Introduction
Non-gonococcal urethritis (NGU) is one of the most
common sexually transmitted infections caused by Chlamydia
trachomatis, Mycoplasma genitalium, and Neisseria
gonorrhoeae (1).
Mycoplasma, the pathogen of NGU, can adhere to the surface
of the genitourinary tract, resulting in an epithelial infection
(2). In recent years, with changes
in life-style and sexual behavior of people, the incidence of NGU
is increasing, and the primary clinical manifestation is
urethritis, generating a serious impact on the quality of life and
health of people (3).
At present, patients with NGU are treated with
azithromycin (a broad-spectrum drug) monotherapy (4). Azithromycin, a macrolide, is useful to
treat infections caused by Gram-positive aerobic bacteria such as
Staphylococcus aureus (5).
However, due to its widespread clinical application in recent
years, several pathogens have developed resistance against
azithromycin. Additionally, azithromycin has many adverse reactions
(6). Therefore, although it has an
effect on NGU, there are still some limitations.
Doxycycline is a long-acting tetracycline-based
broad-spectrum antibiotic and has a high fat solubility (7,8).
Doxycycline prevents protein synthesis by binding to nucleosome 30S
subunit of pathogenic bacteria, and inhibits the replication of
bacterial protein DNA, thus killing the pathogen (7,8).
Previous research has revealed that doxycycline has better
tolerance, a longer half-life period and a stronger antibacterial
activity compared to tetracycline (9). However, due to the wide application of
antibiotics in recent years, common pathogenic bacteria achieve
certain resistance against antibiotics, leading to unsatisfactory
antibiotic monotherapy treatment (10). However, a previous study revealed
that Ureaplasma urealyticum and Mycoplasma hominis
were highly sensitive to doxycycline, and were not resistant
against doxycycline (11). Previous
studies have also revealed that multiple antibiotics are more
effective than monotherapy in treating NGU (12). Therefore, in the present study, the
efficacy of azithromycin and doxycycline combination therapy was
evaluated for the treatment of NGU.
Interleukin-6 (IL-6) is released by a monocyte in
response to a bacterial or viral infection (13). As one of the primary inflammatory
factors, IL-6 can reflect the degree of inflammatory response of
the immune system against the invading pathogen (13). A previous study revealed that
urethral mucosa in patients with non-gonococcal Mycoplasma
urethritis promoted IL-6 secretion which in turn increased
inflammation (14). It is suggested
that IL-6 plays a pivotal role in the inflammatory response towards
NGU.
At present, there are relatively few studies on the
application of azithromycin and doxycycline combination therapy in
NGU and their effects on IL-6. The present study aimed to explore
the efficacy of this combination therapy in NGU and the effects of
doxycycline on the inflammatory response by assessing the
expression of IL-6.
Patients and methods
General data
The present study was conducted from January 2016 to
January 2017, and 98 patients with NGU were prospectively selected,
including 47 males and 51 females, with an average age of 35.2±2.4
years, and the sample size was estimated as previously described
(15). The patients were assigned to
an azithromycin group (46 patients, treated with azithromycin
alone) or a combination group (52 patients, treated with
azithromycin and doxycycline).
Inclusion and exclusion criteria
The inclusion criterion was as follows: Patients
diagnosed with NGU by PCR detection of Mycoplasma and
chlamydia in urethral discharge during their first visit (16).
The exclusion criteria were as follows: i) Patients
with severe organ diseases, such as liver, kidney as well as other
organ diseases; ii) patients who had taken antibiotics 2 weeks
before being enrolled in the study; iii) patients allergic to
azithromycin or doxycycline; iv) patients who had taken doxycycline
and azithromycin; v) patients who had taken drugs such as
cephalosporins with effects onthe efficacy of doxycycline or
azithromycin; vi) patients infected with Neisseria
gonorrhoeae or any other STIs; vii) patients withrepeated
unprotected sex during the study period; viii) patients who did not
receive any physical examination; ix) patients with communication
disorder or cognitive disorders.
All patients and their family members agreed to
participate in the study and signed informed consent documents. The
present study was approved by the Ethics Committee of Xinchang
People's Hospital.
Therapeutic methods
Azithromycin dispersible tablets were administrated
to patients in the azithromycin group once a day at a dose of 1 mg,
2 h after a meal and the dosage was doubled during the first use,
and then it was returned to the normal dosage. Patients in the
combination group were administered enteric-coated doxycycline
hydrochloride tablet at 100 mg once a day along with azithromycin
dispersible tablets. The dosage was doubled during the first use,
and then it was returned to the normal dosage, and the tablet was
taken after meals for a 7-day treatment course. In addition, the
patients were closely observed. The withdrawal time was recorded.
The indexes were evaluated after two courses of treatment. Patients
who failed to respond to the treatment were provided with another
therapy plan.
Observation indicators
Observation indicators were as follows: i) After two
weeks of treatment, the therapeutic efficacy of the two groups was
evaluated. The therapeutic efficacy was divided into recovery,
onset of action, improvement and inefficacy. Efficacy evaluation
criteria were as follows (17): a)
Recovery, disappearance of symptoms, and negative results in the
diagnostic test; b) onset of action, relief in signs and symptoms
and negative results in the diagnostic test; c) improvement,
remission in signs and symptoms and positive results in the
diagnostic test; inefficacy, no improvement in signs and symptoms
with lack of relief and even increased severity of infection, with
positive results in the diagnostic test. The total efficacy rate
was calculated as follows: Total efficacy rate=(Number of recovery
cases + number of onset of action cases)/total number of cases
x100%. ii) The symptom relief time and medication period (2 courses
of treatment each consisting of 7 days) of the two groups were
recorded and compared. iii) The recurrence in the two groups within
one year after treatment was terminated, if any, was recorded and
compared. Patients who failed to respond to the treatment were not
included in the evaluation. The recurrence criteria were symptom
recurrence and positive mycoplasma or chlamydia culture from
urethral/cervical secretion. iv) The enzyme-linked immunosorbent
assay (ELISA) was used to assess and compare serum IL-6 levels in
the two groups of patients before treatment and 1 week after drug
withdrawal. ELISA was performed according to the kit instructions
(Shanghai Enzyme-Linked Biotechnology Co., Ltd., China). v) Adverse
reactions such as headaches, abdominal pain, nausea and vomiting
and rash that occurred during the treatment were recorded and
compared between the two groups. vi) The Quality of life (QoL) core
questionnaire, the QLQ-C30 by EORTC (18), primarily designed for use in cancer
clinical trials, but that can be used in other research, was used
to evaluate the quality of life in patients of the two groups after
2 courses of treatment. This questionnaire is comprised of 5
function scales (role, emotional, somatic, cognitive and social),
with a total of 30 items. A higher score indicated better quality
of life and functioning. The results of the QoL questionnaires were
analyzed according to the specific scoring manual provided by the
EORTC (19), all scores were
expressed on a scale ranging between 0-100.
Statistical analysis
The measured data were expressed as the mean ±
standard deviation. The independent t-test was used to compare data
between the two groups. Paired t-test was used to compare data
before and after the treatment in both groups. Direct counts were
expressed as percentages and measured by the χ2 test.
Differences were considered significant when P<0.05. All
statistical analyses were carried out in SPSS v19.0 (AsiaAnalytics
formerly SPSS China).
Results
Comparison of general data
There were no significant differences in sex and age
as well as other factors between the two groups (all P>0.05;
Table I).
 | Table IGeneral data (n, %). |
Table I
General data (n, %).
| Factors | Azithromycin group
(n=46) | Combination group
(n=52) | t/χ2
tests | P-value |
|---|
| Sex | | | 0.001 | 0.980 |
|
Male | 22 (47.83) | 25 (48.08) | | |
|
Female | 24 (52.17) | 27 (51.92) | | |
| Age (years) | | | 0.002 | 0.960 |
|
≤35 | 21 (45.65) | 24 (46.15) | | |
|
>35 | 25 (54.35) | 28 (53.85) | | |
| BMI
(kg/m2) | | | 0.027 | 0.869 |
|
≤22 | 24 (52.17) | 28 (53.85) | | |
|
>22 | 22 (47.83) | 24 (46.15) | | |
| Marriage | | | 0.010 | 0.922 |
|
Yes | 35 (76.09) | 40 (76.92) | | |
|
No | 11 (23.91) | 12 (23.08) | | |
| Liver function | | | | |
|
Serum total
protein (g/l) | 72.31±2.13 | 72.34±2.11 | 0.070 | 0.944 |
|
Alanine
aminotransferase (µmol/l) | 28.42±4.57 | 27.93±4.52 | 0.533 | 0.595 |
|
Total
bilirubin (µmol/l) | 11.22±2.12 | 11.21±2.04 | 0.024 | 0.981 |
| Renal function | | | | |
|
Creatinine
(µmol/l) | 66.19±3.05 | 67.02±3.12 | 1.328 | 0.187 |
|
Serum urea
(mmol/l) | 5.05±0.41 | 5.06±0.38 | 0.125 | 0.901 |
|
Uric acid
(µmol/l) | 269.11±10.44 | 271.32±10.31 | 1.053 | 0.295 |
Comparison of clinical efficacy
between the two groups
The total effective rate in the azithromycin group
was lower than that in the combination group (52.17 vs. 84.62%,
P<0.001; Table II).
 | Table IIClinical efficacy in the two groups
(n, %). |
Table II
Clinical efficacy in the two groups
(n, %).
| Efficacy | Azithromycin group
(n=46) | Combination group
(n=52) | χ2
test | P-value |
|---|
| Healing
achieved | 14 (30.43) | 28 (39.02) | | |
| Markedly
effective | 10 (21.74) | 16 (31.71) | | |
| Improvement
achieved | 16 (34.78) | 6 (17.07) | | |
| Ineffective | 6 (2.22) | 2 (12.20) | | |
| Total effective
rate | 24 (52.17) | 44 (84.62) | 12.09 | <0.001 |
Comparison of symptom relief time and
medication time in two groups
The symptom relief time and medication period (2
courses of treatment each consisting of 7 days) in the azithromycin
group were 8.12±1.31 and 11.42±2.51 days, respectively. The same
indicators in the combination group were 5.79±1.14 and 8.85±2.11
days, respectively. The symptom relief time and medication period
in the combination group were also significantly lower than in the
azithromycin group (P<0.001; Table
III).
 | Table IIITime course of symptom relief and
medication in the two groups. |
Table III
Time course of symptom relief and
medication in the two groups.
| Parameter | Azithromycin group
(n=46) | Combination group
(n=52) | t-value | P-value |
|---|
| Time course of
symptom relief (days) | 8.12±1.31 | 5.79±1.14 | 9.415 | <0.001 |
| Medication period
(days) | 11.42±2.51 | 8.85±2.11 | 5.506 | <0.001 |
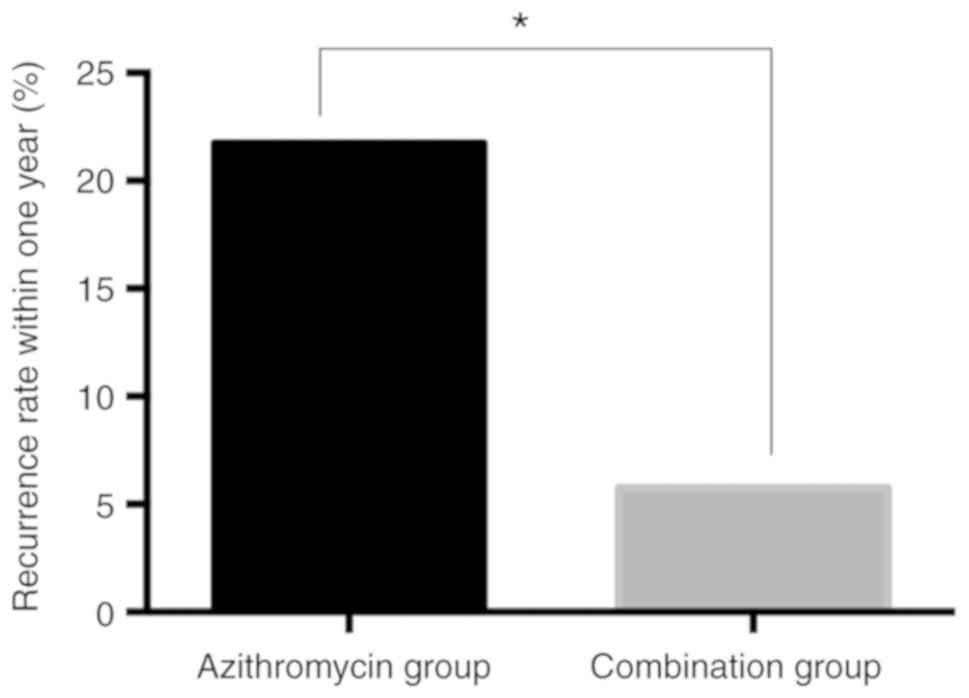
Recurrence after withdrawal in the two
groups
A total of 10 patients in the azithromycin group had
a recurrence within one year after the treatment was terminated,
with a recurrence rate of 21.74%. In the combination group, 3
patients had a recurrence within one year after withdrawal, with a
recurrence rate of 5.77%. The recurrence rate in azithromycin group
was significantly greater than the combination group
(χ2=5.410, P=0.020; Fig.
1).
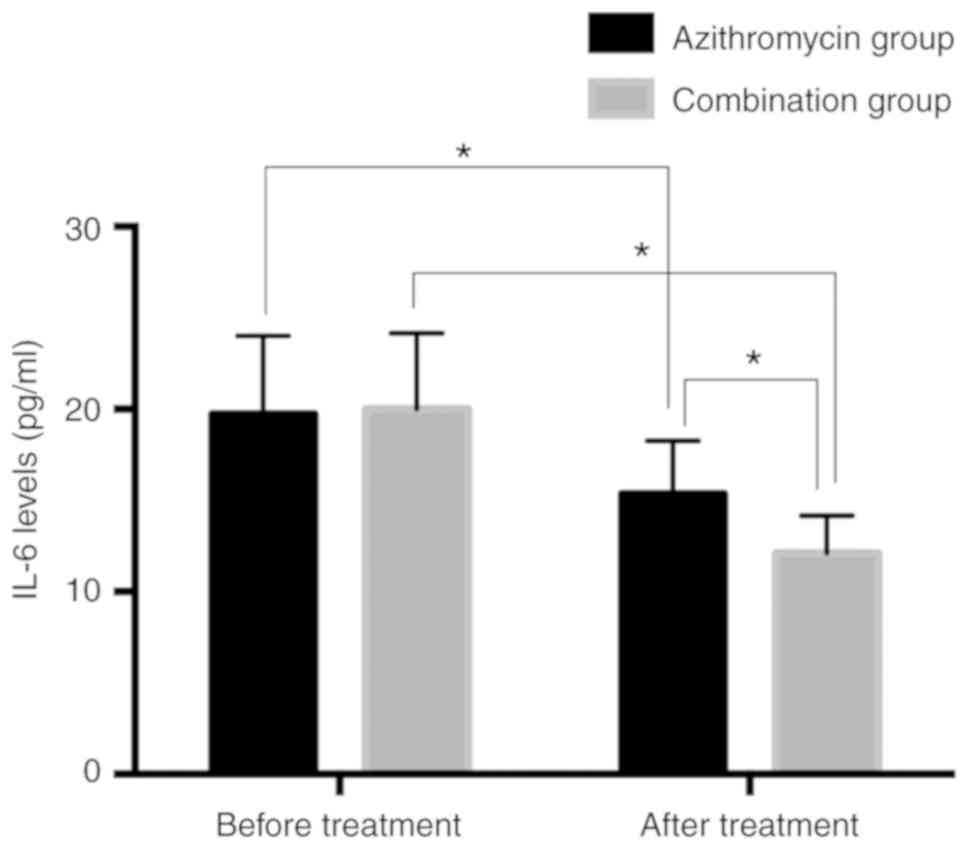
Serum IL-6 levels in patients before
and after treatment in two groups
Serum IL-6 levels in the azithromycin group before
and after treatment were 19.73±4.29 and 15.36±2.91 pg/ml,
respectively. However, serum IL-6 levels in the combination group
before and after treatment were 20.02±4.14 and 12.12±2.05 pg/ml,
respectively. There was no significant difference in the serum IL-6
levels between the two groups before treatment (P>0.05). After
treatment, the levels of serum IL-6 in the two groups were
significantly lower than those before treatment (P<0.05) and
serum IL-6 in the combination group was significantly lower than
that in the azithromycin group (P<0.05; Fig. 2).
Incidence of adverse reactions during
treatment in both groups
The occurrence of headaches, abdominal pain, nausea
and vomiting, and rash in the azithromycin group and combination
group were 1, 2, 1, 2 and 1, 2, 2, 2, respectively, which revealed
no intergroup difference in the incidence of adverse reactions
(13.04% vs. 13.46%, P>0.05; Table
IV).
 | Table IVIncidence of adverse reactions in the
two groups (n, %). |
Table IV
Incidence of adverse reactions in the
two groups (n, %).
| Adverse
reactions | Azithromycin group
(n=46) | Combination group
(n=52) | χ2
test | P-value |
|---|
| Headaches | 1 (2.17) | 1 (1.92) | | |
| Abdominal pain | 2 (4.35) | 2 (3.85) | | |
| Nausea and
vomiting | 1 (2.17) | 2 (3.85) | | |
| Rash | 2 (4.35) | 2 (3.85) | | |
| Total incidence
rate | 6 (13.04) | 7 (13.46) | 0.004 | 0.951 |
Comparison of the quality of life
between the two groups after two courses of treatment
There was no significant difference in the quality
of life score between the two groups before treatment (P>0.05).
After treatment, the quality of life score in the two groups was
significantly greater than that before treatment (P<0.05). After
treatment,the role, emotional and somatic, cognitive and social
functions in the combination group were significantly greater than
those in the azithromycin group (all P<0.05; Table V).
 | Table VComparison of the quality of life
between the two groups. |
Table V
Comparison of the quality of life
between the two groups.
| Parameters | Azithromycin group
(n=46) | Combination group
(n=52) | t-value | P-value |
|---|
| Before
treatment | | | | |
|
Role
function | 56.11±2.56 | 55.99±2.49 | 0.235 | 0.815 |
|
Emotional
function | 55.39±2.61 | 56.12±2.72 | 1.351 | 0.180 |
|
Somatic
function | 55.73±2.55 | 55.59±2.31 | 0.285 | 0.776 |
|
Cognitive
function | 56.02±2.94 | 55.83±2.84 | 0.325 | 0.746 |
|
Social
function | 55.17±2.83 | 55.26±2.79 | 0.158 | 0.875 |
| After
treatment | | | | |
|
Role
function |
72.67±2.12a |
80.45±2.23a | 17.64 | <0.001 |
|
Emotional
function |
70.82±2.23a |
79.91±2.06a | 20.97 | <0.001 |
|
Somatic
function |
70.99±2.18a |
81.47±2.02a | 24.70 | <0.001 |
|
Cognitive
function |
73.68±3.02a |
80.33±2.87a | 11.17 | <0.001 |
|
Social
function |
70.27±2.16a |
81.52±2.46a | 23.91 | <0.001 |
Discussion
NGU is a sexually transmitted disease caused by
Chlamydia trachomatis or Mycoplasma hominis.
Mycoplasma and chlamydia are common sexually transmitted
pathogens in males and females, both of which can cause stubborn
infections that cannot be cured by antibiotics. The resulting
disease from a resistant pathogen is difficult to cure and prone to
recurrence (20,21). Since the primary route of
transmission of NGU is sexual, the incidence of NGU has increased
rapidly in recent years, and it has become primarily a sexually
transmitted disease. It generally affects the health of women, but
it also has a negative impact on the male reproductive system, and
can easily lead to sterility. Hence, it is a threat to the health
of people and general well-being (22,23).
Drug therapy is the treatment of choice for patients with NGU.
However, due to the abuse of antibiotics, drug resistance of
pathogens has significantly increased, which renders treatment of
the disease difficult. It is, therefore, imperative to find
alternative and effective treatment methods for NGU (24,25).
The therapeutic effects of azithromycin monotherapy
and azithromycin combined with doxycycline on NGU were compared in
the present study. It was revealed that the efficacy of the
azithromycin group was significantly lower than that of the
combination group. The symptom relief time and medication period in
the combination group were also significantly lower than in the
azithromycin group. Hence, the combination of azithromycin and
doxycycline was more effective in the treatment of NGU.
Azithromycin is commonly used for the treatment of NGU caused by
Mycoplasma infection, and its irregular use has led to
mutations in drug resistance genes in Mycoplasma (26). In Denmark, azithromycin resistance in
Mycoplasma genitalium isolates have been found to be as high
as 40% (27). Doxycycline is highly
effective as an alternative therapeutic drug for NGU caused by
Mycoplasma. Mycoplasma is also highly sensitive to
doxycycline NGU (26). Although
there are relatively few studies on the efficacy of azithromycin in
combination with doxycycline in the treatment of NGU, the present
study revealed that the combination therapy can be very useful. To
further evaluate the effects of the combination therapy, the
recurrence rate, adverse reactions and serum IL-6 levels before and
after treatment between the two groups were compared. The results
revealed that the recurrence rate and serum IL-6 level in the
combination group were lower than those in the azithromycin group
after one year after the termination of treatment. Moreover, there
was no significant difference in the incidence of adverse reactions
between the two groups. This may, thus, indicate that the addition
of doxycycline to azithromycin monotherapy can not only reduce the
recurrence of NGU of patients, but also alleviate the inflammatory
reaction without causing more adverse events. One previous study
has revealed that 90% of doxycycline is absorbed through the
gastrointestinal tract when taken orally; in addition to having
fewer side effects (28). In
addition, doxycycline, a tetracycline, acts as a powerful
antibiotic by mainly blocking the protein synthesis of pathogens by
binding specifically to ribosomal 30S subunits. It has higher fat
solubility and a strong tissue penetration ability and also causes
fewer side-effects in patients with renal dysfunction (29,30).
Previous studies have indicated that doxycycline may be more
effective than azithromycin in the treatment of rectal chlamydia
infection (31). Although the
authors did not evaluate the combination therapy, they concluded
that doxycycline was more effective than azithromycin. Finally, the
quality of life between the two groups was compared, and it was
revealed that the quality of life score of the azithromycin group
was significantly lower than that of the combination group. Hence,
azithromycin combined with doxycycline was revealed to improve the
therapeutic efficacy and the quality of life of patients.
However, there are some limitations in the present
study. For example, the impact of different courses of treatment on
patients as well as the efficacy of doxycycline monotherapy were
not explored. A previous study revealed that 1 g azithromycin was
more effective than doxycycline used for Mycoplasma
genitalium in treating nongonococcal urethritis (25), however we have yet to investigate
this. In addition, we have not analyzed the effectiveness of IL-6
level and the correlation of IL-6 level with clinical efficacy and
adverse reactions. These topics may be explored in a future
study.
In conclusion, azithromycin combined with
doxycycline was revealed to be more effective than azithromycin
monotherapy in the treatment of non-gonococcal urethritis. It could
effectively alleviate the symptoms of patients and reduce the
expression of inflammatory factors such as IL-6 in the serum of
patients, improve the quality of life of patients, without causing
more adverse reactions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL wrote the manuscript and revised it critically
for important intellectual content. ZL and MH interpreted and
analyzed the patient data. DL and JC designed the study and
performed the experiment. HD and JY were responsible for the
analysis and discussion of the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xinchang People's Hospital. Patients who participated
in this research, signed the informed consent and had complete
clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leeyaphan C, Jiamton S, Chanyachailert P,
Surawan T and Omcharoen V: Treatment outcomes and loss to follow-up
rate of male patients with gonococcal and nongonococcal urethritis
who attended the sexually transmitted disease clinic: An 8-year
retrospective study. Indian J Sex Transm Dis AIDS. 38:37–42.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ong JJ, Sarumpaet A, Chow EP, Bradshaw C,
Chen M, Read T and Fairley CK: Should female partners of men with
non-gonococcal urethritis, negative for Chlamydia
trachomatis and Mycoplasma genitalium, be informed and
treated? Clinical outcomes from a partner study of heterosexual men
with NGU. Sex Transm Dis. 44:126–130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pond MJ, Nori AV, Witney AA, Lopeman RC,
Butcher PD and Sadiq ST: High prevalence of antibiotic-resistant
Mycoplasma genitalium in nongonococcal urethritis: The need
for routine testing and the inadequacy of current treatment
options. Clin Infect Dis. 58:631–637. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ito S, Hatazaki K, Shimuta K, Kondo H,
Mizutani K, Yasuda M, Nakane K, Tsuchiya T, Yokoi S, Nakano M, et
al: Haemophilus influenzae isolated from men with acute urethritis:
Its pathogenic roles, responses to antimicrobial chemotherapies,
and antimicrobial susceptibilities. Sex Transm Dis. 44:205–210.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sethi S, Zaman K and Jain N: Mycoplasma
genitalium infections: Current treatment options and resistance
issues. Infect Drug Resist. 10:283–292. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kissinger PJ, White S, Manhart LE,
Schwebke J, Taylor SN, Mena L, Khosropour CM, Wilcox L, Schmidt N
and Martin DH: Azithromycin treatment failure for Chlamydia
trachomatis among heterosexual men with nongonococcal
urethritis. Sex Transm Dis. 43:599–602. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
González-Lizárraga F, Socías SB, Ávila CL,
Torres-Bugeau CM, Barbosa LR, Binolfi A, Sepúlveda-Díaz JE, Del-Bel
E, Fernandez CO, Papy-Garcia D, et al: Repurposing doxycycline for
synucleinopathies: Remodelling of α-synuclein oligomers towards
non-toxic parallel beta-sheet structured species. Sci Rep.
7(41755)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stein GE, Mummaw NL and Havlichek DH: A
preliminary study of clarithromycin versus doxycycline in the
treatment of nongonococcal urethritis and mucopurulent cervicitis.
Pharmacotherapy. 15:727–731. 1995.PubMed/NCBI
|
|
9
|
Cunha BA, Baron J and Cunha CB:
Similarities and differences between doxycycline and minocycline:
Clinical and antimicrobial stewardship considerations. Eur J Clin
Microbiol Infect Dis. 37:15–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abdolrasouli A and Roushan A:
Corynebacterium propinquum associated with acute, nongonococcal
urethritis. Sex Transm Dis. 40:829–831. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khosropour CM, Manhart LE, Colombara DV,
Gillespie CW, Lowens MS, Totten PA, Golden MR and Simoni J:
Suboptimal adherence to doxycycline and treatment outcomes among
men with non-gonococcal urethritis: A prospective cohort study. Sex
Transm Infect. 90:3–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manhart LE, Gillespie CW, Lowens MS,
Khosropour CM, Colombara DV, Golden MR, Hakhu NR, Thomas KK, Hughes
JP, Jensen NL and Totten PA: Standard treatment regimens for
nongonococcal urethritis have similar but declining cure rates: A
randomized controlled trial. Clin Infect Dis. 56:934–942.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Braune J, Weyer U, Hobusch C, Mauer J,
Brüning JC, Bechmann I and Gericke M: IL-6 regulates M2
polarization and local proliferation of adipose tissue macrophages
in obesity. J Immunol. 198:2927–2934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kinghorn GR: Neisseria gonorrhoeae.
Keywords: Neisseria gonorrhoeae, penicillin sensitivity. Sex
Transm Infect. 54:112–114. 1978.
|
|
15
|
Kondo H, Ito S, Hatazaki K, Horie K,
Nakane K, Mizutani K, Tsuchiya T, Yasuda M, Yokoi S, Nakano M and
Deguchi T: GyrA and/or ParC alterations of haemophilus influenzae
strains isolated from the urethra of men with acute urethritis. J
Infect Chemother. 24:232–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deguchi T: Proposed treatment strategies
for non-gonococcal urethritis. Lancet Infect Dis. 17:1121–1122.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Frølund M, Wikström A, Lidbrink P, Abu
Al-Soud W, Larsen N, Harder CB, Sørensen SJ, Jensen JS and Ahrens
P: The bacterial microbiota in first-void urine from men with and
without idiopathic urethritis. PLoS One.
13(e0201380)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB, de Haes JC, et al: The European organization for research and
treatment of cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fayers PM, Aaronson NK, Bjordal K,
Groenvold M, Curran D and Bottomley A: On behalf of the EORTC
Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd
edition. European Organization for Research and Treatment of
Cancer, Brussels, 2001.
|
|
20
|
Nenoff P, Manos A, Ehrhard I, Krüger C,
Paasch U, Helmbold P and Handrick W: Non-viral sexually transmitted
infections-Epidemiology, clinical manifestations, diagnostics and
therapy: Part 2: Chlamydia and mycoplasma. Hautarzt.
68:50–58. 2017.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
21
|
Handsfield H: Management of herpetic
urethritis and female partners of men with nongonococcal
urethritis. Sex Transm Dis. 44:131–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bradshaw CS, Tabrizi SN, Read TR, Garland
SM, Hopkins CA, Moss LM and Fairley CK: Etiologies of nongonococcal
urethritis: Bacteria, viruses, and the association with orogenital
exposure. J Infect Dis. 193:336–345. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Jensen JS, Bradshaw CS, Tabrizi SN,
Fairley CK and Hamasuna R: Azithromycin treatment failure in
Mycoplasma genitalium-positive patients with nongonococcal
urethritis is associated with induced macrolide resistance. Clin
Infect Dis. 47:1546–1553. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Ito S, Yasuda M, Seike K, Sugawara T,
Tsuchiya T, Yokoi S, Nakano M and Deguchi T: Clinical and
microbiological outcomes in treatment of men with non-gonococcal
urethritis with a 100-mg twice-daily dose regimen of sitafloxacin.
J Infect Chemother. 18:414–418. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mena LA, Mroczkowski TF, Nsuami M and
Martin DH: A randomized comparison of azithromycin and doxycycline
for the treatment of Mycoplasma genitalium-positive
urethritis in men. Clin Infect Dis. 48:1649–1654. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Salado-Rasmussen K and Jensen JS:
Mycoplasma genitalium testing pattern and macrolide
resistance: A Danish nationwide retrospective survey. Clin Infect
Dis. 59:24–30. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yamagoe M, Osada T, Goto M, Nishida S and
Nakamura M: Clinical studies on doxycycline in male nongonococcal
urethritis. Jpn J Antibiot. 38:3156–3168. 1985.PubMed/NCBI(In Japanese).
|
|
28
|
Maohua L, Hongtao J, Xiuxin W, Yu Z, Tao S
and Hua Y: Clinical analysis of 195 cases of non-gonococcal
urethritis treated with doxycycline dispersible tablets. Shanghai
Med Pharm J. 19:34–42. 2019.
|
|
29
|
Aparicio NJ, Muchinik G, Levalle O, Tropea
L, Guitelman A and Grinstein S: The effect of a treatment with
doxycycline on semen of asthenozoospermic patients with
T-mycoplasma genital infection. Andrologia. 12:521–524.
1980.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Totten PA, Jensen NL, Khosopour CM,
Gillespie CW, Jensen JS, Kenny GK, Golden MR and Manhart LE: O19.1
Azithromycin and doxycycline resistance profiles of Mycoplasma
genitalium and association with treatment outcomes. Sex Transm
Infect. 89 (Suppl 1)(A62)2013.
|
|
31
|
Li B, Hocking JS, Bi P, Bell C and Fairley
CK: The efficacy of azithromycin and doxycycline treatment for
rectal chlamydial infection: A retrospective cohort study in South
Australia. Intern Med J. 48:259–264. 2018.PubMed/NCBI View Article : Google Scholar
|