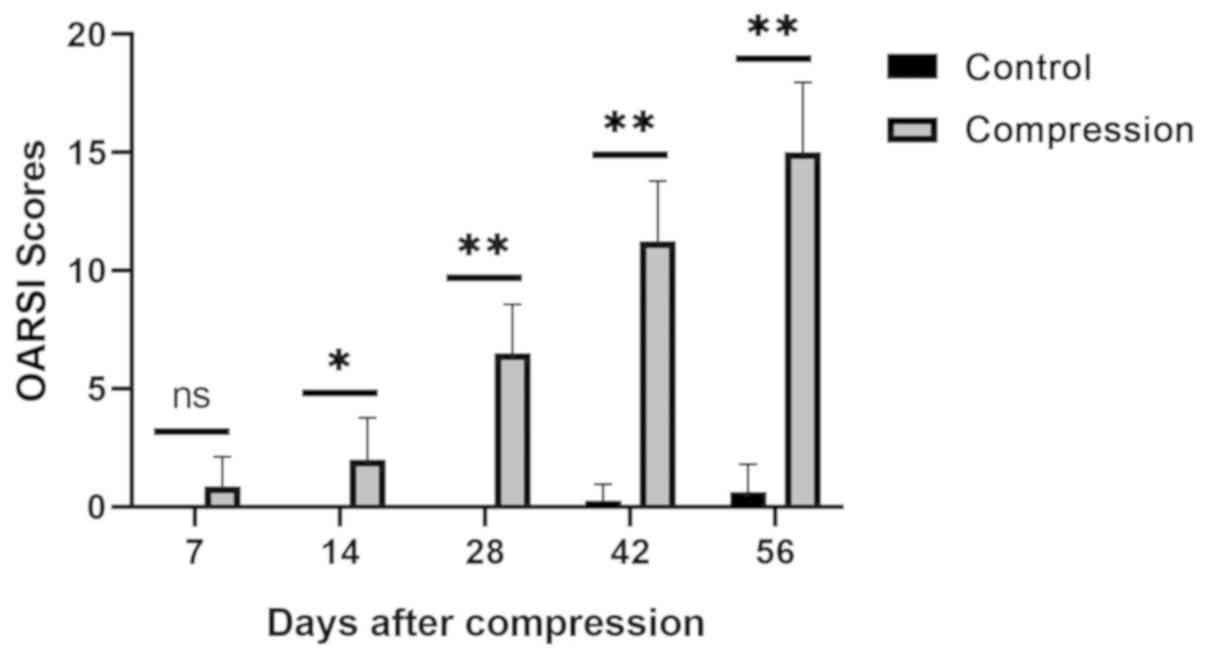
The macroscopic scores of the facet joint cartilage
at each measured day following surgery are presented in Fig. 4. Comparisons between the control and
the compression groups were performed at the indicated days.
Statistically significant differences were observed on days 14, 28,
42 and 56 following surgery (P<0.05 for day 14 and P<0.01 for
all other cases). However, significant differences were not
observed at 7 days following surgery (P>0.05). The results
suggest that after 7 days, the macroscopic scores of the facet
joint cartilage in the compression levels were significantly higher
compared with those in the control levels throughout the testing
period (P<0.05).
Articular cartilage histological
observation
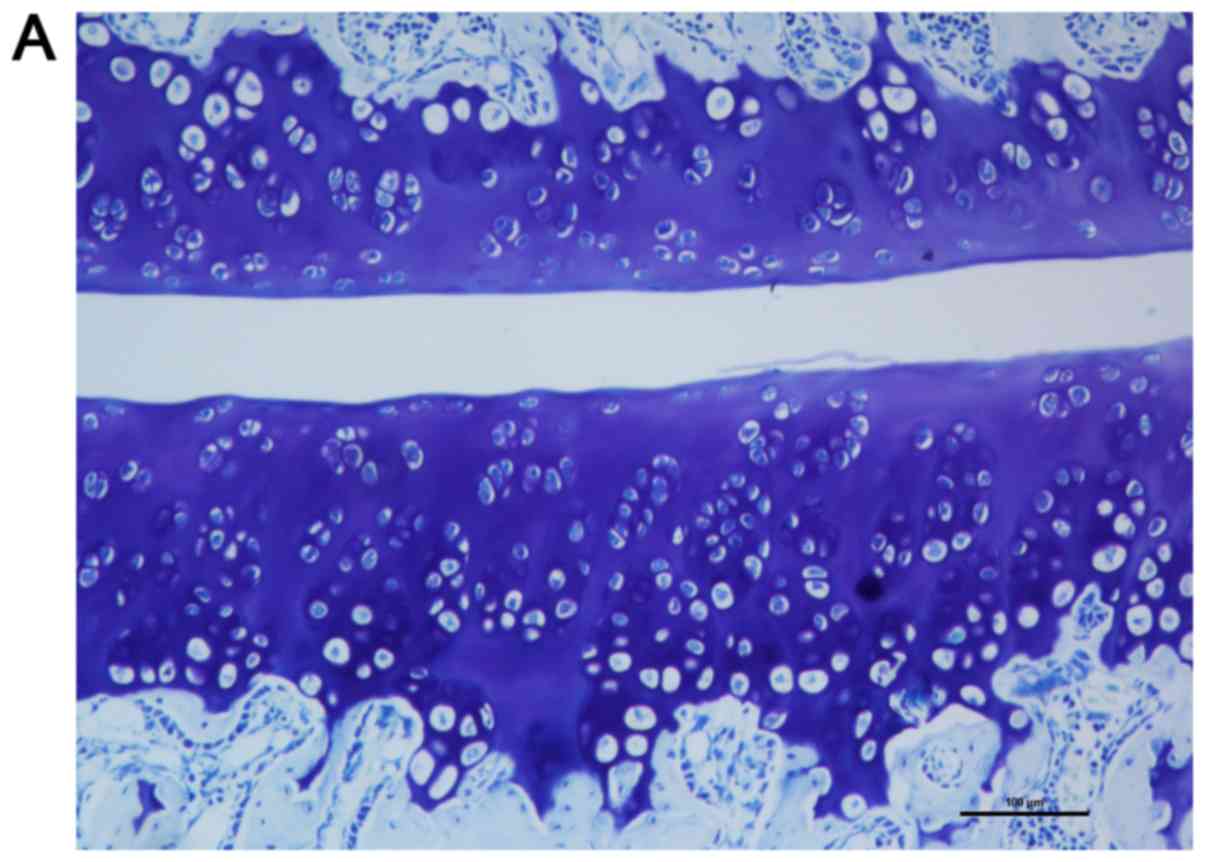
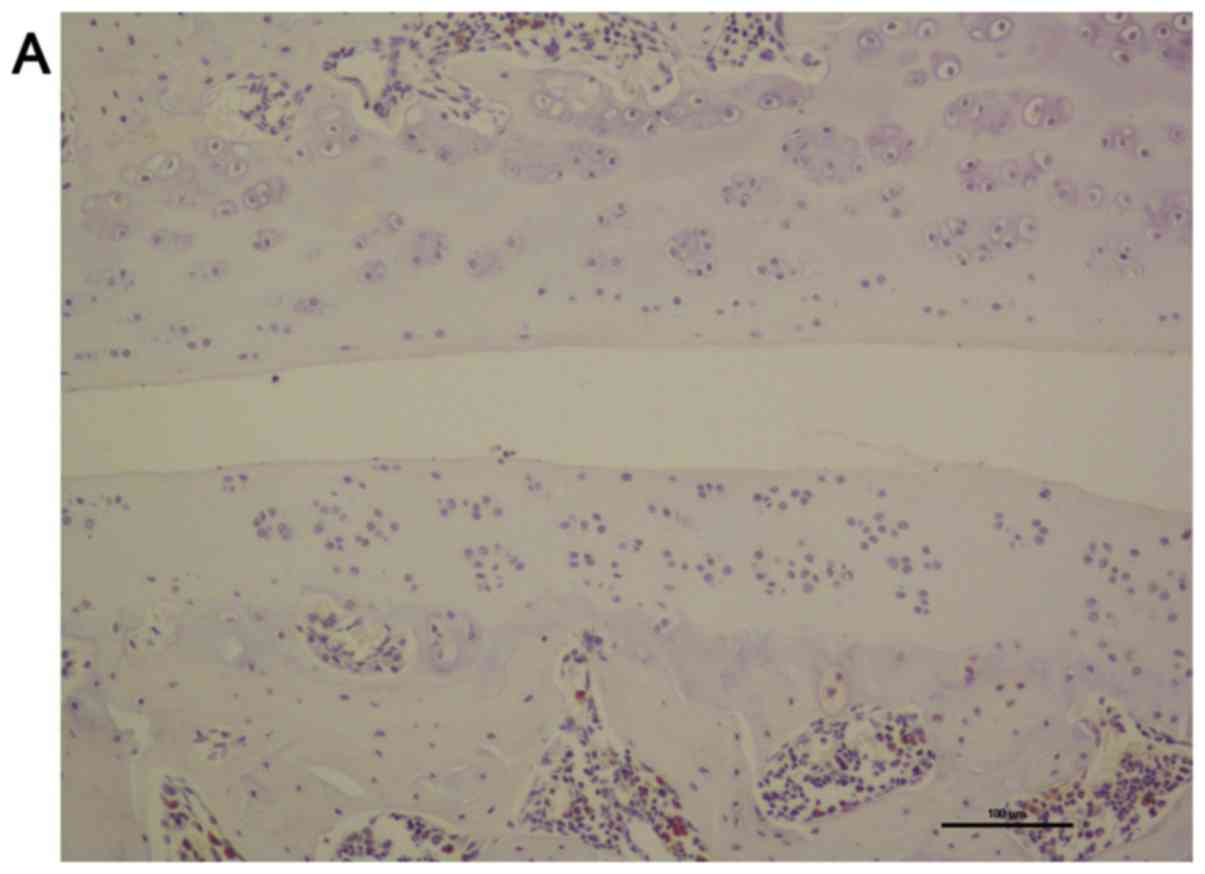
Toluidine blue staining of the articular cartilage
indicated smooth and normal surfaces without cracks, erosions or
ulcerations in the control level. A smooth cartilage surface with
normal cartilage thickness and regular chondrocyte arrangement were
observed in control level at all time points selected (7 and 56
days following surgery) as shown in Fig.
5A and B. There was almost no
difference between the two time points either in quantity or in the
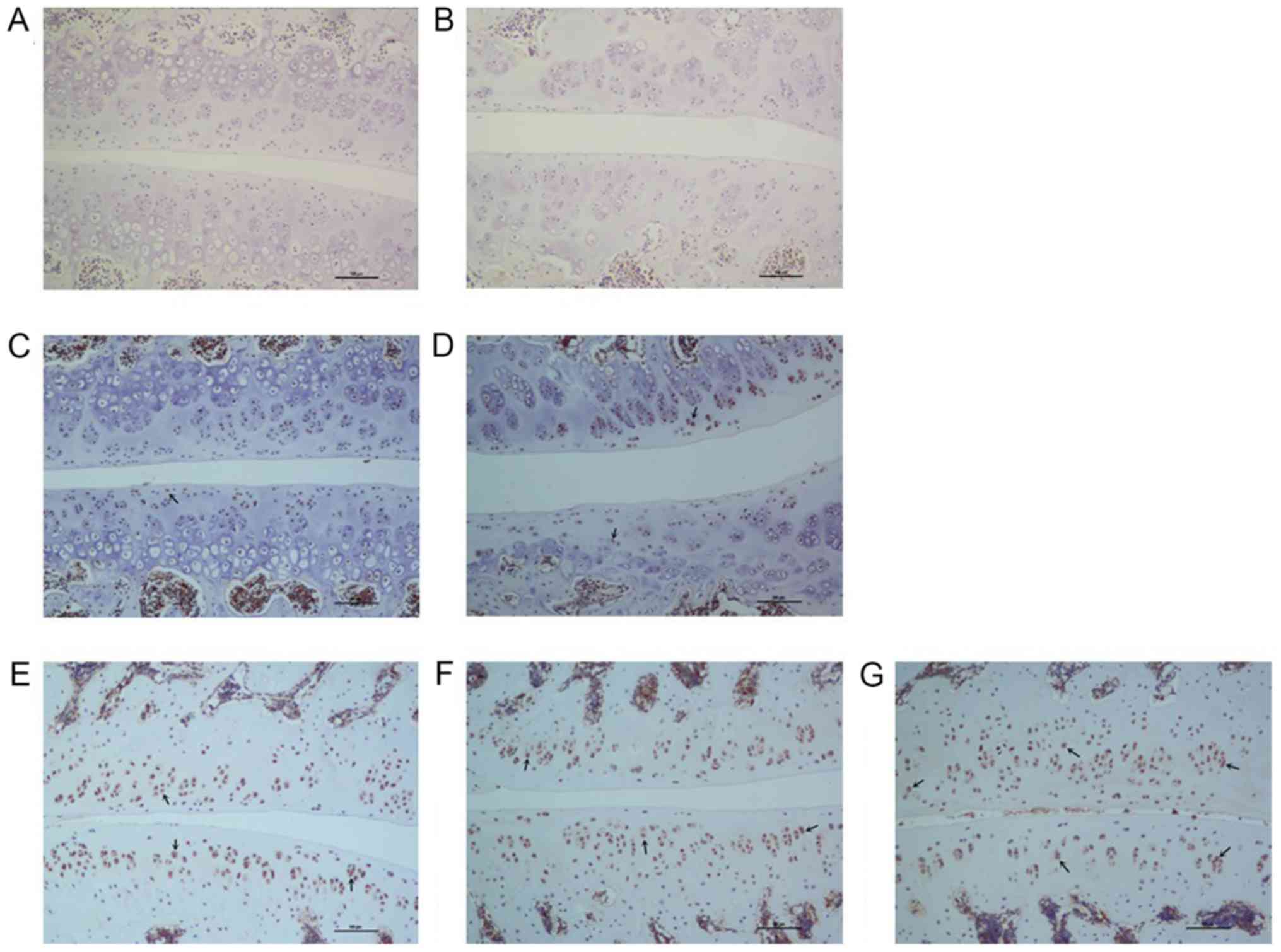
size of chondrocytes. Compared with the control level, the
morphology of articular cartilage of the compression level was
altered over time. At 7 days following surgery, the facet joint
space narrowed (Fig. 5C), whereas at
14 days the cartilage surface became rough, with lighter staining
on the surface (Fig. 5D). At 28
days, the number of chondrocytes in the surface layer were reduced,
with a rougher cartilage surface and reduced thickness (Fig. 5E). At 42 days, the number of
chondrocytes was further reduced and a further reduction in the
thickness of the cartilage surface was observed (Fig. 5F). At 56 days following surgery,
distinct roughness was identified on the articular surface as well
as a decrease in thickness (Fig.
5G).
Articular cartilage degeneration in the control and
compression groups was evaluated according to the OARSI scoring
system. The OARSI scoring results are presented in Fig. 6. Comparison between the control and
compression groups was performed at the indicated days. Significant
differences were observed at all the observed time points
(P<0.05 on day 14 and P<0.01 for all other cases) apart from
day 7 (P>0.05). These results demonstrated that facet joint
articular cartilage degeneration in the compression level was
significantly worse compared with that in the control level at all
the observed time points, except for the first measured
postoperative time point on day 7.
Immunohistochemical staining
The immunohistochemical staining observations of
IL-1β and TNF-α levels in the control group were almost the same in
the indicated time points. Mild IL-1β and TNF-α staining was
observed in the cartilage matrix (Figs.
7A and B as well as 8A and B). In
the cartilage of the facet joint of the compression group, the
percentage of IL-1β and TNF-α-positive chondrocytes gradually
increased from 7 to 56 days following surgery (Figs. 7C-G and 8C-G). At 56 day after surgery, a high
number of IL-1β and TNF-α-positive chondrocytes was observed
throughout the cartilage. Semi-quantitative analysis of
immunohistochemical staining showed that expression of both IL-1β
and TNF-α in the cartilage were significantly increased in the
compression group in comparison with the control group at every
time point (Tables I and II; P<0.01).
 | Table IImmunohistochemical scores of IL-1β
expression in cartilage in the two levels. |
Table I
Immunohistochemical scores of IL-1β
expression in cartilage in the two levels.
| Group | 7 days | 14 days | 28 days | 42 days | 56 days |
|---|
| Control level | 0.37±0.34 | 0.54±0.47 | 0.51±0.55 | 0.69±0.72 | 0.63±0.19 |
| Compression
level |
1.76±1.03a |
3.32±1.29a |
5.14±2.37a |
8.14±2.25a |
8.23±1.36a |
| T-value | 5.43 | 7.78 | 10.34 | 13.49 | 15.31 |
 | Table IIImmunohistochemical scores of TNF-α
expression in cartilage in the two levels. |
Table II
Immunohistochemical scores of TNF-α
expression in cartilage in the two levels.
| Group | 7 days | 14 days | 28 days | 42 days | 56 days |
|---|
| Control level | 0.45±0.38 | 0.59±0.83 | 0.66±0.35 | 0.50±0.71 | 0.63±1.09 |
| Compression
level |
1.39±0.76a |
4.03±1.99a |
4.51±3.13a |
7.36±3.14a |
8.98±2.21a |
| T-value | 3.98 | 6.95 | 7.66 | 9.93 | 14.96 |
Discussion
Lumbar facet joint osteoarthritis is a common
pathological condition during lumbar degeneration that is
characterized by the presence of synovial inflammation, a narrow
facet joint space and degeneration of the cartilage and the
subchondral bone (14). This
progressive degenerative change is symptomatic and may result in
lower back pain (18). However, the
mechanism of pathogenesis underlying lumbar facet joint
osteoarthritis remains unclear (19). Animal models can provide valuable
insights into the mechanisms of action behind the symptomatic
degeneration of the lower spine. To investigate the pathological
mechanisms behind lumbar facet joint osteoarthritis further, in
addition to discovering potentially novel prevention and treatment
measures for this disease, the construction of an effective animal
model is vital (20).
Until recently, two types of animal models have been
established to simulate lumbar facet joint osteoarthritis, namely
by chemical or mechanical induction (13). To the best of our knowledge, the
majority of previous studies have focused on the chemically-induced
animal models. In the chemically-induced animal models, different
types of chemical agents are injected into the facet joints to
induce autoimmune reactions, including collagenase, monosodium
iodoacetate and complete Freund's adjuvant (21-26).
The rapid onset of the autoimmune-induced inflammatory reaction is
mediated by an intra-articular injection of exogenous chemical
stimulators like the above in the facet joint. Chemically-induced
animal models exhibit a rapid onset of facet joint osteoarthritis
that is followed by the development of numerous cracks and defects
in the cartilage (22,26). In contrast, the corresponding
pathological process noted in humans is reasonably slow with few
cracks or defects. The aforementioned difference in the results is
attributed to the different pathogenic mechanisms of action
underlying facet joint osteoarthritis between humans and animals.
Therefore, in contrast to human facet joint osteoarthritis, the
cause of which is associated with mechanical stress,
chemically-induced animal models of osteoarthritis may be caused
solely by chemical reactions.
Previous studies have demonstrated that compression
on synovial joints results in pathological changes in
osteoarthritis (27,28). The lumbar facet joint exhibits
similar pathological changes following compression injury. The
human lumbar facet joint is burdened with axial compression force
and shearing stress applied to the articular surface. Although
normal amounts of loading stress provide the chondrocytes within
facet joints with a natural nutritional supply for cell survival
under physiological conditions, excessive stress loading on the
facet joints can induce cartilage degeneration due to injury to the
chondrocytes and matrix structure (28).
The first animal model of mechanically-induced facet
joint pain was constructed by Henry et al (29) using a brief compression of the L5/6
facet joint. In this previously established novel rat model,
following surgical exposure of the lumbar facet joints, modified
clamps were applied for 3 min with an average 400 grams force to
compress the spinal segments by ~1 mm. The end result was injury to
the facet joint and increased pressure sensitivity from algometry
at the operated area and mechanical allodynia at the hind paw.
Unlike the chemically-induced models, this was a
mechanically-induced model of lumbar facet joint osteoarthritis
which simulated the pathology observed in humans during
osteoarthritis more accurately. The disadvantage of this model may
be the variation between the model's short-time compression and the
human's chronic low back pain pathogenesis. To overcome this
limitation, this present study used intraspinal compression springs
that were fixed between spinous processes to provide a persistent
force of compression. Since the compression springs were implanted
between the spinous processes, the compression force line direction
was parallel to the spinal axis and not to the facet joint. When
the springs were elongated, lumbar facet joints experience stress
from both axial compression force and shearing. The compression
stress was a dynamic process, suggesting that the magnitude of the
applied force varied according to the rat's lumbar position. These
observations suggest that the animal model established in the
present study presented higher accuracy and was more consistent
with the human pathology (30,31). In
addition, this present study demonstrated that persistent
mechanical compression injury of the facet joints in the rat model
induced intra-articular osteoarthritis. This observation was
substantiated by the considerable degradation observed in the facet
joint cartilage compared with those noted in the cartilage of the
control group. The pathological changes included synovitis,
narrowing of the joint space, thinning of the cartilage and an
increase in the expression of inflammatory factors. In addition,
these changes appeared to be aggravated over time, suggesting a
time-dependent association between the severity of osteoarthritis
and compression injury in the facet joints.
The increased expression of the inflammatory
cytokines, including TNF-α and notably IL-1β, in the facet joint
tissues of degenerative lumbar spinal disorders was first reported
by Igarashi et al (32). This
finding suggested that inflammatory cytokines in the degenerated
facet joints may be associated with the cause of pain in
degenerative lumbar disorders. In a number of previously
established chemically-induced lumbar facet joint osteoarthritis
animal models, increased expression levels of IL-1β and TNF-α were
observed (22,24-26).
Additionally, a strong correlation between the expression levels of
inflammatory cytokines and facet joint degeneration-induced low
back pain has also been previously demonstrated. These
aforementioned findings were confirmed by the association between
the expression levels of the inflammatory cytokines and the
pain-associated behavioral changes (29,33). In
the present study, a time-dependent increase in IL-1β and TNF-α
positive chondrocytes was also demonstrated as a result of the
overloading of the lumbar facet joints. In contrast to this
observation, late stage chemically-induced lumbar facet joint
osteoarthritis animal models was accompanied with the induction of
severe osteoarthritis, where the expression levels of the
inflammatory cytokines were reduced due to mass chondrocyte
apoptosis and cartilage matrix degradation (33). This observation was inconsistent with
those of the present study, where the upregulation of inflammatory
cytokine expression was detected. IL-1β and TNF-α serve key roles
in synovitis and cartilage degeneration (32,34,35).
Therefore, the mechanically-induced lumbar facet joint
osteoarthritis animal model presented in this present study appears
to be more representative to the corresponding human disease
compared with the chemically-induced models. Although chemical
induction induces a rapid onset of severe osteoarthritis (22,26), the
pathological processes noted in these cases were considerably
different compared with those noted in humans.
In conclusion, in the present study a novel rat
model of lumbar facet joint osteoarthritis was successfully
established by persistent mechanical compression injury. In
addition, a time-dependent pathological process of osteoarthritis
was confirmed as a result of facet joint overload, which stimulated
the natural course of facet joint degeneration in humans more
efficiently compared with other models such as chemically-induced
and briefly mechanically-induced models. This present study can be
used as a starting point for the investigation into the
understanding and development of novel treatments for lower back
pain associated with ailments in the lumbar facet joint.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and-or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and SH provided, conceived and designed the
current study. YL and SP performed the experiments and analyzed the
data. YL wrote, edited and reviewed the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were reviewed and
approved by the Sixth Medical Center of the Chinese PLA General
Hospital Animal Ethics Board (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balagué F, Mannion AF, Pellisé F and
Cedraschi C: Non-specific low back pain. Lancet. 379:482–491.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hartvigsen J, Hancock MJ, Kongsted A, Louw
Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J,
et al: Lancet Low Back Pain Series Working Group: What low back
pain is and why we need to pay attention. Lancet. 391:2356–2367.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Deyo RA and Weinstein JN: Low back pain. N
Engl J Med. 344:363–370. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Langevin HM and Sherman KJ:
Pathophysiological model for chronic low back pain integrating
connective tissue and nervous system mechanisms. Med Hypotheses.
68:74–80. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin BI, Deyo RA, Mirza SK, Turner JA,
Comstock BA, Hollingworth W and Sullivan SD: Expenditures and
health status among adults with back and neck problems. JAMA.
299:656–664. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nguyen C, Poiraudeau S and Rannou F: From
Modic 1 vertebral-endplate subchondral bone signal changes detected
by MRI to the concept of 'active discopathy'. Ann Rheum Dis.
74:1488–1494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Katz JN: Lumbar disc disorders and
low-back pain: Socioeconomic factors and consequences. J Bone Joint
Surg Am. 88 (Suppl 2):21–24. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jensen MC, Brant-Zawadzki MN, Obuchowski
N, Modic MT, Malkasian D and Ross JS: Magnetic resonance imaging of
the lumbar spine in people without back pain. N Engl J Med.
331:69–73. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine. 25:487–492.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Manchikanti L, Pampati V, Beyer C, Damron
K and Barnhill RC: Evaluation of psychological status in chronic
low back pa in: Comparison with general population. Pain Physician.
5:149–155. 2002.PubMed/NCBI
|
|
11
|
Lyu Y, Chen H and Zheng ZM: Research
progress of animal model of disc degeneration. Zhongguo Jizhu Jisui
Zazhi. 16:68–71. 2006.
|
|
12
|
Perolat R, Kastler A, Nicot B, Pellat JM,
Tahon F, Attye A, Heck O, Boubagra K, Grand S and Krainik A: Facet
joint syndrome: From diagnosis to interventional management.
Insights Imaging. 9:773–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gellhorn AC, Katz JN and Suri P:
Osteoarthritis of the spine: The facet joints. Nat Rev Rheumatol.
9:216–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu Y and Hou S: Progress on the research
of the low back pain animal model. Chin J Bone Jt. 7:146–149.
2018.
|
|
15
|
Sawa AG and Crawford NR: The use of
surface strain data and a neural networks solution method to
determine lumbar facet joint loads during in vitro spine testing. J
Biomech. 41:2647–2653. 2008.
|
|
16
|
Pelletier JP, Jovanovic D, Fernandes JC,
Manning P, Connor JR, Currie MG, Di Battista JA and
Martel-Pelletier J: Reduced progression of experimental
osteoarthritis in vivo by selective inhibition of inducible nitric
oxide synthase. Arthritis Rheum. 41:1275–1286. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Boswell MV, Singh V, Staats PS and Hirsch
JA: Accuracy of precision diagnostic blocks in the diagnosis of
chronic spinal pain of facet or zygapophysial joint origin. Pain
Physician. 6:449–456. 2003.PubMed/NCBI
|
|
19
|
Kalichman L, Li L, Kim DH, Guermazi A,
Berkin V, O'Donnell CJ, Hoffmann U, Cole R and Hunter DJ: Facet
joint osteoarthritis and low back pain in the community-based
population. Spine. 33:2560–2565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Winkelstein BA: How can animal models
inform on the transition to chronic symptoms in whiplash? Spine. 36
(Suppl):S218–S225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yeh TT, Wu SS, Lee CH, Wen ZH, Lee HS,
Yang Z, Nimni ME and Han B: The short-term therapeutic effect of
recombinant human bone morphogenetic protein-2 on
collagenase-induced lumbar facet joint osteoarthritis in rats.
Osteoarthritis Cartilage. 15:1357–1366. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yeh TT, Wen ZH, Lee HS, Lee CH, Yang Z,
Jean YH, Wu SS, Nimni ME and Han B: Intra-articular injection of
collagenase induced experimental osteoarthritis of the lumbar facet
joint in rats. Eur Spine J. 17:734–742. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Adães S, Mendonça M, Santos TN,
Castro-Lopes JM, Ferreira-Gomes J and Neto FL: Intra-articular
injection of collagenase in the knee of rats as an alternative
model to study nociception associated with osteoarthritis.
Arthritis Res Ther. 16(R10)2014.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Kim JS, Kroin JS, Buvanendran A, Li X, van
Wijnen AJ, Tuman KJ and Im HJ: Characterization of a new animal
model for evaluation and treatment of back pain due to lumbar facet
joint osteoarthritis. Arthritis Rheum. 63:2966–2973.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gong K, Shao W, Chen H, Wang Z and Luo ZJ:
Rat model of lumbar facet joint osteoarthritis associated with
facet-mediated mechanical hyperalgesia induced by intra-articular
injection of monosodium iodoacetate. J Formos Med Assoc.
110:145–152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shuang F, Zhu J, Song K, Hou S, Liu Y,
Zhang C and Tang J: Establishment of a rat model of
adjuvant-induced osteoarthritis of the lumbar facet joint. Cell
Biochem Biophys. 70:1545–1551. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kohatsu ND and Schurman DJ: Risk factors
for the development of osteoarthrosis of the knee. Clin Orthop
Relat Res. 261:242–246. 1990.PubMed/NCBI
|
|
28
|
Inoue N, Orías AAE and Segami K:
Biomechanics of the lumbar facet joint. Spine Surg Relat Res.
4:1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Henry JL, Yashpal K, Vernon H, Kim J and
Im HJ: Lumbar facet joint compressive injury induces lasting
changes in local structure, nociceptive scores, and inflammatory
mediators in a novel rat model. Pain Res Treat.
2012(127636)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Varlotta GP, Lefkowitz TR, Schweitzer M,
Errico TJ, Spivak J, Bendo JA and Rybak L: The lumbar facet joint:
A review of current knowledge: Part 1: Anatomy, biomechanics, and
grading. Skeletal Radiol. 40:13–23. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rajeev A, Choudhry N, Shaikh M and Newby
M: Lumbar facet joint septic arthritis presenting atypically as
acute abdomen - A case report and review of the literature. Int J
Surg Case Rep. 25:243–245. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Igarashi A, Kikuchi S, Konno S and
Olmarker K: Inflammatory cytokines released from the facet joint
tissue in degenerative lumbar spinal disorders. Spine.
29:2091–2095. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shuang F, Hou SX, Zhu JL, Liu Y, Zhou Y,
Zhang CL and Tang JG: Establishment of a rat model of lumbar facet
joint osteoarthritis using intraarticular injection of urinary
plasminogen activator. Sci Rep. 5(9828)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Apkarian AV, Lavarello S, Randolf A, Berra
HH, Chialvo DR, Besedovsky HO and del Rey A: Expression of IL-1beta
in supraspinal brain regions in rats with neuropathic pain.
Neurosci Lett. 407:176–181. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tachihara H, Kikuchi S, Konno S and
Sekiguchi M: Does facet joint inflammation induce radiculopathy?:
An investigation using a rat model of lumbar facet joint
inflammation. Spine. 32:406–412. 2007.PubMed/NCBI View Article : Google Scholar
|