Introduction
Epilepsy is a common chronic disabling neurological
disease that affects more than 1% of the population of the world
(1,2). It is mainly caused by abnormal
discharge of brain neurons, characterized by transient dysfunction
of the nervous system (3). If
seizures of patients are not controlled for a long time and reoccur
frequently, they are likely to cause brain damage (4). Advancements in medical technology have
contributed to great accuracy in the diagnosis of epilepsy.
Conventional anti-epileptic first-line drugs can control the
seizures of most patients with epilepsy, but persistent seizures
will occur in more than 30% of patients according to relevant
statistics (2,5).
Clinically, an epileptic seizure is generally
controlled by symptomatic treatment with long-term medication
(6). Traditional antiepileptic drugs
have adverse effects on the skeletal system of middle-aged and
elderly patients, and the degree of abnormal bone mineral density
(BMD) and bone metabolism increases with the medication time,
leading to a higher risk of fracture (7,8).
Levetiracetam, a pyrrolidone derivative with high water solubility
and high permeability, can inhibit the spread of lesions by
increasing the excitability threshold of normal brain tissue cells
(9,10). It is quickly absorbed after the oral
administration, exhibiting good efficacy for preventing seizures
(9,10). Lacosamide (LCM) is a newly developed
antiepileptic drug, which has been used as an adjuvant treatment
for partial or systemic epilepsy in numerous countries in recent
years (11). Unlike traditional
sodium channel blockers, LCM is novel because it can selectively
enhance the slow inactivation of voltage-gated sodium channels
without affecting its rapid inactivation (12). In addition, it can selectively
attenuate collapsin response mediator protein 2 (CRMP2)-induced
tubulin polymerization (13) to
exert an antiepileptic effect.
However, to date, levetiracetam combined with LCM
has rarely been used in the treatment of senile epilepsy. In
addition, the effects of this combination therapy on the efficacy
and neural function of patients are not clear. In the present
study, the therapeutic effect of levetiracetam combined with LCM in
patients with epilepsy was explored, aiming to inspire new
treatment options for epilepsy.
Patients and methods
Basic information
We assigned 252 patients with refractory partial
seizures admitted to the 5th People's Hospital of Qingdao (Qingdao,
China) to receive either levetiracetam tablets [120 patients, the
control group (CG)] or levetiracetam tablets combined with LCM [132
patients, the joint group (JG)]. The CG was comprised of 64 males
and 56 females, aged 55.42±4.98 years, with an average course of
disease of 8.01±3.31 years, while the JG was comprised of 69 males
and 63 females, aged 56.13±5.68 years, with an average course of
disease of 8.57±3.17 years. The present study was carried out under
the approval of the Ethics Committee of the 5th People's Hospital
of Qingdao (Qingdao, China) (approval no. QD31344). All patients
and their families signed the written informed consent.
Inclusion criteria were as follows: i) Patients
diagnosed with refractory partial seizures; ii) patients older than
18 years; iii) patients with more than 4 seizures per month; iv)
patients exhibiting a poor response to a stable administration of
one or more first-line antiepileptic drugs; v) patients undergoing
no adjustment in the drug treatment within 6 months prior to this
study; and vi) patients whose plasma-drug concentrations were
within the effective range.
Exclusion criteria were as follows: i) Patients with
poor compliance with the treatment or the follow-up; ii) patients
not accompanied by family members at the time of admission; iii)
patients with other diseases or complications affecting the results
of the study; iv) patients with incomplete clinical data; v)
patients who were pregnant or lactating women; vi) patients with a
known history of drug addiction or abuse; vii) patients with
abnormal expression levels of indicators for liver and renal
function.
Treatment plan
For the CG, on the basis of conventional treatment,
patients were treated with levetiracetam tablets (UCB Pharma S.A.;
China Food and Drug Administration Approval no. J20160085) at an
initial dose of 250 mg, twice a day (500 mg/day), which was
increased to 500 mg, twice a day (1,000 mg/day) after two weeks,
and then increased to 1,000 mg, twice a day (2,000 mg/day) after
another two weeks. Then the dose was maintained at 1,000 mg and
adjusted according to the conditions of the patients.
For the JG, in addition to the levetiracetam tablets
designed for the CG, patients were also treated with LCM (Aesica
Pharmaceuticals GmbH; China Food and Drug Administration Approval
no. H20180069) at an initial dose of 50 mg, twice daily. According
to the response and tolerance of patients, the dose was increased
by 100 mg (twice a day) every other week, and was maintained at 200
to 400 mg per day.
Detection method
Fasting venous blood samples were collected from all
patients. Blood calcium (Ca) and blood phosphorus (P) were detected
on the Beckman Coulter AU2700 Chemistry Analyzer. Serum Glial
fibrillary acidic protein (GFAP), neuron-specific enolase (NSE),
alkaline phosphatase (ALP), and parathyroid hormone (PTH), S-100β
protein (S-100β) were tested using the enzyme-linked immunosorbent
assay (ELISA). GFAP, NSE, ALP, and PTH ELISA kits were purchased
from BioSwamp Life Science Lab (cat. nos. HM10951, HM10786,
HM10232, and HM10797, respectively). The S-100β ELISA kit was from
Wuhan Yipu Biotechnology Co., Ltd. (cat. no. MM-13258H1). The
results were analyzed on the ELISA analyzer manufactured by Beijing
Linmao Technology Co., Ltd. (cat. no. BS-1101). According to the
kit instructions, standards (50 µl) were added at various
concentrations to the standard wells, 10 µl of samples and 40 µl of
the diluent were added to the sample wells, and 50 µl of distilled
water to the blank well. Then, 50 µl of enzyme-labeled reagent was
added to the standard wells and the sample well, the reaction wells
covered were covered with a sealer, and incubated for 1 h in a 37˚C
water bath or incubator. Next, 50 µl of color reagents A and B were
added to each well, the plate was shaken gently, and placed in a
dark place at 37˚C for 15 min for color development. Finally, 50 µl
stop solution was added to each well, the ELISA Microwell Plate
Reader was adjusted to zero using the blank well within 15 min, and
then the OD value of each well was measured at 450 nm within 25
min. All test procedures strictly followed the instructions of the
kit.
Outcome measures
The following indicators were recorded at 6 months
before and after treatment: i) The treatment efficacy in the two
groups was assessed. A marked response indicated a complete
remission of clinical symptoms, great improvements in vital signs,
and a decrease in seizure frequency by >70%. A moderate response
indicated a partial remission of clinical symptoms, moderate
improvements in vital signs, and a decrease in seizure frequency by
30-70%. No response indicated no improvements in the clinical
symptoms or vital signs, and a decrease in seizure frequency by
<30% or even no decrease. The total response rate was calculated
as follows: Total response rate=percentage of patients with a
marked response + percentage of patients with a moderate response.
ii) The frequency of seizures was recorded and compared between the
two groups. iii) Serum levels of markers for neural function
including GFAP, NSE, and S-100β in the two groups were assessed.
iv) The BMD of different parts of the body (femoral neck, lumbar
vertebra L2-4, femoral trochanter, and Ward triangle) and the
expression levels of bone metabolism indexes (Ca, P, ALP, PTH) were
monitored. v) Adverse reactions during the treatment and the
quality of life scores were recorded. The quality of life was
assessed using the Quality of Life in Epilepsy Inventory (QOLIE-31)
(14), which is a 100-point scale
assessing 6 items: Worries about seizures, emotional health, mental
state, cognitive function, drug influence, and social function. A
higher total score indicated a better quality of life. vi) Patients
were followed up on the telephone or through the outpatient
service, and the medication was recorded.
Statistical analysis
The statistical analysis was performed on SPSS v20.0
(IBM Corp.). Count data were expressed as [n (%)] and compared
between the two groups by the chi-square test. Measurement data
were expressed as the mean ± standard deviation (SD) and compared
between the two groups by the independent sample t-test. Multiple
comparisons between the two groups before and after treatment were
analyzed using the one-way ANOVA, and the LSD t-test was used for
the post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of general information
Details of the general information of patients are
presented in Table I. Patients from
the JG and patients from the CG were comparable since they were not
markedly different in sex, age, body mass index (BMI), place of
residence, course of the disease, duration of education,
creatinine, and urine urea nitrogen (P>0.05).
 | Table IComparison of the clinical general
information (mean ± SD)/[n (%)]. |
Table I
Comparison of the clinical general
information (mean ± SD)/[n (%)].
| | JG (n=132) | CG (n=120) | χ2/t | P-value |
|---|
| Sex | | | 0.028 | 0.866 |
|
Male | 69 (52.27) | 64 (53.33) | | |
|
Female | 63 (47.73) | 56 (46.67) | | |
| Age (years) | | | 0.115 | 0.735 |
|
≤55 | 60 (45.45) | 52 (43.33) | | |
|
>55 | 72 (54.55) | 68 (56.67) | | |
| Average age
(years) | 56.13±5.68 | 55.42±4.98 | 1.051 | 0.295 |
| BMI
(kg/m2) | 23.61±2.78 | 23.97±2.69 | 1.043 | 0.298 |
| Place of
residence | | | 0.037 | 0.847 |
|
Urban
area | 71 (53.79) | 66 (55.00) | | |
|
Rural
area | 61 (46.21) | 54 (45.00) | | |
| Course of the
disease (years) | 8.57±3.17 | 8.01±3.31 | 1.371 | 0.172 |
| Duration of
education (years) | 10.07±2.13 | 10.24±2.53 | 0.579 | 0.563 |
| Creatinine
(µmol/l) | 63.48±8.74 | 65.37±9.02 | 1.689 | 0.093 |
| Urine urea nitrogen
(mmol/l) | 6.03±1.51 | 6.13±1.71 | 0.493 | 0.623 |
Comparison of treatment efficacy
Details of the treatment efficacy in the two groups
are presented in Table II. The
total response rate was markedly higher in the JG than in the CG
(90.15% vs. 80.83%, P<0.05).
 | Table IIComparison of treatment efficacy n
(%). |
Table II
Comparison of treatment efficacy n
(%).
| Response | JG (n=132) | CG (n=120) | χ2 | P-value |
|---|
| Marked | 67 (50.76) | 50 (41.67) | | |
| Moderate | 52 (39.39) | 47 (39.16) | | |
| No response | 13 (9.85) | 23 (19.17) | | |
| Total response
rate | 90.15% | 80.83% | 4.457 | 0.035 |
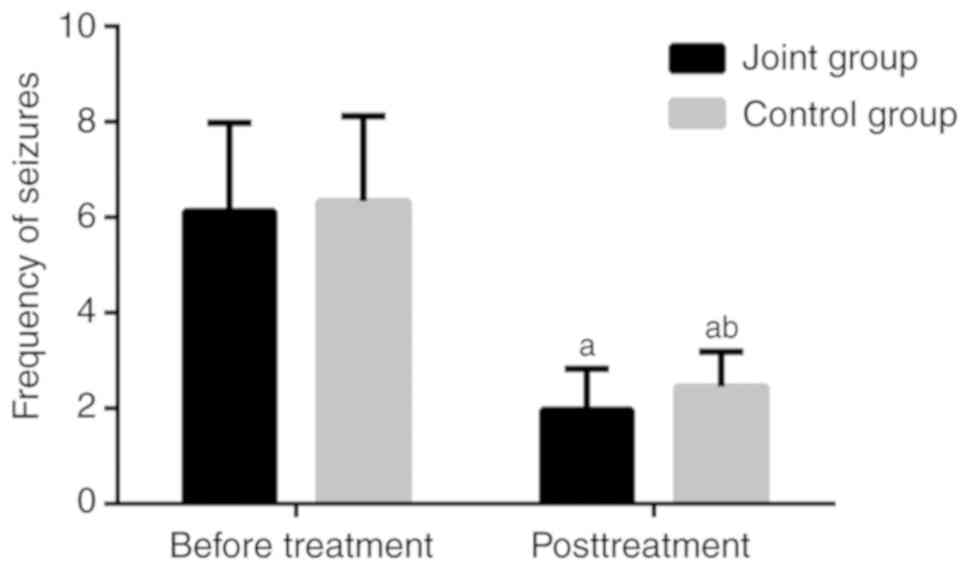
Comparison of the frequency of
seizures before and after treatment
Details of the frequency of seizures in the two
groups are presented in Fig. 1. All
patients met the inclusion criteria for the frequency of seizures.
The JG and CG were not different in the frequency of seizures
before treatment (P>0.05). After 6 months of treatment, the
frequency of seizures decreased in both groups, with a slightly
higher frequency in the CG than in the JG (P<0.05).
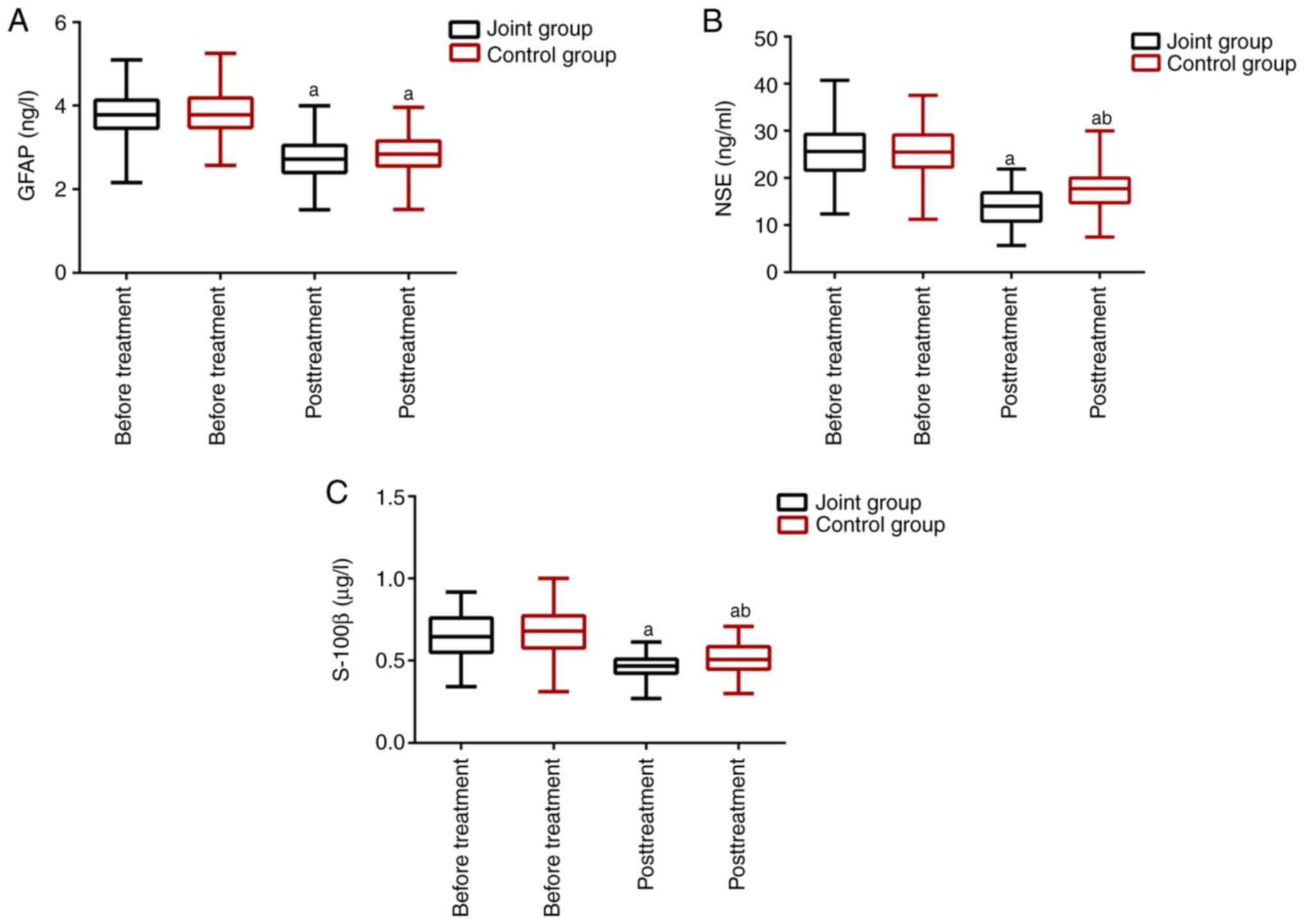
Comparison of neural function markers
before and after treatment
The expression levels of neural function markers are
presented in Fig. 2. The two groups
were not different in the neural function before treatment. The
expression levels of NSE, S-100β, and GFAP significantly decreased
in the two groups after treatment (P<0.05), with lower NSE and
S-100β levels in the JG than in the CG (P<0.05).
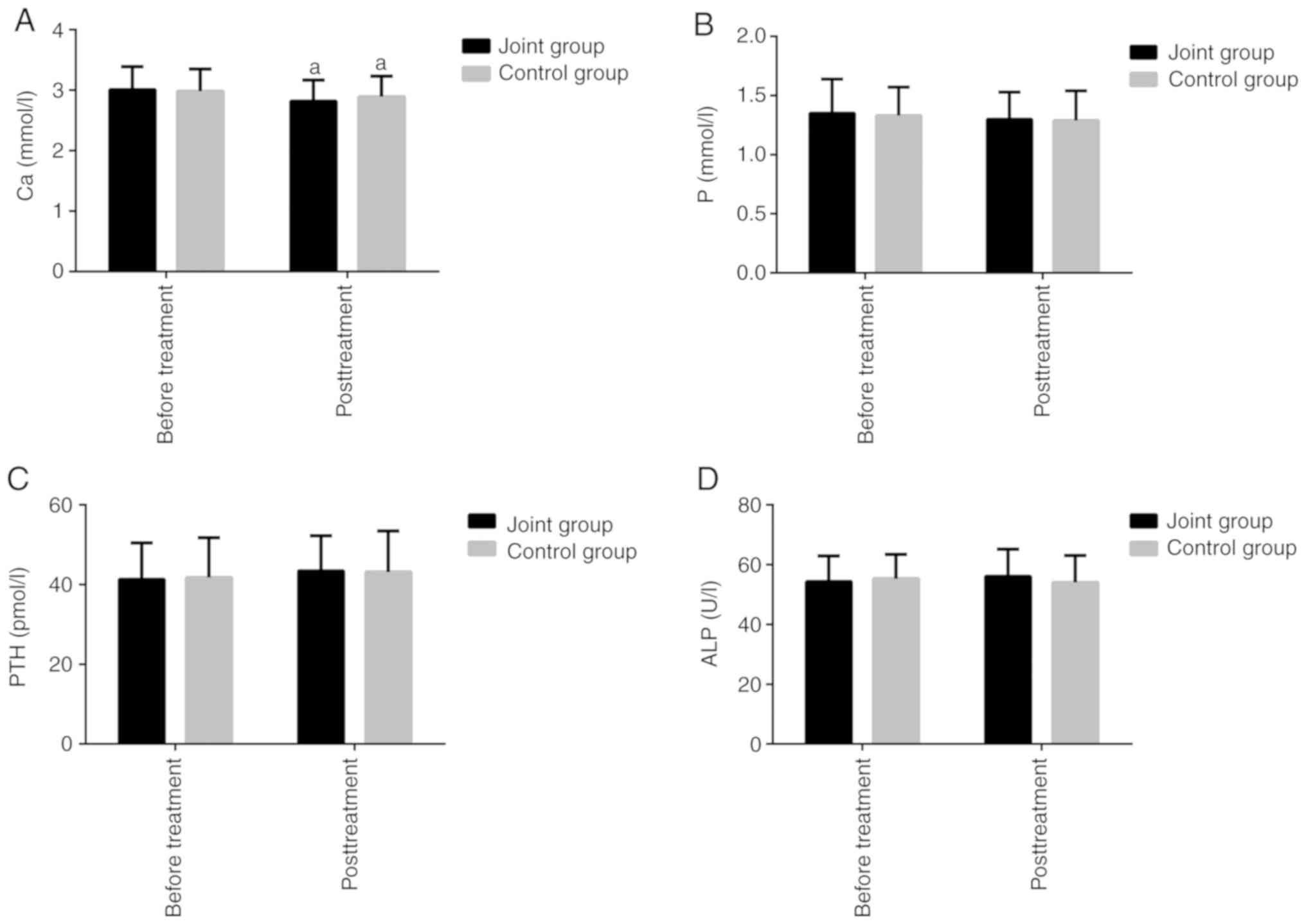
Comparison of BMD and bone metabolism
before and after treatment
The BMDs of all patients after 6 months of treatment
are presented in Table III. The JG
and the CG were not obviously different in the BMD of different
body parts before treatment (P>0.05). The BMD of the femoral
neck decreased in both groups after a period of treatment
(P<0.05), but there were no differences between the two groups
(P>0.05). The comparison of the bone metabolism indexes are
presented in Fig. 3. The JG and the
CG were not obviously different in the expression levels of bone
metabolism indexes before treatment. There were no marked
differences in the P and PTH levels between the two groups before
and after treatment (P>0.05). In the JG, the ALP level after
treatment was higher than that before treatment, but the difference
was not statistically significant. In the CG, the ALP level after
treatment was lower than that before treatment, but the difference
was not statistically significant (P>0.05). The Ca level
decreased in both groups after treatment (P<0.05), but there was
no significant difference between the two groups after treatment
(P>0.05).
 | Table IIIComparison of BMD between the two
groups (mean ± SD). |
Table III
Comparison of BMD between the two
groups (mean ± SD).
| | JG (n=132) | GC (n=120) |
|---|
| Body part | Before
treatment | After
treatment | t-value | P-value | Before
treatment | After
treatment | t-value | P-value |
|---|
| Femoral neck | 0.73±0.16 | 0.67±0.21 | 2.828 | 0.005 | 0.71±0.15 | 0.66±0.16 | 2.247 | 0.025 |
| Lumbar vertebra
L2-4 | 0.72±0.18 | 0.70±0.23 | 0.834 | 0.405 | 0.74±0.20 | 0.71±0.16 | 1.193 | 0.234 |
| Femoral
trochanter | 0.65±0.14 | 0.63±0.19 | 0.923 | 0.367 | 0.64±0.17 | 0.60±0.20 | 1.760 | 0.080 |
| Ward triangle | 0.66±0.18 | 0.64±0.22 | 0.876 | 0.382 | 0.67±0.19 | 0.63±0.14 | 1.670 | 0.096 |
Comparison of adverse reactions during
treatment
Adverse reactions during the medication period are
presented in Table IV. Adverse
reactions occurring in the present study included nausea and
vomiting, diarrhea, rash, leukocytosis, dizziness, and decreased
appetite. The comparison of the total incidence of adverse
reactions between the JG and the CG revealed no marked difference
(20.46% vs. 25.83%, P>0.05).
 | Table IVComparison of adverse reactions, n
(%). |
Table IV
Comparison of adverse reactions, n
(%).
| Adverse
reactions | JC (n=132) | CG (n=120) | χ2 | P-value |
|---|
| Nausea and
vomiting | 9 (6.82) | 11 (9.17) | | |
| Diarrhea | 4 (3.03) | 3 (2.50) | | |
| Rash | 4 (3.03) | 6 (5.00) | | |
| Leukopenia | 2 (1.52) | 3 (2.50) | | |
| Dizziness | 3 (2.27) | 1 (0.83) | | |
| Decreased
appetite | 5 (3.79) | 7 (5.83) | | |
| Total
incidence | 20.46% | 25.83% | 1.026 | 0.311 |
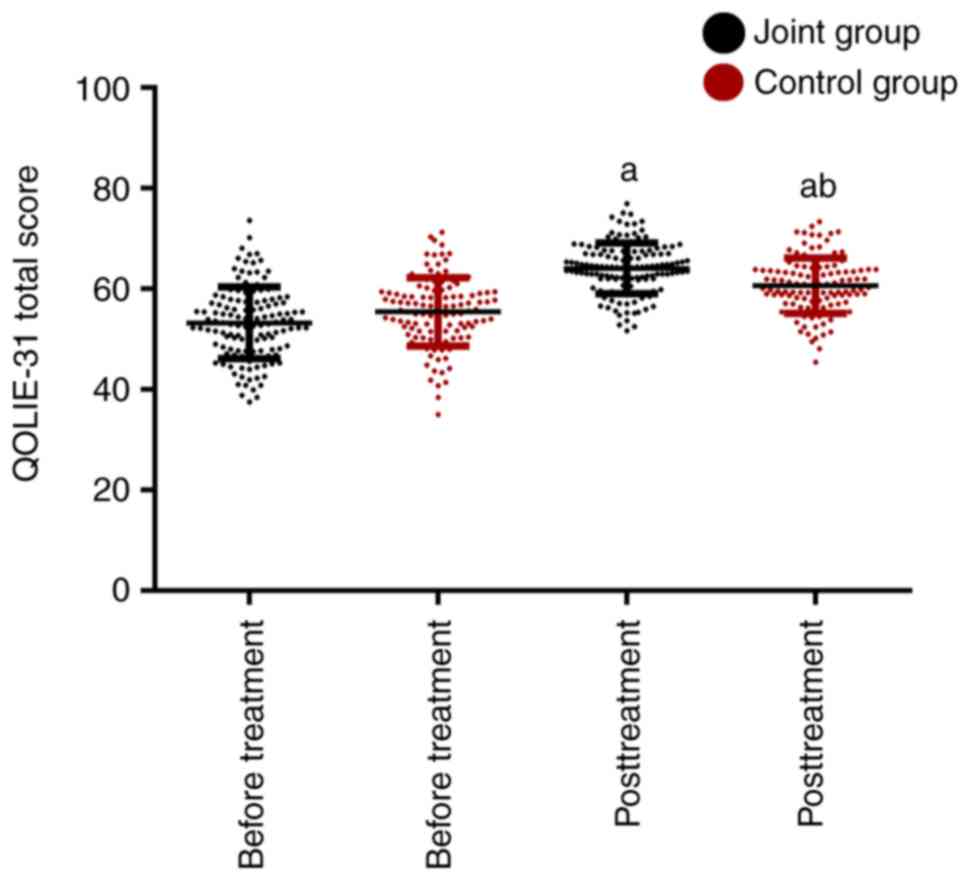
Comparison of the quality of life
before and after treatment
The scores of the quality of life before and after
treatment are presented in Fig. 4.
The JG and CG were not markedly different in the QOLIE-31 score
before treatment (P>0.05). After treatment, the QOLIE-31 score
significantly increased in the two groups, with a slightly higher
QOLIE-31 score in the JG than in the CG, and the difference was
statistically significant (P<0.05).
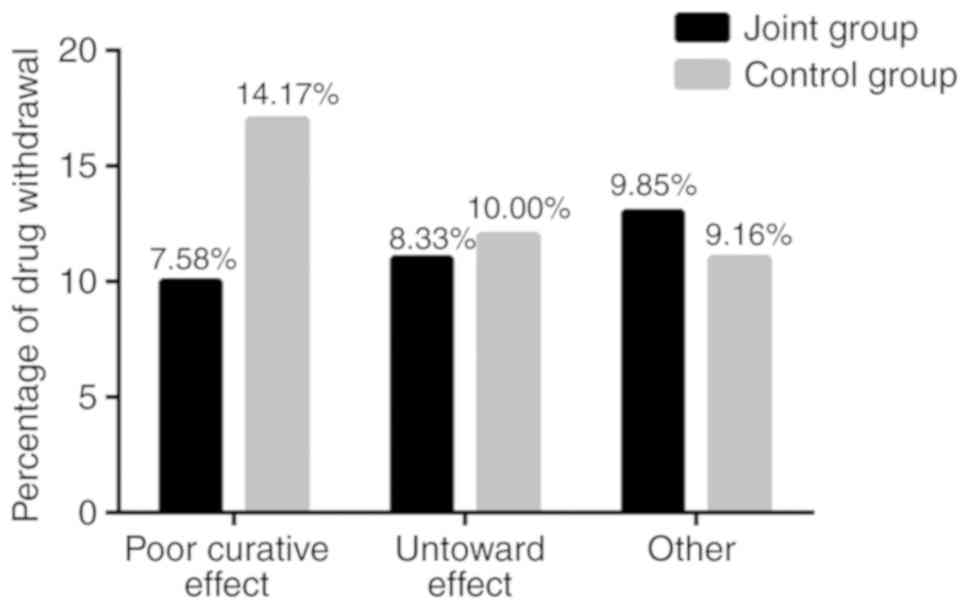
One-year drug retention rate and
causes of drug withdrawal in the two groups
The 1-year drug retention rates in the two groups
are presented in Table V and causes
of drug withdrawal are presented in Fig.
5. The 1-year drug retention rate was 74.24% in the JG, with 34
cases of drug withdrawal (10 cases were caused by the poor curative
effect, 11 by the untoward effect, and 13 by other reasons). The
1-year drug retention rate was 66.67% in the JG, with 40 cases of
drug withdrawal (17 cases were caused by the poor curative effect,
12 by the untoward effect, and 11 by other reasons). The 1-year
drug retention rate was higher in the JG than in the CG.
 | Table VComparison of drug retention, n
(%). |
Table V
Comparison of drug retention, n
(%).
| Drug use | JG (n=132) | CG (n=120) | χ2 | P-value |
|---|
| Drug retention
rate | 98 (74.24) | 80 (66.67) | 42.460 | <0.001 |
| Drug withdrawal
rate | 34 (25.76) | 40 (33.33) | 42.460 | <0.001 |
Discussion
Epilepsy is divided into systemic and partial
seizures (15). Epilepsy is
characterized by an acute but short onset, diverse clinical
manifestations, a high risk of recurrence, and a complicated
mechanism of seizures. Patients with recurrent seizures, whatever
the cause is, are often attacked by a variety of psychological,
physical, and social diseases that seriously impair their physical
and mental health and quality of life (16,17).
In the present study, the treatment responses
between two groups were compared to analyze the therapeutic effect
of levetiracetam combined with LCM in patients with epilepsy. The
results of treatment efficacy in patients with refractory partial
seizures in the two groups after 6 months of treatment revealed
that patients in the JG had improved treatment efficacy and
markedly lower frequency of seizures. A previous study revealed
that, in the cortical brain tissue of patients with refractory
seizures, LCM can target GABAA receptors and play a synergistic
effect with levetiracetam to relieve GABA functional impairment
(18). LCM can reduce the frequency
of seizures and improve the efficacy in the treatment for children
with intractable epilepsy as an adjuvant drug (19). According to the results of the
present study and the aforementioned studies, it was theorized that
levetiracetam can cooperate with LCM to expand the coverage of
treatment of epilepsy, protect the neural function of patients, and
improve the treatment efficacy. When a patient has a seizure, the
nerve tissues are damaged, which causes a mass release of specific
factors in the neuron and prompts those specific factors to enter
the blood circulation through the damaged blood-brain barrier. The
detection of serum nerve injury-related factors can quantitatively
reflect the degree of nerve damage caused by seizures (20,21).
GFAP is a specific marker for astrocytes, whose mass release can
cause abnormal excitation of neurons and aggravate the progression
of epilepsy (22). NSE that is
present in nerve tissues and S-100β that is present in glial cells,
which are upregulated at the onset of a seizure, can reflect the
degree of neuronal damage (23). In
the present study, the levels of NSE, S-100β, and GFAP were
significantly reduced after treatment in the two groups, with a
more pronounced reduction in the JG than in the CG. These results
suggest that levetiracetam combined with LCM can significantly
improve neural function, reduce neural tissue damage, and better
protect the brain.
The metabolism of bone is active. In the normal
physiological environment, the formation and absorption of bone
tissues are in a dynamic equilibrium (24,25). The
maintenance of this equilibrium depends on the balance of multiple
hormones or trace elements, including PTH, ALP, vitamin D, as well
as other steroid hormones (24,25).
Fractures in patients with epilepsy are most likely to be caused by
a long-term use of antiepileptic drugs which can reduce BMD
(26). Therefore, the exploration of
the effects of long-term drug treatment on BMD is crucial to the
prevention of adverse consequences. In the present study, the BMD
and the expression levels of bone metabolism indicators were
assessed in the two groups after 6 months of treatment. The results
revealed that levetiracetam monotherapy caused a decrease in
femoral BMD and Ca content, but caused no obvious changes in the
BMD of other body parts or the expression levels of bone metabolism
indicators. There was no significant difference between the two
groups in the expression levels of bone metabolism indicators after
treatment. A study by Beniczky et al (27) suggested that the BMD in patients
treated with levetiracetam monotherapy is significantly reduced.
However, in an animal model study by Anwar et al (28), they revealed that levetiracetam did
not cause changes in BMD. The latest research has revealed a marked
reduction in the BMD and levels of bone metabolism markers (ALP,
Ca) in patients receiving levetiracetam monotherapy, and suggested
that levetiracetam may alter bone marrow density by affecting the
optimal mineralization of cartilage and thereby interfering with
the maturation of bone tissue (29).
To date, there have been few studies on the effect of LCM on the
BMD and metabolism in epileptic patients. According to the results
of the present study, it was theorized that LCM does not aggravate
the adverse effects on BMD and bone metabolism caused by
levetiracetam.
In the present study, the incidence of adverse
reactions was slightly lower in the JG than in the CG, but the
difference was not statistically significant. Previous studies
(30,31) have revealed that LCM does not
aggravate the side effects of levetiracetam in the treatment of
epilepsy, suggesting that there are no adverse effects of the
combination of the two. But whether LCM can relieve the side
effects of levetiracetam remains to be further studied. QOLIE-31
was used to assess the quality of life of patients after treatment.
The results revealed that the quality of life of patients was
improved in both groups after treatment, with a higher QOLIE-31
score in the JG. A former study concluded that LCM as an adjuvant
treatment for levetiracetam can significantly increase the QOLIE-10
score of patients with refractory epilepsy and reduce the
occurrence of anxiety and depression in patients (32). In addition, LCM as an adjuvant drug
can improve cognitive function and the mental state of patients
with epilepsy (33). The results of
the present study and the aforementioned studies indicated that
levetiracetam treatment supplemented by LCM can improve the mood
and quality of life of patients with epilepsy. In the present
study, the 1-year drug retention rate was 74.24% in the JG and
66.67% in the CG. According to previous studies, the 1-year drug
retention rate was approximately 62.0% after long-term LCM
monotherapy (34) and approximately
34.4% after long-term treatment with levetiracetam (35). In the present study, the 1-year drug
retention rate was enhanced in patients with refractory partial
seizures treated with levetiracetam combined with LCM. It is
surmised that the increased 1-year drug retention rate may be due
to the improved efficacy of levetiracetam treatment supported by
LCM.
The present study mainly explored the effects of
levetiracetam tablets and LCM on therapeutic efficacy and neural
function in patients with epilepsy. However, at present only the
related outcome measures after 6 months of treatment were assessed
and a long-term follow-up was not conducted. Patients included in
this study were middle-aged and elderly people. Therefore, whether
levetiracetam and LCM can affect the BMD and bone metabolism in
younger patients, and whether the BMD and bone metabolism vary
among patients with different sexes and ages should be further
explored. In the future, we will address these issues to provide a
reference for future clinical treatment of epilepsy.
In summary, levetiracetam tablets combined with LCM
significantly enhanced the therapeutic effect and improved the
neural function in patients with refractory partial seizures, but
it may cause a slight adverse effect on BMD and bone metabolism in
the short term.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL conceived and designed the study. AL, QG and MW
were responsible for the collection, analysis and interpretation of
the data. QG drafted the manuscript. AL revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the 5th People's Hospital of Qingdao (approval no.
QD31344). Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilliam FG, Black KJ, Carter J, Freedland
KE, Sheline YI, Tsai WY and Lustman PJ: A trial of sertraline or
cognitive behavior therapy for depression in epilepsy. Ann Neurol.
86:552–560. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Englot DJ, Rolston JD, Wright CW, Hassnain
KH and Chang EF: Rates and predictors of seizure freedom with vagus
nerve stimulation for intractable epilepsy. Neurosurgery.
79:345–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kanner AM, Ashman E, Gloss D, Harden C,
Bourgeois B, Bautista JF, Abou-Khalil B, Burakgazi-Dalkilic E,
Llanas Park E, Stern J, et al: Practice guideline update summary:
Efficacy and tolerability of the new antiepileptic drugs II:
Treatment-resistant epilepsy: Report of the guideline development,
dissemination, and implementation subcommittee of the American
academy of neurology and the American epilepsy society. Neurology.
91:82–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen Z, Brodie MJ, Liew D and Kwan P:
Treatment outcomes in patients with newly diagnosed epilepsy
treated with established and new antiepileptic drugs: A 30-year
longitudinal cohort study. JAMA Neurol. 75:279–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bouwens van der Vlis TAM, Schijns OEMG,
Schaper FLWVJ, Hoogland G, Kubben P, Wagner L, Rouhl R, Temel Y and
Ackermans L: Deep brain stimulation of the anterior nucleus of the
thalamus for drug-resistant epilepsy. Neurosurg Rev. 42:287–296.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grinspan ZM, Shellhaas RA, Coryell J,
Sullivan JE, Wirrell EC, Mytinger JR, Gaillard WD, Kossoff EH,
Valencia I, Knupp KG, et al: Comparative effectiveness of
levetiracetam vs phenobarbital for infantile epilepsy. JAMA
Pediatr. 172:352–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shiek Ahmad B, O'Brien TJ, Gorelik A, Hill
KD and Wark JD: Bone mineral changes in epilepsy patients during
initial years of antiepileptic drug therapy. J Clin Densitom.
19:450–456. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Phabphal K, Geater A, Limapichart K,
Sathirapanya P, Setthawatcharawanich S, Witeerungrot N,
Thammakumpee N and Leelawattana R: The association between BsmI
polymorphism and bone mineral density in young patients with
epilepsy who are taking phenytoin. Epilepsia. 54:249–255.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gupta M: Levetiracetam-induced
leukocytoclastic vasculitis. Indian J Pharmacol. 49:124–126.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arican P, Gencpinar P, Cavusoglu D and
Olgac Dundar N: Levetiracetam monotherapy for the treatment of
infants with epilepsy. Seizure. 56:73–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kellinghaus C: Lacosamide as treatment for
partial epilepsy: Mechanisms of action, pharmacology, effects, and
safety. Ther Clin Risk Manag. 5:757–766. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Niespodziany I, Leclère N, Vandenplas C,
Foerch P and Wolff C: Comparative study of lacosamide and classical
sodium channel blocking antiepileptic drugs on sodium channel slow
inactivation. J Neurosci Res. 91:436–443. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wilson SM and Khanna R: Specific binding
of lacosamide to collapsin response mediator protein 2 (CRMP2) and
direct impairment of its canonical function: Implications for the
therapeutic potential of lacosamide. Mol Neurobiol. 51:599–609.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saadi A, Patenaude B and Mateen FJ:
Quality of life in epilepsy-31 inventory (QOLIE-31) scores: A
global comparison. Epilepsy Behav. 65:13–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Linane A, Lagrange AH, Fu C and
Abou-Khalil B: Generalized onset seizures with focal evolution
(GOFE)-A unique seizure type in the setting of generalized
epilepsy. Epilepsy Behav. 54:20–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xue-Ping W, Hai-Jiao W, Li-Na Z, Xu D and
Ling L: Risk factors for drug-resistant epilepsy: A systematic
review and meta-analysis. Medicine (Baltimore).
98(e16402)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weisman H, Fried I, Gilboa T, Bennett-Back
O, Ekstein D, Shweiki M, Shoshan Y and Benifla M: Prevalence,
characteristics, and long-term prognosis of epilepsy associated
with pediatric brain tumors. World Neurosurg. 109:e594–e600.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ruffolo G, Di Bonaventura C, Cifelli P,
Roseti C, Fattouch J, Morano A, Limatola C, Aronica E, Palma E and
Giallonardo AT: A novel action of lacosamide on GABAA currents sets
the ground for a synergic interaction with levetiracetam in
treatment of epilepsy. Neurobiol Dis. 115:59–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gulati P, Cannell P, Ghia T, Bint L, Walsh
P, Ghosh S and Nagarajan L: Lacosamide as adjunctive therapy in
treatment-resistant epilepsy in childhood. J Paediatr Child Health.
51:794–797. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kaciński M, Budziszewska B, Lasoń W, Zajac
A, Skowronek-Bała B, Leśkiewicz M, Kubik A and Basta-Kaim A: Level
of S100B protein, neuron specific enolase, orexin A, adiponectin
and insulin-like growth factor in serum of pediatric patients
suffering from sleep disorders with or without epilepsy. Pharmacol
Rep. 64:1427–1433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang LG, Zhai QX, Tang ZH, Zhang YX, Guo
YX, Chen ZH, Wang C, Zhuo MQ, Zeng XL and Zhang JW: Effect of
ilepcimide combined western drugs on serum level of neuron specific
enolase in treating epilepsy children patients. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 36:912–915. 2016.PubMed/NCBI(In Chinese).
|
|
22
|
Xu Z, Xue T, Zhang Z, Wang X, Xu P, Zhang
J, Lei X, Li Y, Xie Y, Wang L, et al: Role of signal transducer and
activator of transcription-3 in up-regulation of GFAP after
epilepsy. Neurochem Res. 36:2208–2215. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chang CC, Lui CC, Lee CC, Chen SD, Chang
WN, Lu CH, Chen NC, Chang AY, Chan SH and Chuang YC: Clinical
significance of serological biomarkers and neuropsychological
performances in patients with temporal lobe epilepsy. BMC Neurol.
12(15)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fu J, Peng L, Li J, Tao T and Chen Y:
Effects of second-generation antiepileptic drugs compared to
first-generation antiepileptic drugs on bone metabolism in patients
with epilepsy: A meta-analysis. Horm Metab Res. 51:511–521.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mintzer S, Boppana P, Toguri J and
DeSantis A: Vitamin D levels and bone turnover in epilepsy patients
taking carbamazepine or oxcarbazepine. Epilepsia. 47:510–515.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fernandez H, Cooke M and Patel T: Epilepsy
and lifestyle behaviors related to bone health. Epilepsia.
60:2306–2313. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Beniczky SA, Viken J, Jensen LT and
Andersen NB: Bone mineral density in adult patients treated with
various antiepileptic drugs. Seizure. 21:471–472. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Anwar MJ, Radhakrishna KV and Vohora D:
Phenytoin and sodium valproate but not levetiracetam induce bone
alterations in female mice. Can J Physiol Pharmacol. 92:507–511.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Haggar SM, Mostafa TM, Allah HMS and
Akef GH: Levetiracetam and lamotrigine effects as mono- and
polytherapy on bone mineral density in epileptic patients. Arq
Neuropsiquiatr. 76:452–458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Robins A, Patel M and Azim A: A case of
physical and mental adverse drug reactions associated with
levetiracetam in post-stroke epilepsy. J Am Geriatr Soc.
60:159–160. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen J, Liu XM, Yue X and Chen SZ: The
clinical efficacy and safety of levetiracetam add-on therapy for
child refractory epilepsy. Eur Rev Med Pharmacol Sci. 20:2689–2694.
2016.PubMed/NCBI
|
|
32
|
Rocamora R, Ley M, Molins A, Toledo M,
Sansa G, Bertol V, Becerra JL, Carreño M and Mauri JÁ: Effect of
lacosamide on depression and anxiety symptoms in patients with
focal refractory epilepsy: A prospective multicenter study.
Epilepsy Behav. 79:87–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakhutina L, Kunnakkat SD, Coleman M,
Lushbough C, Arnedo V, Soni N and Grant AC: Effects of adjunctive
lacosamide on mood and quality of life in patients with epilepsy.
Epilepsy Behav. 73:90–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Böttcher S, Lutz MT and Mayer T:
Lacosamide in the treatment of patients with epilepsy and
intellectual disabilities: A long-term study of 136 patients.
Epilepsia. 58:1749–1754. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sunwoo JS, Park BS, Ahn SJ, Hwang S, Park
CY, Jun JS, Kim DW, Lee ST, Jung KH, Park KI, et al: Three-year
retention rates of levetiracetam, topiramate, and oxcarbazepine: A
retrospective hospital-based study. Clin Neuropharmacol. 40:56–62.
2017.PubMed/NCBI View Article : Google Scholar
|