Introduction
Lichen planus is a chronic inflammatory disease that
effects the skin and oral mucosa (1). It affects ~1-2% of people over 40
years worldwide (2). As with oral
mucosa, buccal mucosa, the tongue and gingiva are the main tissues
affected (3). Oral lichen planus
(OLP) is commonly affected by bilateral lesions and presents
clinically in multiple forms, such as reticular, papular,
plaque-like, erosive, atrophic and bullous (4). OLP is a potentially malignant disorder
of the oral mucosa (5), indicating
that it may undergo malignant transformation (6). Repeated biopsies are required for
suspected lesions. Consequently, a non-invasive tool for assisting
in the follow-up of ongoing diseases is urgently required within
clinics. A number of non-invasive techniques have been developed,
such as toluidine blue and Lugol's iodine vital staining,
autofluorescence, chemiluminescence and brush biopsy (7). The use of these adjuncts is useful and
helps to increase clinical diagnostic sensitivity and specificity
of OLP compared to clinical examination alone, even if not markedly
(7).
Reflectance confocal microscopy (RCM) is a novel
in vivo infrared optical detection device that has been
widely used in the examination of dermatological (8,9) and
oral mucosal diseases (10,11) in recent years. RCM provides
real-time horizontal frame images at the cellular level, which can
reach the superficial dermis (12).
The contrast of RCM images relies on the different refractive index
of subcellular components (13). A
number of studies assessing the application of RCM in OLP have been
previously reported and RCM cellular and architectural findings
have been described (14-17).
However, to the best of our knowledge, relevant studies in a
Chinese cohort have not been performed. The present study aimed to
observe the RCM features of OLP in a Chinese population.
Materials and methods
Patients
A total of 47 patients (25 males and 22 females) who
were clinically diagnosed with OLP, with a mean age of 49.4 years
(range, 29-74), were enrolled at the Ninth People's Hospital,
Shanghai Jiao Tong University, School of Medicine between April
2015 and June 2016 (Table I). The
patients received an oral dose of fluconazole 50 mg once a day and
a 1% sodium bicarbonate mouth wash three times a day for two weeks.
Inclusion criteria were patients with newly diagnosed OLP
exhibiting a reticular pattern, which was clinically diagnosed by
an oral medicine specialist and confirmed by subsequent
histopathological examination according to modified World Health
Organization (WHO) diagnostic criteria (18). Exclusion criteria were lesions with
erosion and ulceration. All patients were informed about the
purpose and procedure of the study and provided written informed
consent. Ethical approval for this investigation was obtained from
the Ethics Committee of Shanghai Ninth People's Hospital, Shanghai
Jiao Tong University School of Medicine (approval no. 2014038).
 | Table IPatient demographics. |
Table I
Patient demographics.
| Demographic | Value |
|---|
| Sex (no.) |
|
Male | 25 |
|
Female | 22 |
| Mean age (years) | 49.4±13.3 |
| Localization
(no.) |
|
Lower lip
(dysplasia) | 11(1) |
|
Upper
lip | 4 |
|
Dorsal
tongue (dysplasia) | 21(4) |
|
Ventral
surface of the tongue | 5 |
|
Buccal
mucosa (dysplasia) | 6(2) |
In vivo reflectance confocal
microscopy (RCM)
A commercial RCM (Vivascope 3000; Lucid, Inc.) with
a light source of a near-infrared wavelength (830 nm) stimulated by
a diode laser at the variable power of 0-22 mW was used in the
present study. The system included a 30x water immersion objective
lens with a numerical aperture of 0.9. A tissue ring was attached
to the lens after ultrasound gel was interposed between the lens
and the coverglass. The images were displayed in 1,000x1,000-pixel
horizontal sections. The lateral resolution could reach 0.5-1 µm.
The Vivastack mode was taken to obtain sequential sections of vital
tissue at intervals of 5 mm from the superficial layers to the
submucosa. Patients were examined using RCM and the healthy
contralateral sides of the lesion area were also examined using RCM
as a control.
RCM features, including parakeratosis, acanthosis,
liquefaction degeneration, inflammatory cell infiltration and
dilated blood vessels were evaluated. In addition, RCM features
were analyzed for their sensitivity and specificity for evaluating
the accuracy of RCM. Sensitivity is presented as the percentage of
OLP lesions with the RCM features and specificity is presented as
the percentage of healthy areas without RCM features.
Biospy preparation
Each lesion underwent an incisional biopsy (~5x5 mm)
after two weeks of treatment, using a scalpel blade under local
anesthesia (2% lidocaine with epinephrine) at the same lesion site
that was used for the RCM analysis. Biopsy specimens were routinely
fixed in 10% phosphate-buffered neutral formalin for 24 h at room
temperature (20-25˚C), embedded in paraffin and sectioned
vertically in the traditional manner. Subsequently, 5-mm thick
sections were stained with hematoxylin for 5-10 min and eosin for
1-5 min at room temperature (20-25˚C). Two experienced pathologists
performed histopathological evaluations using a light microscope
(BX51; Olympus Corporation) with a digital camera (DP71; Olympus
Corporation) under x100, x200 and x400 magnification.
Results
Study population
The present study was comprised of 25 males and 22
females. A total of 47 lesions and 47 perilesional healthy areas
were examined: 11 on the lower lip, 4 on the upper lip, 21 on the
dorsal tongue, 5 on the ventral surface of the tongue, 6 on the
buccal mucosa and 0 on the gingiva. A total of 7 patients presented
with histopathological dysplasia. Table
I presents the demographic characteristics of the patients that
were enrolled.
RCM features
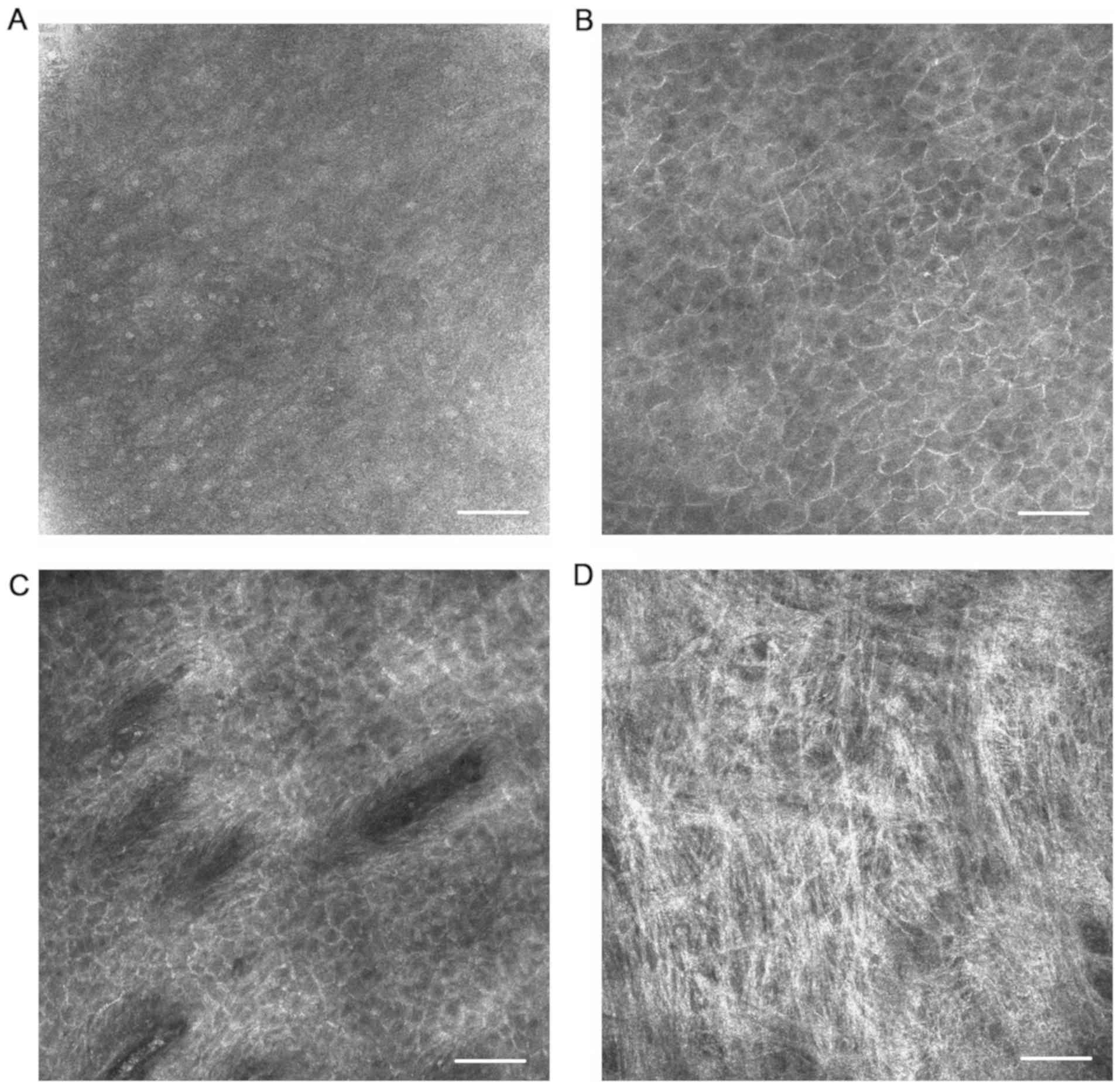
In the healthy tissue sites, keratinocytes were
identified by their outlines and the rounded highly refractive
nucleolus was observed within the cells of the thin superficial
layer (Fig. 1A). At the stratum
spinosum, smaller cells presented a honeycomb-like architecture
(Fig. 1B). At the level of the
epithelial-connective tissue junction, connective tissue papillae
and blood flowing inside the vessels could be seen in vivo,
especially on the lips (Fig. 1C).
Connective tissue could also be observed in the dermis, although
not very clearly (Fig. 1D). In the
specialized lingual epithelium, filiform papillae exhibited
elongated sickle-like structures and the taste buds consisted of
large, spindle-shaped cells.
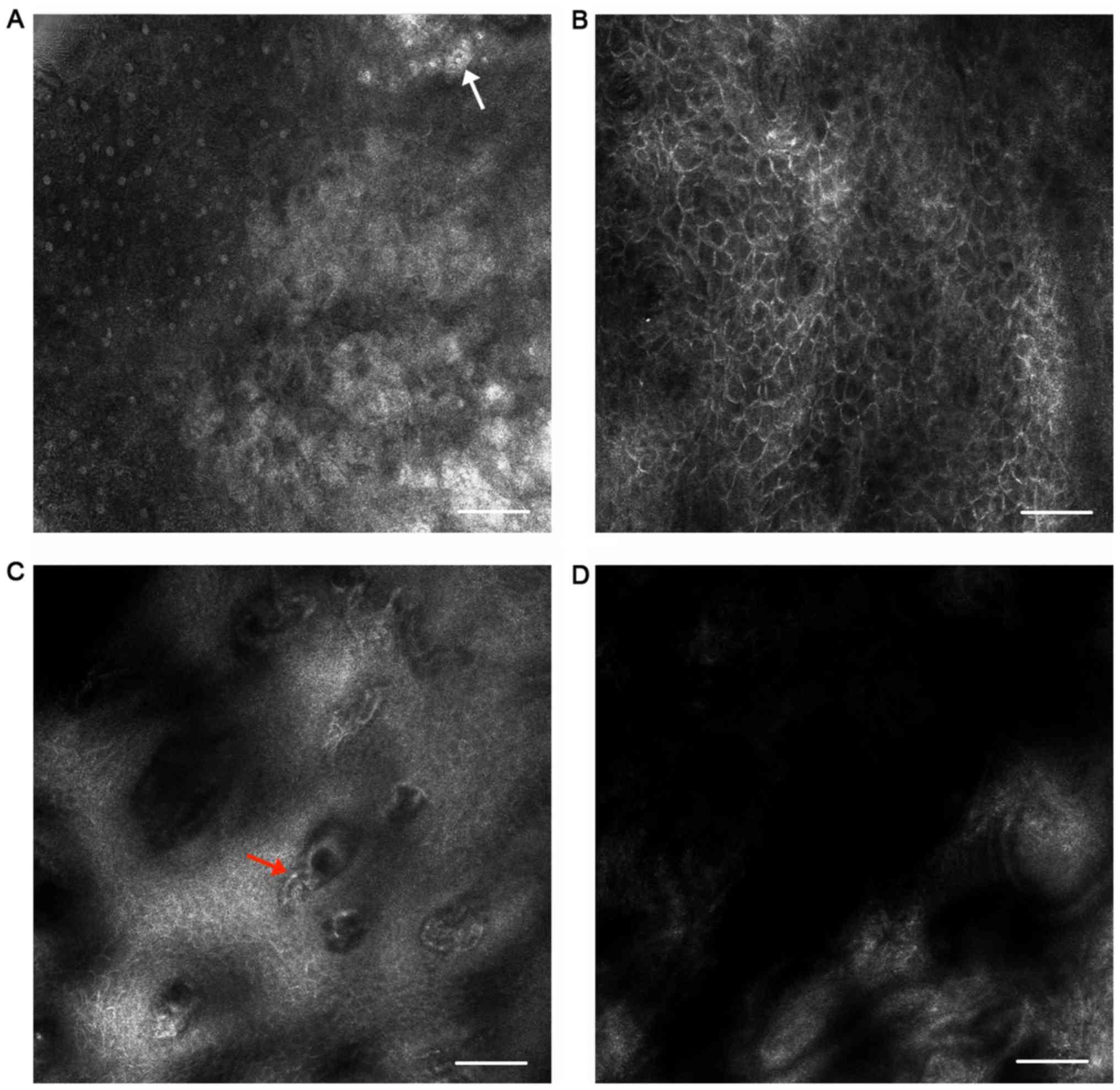
In OLP, at the superficial layers, hyperkeratosis
could be detected (Fig. 2A). A
greater number of layers were identified in the stratum spinosum in
OLP lesions than in normal sites using the Vivastack mode, which
meant that acanthosis could be observed (Fig. 2B). Keratinocytes presented larger at
this layer. At the epithelial-connective tissue junction, the
ring-like bright structures disappeared due to necrosis of the
keratinocytes. Non-rimmed papillae were obscured by roundish, small
inflammatory cells (Fig. 2C). Dark
lumen structures with bright round cells located inside these
structures were visible in the papillary dermis, which corresponded
to dilated vessels with inflammatory cell infiltration (Fig. 2D). Bright large dentritic
structures, which were interpreted to be melanophages, could be
seen in the dermis.
Statistical analysis
Sensitivity and specificity were calculated. The
sensitivity and specificity of inflammatory cells were 0.70 and
0.87, respectively. The values of non-rimmed papillae were 0.47 and
1.00, respectively. The sensitivity of non-rimmed papillae of
lesions on the dorsal tongue was reduced to 0.29 (Table II).
 | Table IIFeatures of oral lichen planus
indicated by reflectance confocal microscopy, sensitivity and
specificity analysis. |
Table II
Features of oral lichen planus
indicated by reflectance confocal microscopy, sensitivity and
specificity analysis.
| | Lower lip (n=11) | Upper lip (n=4) | Dorsal tongue
(n=21) | Ventral tongue
(n=5) | Buccal (n=6) | Total (n=47) |
|---|
| Features | n | Sensitivity (%) | Specificity (%) | n | Sensitivity (%) | Specificity (%) | n | Sensitivity (%) | Specificity (%) | n | Sensitivity (%) | Specificity (%) | n | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) |
|---|
| Hyperkeratosis | 2 | 18 | 100 | 0 | 0 | 75 | 5 | 24 | 62 | 1 | 20 | 60 | 1 | 17 | 83 | 19 | 74 |
| Acanthosis | 7 | 64 | 91 | 3 | 75 | 25 | 17 | 81 | 29 | 2 | 40 | 60 | 3 | 50 | 67 | 68 | 51 |
| Nonrimmed
papillae | 7 | 64 | 100 | 1 | 25 | 100 | 6 | 29 | 100 | 3 | 60 | 100 | 5 | 83 | 100 | 47 | 100 |
| Inflammatory
cells | 8 | 73 | 73 | 1 | 25 | 75 | 15 | 71 | 95 | 5 | 100 | 100 | 4 | 67 | 83 | 70 | 87 |
| Increased
vascularity | 8 | 73 | 64 | 1 | 25 | 50 | 6 | 29 | 95 | 3 | 60 | 100 | 4 | 67 | 83 | 47 | 83 |
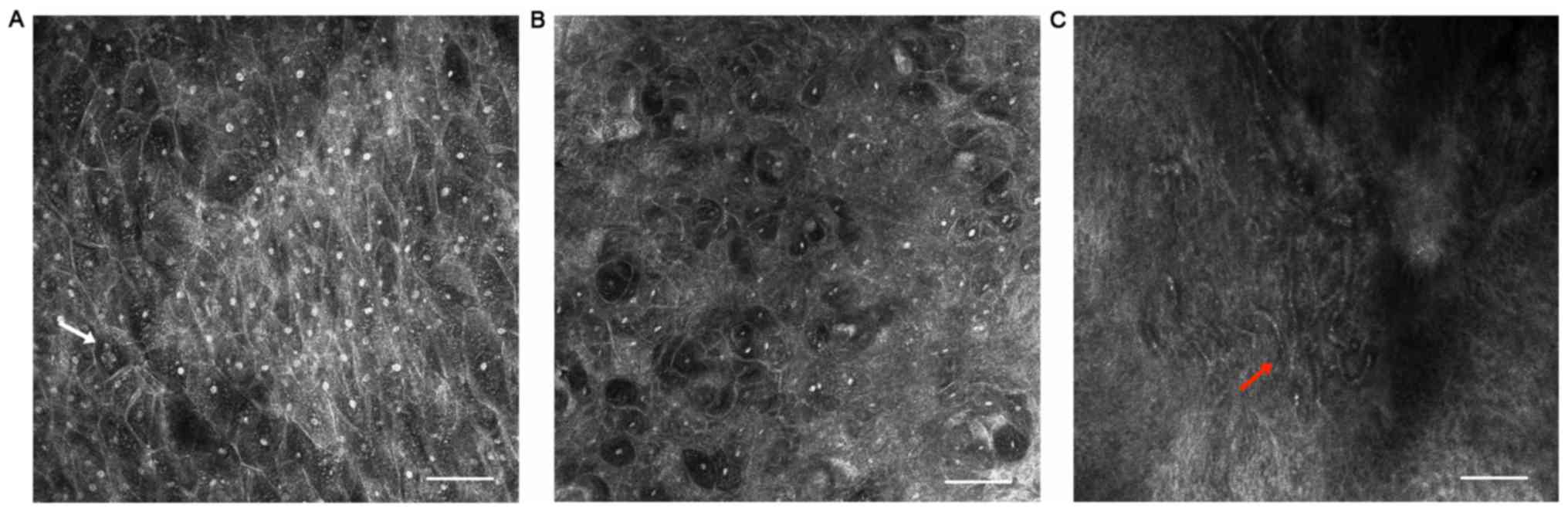
Epithelial dysplasia
In a total of 7 cases, histopathological examination
revealed various degrees of dysplasia. Corresponding features were
also identified using RCM imaging, such as keratinocytes which
varied in size, disarrangement at the stratum spinosum (Fig. 3B) and multi-nucleolated cells
(Fig. 3A) being occasionally
detected. Intercellular dark spaces and neoangiogenesis were also
observed (Fig. 3C).
Discussion
OLP is a chronic inflammatory condition that is
caused by autoimmune disorders (19). OLP may manifest in various ways,
such as in a reticular-plaque pattern or as erosive lesions and
plaques (20). The plaque type of
OLP can resemble leukoplakia by visual inspection and palpation
(21). Furthermore, OLP is
considered to be a disease that results in a significantly
increased risk of developing cancer (22). As such, it is essential for patients
with OLP to visit a doctor regularly. A rapid noninvasive tool
would be beneficial for clinical diagnostic evaluations during
patient follow-ups. RCM has been used recently for oral mucosal
diseases.
A number of studies have been published
investigating the applications for RCM in OLP. Contaldo et
al (14) reported
parakeratosis, hypergranulosis, acanthosis, necrotic keratinocytes,
disrupted connective tissue papillae and inflammatory cell
infiltration in RCM images of OLP tissue. Alessi et al
(16) corroborated these findings
and described melanophages as bright, large dendritic cells in five
cases. In the present study, hyperkeratosis, parakeratosis and
acanthosis were observed. At the level of the epithelial-connective
tissue junction, the disappearance of bright ring-like structures
indicated the destruction of the basal layer (14), which corresponded with the observed
histopathological characteristics (23). Papillary rims may be obscured by the
presence of inflammatory cells, which present as brightly
refractive, roundish structures, but unlike in histopathology it
was not possible to further distinguish the inflammatory cells
(24). Dilated vessels and
melanophages were also identified in the upper dermis.
Maturational disorders may occur in OLP due to its
chronic inflammatory condition (25). Contaldo et al (14) briefly described multi-nucleolated
cells and structures that resembled keratin pearls, which were
identified in two lesions upon RCM examination. The WHO has deemed
OLP as a premalignant disease (25). A previous study also demonstrated
that the reticular type of OLP had the highest rate of malignant
transformation (26). In the
present study, a total of 7 biopsy samples were revealed to exhibit
various degrees of epithelial dysplasia. Cellular disarrangement,
pleomorphism, dispersed nuclei and large intercellular spaces were
also identified. In 2019, Contaldo et al (15) reported that the most characteristic
RCM criteria for malignancies were cytological and architectural
disarray in well-differentiated oral squamous cell carcinoma, as
well as cellular pleomorphism, increased nuclear-cytoplasmic ratios
and multi-nucleolated keratinocytes in moderately/poorly
differentiated oral squamous cell carcinoma. These findings were
similar to those in the present study, which indicated that these
features may suggest underlying cancerous changes in OLP lesions.
However, only few features of dysplasia were detected by RCM in the
present study. Further work is required to assess the use of RCM in
the diagnosis of precancerous lesions.
In the present study, the sensitivity and
specificity of the RCM features on the dorsal tongue were lower
compared with other sites, such as lips and buccal mucosa, due to
the disturbance of the tongue papillae and taste buds.
Hyperkeratosis and acanthosis also interfere with the display
resolution of RCM (27). The
features of RCM are difficult to identify in the deeper layer of
the sections due to fact the upper tissue layer interferes with
image formation (28). It has also
been reported that in strongly keratinized lesions, images cannot
be clearly identified (15).
Subclinical inflammation and alteration may be present on the
clinically unaffected areas which would also interfere with
specificity values. Otherwise, it has been reported that it is
difficult to distinguish between the range and type of infiltrating
inflammatory cells (29), as well
as between melanocytes and Langerhans cells (30). Furthermore, a more portable device
would be helpful during manipulation (31). Recently a new video-mosaicking
approach was reported for intraoral imaging, meaning this technique
may be able to overcome the limitation of original confocal
microscopy (32).
In conclusion, RCM may be a non-invasive, adjunctive
tool that can be used to evaluate OLP in real-time and may be of
use for the diagnosis of OLP to avoid unnecessary invasive
procedures. Furthermore, RCM may potentially be used as an
auxiliary tool to reduce the area of resection required when there
are epithelial alterations that are not visible to the naked eye.
The diagnostic value of RCM criteria needs to be further confirmed
through a blinded evaluation comparing OLP and non-OLP lesions.
Further research is necessary to determine the characteristics and
use of RCM in OLP lesions for future clinical applications.
Acknowledgements
The authors would like to thank Dr Zhen Tian and Dr
Lizhen Wang (Department of Oral Pathology, Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine) for helping with
pathological assessments.
Funding
The current study was supported by the National
Nature Science Foundation of China (grant no. 81802694) and Seed
Funding of Shanghai Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine (grant no. JYZZ046).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and GZ designed the study. HP, GZ and LS
collected the clinical data. HP and GZ performed the experiments
and analyzed the data. HP and YWwrote the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for this investigation was obtained
from the Ethics Committee of Shanghai Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine (approval no.
2014038).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skrinjar I, Vidranski V, Brzak BL, Vidovic
Juras D, Andabak Rogulj A, Brailo V and Vucicevic Boras V: Salivary
cortisol levels in patients with oral lichen planus-A pilot
case-control study. Dent J (Basel). 7(59)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lavanya N, Jayanthi P, Rao UK and
Ranganathan K: Oral lichen planus: An update on pathogenesis and
treatment. J Oral Maxillofac Pathol. 15:127–132. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ismail SB, Kumar SK and Zain RB: Oral
lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis,
management and malignant transformation. J Oral Sci. 49:89–106.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jones KB and Jordan R: White lesions in
the oral cavity: Clinical presentation, diagnosis, and treatment.
Semin Cutan Med Surg. 34:161–170. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Warnakulasuriya S, Reibel J, Bouquot J and
Dabelsteen E: Oral epithelial dysplasia classification systems:
Predictive value, utility, weaknesses and scope for improvement. J
Oral Pathol Med. 37:127–133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mendes SF, de Oliveira Ramos G, Rivero ER,
Modolo F, Grando LJ and Meurer MI: Techniques for precancerous
lesion diagnosis. J Oncol. 2011(326094)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
White WM, Rajadhyaksha M, González S,
Fabian RL and Anderson RR: Noninvasive imaging of human oral mucosa
in vivo by confocal reflectance microscopy. Laryngoscope.
109:1709–1717. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
González S, Sánchez V, González-Rodríguez
A, Parrado C and Ullrich M: Confocal microscopy patterns in
nonmelanoma skin cancer and clinical applications. Actas
Dermosifiliogr. 105:446–458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maher NG, Collgros H, Uribe P, Ch'ng S,
Rajadhyaksha M and Guitera P: In vivo confocal microscopy for the
oral cavity: Current state of the field and future potential. Oral
Oncol. 54:28–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dental Supplement, Romano A, Santarelli A,
Lajolo C, Della Vella F, Mascitti M, Serpico R and Contaldo M:
Analysis of oral mucosa erosive-ulcerative lesions by reflectance
confocal microscopy. J Biol Regul Homeost Agents. 33 (Suppl
1):S11–S17. 2019.PubMed/NCBI
|
|
12
|
Rajadhyaksha M, González S, Zavislan JM,
Anderson RR and Webb RH: In vivo confocal scanning laser microscopy
of human skin II: Advances in instrumentation and comparison with
histology. J Invest Dermatol. 113:293–303. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dental Supplement, Contaldo M, Lajolo C,
Di Petrillo M, Ballini A, Inchingolo F, Serpico R and Romano A:
Analysis of lip pigmentations by reflectance confocal microscopy:
Report of two cases. J Biol Regul Homeost Agents. 33 (Suppl
1):S19–S25. 2019.PubMed/NCBI
|
|
14
|
Contaldo M, Di Stasio D, Petruzzi M,
Serpico R and Lucchese A: In vivo reflectance confocal microscopy
of oral lichen planus. Int J Dermatol. 58:940–945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Contaldo M, Lauritano D, Carinci F, Romano
A, Di Stasio D, Lajolo C, Della Vella F, Serpico R and Lucchese A:
Intraoral confocal microscopy of suspicious oral lesions: A
prospective case series. Int J Dermatol: Jul 9, 2019 (Epub ahead of
print).
|
|
16
|
Alessi SS, Nico MM, Fernandes JD and
Lourenco SV: Reflectance confocal microscopy as a new tool in the
in vivo evaluation of desquamative gingivitis: Patterns in mucous
membrane pemphigoid, pemphigus vulgaris and oral lichen planus. Br
J Dermatol. 168:257–264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ardigo M, Donadio C, Franceschini C,
Catricala C and Agozzino M: Interest of reflectance confocal
microscopy for inflammatory oral mucosal diseases. J Eur Acad
Dermatol Venereol. 29:1850–1853. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van der Meij EH and van der Waal I: Lack
of clinicopathologic correlation in the diagnosis of oral lichen
planus based on the presently available diagnostic criteria and
suggestions for modifications. J Oral Pathol Med. 32:507–512.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kurago ZB: Etiology and pathogenesis of
oral lichen planus: An overview. Oral Surg Oral Med Oral Pathol
Oral Radiol. 122:72–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sugerman PB and Sabage NW: Oral lichen
planus: Causes, diagnosis and management. Aust Dent J. 47:290–297.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Edwards PC and Kelsch R: Oral lichen
planus: Clinical presentation and management. J Can Dent Assoc.
68:494–499. 2002.PubMed/NCBI
|
|
22
|
Thongprasom K, Carrozzo M, Furness S and
Lodi G: Interventions for treating oral lichen planus. Cochrane
Database Syst Rev. 7(CD001168)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moscarella E, González S, Agozzino M,
Sánchez-Mateos JL, Panetta C, Contaldo M and Ardigò M: Pilot study
on reflectance confocal microscopy imaging of lichen planus: A
real-time, non-invasive aid for clinical diagnosis. J Eur Acad
Dermatol Venereol. 26:1258–1265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Agozzino M, González S and Ardigò M:
Reflectance confocal microscopy for inflammatory skin diseases.
Actas Dermosifiliogr. 107:631–639. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
25
|
Irani S, Esfahani AM and Ghorbani A:
Dysplastic change rate in cases of oral lichen planus: A
retrospective study of 112 cases in an Iranian population. J Oral
Maxillofac Pathol. 20:395–399. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mattsson U, Jontell M and Holmstrup P:
Oral lichen planus and malignant transformation: Is a recall of
patients justified? Crit Rev Oral Biol Med. 13:390–396.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Incel P, Gurel MS and Erdemir AV: Vascular
patterns of nonpigmented tumoral skin lesions: Confocal
perspectives. Skin Res Technol. 21:333–339. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Contaldo M, Poh CF, Guillaud M, Lucchese
A, Rullo R, Lam S, Serpico R, MacAulay CE and Lane PM: Oral mucosa
optical biopsy by a novel handheld fluorescent confocal microscope
specifically developed: Technologic improvements and future
prospects. Oral Surg Oral Med Oral Pathol Oral Radiol. 116:752–758.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ardigò M, Maliszewski I, Cota C, Scope A,
Sacerdoti G, Gonzalez S and Berardesca E: Preliminary evaluation of
in vivo reflectance confocal microscopy features of discoid lupus
erythematosus. Br J Dermatol. 156:1196–1203. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hashemi P, Pulitzer MP, Scope A,
Kovalyshyn I, Halpern AC and Marghoob AA: Langerhans cells and
melanocytes share similar morphologic features under in vivo
reflectance confocal microscopy: A challenge for melanoma
diagnosis. J Am Acad Dermatol. 66:452–462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lucchese A, Gentile E, Romano A, Maio C,
Laino L and Serpico R: The potential role of in vivo reflectance
confocal microscopy for evaluating oral cavity lesions: A
systematic review. J Oral Pathol Med. 45:723–729. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Peterson G, Zanoni DK, Ardigo M, Migliacci
JC, Patel SG and Rajadhyaksha M: Feasibility of a video-mosaicking
approach to extend the field-of-view for reflectance confocal
microscopy in the oral cavity in vivo. Lasers Surg Med: May 8, 2019
(Epub ahead of print).
|