Introduction
The main function of the intestinal epithelium is to
absorb water and nutrients to maintain life, and the transport of
these substances should be tightly regulated in order to maintain
homeostasis (1). The intestinal
epithelium is an important structure, responsible for transporting
water and nutrients, and constitutes a physical barrier regulating
the selective paracellular permeability to ions and molecules
(2). Adjacent intestinal epithelial
cells are tightly packed together and are connected via three types
of junctional complexes, namely desmosomes, adherens junctions and
tight junctions (TJs) (3). TJs are
the most apical junctions along the lateral surface, and are the
main structure regulating epithelial barrier function (4). TJs are composed of transmembrane
proteins, which include claudins, occludin and tricellulin, and
cytosolic scaffold proteins, such as zona occludens proteins
(ZO)1-3, Afadin (AF-6) and cingulin (2).
Ulcerative colitis (UC) is a chronic
gastrointestinal disorder characterized by diffuse mucosal
inflammation of the colon. In UC, pathological lesions are mainly
located in the rectum, affecting a part or the whole large
intestine (5). Moreover, the
clinical course of the disease is characterized by remissions and
exacerbations (6). It has been
reported that dysregulation of the intestinal mucosal barrier
contributes to the development, perpetuation and severity of
inflammatory bowel disease (IBD) (7). At the same time, IBD may also
contribute to a leaky epithelial barrier due to the inflammatory
microenvironment (8). In UC,
impaired complexity of TJs and downregulation of TJ-related
proteins have been considered as the main mechanisms mediating the
dysfunction of the intestinal mucosal barrier (9,10).
Commonly used drugs for UC treatment include 5-aminosalicylic acid,
immunosuppressants and biological agents (5). Furthermore, given the continuous
progress in the research on intestinal microorganisms, probiotics
have become a novel treatment strategy for UC.
The gastrointestinal tract contains a large and
complex ecology of microorganisms. For example, it is estimated
that ~100 trillion microbes reside in the human adult gut,
collectively known as the gut microbiota (11). The mutually beneficial relationship
between the host and its gut microbiota contributes to host health,
as gut microbiota are involved in several biological processes,
including the absorption of nutrients, protection against
infections, maturation of the immune system and regulation of
metabolism (12). The International
Symposium of the International Scientific Association of Probiotics
and Prebiotics defined probiotics as ‘the living microorganisms
that when administered in adequate amounts, confer health benefits
on the host’ (13). Clostridium
butyricum (CB) is a gram-positive obligate anaerobic
bacillus. Compared with other non-sporulating probiotics, CB
is resistant to the effects of gastric acid and temperature, thus
making its investigation and use easier (14,15).
It has been reported that butyrate, an important type of short
chain fatty acid (SCFA) produced by CB, exhibits positive
effects on the maintenance of intestinal barrier function (16,17).
Therefore, CB is effectively used in clinical practice to
improve the intestinal barrier function and to treat UC. However,
its exact underlying mechanisms require further investigation.
In the present study, a dextran sodium sulfate
(DSS)-induced colitis model was established in C57BL/6 mice to
investigate the potential effects of CB on the expression
levels of TJ-related proteins, intestinal inflammation and
oxidative stress. It has been well established that activation of
the Akt/mTOR pathway and its downstream molecules, including p70
ribosomal protein S6 kinase (S6K) and eukaryotic translation
initiation factor 4E binding protein 1 (4E-BP1), regulate protein
synthesis (18). Therefore, the
present study further investigated the role of CB on the
regulation of TJ-related protein expression levels via the Akt/mTOR
pathway.
Materials and methods
Animals and mouse model
A total of 45 C57BL/6 mice (males; age, 6 weeks;
weight, 16-20 g) were obtained from the Beijing Vital River
Laboratory Animal Technology Co., Ltd. Animals were housed in a
specific pathogen-free room at a temperature of 25˚C, a humidity of
45-50% and a 12 h light/dark cycle. All animals had free access to
food and water, and were allowed to acclimatize to laboratory
conditions for 7 days. All animal experiments were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and were approved by the Ethics Committee of Renmin
Hospital of the Wuhan University.
Colitis was induced in C57BL/6 mice following
treatment with 3% DSS [molecular weight (MW), 35,000-50,000 Da; MP
Biomedicals, LLC] dissolved in drinking water for 7 days, as
previously described (19). The
progression of DSS-induced colitis was monitored daily by measuring
the disease activity index (DAI), as previously described (19). A freeze-dried powder containing
CB bacteria of 5x109 colony forming units (CFU)/g
was purchased from Kexing Biopharm Co., Ltd.
Experimental design
Mice were randomly divided into three experimental
groups (n=15 mice/group: Control, DSS and DSS + CB groups.
In the DSS and DSS + CB mice groups, animals were treated
with 3% DSS dissolved in drinking water for 7 days, while mice in
the control group received standard laboratory water ad
libitum. During the induction of colitis, mice in the DSS +
CB group were co-treated daily with 200 µl (2x108
CFU) CB solution via gavage, as previously described
(20). Mice in the control and DSS
groups received 200 µl normal saline via gavage. All mice were
anesthetized with pentobarbital solution (50 mg/kg) via
intraperitoneal injection 7 days after standard laboratory water or
DSS treatment. Following anesthesia, 0.5 ml blood was collected
from the mice via eyeball enucleation. To isolate colon tissue,
mice were sacrificed by cervical dislocation. Following a 2 min
observation, mice were confirmed to be dead without respiration and
corneal reflex. A 0.5-cm segment of the distal colon was cut and
isolated, rinsed with PBS, fixed for 48 h in 4% (w/v)
paraformaldehyde at 4˚C, dehydrated and embedded in paraffin.
Intestinal permeability assay
The intestinal permeability of mice with DSS-induced
colitis was measured as previously described (21). A total of 44 mg/100 g body weight
FITC-labeled 4-kDa dextran (FD-4; MW, 4,000 Da; Sigma-Aldrich;
Merck KGaA) was administrated to each mouse via gavage following
fasting for 4 h. Following FD-4 administration for 5 h, 400 µl
blood was collected and centrifuged at 2,500 x g for 10 min at 4˚C
to isolate serum. The serum fluorescence intensity was determined
using a multimode reader (PerkinElmer, Inc.) at excitation
wavelength of 485 nm and emission wavelength of 520 nm.
Subsequently, a standard curve was generated using serial dilutions
of FD-4.
Histology
Acolon segment was cut and isolated, its length was
measured and analyzed using histology methods. Rectum tissues were
fixed for 48 h with 4% (w/v) paraformaldehyde at 4˚C, dehydrated
and embedded in paraffin. Subsequently, the tissue samples were
sectioned (thickness, 4 µm), stained with hematoxylin and eosin
(HE) for 97 min at room temperature and observed under an light
microscope, at X100 and X200 magnification. Intestinal inflammation
grading was performed as previously described (22). Histologic score was assessed in a
blinded manner by two independent researchers.
Western blotting
To investigate the protein expression levels of
claudin-1, claudin-2, occludin, and ZO-1, and the activation status
of the Akt/mTOR signaling pathway, total protein samples were
extracted from murine colon tissues using radioimmunoprecipitation
assay lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology), containing phenylmethanesulfonyl fluoride (cat. no.
P105539; Shanghai Aladdin Bio-Chem Technology Co., Ltd.) and
phosphoesterase inhibitors (cat. no. S1873; Beyotime Institute of
Biotechnology). The concentration of the extracted proteins was
measured using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Subsequently, total protein extracts
were dissolved in 5X SDS-PAGE loading buffer and heated in a
boiling water bath for 10 min. A total of 40 µg proteins/lane were
loaded onto an SDS-PAGE (10% gel) and electrophoresis was performed
for 1.5 h prior to protein transfer onto PVDF membranes. Following
transfer, membranes were blocked with 5% skimmed milk in TBS-0.1%
Tween 20 (TBST) buffer for 2 h at room temperature and then
incubated with primary antibodies overnight at 4˚C. The following
primary antibodies were used: Rabbit polyclonal anti-claudin-1
(cat. no. AF0127; 1:1,500), anti-claudin-2 (cat. no. AF0128;
1:1,500), anti-occludin (cat. no. DF7504; 1:1,500), anti-ZO-1 (cat.
no. AF5145; 1:1,000; all from Affinity Biosciences, Inc.),
anti-total-Akt (cat. no. 10176-2-AP; 1:1,000; ProteinTech Group,
Inc.), anti-phosphorylated (p)-Akt (cat. no. 4060p; 1:1,000),
anti-total-mTOR (cat. no. 2983; 1:1,000), anti-total-S6K (cat. no.
2708; 1:1,000), anti-p-S6K (cat. no. 9234; 1:1,000; all from Cell
Signaling Technology, Inc.), anti-GAPDH (cat. no. AB-P-R001;
1:1,000; Hangzhou Goodhere Biotechnology Co., Ltd.). After washing
five times with TBST for 5 min each, membranes were further
immunoblotted with an HRP-conjugated AffiniPure goat anti-rabbit
IgG (cat. no. BA1054; 1:50,000; Wuhan Boster Biological Technology,
Ltd.) secondary antibody for 2 h at 37˚C. Subsequently, membranes
were washed again with TBS-T for five times (5 min/time), treated
with an ECL plus kit (cat. no. P1050; Applygen Technologies Inc.)
for ~5 min at room temperature and the grayscale values of each
band on the blots were analyzed with the BandScan v5.0 software
(Glyko Biomedical Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression levels of claudin-1, claudin-2,
occludin and ZO-1 were determined using RT-qPCR. Colon tissues were
homogenized and total RNA was extracted with TRIzol®
reagent (cat. no. 15596-026; Ambion; Thermo Fisher Scientific,
Inc.). Subsequently, total RNA was reverse transcribed into cDNA
using a Hiscript RT reagent kit (cat. no. R101-01/02; Vazyme
Biotech Co., Ltd.) under the following conditions: 25˚C for 5 min,
50˚C for 15 min, 85˚C for 5 min and 4˚C for 10 min. The qPCR step
was performed using a SYBR Green Master Mix kit (cat. no. Q111-02;
Vazyme Biotech Co., Ltd.), according to the manufacturer's
instructions, on a QuantStudio 6 Flex Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
conditions: 50˚C for 2 min, 95˚C for 10 min; 40 cycles of 95˚C for
30 sec and 60˚C for 30 sec. The data was analyzed with the
2-ΔΔCq method (23).
GAPDH served as the endogenous control. The primer sequences and
corresponding amplicon sizes are listed in Table I.
 | Table IList of PCR primers and amplicon
size. |
Table I
List of PCR primers and amplicon
size.
| Gene name | Primer | Sequence | Size, bp |
|---|
| Mus
GAPDH | Forward |
5'-ATGGGTGTGAACCACGAGA-3' | 229 |
| | Reverse |
5'-CAGGGATGATGTTCTGGGCA-3' | |
| Mus
claudin-1 | Forward |
5'-GGCTGATCGCAATCTTTGT-3' | 131 |
| | Reverse |
5'-TAATGTCGCCAGACCTGAA-3' | |
| Mus
claudin-2 | Forward |
5'-CCGTGTTCTGCCAGGATT-3' | 199 |
| | Reverse |
5'-GCTGAGATGATGCCCAAG-3' | |
| Mus
occludin | Forward |
5'-TGGATCTATGTACGGCTCAC-3' | 203 |
| | Reverse |
5'-CCATCTTTCTTCGGGTTT-3' | |
| Mus
ZO-1 | Forward |
5'-CCAGCAACTTTCAGACCACC-3' | 154 |
| | Reverse |
5'-TTGTGTACGGCTTTGGTGTG-3' | |
ELISA
Murine blood (0.5 ml) was collected from the orbital
venous plexus, as aforementioned, and centrifuged at 2,500 x g for
10 min at 4˚C. The supernatants were subsequently collected and
stored at -20˚C. The cytokines, TNF-α (cat. no. E-EL-M0049c), IL-1β
(cat. no. E-EL-M 0037c), IL-6 (cat. no. E-EL-M0044c), IL-10 (cat.
no. E-EL-M0037c) and IL-13 (cat. no. E-EL-M0727c) (all Elabscience,
Inc.), were quantitatively measured using ELISA (Multiskan MK3;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The absorbance (optical density value) was measured on a
microplate reader and the corresponding concentration of each
sample was converted from the standard curve.
Statistical analysis
The experiments were repeated three times.
Statistical analyses were performed using SPSS 20.0 software (IBM
Corp.). Data are presented as the mean ± SD. All data were analyzed
using mixed and one-way ANOVA and followed by Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
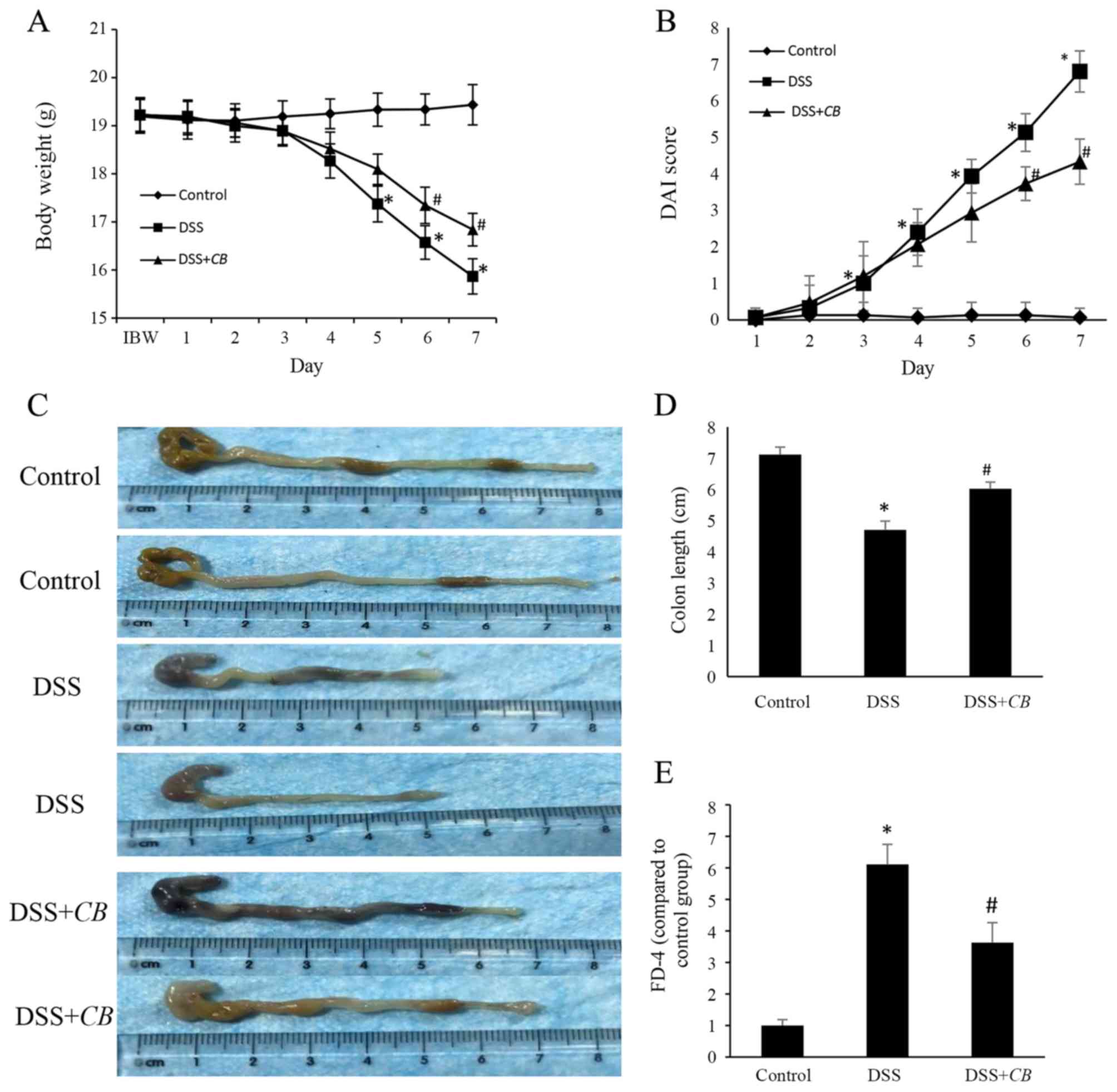
CB ameliorates DSS-induced
colitis
To investigate the therapeutic efficacy of CB
treatment in DSS-induced colitis, the alterations in body weight of
mice were detected. No significant changes in body weight were
observed in the control group during the experiment (Fig. 1). However, mice in the DSS group
exhibited significant weight loss from the 5th day, which was
reversed following treatment with CB from the 6th day.
Subsequently, to assess the symptoms of DSS-induced colitis, the
DAI score was determined in each group (Fig. 1B). The DAI score in the healthy
control group was ~0 during the 7-day experimental period. However,
mice in the DSS and DSS + CB groups developed obvious
symptoms of colitis, including activity reduction, diarrhea and
rectal bleeding. These symptoms appeared at the 3rd day after
colitis induction, reaching peak on the 7th day. Compared with the
DSS group, mice in the DSS + CB group also exhibited a
significantly lower DAI score from day 6. Furthermore, the colon
length was significantly decreased in the DSS group compared with
the control group; however, the colon length was significantly
increased in the DSS + CB group compared with the DSS group
(Fig. 1C and D). These findings indicated that CB
administration effectively alleviated DSS-induced colitis.
CB restores intestinal barrier injury
in the DSS-induced colitis mouse model
FD-4 was used to assess the intestinal permeability
and the effects of CB on intestinal barrier function. Mice
in the DSS group demonstrated 6-fold increased FD-4 serum levels
compared with those in the control group. This finding suggested
that the intestinal barrier function was severely impaired in mice
with DSS-induced colitis. Additionally, compared with the DSS
group, serum FD-4 levels were 1.7-fold lower in the DSS + CB
group (Fig. 1E). The aforementioned
data indicated that CB improved the intestinal epithelial
barrier function in DSS-induced colitis.
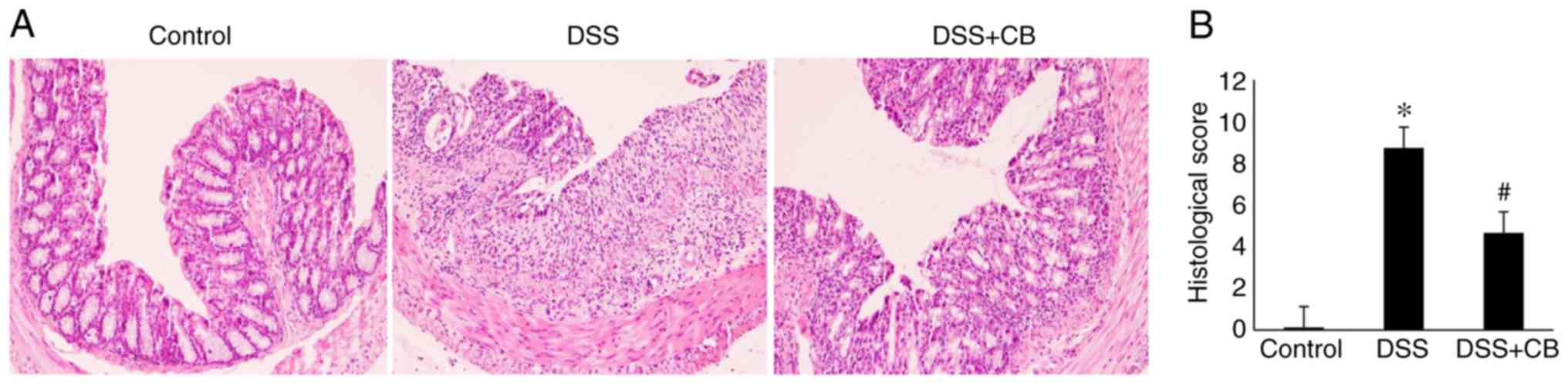
Effects of CB on colon histology in
the DSS-induced colitis mouse model
HE staining demonstrated that CB ameliorated
the histopathologic changes in DSS-induced colitis. In the control
group, the colonic mucosa was intact, glandular cells were arranged
orderly and no inflammatory cell infiltration in the normal crypts
was observed. However, compared with the control group, HE staining
in the DSS group revealed epithelial defects, destroyed crypts and
extensive inflammatory cell infiltration. Furthermore, compared
with the DSS group, in the DSS + CB group, the colonic
mucosa injury was mild, with more intact glands and reduced number
of inflammatory infiltrate cells apparent in the crypts (Fig. 2A). The histological score among the
three groups was significantly different; the score was
significantly increased in the DSS group compared with the control
group; however, the score was significantly decreased in the DSS +
CB group compared with the DSS group (Fig. 2B).
Effects of CB on intestinal
inflammation and oxidative stress
The ELISA results indicated that treatment with DSS
significantly elevated the secretion of proinflammatory cytokines,
including TNF-α, IL-1β and IL-13. However, the increased cytokine
serum levels were reversed following co-treatment with CB.
On the contrary, IL-10 secretion levels were attenuated in the DSS
group compared with those in the control group, but its levels were
increased in CB-treated mice. Moreover, compared with the
control group, IL-6 secretion levels in the DSS group were
significantly elevated, but there was no significant difference
between the DSS and DSS +CB group (Fig. 3A).
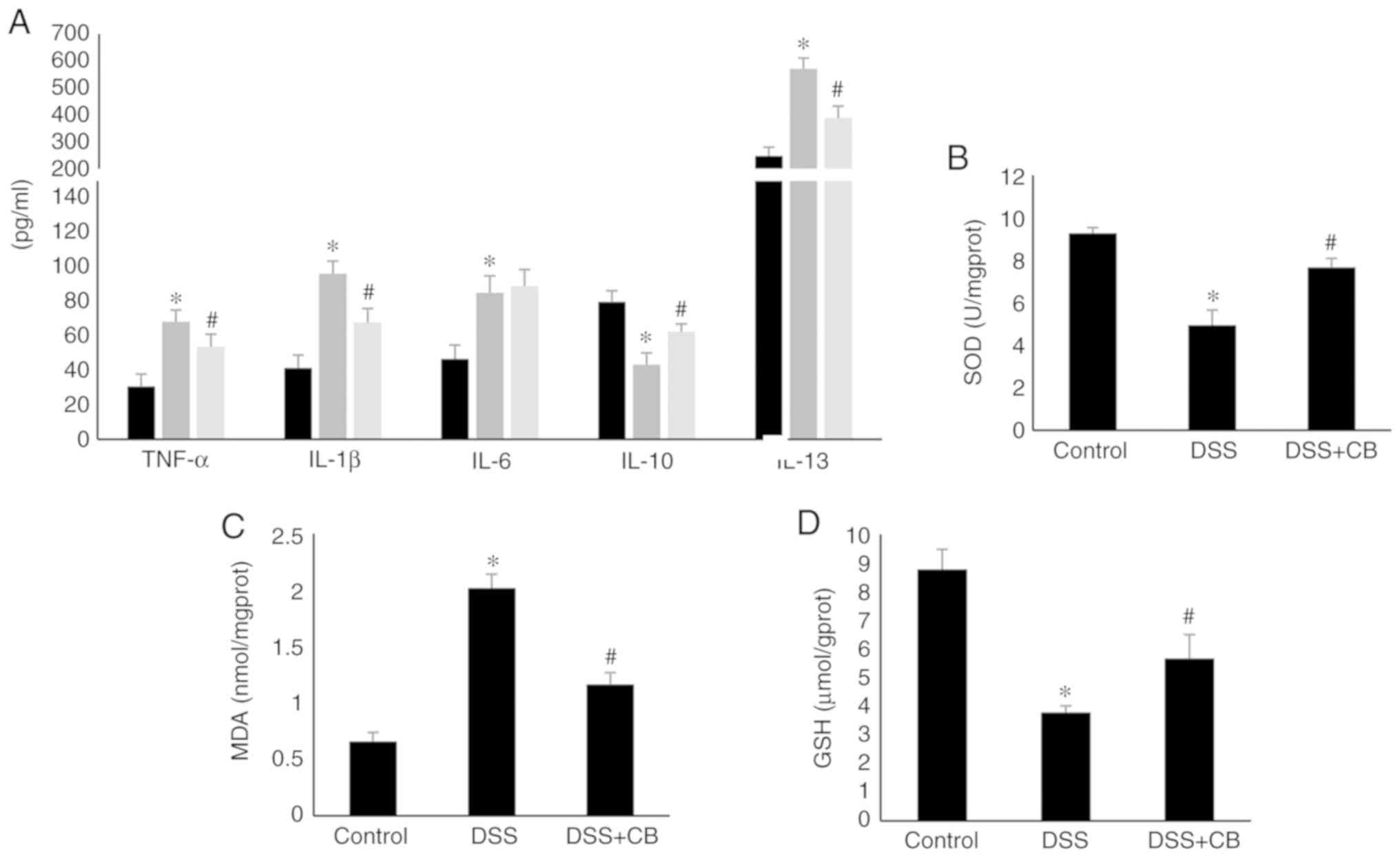 | Figure 3Effect of CB on cytokines
secretion levels and oxidative stress indicators. (Α) Serum levels
of TNF-α, IL-1β, IL-6, IL-10 and IL-13 in different groups. Levels
of oxidative stress indicators, (B) SOD, (C) MDA and (D) GSH in
different groups. Data are presented as the mean ± SD.
*P<0.05 vs. control group; #P<0.05 vs.
DSS group. CB, Clostridium butyricum; TNF-α, tumor
necrosis factor α; IL, interleukin; DSS, dextran sodium sulfate;
MDA, malondialdehyde; GSH, glutathione; SOD, superoxide
dismutase. |
The results demonstrated that CB treatment
attenuated the DSS-induced alterations in lipid peroxidation and
antioxidant enzymatic status in colon tissues. Therefore, following
treatment with DSS, MDA levels were significantly increased, while
those of GSH and SOD were decreased compared with the control
group. However, co-treatment with CB significantly restored
the levels of MDA and GSH, and the enzymatic activity of SOD
(Fig. 3B-D).
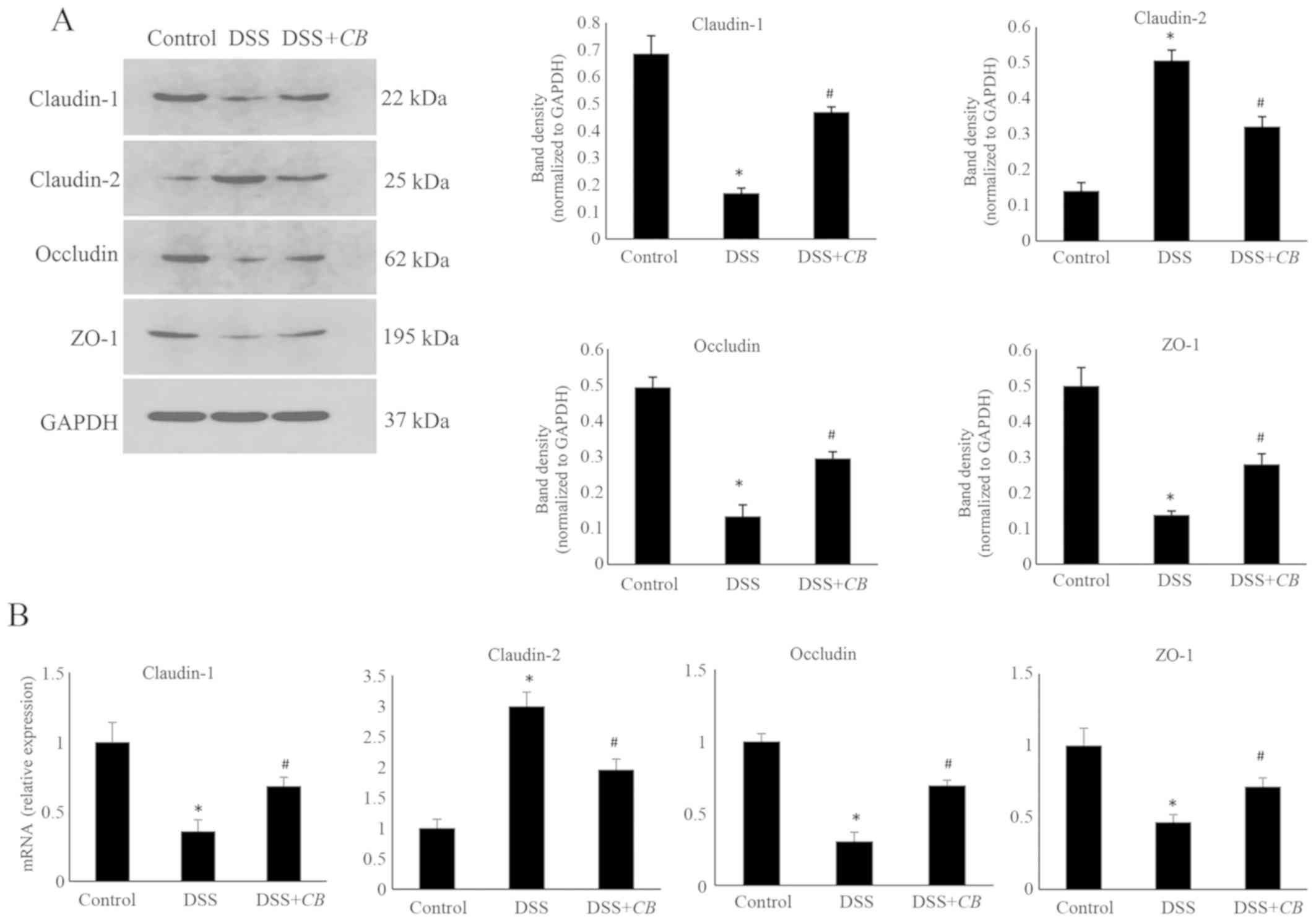
Effects of CB on the expression levels
of claudin-1, claudin-2, occludin and ZO-1
The effects of CB on the protein and mRNA
expression levels of the TJ-related proteins, claudin-1, claudin-2,
occludin and ZO-1, were assessed using western blot analysis
(Fig. 4A) and RT-qPCR (Fig. 4B), respectively. The protein
expression levels of claudin-1, occludin and ZO-1 were decreased,
and those of claudin-2 were elevated in the DSS group compared with
the control group. However, the expression levels of claudin-1,
occludin, ZO-1 and claudin-2 were significantly reversed following
co-treatment with CB. Consistent with the western blotting
results, the relative mRNA synthesis findings obtained via RT-qPCR,
indicated that CB increased the mRNA expression levels of
claudin-1, occludin and ZO-1, and decreased those of claudin-2
(P<0.05).
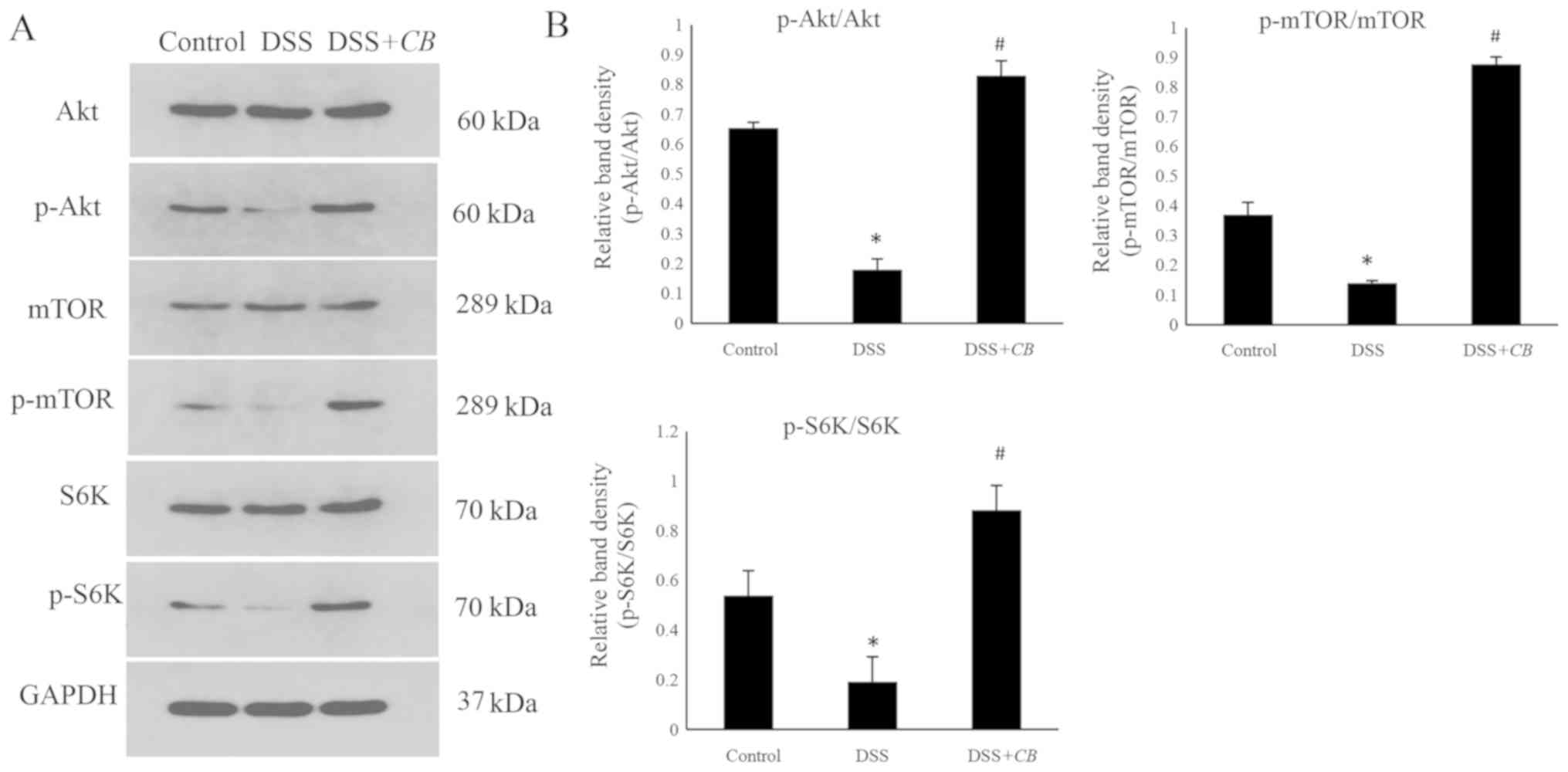
Effects of CB on the activation of the
Akt/mTOR signaling pathway
Further experiments were performed to investigate
whether CB promoted the expression levels of TJ-related
proteins via the Akt/mTOR signaling pathway. The results
demonstrated that in the DSS-induced colitis group p-Akt protein
levels were significantly reduced compared with the control group;
however, in the DSS+CB group, p-Akt protein levels were
significantly increased compared with the DSS group (Fig. 5). Subsequently, the protein
expression levels of p-mTOR and p-S6K, two downstream molecules of
the Akt/mTOR signaling pathway, were detected. Mice in the DSS
group exhibited significantly decreased p-mTOR and p-S6K protein
expression levels compared with the control group. However,
pretreatment with CB elevated both p-mTOR and p-S6K protein
expression levels, and the p-mTOR/mTOR and p-S6K/S6K ratios
compared with the DSS group (Fig.
5). These findings suggested that DSS inhibited the activation
of the Akt downstream signaling molecules, p-mTOR and S6K, while
CB restored the DSS-induced suppression of protein
synthesis.
Discussion
UC and Crohn's disease are the two main forms of IBD
which has been shown to have a complex genetic basis and is
affected by several risk factors, including environmental factors,
the intestinal microbiota and the host immune system (24). Moreover, increasing evidence has
revealed that the impaired intestinal barrier is an important
feature of colitis (25,26). The gut microbiota and the host
immune system constantly communicate with each other to maintain
gut homeostasis and host health (24). CB is a strictly anaerobic
gram-positive bacterium, which produces acetic and butyric acid,
two important components of SCFAs synthetized by gut microbiota
(27). Furthermore, CB
commonly exists in the intestines of humans and animals, and has
been proved effective to treat intestinal inflammation (20,28,29).
CB also serves an important role in providing nutrients for
the host and maintaining the microbial balance in the intestine,
while exhibiting a high tolerance to several antibiotics (30,31).
Therefore, present study aimed to investigate the specific
molecular mechanisms of CB in protecting the intestinal
mucosal barrier function in DSS-induced colitis.
Currently, the DSS-induced colitis model remains the
most widely used mouse model of colitis mimicking human UC due to
its similarities in terms of symptoms and histopathological changes
(19). In the present study,
C57BL/6 mice were treated with 3% DSS for 7 days to establish the
acute colitis mouse model. Colitis in the DSS-induced model was
accompanied by several symptoms, including diarrhea, weight loss,
hematochezia and histological damage, such as surface epithelial
loss, crypt destruction and increased inflammatory cell
infiltration. The present results demonstrated that co-treatment of
DSS-treated mice with CB relieved colitis symptoms and
protected intestinal barrier function. Determining body weight, DAI
and histological grading score in mice in the DSS, DSS + CB
and control groups identified that CB not only alleviated
colitis-related symptoms, maintained body weight and reduced DAI
score, but also prevented histological damage by decreasing
neutrophil infiltration, promoting the restoration of epithelial
cell damage and reducing the rectal histological score.
Furthermore, FD-4 serum levels were significantly decreased in the
DSS + CB group compared with the DSS group.
Proinflammatory cytokines are associated with the
intestinal barrier dysfunction in UC. For example, it has been
reported that TNF-α increases TJ permeability by modulating myosin
light chain kinase (MLCK) gene expression via the NF-κB signaling
pathway in Caco-2 cells (32). In
addition, IL-1β enhances intestinal TJ permeability by regulating
p38 kinase activation of activating transcription factor 2 (ATF-2)
and via ATF-2 regulation of MLCK gene activity (33). IL-13 is considered an important
effector cytokine in patients with UC, which may lead to epithelial
barrier dysfunction by regulating epithelial apoptosis, TJs and
restitution velocity (34). IL-10,
an anti-inflammatory cytokine, serves a key role in the intestinal
immune system and is closely associated with IBD. A previous study
has shown that IL10-/- mice spontaneously develop
colitis, which may be alleviated by the administration of
IL-10(35). Furthermore, a
genome-wide association study in humans revealed that a
single-nucleotide polymorphism in the IL-10 gene was closely
associated with IBD (36). Kuhn
et al (37), demonstrated in
two murine models of bowel injury that IL-6 was induced soon after
injury in multiple cell types, while its inhibition resulted in
impaired wound healing. Additionally, these authors found that IL-6
mRNA transcripts were enriched within the surrounding area of colon
perforation in human patients, thus suggesting that this
IL-6-driven wound healing mechanism could be important in humans.
Previous studies have reported that butyrate inhibits the secretion
of TNF-α, interferon-γ, IL-12, IL-5 and IL-13 (38,39),
while CB promotes IL-10 production via intestinal
macrophages in the inflamed mucosa of mice with experimental
colitis (28). In the present
study, treatment with CB also inhibited the expression
levels of the proinflammatory cytokines TNF-α, IL-1β and IL-13, and
promoted that of IL-10. Consistent with previous studies, the
current results suggested that IL-6 secretion levels in mice with
DSS-induced colitis were increased compared with the control group.
However, the differences in IL-6 secretion levels between the DSS
and DSS + CB groups were not significant, indicating that
CB had no significant effect on IL-6 secretion. Therefore,
the role of IL-6 in wound healing should be further
investigated.
MDA, a marker for lipid peroxidation, is commonly
used as an indicator for oxidative damage to cells and tissues
(40). The present study
demonstrated that in DSS-treated animals, MDA levels were
increased, while co-treatment with CB significantly
alleviated its levels. Furthermore, GSH, an important indicator of
the body's antioxidant capacity, contributes to the antioxidant
defense system (41). It has also
been reported that GSH eliminates free radicals and protects the
gastrointestinal mucosal epithelium by preventing injuries caused
by inflammation, local ischemia and oxidants (42). SOD is an important antioxidant
enzyme that attenuates tissue oxidative damage and acts as a free
radical scavenger in defense against oxidative cell injury
(43). In the current study it was
identified that co-treatment of DSS-treated mice with CB
significantly elevated GSH levels and SOD enzyme activities.
Inflammation and oxidative stress are two closely
associated and tightly linked processes that provoke cell injury
(44). It has been reported that
the anti-inflammatory and antioxidative effects of CB may be
associated with the metabolism of SCFAs. These molecules serve a
critical role in colonic health by acting as important energetic
substrates for epithelial cells (45), modulating immune cell adhesion
molecules and affecting chemotaxis, cytokine release and leukocyte
migration (46). In addition, SCFAs
may modulate oxidative stress via inhibiting histone deacetylases
(47). An in vitro study has
shown that butyrate protects colonocytes from reactive oxygen
species-induced DNA damage (48).
The present study demonstrated that CB, as a major source of
butyrate, inhibited the secretion of proinflammatory cytokines and
reduced oxidative stress.
The present results suggested that CB could
protect the intestinal barrier function via upregulating the
expression of TJ-related proteins, including claudin-1, occluding
and ZO-1, at both protein and mRNA levels. However, the mRNA and
protein levels of claudin-2 were decreased in the DSS + CB
group. Claudins serve an important role in intestinal barrier
function and regulate the permeability of epithelial layers to ions
and water by upregulating the expression of ≥1 of the 27 claudin
isoforms (49). Each claudin
molecule consists of four right helical folded transmembrane
domains and two extracellular rings (50). The amino- and carboxyl-terminal
groups reside in the cytosol and are linked to ZOs via the
post-synaptic density 95/Drosophila discs large/zona-occludens 1
binding domain. Moreover, these two extracellular rings are the
structural basis of TJs and affect the selective permeability to
ions and water (50). Previous
studies have reported that claudin-1, -3, -4, -5 and -8 exhibit
sealing functions and protect the intestinal barrier function
(51-55).
Additionally, claudin-2, -7,-12 and -15 provide paracellular
channels to promote the charge-selective passage of small ions and
increase the permeability of intestinal mucosa (56-58).
Occludin regulates the localization and function of
TJ complex via phosphorylation, which affects TJ permeability.
Furthermore, two subtypes of occludin have been identified,
characterized by the formation of two outer rings via four
transmembrane domains; these extracellular rings are responsible
for binding between adjacent cells (59). Amino acid residues in the
intracellular domain of occludin directly bind to ZOs and
subsequently interact with cytoskeleton proteins via cytoplasmic
proteins (59). ZOs belong to the
membrane associated guanylate kinase family and consists of three
isoforms: ZO-1, -2 and -3. ZOs interact with several transmembrane
proteins, and actin cytoskeleton and cytoskeleton-associated
proteins via the N- and C-terminal regions, respectively (1). A previous study has revealed that
CB upregulated ZO-1, claudin-1 and occludin expression
levels in vitro in lipopolysaccharide (LPS)-treated IPEC-J2
cells and inhibited LPS-induced apoptosis (60). Moreover, Daly and Shirazi-Beeche
(61) observed that butyrate
downregulated claudin-2 expression in butyrate-treated colonic
epithelial cells, using microarray analysis. Consistent with
previous studies, the present results suggested that the protein
and mRNA expression levels of claudin-1, occludin and ZO-1 were
significantly increased in the CB-treated group.
Furthermore, claudin-2 expression was higher in the DSS group
compared with the control group, while CB could reverse this
trend.
Akt, a serine/threonine kinase, is an essential
intracellular signaling molecule that promotes cell survival,
growth, proliferation and metabolism. It has also been reported
that activation of Akt may lead to phosphorylation of mTOR, which
in turn activates the downstream signaling molecules, S6K and
4E-BP1(62). Furthermore, activated
S6K and 4E-BP1 directly regulate protein synthesis (63). Previous studies have revealed that
the increased expression of TJ-related proteins in intestinal
epithelial cells is closely associated with the activation of the
Akt/mTOR signaling pathway (64).
Yan et al (65) investigated
the effect of butyrate on TJ permeability in a model of LPS-induced
inflammation in IPEC-J2 cells, and found that butyrate elevated
both mRNA and protein expression levels of TJ-related proteins via
activating the Akt/mTOR-mediated protein synthesis. In addition, a
recent study showed that microbiota metabolites, SCFA, activated
the mTOR signaling pathway and promoted intestinal epithelial cell
expression of RegIIIr and β-defensins in a G protein-coupled
receptor 43-dependent manner (66).
In the present study, p-Akt and total Akt protein expression levels
were alleviated in DSS-treated mice, thus resulting in p-mTOR and
p-S6K downregulation. This finding indicated that the Akt/mTOR
signaling pathway was inhibited in DSS-induced colitis, and as a
result, TJ-related protein synthesis was inhibited, leading to the
disruption of the epithelial barrier function. However, treatment
with CB restored p-Akt, p-mTOR and p-S6K protein expression
levels, suggesting that CB could promote TJ-related protein
synthesis via activating the Akt/m-TOR signaling pathway.
The current study has certain limitations. It
remains to be investigated whether oral administration of CB
increased the content of intestinal SCFAs or affected the gut
microbiome. The safety of CB also requires further study.
Moreover, animal studies have indicated that CB and SCFAs
have a beneficial impact on colonic diseases; however, evidence in
humans is currently lacking. Therefore, future studies are required
to further determine the effects of combination therapy with
anti-inflammatory drugs, prebiotics or probiotics in clinical
trials. Due to funding constraints, the protective effect of
CB on intestinal functional barrier will require further
research.
In conclusion, previous studies have shown that
butyrate exhibits anti-inflammatory, barrier-protective and
anti-carcinogenic effects in colon. The present results further
demonstrated that CB alleviated colon inflammation and
restored intestinal barrier function in DSS-induced colitis, by
promoting TJ-related proteins expression via the activation of the
Akt/mTOR-mediated protein synthesis pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81800481).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TD designed the experiments, interpreted and
analyzed the data, and revised the manuscript. ML and WX designed
and performed the experiments, and wrote the manuscript. XW was
responsible for data and statistical analyses. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments in the present study were performed
in accordance with the Guidelines on Animal Experiments from The
Committee of Medical Ethics, The National Health Department of
China. This study and all experimental protocols were approved by
the Laboratory Animals Welfare and Ethics Committee of Renmin
Hospital of the Wuhan University (approval no. 20190608).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sanchez de Medina F, Romero-Calvo I,
Mascaraque C and Martinez-Augustin O: Intestinal inflammation and
mucosal barrier function. Inflamm Bowel Dis. 20:2394–2404.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suzuki T: Regulation of the intestinal
barrier by nutrients: The role of tight junctions. Anim Sci J.
91(e13357)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Andrews C, McLean MH and Durum SK:
Cytokine tuning of intestinal epithelial function. Front Immunol.
9(1270)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rubin DT, Ananthakrishnan AN, Siegel CA,
Sauer BG and Long MD: ACG Clinical Guideline: Ulcerative colitis in
adults. Am J Gastroenterol. 114:384–413. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kornbluth A and Sachar DB: Ulcerative
colitis practice guidelines in adults: American College Of
Gastroenterology, Practice Parameters Committee. Am J
Gastroenterol. 105:501–523; quiz 524. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chang J, Leong RW, Wasinger VC, Ip M, Yang
M and Phan TG: Impaired intestinal permeability contributes to
ongoing bowel symptoms in patients with inflammatory bowel disease
and mucosal healing. Gastroenterology. 153:723–731.e1.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Luissint AC, Parkos CA and Nusrat A:
Inflammation and the intestinal barrier: Leukocyte-epithelial cell
interactions, cell junction remodeling, and mucosal repair.
Gastroenterology. 151:616–632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Buckley A and Turner JR: Cell biology of
tight junction barrier regulation and mucosal disease. Cold Spring
Harb Perspect Biol. 10(a029314)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suau A, Bonnet R, Sutren M, Godon JJ,
Gibson GR, Collins MD and Doré J: Direct analysis of genes encoding
16S rRNA from complex communities reveals many novel molecular
species within the human gut. Appl Environ Microbiol. 65:4799–4807.
1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nicholson JK, Holmes E, Kinross J,
Burcelin R, Gibson G, Jia W and Pettersson S: Host-gut microbiota
metabolic interactions. Science. 336:1262–1267. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The international scientific
association for probiotics and prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schönherr-Hellec S, Klein G, Delannoy J,
Ferraris L, Friedel I, Rozé JC, Butel MJ and Aires J: Comparative
phenotypic analysis of ‘Clostridium neonatale’ and Clostridium
butyricum isolates from neonates. Anaerobe. 48:76–82.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kong Q, He GQ, Jia JL, Zhu QL and Ruan H:
Oral administration of Clostridium butyricum for modulating
gastrointestinal microflora in mice. Curr Microbiol. 62:512–517.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang HB, Wang PY, Wang X, Wan YL and Liu
YC: Butyrate enhances intestinal epithelial barrier function via
up-regulation of tight junction protein Claudin-1 transcription.
Dig Dis Sci. 57:3126–3135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peng L, Li ZR, Green RS, Holzman IR and
Lin J: Butyrate enhances the intestinal barrier by facilitating
tight junction assembly via activation of AMP-activated protein
kinase in Caco-2 cell monolayers. J Nutr. 139:1619–1625.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of acute and chronic intestinal inflammation. Nat
Protoc. 12:1295–1309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Gu Y, Fang K, Mao K, Dou J, Fan H,
Zhou C and Wang H: Lactobacillus acidophilus and Clostridium
butyricum ameliorate colitis in murine by strengthening the gut
barrier function and decreasing inflammatory factors. Benef
Microbes. 9:775–787. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dawson PA, Huxley S, Gardiner B, Tran T,
McAuley JL, Grimmond S, McGuckin M and Markovich D: Reduced mucin
sulfonation and impaired intestinal barrier function in the
hyposulfataemic NaS1 null mouse. Gut. 58:910–919. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391.
1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matsuoka K and Kanai T: The gut microbiota
and inflammatory bowel disease. Semin Immunopathol. 37:47–55.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zeissig S, Bürgel N, Günzel D, Richter J,
Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M and
Schulzke JD: Changes in expression and distribution of claudin 2, 5
and 8 lead to discontinuous tight junctions and barrier dysfunction
in active Crohn's disease. Gut. 56:61–72. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Martini E, Krug SM, Siegmund B, Neurath MF
and Becker C: Mend your fences: The epithelial barrier and its
relationship with mucosal immunity in inflammatory bowel disease.
Cell Mol Gastroenterol Hepatol. 4:33–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Okamoto T, Sasaki M, Tsujikawa T, Fujiyama
Y, Bamba T and Kusunoki M: Preventive efficacy of butyrate enemas
and oral administration of Clostridium butyricum M588 in
dextran sodium sulfate-induced colitis in rats. J Gastroenterol.
35:341–346. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hayashi A, Sato T, Kamada N, Mikami Y,
Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et
al: A single strain of Clostridium butyricum induces
intestinal IL-10-producing macrophages to suppress acute
experimental colitis in mice. Cell Host Microbe. 13:711–722.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kanai T, Mikami Y and Hayashi A: A
breakthrough in probiotics: Clostridium butyricum regulates
gut homeostasis and anti-inflammatory response in inflammatory
bowel disease. J Gastroenterol. 50:928–939. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Seki H, Shiohara M, Matsumura T, Miyagawa
N, Tanaka M, Komiyama A and Kurata S: Prevention of
antibiotic-associated diarrhea in children by Clostridium
butyricum MIYAIRI. Pediatr Int. 45:86–90. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen L, Li S, Zheng J, Li W, Jiang X, Zhao
X, Li J, Che L, Lin Y, Xu S, et al: Effects of dietary
Clostridium butyricum supplementation on growth performance,
intestinal development, and immune response of weaned piglets
challenged with lipopolysaccharide. J Anim Sci Biotechnol.
9(62)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ye D and Ma TY: Cellular and molecular
mechanisms that mediate basal and tumour necrosis
factor-alpha-induced regulation of myosin light chain kinase gene
activity. J Cell Mol Med. 12:1331–1346. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Al-Sadi R, Guo S, Ye D, Dokladny K,
Alhmoud T, Ereifej L, Said HM and Ma TY: Mechanism of IL-1β
modulation of intestinal epithelial barrier involves p38 kinase and
activating transcription factor-2 activation. J Immunol.
190:6596–6606. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Heller F, Florian P, Bojarski C, Richter
J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm
M, et al: Interleukin-13 is the key effector Th2 cytokine in
ulcerative colitis that affects epithelial tight junctions,
apoptosis, and cell restitution. Gastroenterology. 129:550–564.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kühn R, Löhler J, Rennick D, Rajewsky K
and Müller W: Interleukin-10-deficient mice develop chronic
enterocolitis. Cell. 75:263–274. 1993.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Franke A, Balschun T, Karlsen TH,
Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M,
Nothnagel M, Ellinghaus D, et al: Sequence variants in IL10, ARPC2
and multiple other loci contribute to ulcerative colitis
susceptibility. Nat Genet. 40:1319–1323. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kuhn KA, Manieri NA, Liu TC and
Stappenbeck TS: IL-6 stimulates intestinal epithelial proliferation
and repair after injury. PLoS One. 9(e114195)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Inan MS, Rasoulpour RJ, Yin L, Hubbard AK,
Rosenberg DW and Giardina C: The luminal short-chain fatty acid
butyrate modulates NF-kappaB activity in a human colonic epithelial
cell line. Gastroenterology. 118:724–734. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nancey S, Bienvenu J, Coffin B, Andre F,
Descos L and Flourie B: Butyrate strongly inhibits in vitro
stimulated release of cytokines in blood. Dig Dis Sci. 47:921–928.
2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mano T, Sinohara R, Sawai Y, Oda N,
Nishida Y, Mokumo T, Asano K, Ito Y, Kotake M, Hamada M, et al:
Changes in lipid peroxidation and free radical scavengers in the
brain of hyper- and hypothyroid aged rats. J Endocrinol.
147:361–365. 1995.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Orient A, Donkó A, Szabó A, Leto TL and
Geiszt M: Novel sources of reactive oxygen species in the human
body. Nephrol Dial Transplant. 22:1281–1288. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mårtensson J, Jain A and Meister A:
Glutathione is required for intestinal function. Proc Natl Acad Sci
USA. 87:1715–1719. 1990.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kyle ME, Miccadei S, Nakae D and Farber
JL: Superoxide dismutase and catalase protect cultured hepatocytes
from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun.
149:889–896. 1987.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tian T, Wang Z and Zhang J:
Pathomechanisms of oxidative stress in inflammatory bowel disease
and potential antioxidant therapies. Oxid Med Cell Longev.
2017(4535194)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kelly CJ, Zheng L, Campbell EL, Saeedsolz
CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et
al: Crosstalk between microbiota-derived short-chain fatty acids
and intestinal epithelial HIF augments tissue barrier function.
Cell Host Microbe. 17:662–671. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kaczmarczyk MM, Miller MJ and Freund GG:
The health benefits of dietary fiber: Beyond the usual suspects of
type 2 diabetes mellitus, cardiovascular disease and colon cancer.
Metabolism. 61:1058–1066. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shimazu T, Hirschey MD, Newman J, He W,
Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD,
et al: Suppression of oxidative stress by beta-hydroxybutyrate, an
endogenous histone deacetylase inhibitor. Science. 339:211–214.
2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rosignoli P, Fabiani R, De Bartolomeo A,
Spinozzi F, Agea E, Pelli MA and Morozzi G: Protective activity of
butyrate on hydrogen peroxide-induced DNA damage in isolated human
colonocytes and HT29 tumour cells. Carcinogenesis. 22:1675–1680.
2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Citi S: Intestinal barriers protect
against disease. Science. 359:1097–1098. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Suzuki H, Tani K and Fujiyoshi Y: Crystal
structures of claudins: Insights into their intermolecular
interactions. Ann N Y Acad Sci. 1397:25–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Inai T, Kobayashi J and Shibata Y:
Claudin-1 contributes to the epithelial barrier function in MDCK
cells. Eur J Cell Biol. 78:849–855. 1999.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Milatz S, Krug SM, Rosenthal R, Günzel D,
Müller D, Schulzke JD, Amasheh S and Fromm M: Claudin-3 acts as a
sealing component of the tight junction for ions of either charge
and uncharged solutes. Biochim Biophys Acta. 1798:2048–2057.
2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Van Itallie C, Rahner C and Anderson JM:
Regulated expression of claudin-4 decreases paracellular
conductance through a selective decrease in sodium permeability. J
Clin Invest. 107:1319–1327. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Amasheh S, Schmidt T, Mahn M, Florian P,
Mankertz J, Tavalali S, Gitter AH, Schulzke JD and Fromm M:
Contribution of claudin-5 to barrier properties in tight junctions
of epithelial cells. Cell Tissue Res. 321:89–96. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yu AS, Enck AH, Lencer WI and Schneeberger
EE: Claudin-8 expression in Madin-Darby canine kidney cells
augments the paracellular barrier to cation permeation. J Biol
Chem. 278:17350–17359. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Amasheh S, Meiri N, Gitter AH, Schöneberg
T, Mankertz J, Schulzke JD and Fromm M: Claudin-2 expression
induces cation-selective channels in tight junctions of epithelial
cells. J Cell Sci. 115:4969–4976. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alexandre MD, Jeansonne BG, Renegar RH,
Tatum R and Chen YH: The first extracellular domain of claudin-7
affects paracellular Cl-permeability. Biochem Biophys Res Commun.
357:87–91. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fujita H, Chiba H, Yokozaki H, Sakai N,
Sugimoto K, Wada T, Kojima T, Yamashita T and Sawada N:
Differential expression and subcellular localization of claudin-7,
-8, -12, -13, and -15 along the mouse intestine. J Histochem
Cytochem. 54:933–944. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dörfel MJ and Huber O: Modulation of tight
junction structure and function by kinases and phosphatases
targeting occludin. J Biomed Biotechnol.
2012(807356)2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xiao Z, Liu L, Tao W, Pei X, Wang G and
Wang M: Clostridium tyrobutyricum protect intestinal barrier
function from LPS-induced apoptosis via P38/JNK signaling pathway
in IPEC-J2 Cells. Cell Physiol Biochem. 46:1779–1792.
2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Daly K and Shirazi-Beechey SP: Microarray
analysis of butyrate regulated genes in colonic epithelial cells.
DNA Cell Biol. 25:49–62. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W,
Wang B, He B, Zhang Q, Dai Z and Wu Z: l-tryptophan activates
mammalian target of rapamycin and enhances expression of tight
junction proteins in intestinal porcine epithelial cells. J Nutr.
145:1156–1162. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yan H and Ajuwon KM: Butyrate modifies
intestinal barrier function in IPEC-J2 cells through a selective
upregulation of tight junction proteins and activation of the Akt
signaling pathway. PLoS One. 12(e0179586)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ,
Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, et al: GPR43
mediates microbiota metabolite SCFA regulation of antimicrobial
peptide expression in intestinal epithelial cells via activation of
mTOR and STAT3. Mucosal Immunol. 11:752–762. 2018.PubMed/NCBI View Article : Google Scholar
|