Introduction
Lung cancer poses §a serious threat to human health
and has become a major concern worldwide (1). There are several subtypes of lung
cancer, but it is classified into two main groups: Non-small-cell
lung carcinoma (NSCLC) and small-cell lung carcinoma (SCLC)
(2). NSCLC is the major subtype of
epithelial lung malignancies, which is responsible for the increase
in lung cancer-related deaths (3).
Surgery is the most common treatment strategy for NSCLC, but it is
only suitable for patients with stage I and II tumors; therefore,
the incidence and mortality rate of patients with advanced stage
remain poor. As a consequence, there is an urgent need to explore
novel biomarkers for the diagnosis and prognosis of NSCLC.
Previous studies have reported a number of genes and
signaling pathways that can serve as biomarkers during the
progression of NSCLC, such as NF-kB, tumor suppressor candidate 3
and programmed death-ligand 1 (4-6).
The role of microRNAs (miRNAs/miRs) in the prognosis and
progression of various types of cancer has received increasing
attention (7,8). miRNAs are small non-coding RNAs that
primarily mediate gene expression at the post-transcriptional level
(9,10). In the past decades, hundreds of
miRNAs have been identified, among which a number of miRNAs have
been determined as key molecules in numerous types of cancer. For
example, miR-505 was downregulated in breast cancer and inhibited
cell biological functions, which indicated the potential
therapeutic target role of miR-505(11). In gastric cancer, miR-574-5p
promotes angiogenesis by targeting protein tyrosine phosphatase
non-receptor type 3(12). In NSCLC,
several miRNAs have been reported to exert effects on cancer
progression, especially dysregulated miRNAs. For instance, miR-374a
serves as a prognostic marker for NSCLC, where its downregulation
has been previously associated with poor patient survival (13). In another study, the downregulated
miR-449b-3 expression in NSCLC has been reported to regulate
epithelial-mesenchymal transition in NSCLC cells by targeting
IL-6(14).
miR-4728 is downregulated in NSCLC, which indicates
that it may serve as a biomarker for the disease (15,16).
The role of miR-4728 has been identified in a number of other types
of cancer. miR-4728 is downregulated in papillary thyroid cancer
and inhibits the progression of the disease by mediating the
mitogen-activated protein kinase (MAPK) signaling pathway (17). In breast cancer, miR-4728 may serve
as a marker of erb-b2 receptor tyrosine kinase 2 (HER-2), which is
an important indicator of breast cancer (18). However, whether dysregulated
miR-4728 expression also serves a pivotal role in the progression
of NSCLC is not completely understood. The present study aimed to
investigated the role of miR-4728 in the diagnosis and prognosis of
NSCLC, and to explore the functional role of miR-4728 in tumor
progression by assessing its effect on tumor cell proliferation,
migration and invasion. The results of the present study may aid
with understanding the pathogenesis of NSCLC and identifying a
potential biomarker and therapeutic target for NSCLC.
Materials and methods
Patients and tissues
In the present study, 122 patients with NSCLC that
underwent surgery at Qilu Hospital Huantai Branch were recruited
between April 2011 and March 2013. During the same time period, 58
age- and gender-matched healthy volunteers (age range, 45-78 years;
sex, 36 males and 22 females), who had no history of malignancies,
were enrolled as healthy controls. All participants provided
written informed consent. All patients had not received anti-tumor
therapy prior to recruitment. Blood samples (5 ml) were collected
before surgery, cancerous tissues and matched adjacent
non-cancerous tissues, which were collected at ≥3 cm from the edge
of tumor, were collected during surgery. The tissues were
histopathologically diagnosed by two experimenced histopathologists
according to the diagnosis criteria by World Health Organization
(19). Serum samples were isolated
from the collected blood using centrifugation at 1,500 x g at 4˚C
for 10 min. All clinical samples were stored at -80˚C until further
use. Table I presents the
demographic and clinicopathologic data of patients. A 5-year
follow-up survey was conducted after surgery, and the survival
information of patients was obtained via telephone communication.
The present study was approved by the Ethics Committee of Qilu
Hospital Huantai Branch (approval no. HTh-200145).
 | Table IAssociation between miR-4728
expression levels and the clinicopathologic parameters of patients
with non-small cell lung cancer. |
Table I
Association between miR-4728
expression levels and the clinicopathologic parameters of patients
with non-small cell lung cancer.
| | Serum miR-4728 | | Tissue miR-4728 | |
|---|
| Parameter | Total (n=122) | Low (n=65) | High (n-57) | P-value | Low (n=67) | High (n=55) | P-value |
|---|
| Age | | | | 0.796 | | | 0.596 |
|
<60 | 52 | 27 | 25 | | 30 | 22 | |
|
≥60 | 70 | 38 | 32 | | 37 | 33 | |
| Gender | | | | 0.257 | | | 0.154 |
|
Male | 75 | 43 | 32 | | 45 | 30 | |
|
Female | 47 | 22 | 25 | | 22 | 25 | |
| Tumour size
(cm) | | | | 0.043 | | | 0.042 |
|
<4 | 63 | 28 | 35 | | 29 | 34 | |
|
≥4 | 59 | 37 | 22 | | 38 | 21 | |
| Smoking
history | | | | 0.596 | | | 0.541 |
|
No | 48 | 27 | 21 | | 28 | 20 | |
|
Yes | 74 | 38 | 36 | | 39 | 35 | |
|
Differentiation | | | | 0.519 | | | 0.743 |
|
Well +
moderate | 69 | 35 | 34 | | 37 | 32 | |
|
Poor | 53 | 30 | 23 | | 30 | 23 | |
| Lymph node
metastasis | | | | 0.002 | | | 0.002 |
|
Negative | 72 | 30 | 42 | | 31 | 41 | |
|
Positive | 50 | 35 | 15 | | 36 | 14 | |
| TNM stage | | | | 0.001 | | | 0.001 |
|
I-II | 71 | 29 | 42 | | 30 | 41 | |
|
III-IV | 52 | 36 | 15 | | 37 | 14 | |
Cell culture and transfection
NSCLC cell lines (A549, HCC827, NCI-H1299 and
NCI-H1650) and a normal human lung epithelial cell line (BEAS-2B)
were purchased from American Type Culture Collection. Cells were
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37˚C with 5% CO2. Following overnight
incubation in six-well plates, A549 and HCC827 cells (inoculum
density of 2x106 cell/well) were cultured to ~80%
confluence before they were transfected with 50 nM miR-4728 mimic,
100 nM miR-4728 inhibitor, 50 nM mimic negative control (NC) or 100
nM inhibitor NC using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The subsequent cell experiments were peformed 48 h
post-transfection. All mimics and inhibitors were synthesized by
Shanghai GenePharma Co., Ltd. and the sequences were as follows:
miR-4728 mimic, 5'-CAUGCUGACCUCCCUCCUGCCCCAG-3'; miR-4728
inhibitor, 5'-CUGGGGCAGGAGGGAGGUCAGCAUG-3'; mimic NC,
5'-UUCUCCGAACGUGUCACGU-3'; inhibitor NC,
5'-CAGUACUUUUGUGUAGUACAA-3'.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from serum samples, tissues
and cell lines using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Total RNA was reverse transcribed into cDNA using a High Capacity
cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the following conditions: 42˚C for 30 min
and 85˚C for 5 sec. Subsequently, qPCR was performed using the
SYBR-green I Master mix kit (Invitrogen; Thermo Fisher Scientific,
Inc.) in a 7300 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 10 min followed by 40
cycles of denaturation at 95˚C for 30 sec, annealing at 60˚C for 20
sec and elongation at 72˚C for 30 sec, before final extension at
72˚C for 10 min. miRNA expression levels were quantified using the
2-ΔΔCq method (20) and
normalized to the internal reference gene U6. The primer sequences
used were as follows: miR-4728 forward, 5'-GCCGAGCATGCTGACCTCCC-3'
and reverse, 5'-CTCAACTGGTGTCGTGGA-3'; U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was assessed using a CCK-8 assay
(Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. In brief, transfected cells
(5x103 cells/well) were seeded into 96-well plates.
Following incubation for 0, 24, 48 or 72 h, cell proliferation was
assessed by incubating cells with 10 µl CCK-8 reagent for 4 h at
37˚C with 5% CO2. The absorbance of each well was
measured at a wavelength of 450 nm using a microplate reader.
Transwell assay
A Transwell assay (Corning, Inc.) was conducted
using 8-µm pore membranes to assess NSCLC cell migration and
invasion. To assess cell migration, transfected cells
(2x105) in serum-free DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) were seeded into the upper chamber. DMEM
supplemented with 5% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to the lower chamber as a chemoattractant.
Following incubation for 24 h, migratory cells were stained with
0.1% crystal violet at room temperature for 10 min and quantified
using a light microscope (magnification, x200). For cell invasion,
the upper chambers were precoated with Matrigel (BD Biosciences) at
37˚C for 6 h prior to Transwell assay.
Western blotting
Total protein was extracted from A549 cells using
RIPA buffer (Beyotime Institute of Biotechnology) and quantified
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg/lane) were separated via 10%
SDS-PAGE and transferred to PVDF membranes (EMD Millipore), which
were blocked with 5% non-fat milk at room temperature for 2 h.
Subsequently, the membranes were incubated at 4˚C overnight with
the following primary antibodies: Anti-phosphorylated (p)-ERK1/2
(1:1,000; cat. no. ab214362; Abcam), anti-ERK1/2 (1:10,000; cat.
no. ab184699; Abcam), anti-Bcl-2 (1:1,000; cat. no. ab32124;
Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-β-actin
(1:500; cat. no. ab8229; Abcam). Following primary incubation, the
membranes were incubated with a horseradish peroxidase-conjugated
secondary antibody (1:5,000; cat. no. 715-035-151; Jackson
ImmunoResearch Laboratories, Inc.) at room temperature for 1 h.
Protein bands were visualized using an enhanced chemiluminescent
substrate (Bio-Rad Laboratories, Inc.) and ImageLab version 4.1
software (Bio-Rad Laboratries, Inc.). β-actin was used as the
loading control.
Statistical analysis
Data are presented as the mean ± SD of triplicate
experiments. Differences between groups were analyzed using the
χ2 test, t-test or one-way ANOVA followed by Tukey's
post hoc test. The paired Student's t-test was used to analyze
miR-4728 expression between tumor and normal tissues, whilst the
unpaired Student's t-test was used to compare the differences in
serum miR-4728 expression between patients with NSCLC and healthy
individuals. Statistical analyses were conducted using SPSS
(version 20.0; IBM Corp.) and GraphPad Prism (version 5.0; GraphPad
Software, Inc.) software. A receiver operating characteristic (ROC)
curve was plotted to evalute the diagnsotic value of miR-4728. The
Kaplan-Meier method and log-rank test were used to plot survival
curves and compare the different distributions between the curves,
and Cox regression analysis was used to assess the prognostic
ability of miR-4728. P<0.05 was considered to indicate a
statistically significant difference.
Results
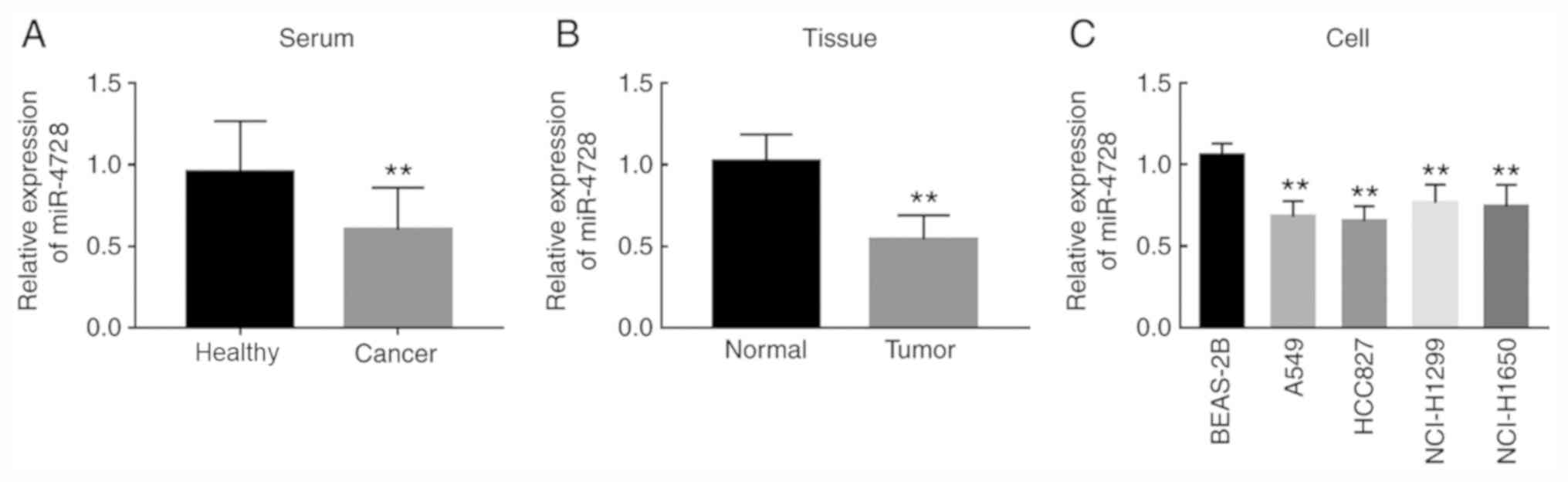
miR-4728 is downregulated in patients
with NSCLC and NSCLC cells
The present study evaluated the relative expression
of miR-4728 in NSCLC serum samples, tissues and cell lines.
Compared with healthy controls, patients with NSCLC displayed
significantly decreased miR-4728 serum expression levels
(P<0.01; Fig. 1A). The
expression of miR-4728 in tumor tissues was also significantly
lower compared with adjacent noncancerous tissues (P<0.01;
Fig. 1B). In addition, the
expression of miR-4728 was decreased in the four NSCLC cell lines
compared with normal BEAS-2B cells (all P<0.01; Fig. 1C).
miR-4728 is associated with the TNM
stage of patients
The clinicopathological features of patients are
presented in Table I. The TNM
stages of the pateints were determined based on the criteria set by
the American Joint Committee on Cancer classification (21). According to the median expression
values (serum expression, 0.642; tissue expression, 0.547),
patients were divided into high and low miR-4728 expression level
groups. The results indicated that the expression of miR-4728 in
serum and tissue samples was significantly associated with tumor
size, lymph node metastasis and TNM stage (all P<0.05) in
patients with NSCLC, but there was no significant association
between miR-4728 expression and other clinicopathological features
(all P>0.05; Table I).
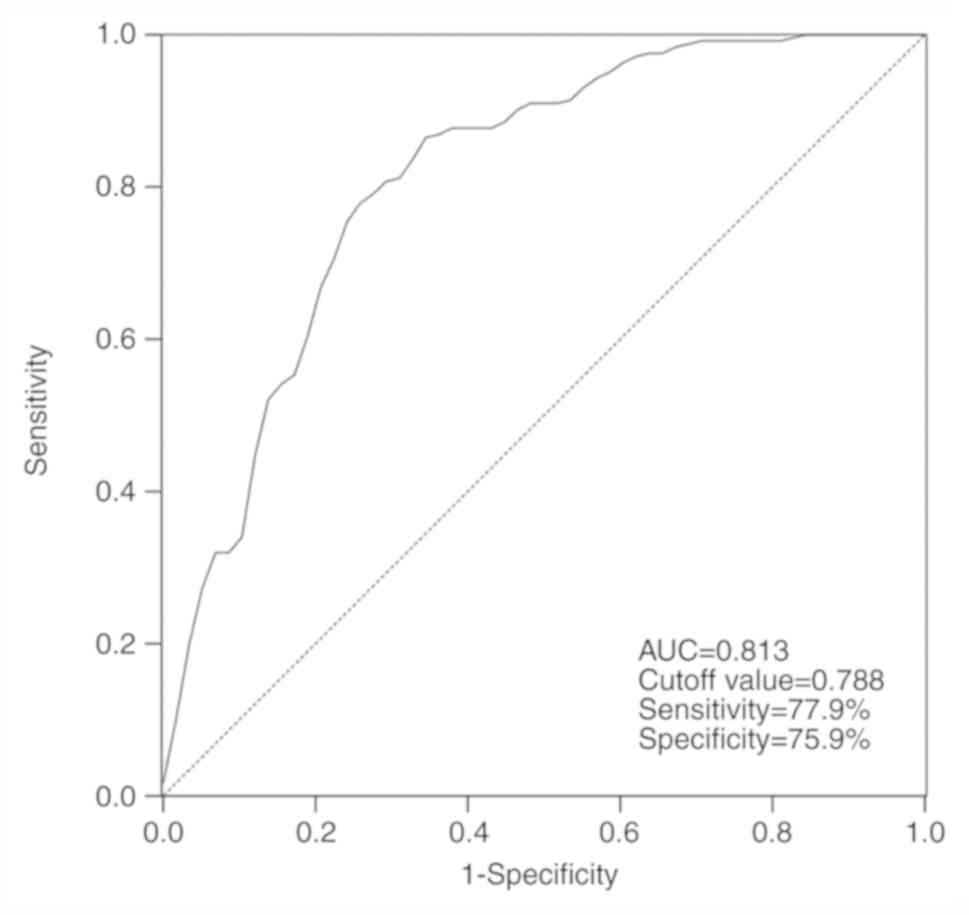
Diagnostic performance of serum
miR-4728 in patients with NSCLC
By assessing the serum levels of miR-4728, the ROC
curve suggested that the area under the curve was 0.813, indicating
that miR-4728 was a relatively accurate diagnostic marker in
patients with NSCLC (Fig. 2). The
diagnosis sensitivity and specificity of miR-4728 were 77.9 and
75.9%, respectively, with a cutoff value of 0.788.
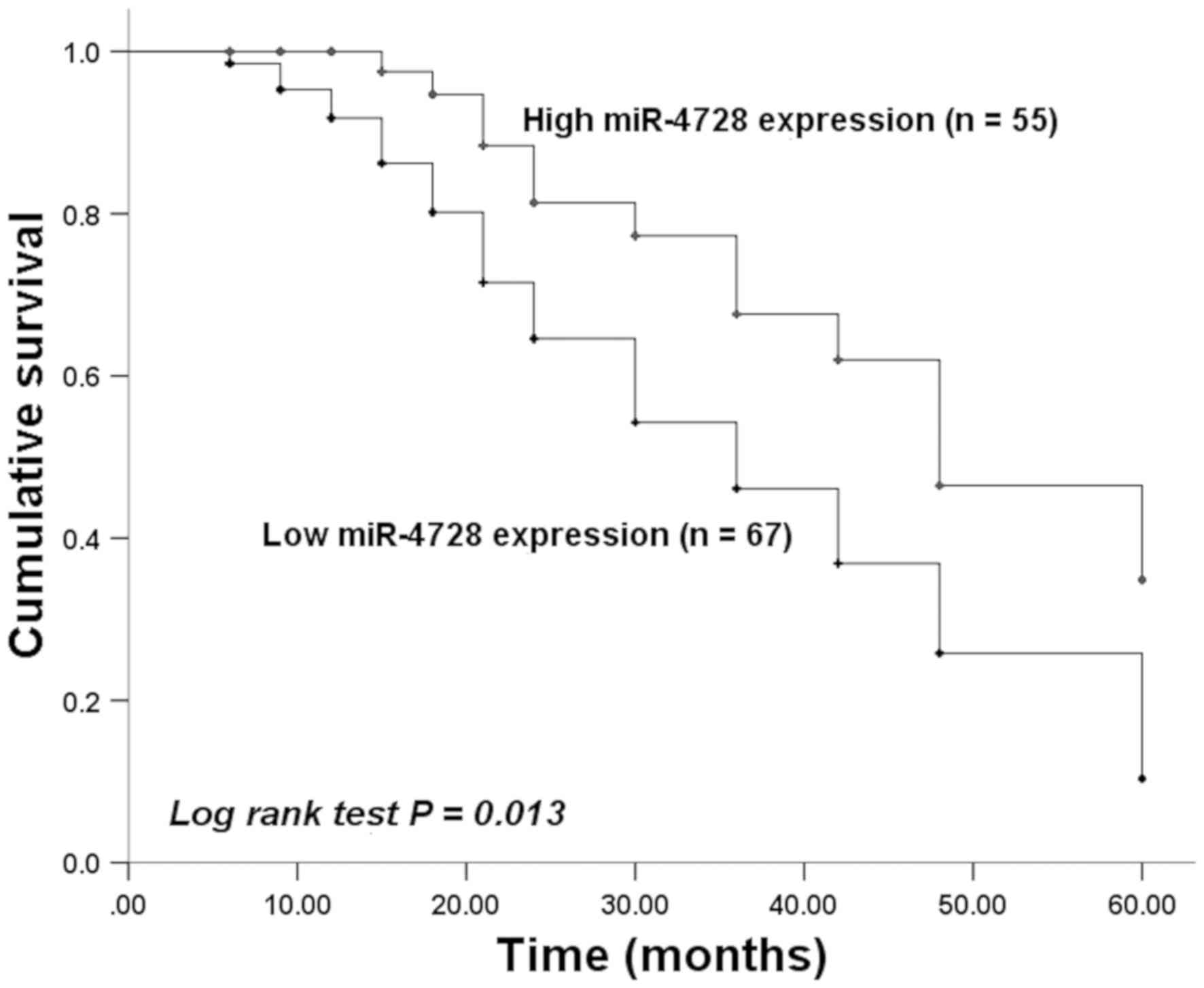
miR-4728 is an independent prognostic
factor in patients with NSCLC
Kaplan-Meier curves were plotted based on survival
information obtained by conducting a 5-year follow-up survey.
Patients with low tissue miR-4728 expression displayed a worse
survival time compared with patients with high tissue miR-4728
expression (P=0.013; Fig. 3). The
prognosis value of miR-4728 was evaluated by performing Cox
regression analysis. The results indicated that the independent
prognostic value of miR-4728 expression had a hazard ratio value of
2.030 (95% confidence interval =1.012-4.072; P=0.042; Table II).
 | Table IICox regression analysis in patients
with non-small cell lung cancer. |
Table II
Cox regression analysis in patients
with non-small cell lung cancer.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| MicroRNA-4728 | 2.030 | 1.012-4.072 | 0.042 |
| Age | 1.249 | 0.654-2.384 | 0.500 |
| Gender | 1.224 | 0.665-2.252 | 0.515 |
| Tumor size | 1.504 | 0.822-2.752 | 0.186 |
| Smoking
history | 1.485 | 0.728-2.699 | 0.248 |
|
Differentiation | 1.656 | 0.893-3.072 | 0.109 |
| Lymph node
metastasis | 1.523 | 0.807-2.873 | 0.194 |
| TNM stage | 1.668 | 0.907-3.066 | 0.059 |
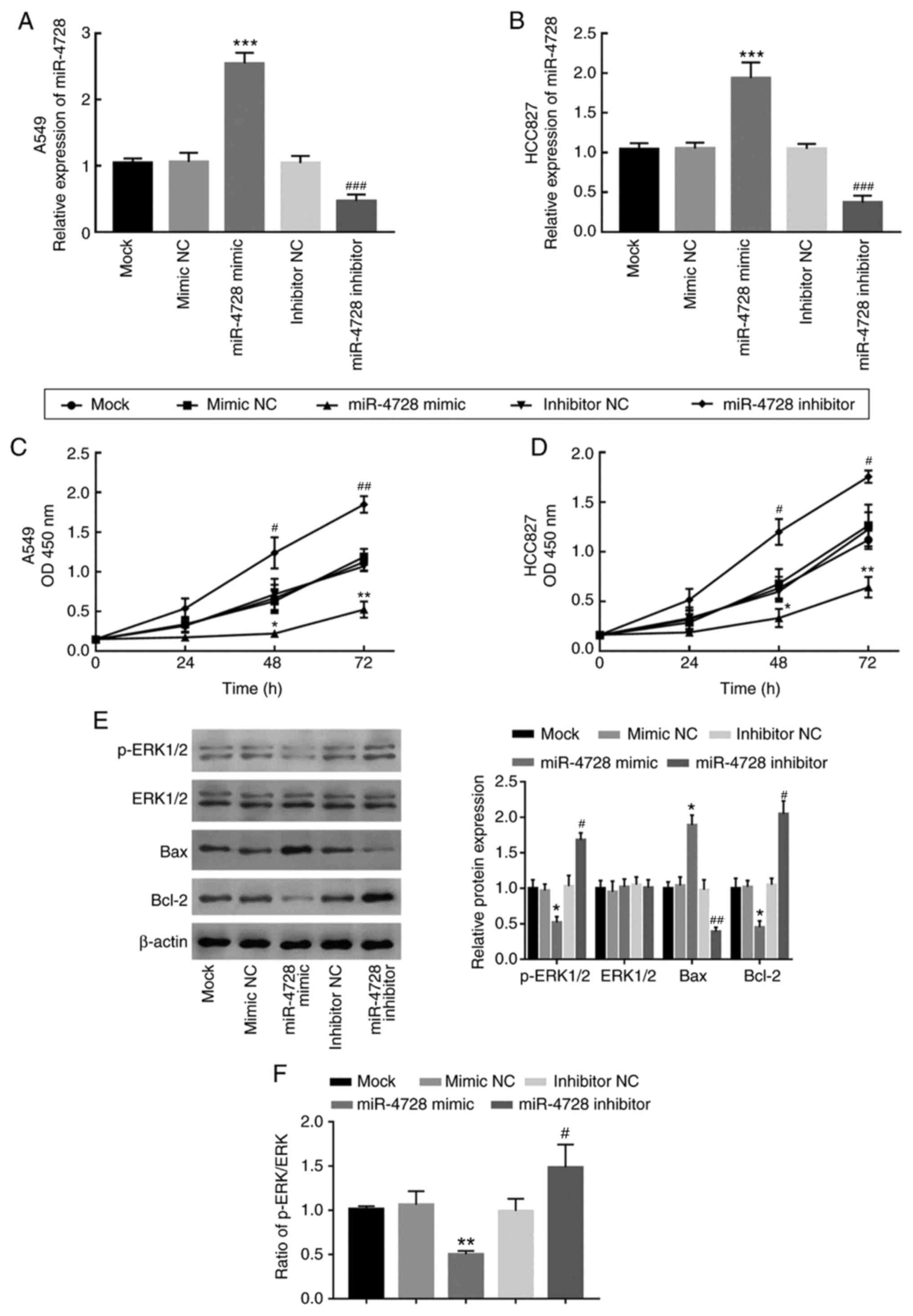
Regulatory effect of miR-4728 on NSCLC
cell proliferation
miR-4728 expression was overexpressed and knocked
down by transfecting A549 and HCC827 cells with miR-4728 mimic and
miR-4728 inhibitor, respectively (P<0.001; Fig. 4A and B). The effect of miR-4728 overexpression
or knockdown on A549 and HCC827 cell proliferation was assessed by
performing the CCK-8 assay. Compared with that in the corresponding
NC groups, cell proliferation was significantly inhibited by
miR-4728 overexpression, but significantly enhanced by miR-4728
knockdown, respectively (P<0.05; Fig. 4C and D). To further investigate the regulatory
effect of miR-4728 on NSCLC cell viability, the activity of the ERK
signaling pathway and apoptosis-related protein expression levels
were analyzed in A549 cells. The results indicated that, compared
with the corresponding NC groups, miR-4728 overexpression inhibited
the ERK signaling pathway, whereas miR-4728 knockdown promoted the
ERK signaling pathway, as indicated by alterations to the p-ERK/ERK
ratio (P<0.05; Fig. 4E and
F). In addition, miR-4728
overexpression significantly increased Bax expression and decreased
Bcl-2 expression compared with the mimic NC group (all P<0.05;
Fig. 4E). By contrast, miR-4728
knockdown displayed the opposite effects on Bax and Bcl-2
expression levels compared with the inhibitor NC group (all
P<0.05; Fig. 4E). The results
demonstrated that miR-4728 might suppress NSCLC cell viability.
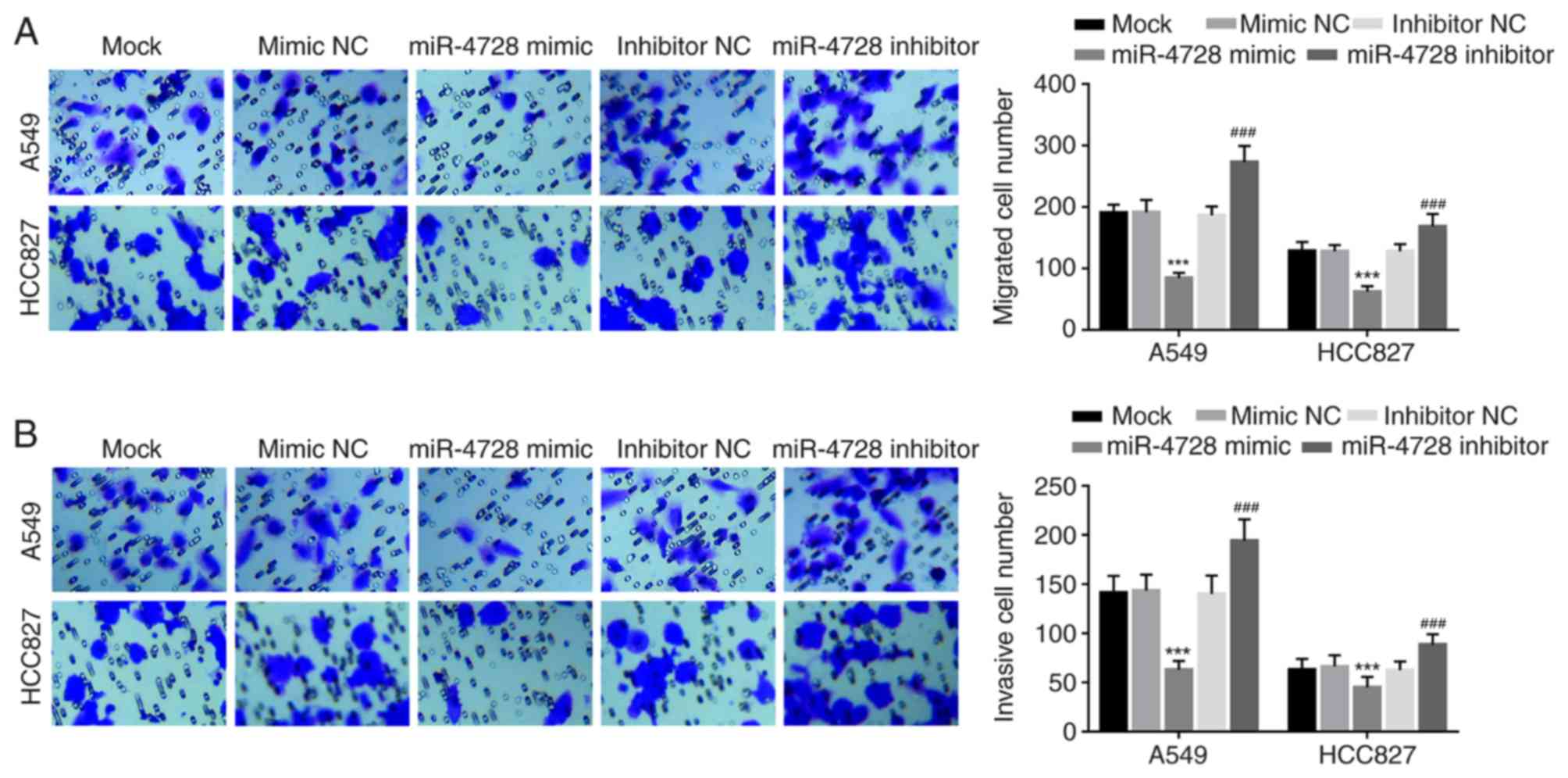
miR-4728 inhibits NSCLC cell migration
and invasion
The effect of miR-4728 on A549 and HCC827 cell
migration and invasion was analyzed by performing Transwell assays.
miR-4728 overexpression significantly inhibited A549 and HCC827
cell migration compared with the mimic NC group, whereas miR-4728
knockdown significantly promoted A549 and HCC827 cell migration
compared with the inhibitor NC group (all P<0.001; Fig. 5A). Similarly, the number of invasive
cells was significantly decreased by miR-4728 overexpression
compared with the mimic NC group, but significantly increased by
miR-4728 knockdown compared with the inhibitor NC group
(P<0.001; Fig. 5B).
Discussion
NSCLC is one of the most common malignant tumors
that threatens human health, with an increasing number of cases and
rising death rate (22). Although
therapeutic strategies have been developed in the past few decades,
the prognosis of NSCLC remains poor (23-25).
Previously, numerous biomarkers have been demonstrated to serve
roles in the prognosis and progression of NSCLC, including a
variety of miRNAs, especially dysregulated miRNAs. For example,
miR-593 was reduced in NSCLC, and inhibited NSCLC cell migration
and invasion by targeting snail family transcriptional repressor
2-related signaling pathways (26).
miR-449b could inhibit NSCLC cell proliferation and invasion by
targeting leucine rich repeat containing G protein-coupled receptor
4(27). Moreover, miR-650 was
upregulated and associated with an unfavorable survival rate in
lung cancer, which enhanced cell proliferation and invasion
(28). Additionally, exploring
miRNAs that serve roles in NSCLC is important for the
identification of novel therapeutic targets for NSCLC.
miRNAs that are associated with the progression and
metastasis of cancer serve as biomarkers in multiple types of
cancer and tumors. miR-216b is downregulated in cervical cancer and
correlated with poor prognosis, which inhibits cell proliferation,
migration and invasion by targeting forkhead box M1(29). miR-153 overexpression is correlated
with poor prognosis and key clinical features in patients with
prostate cancer, which indicated that miR-153 may serve as a
biomarker for prostate cancer (30). miR-4728 has been described as a
suppressor of breast cancer by inhibiting MAPK signaling pathway,
and the expression of miR-4728 was associated with the status of
HER-2 (18,31). miR-4728 also regulates estrogen
receptor 1 via a non-canonical internal seed interaction (32). In papillary thyroid cancer, miR-4728
was identified as a therapeutic target for its dysregulation and
function during the prognosis and progression of papillary thyroid
cancer (17).
In the present study, miR-4728 was downregulated in
NSCLC serum samples and tissues compared with healthy controls.
miR-4728 expression was also significantly associated with tumor
size, lymph node metastasis and TNM stage in patients with NSCLC.
Similarly, in NSCLC cell lines, miR-4728 was downregulated compared
with BEAS-2B cells. Moreover, miR-4728 knockdown promoted NSCLC
cell proliferation, migration and invasion compared with the mimic
NC group. The ROC analysis results indicated that serum miR-4728
expression had relatively high diagnostic accuracy in patients with
NSCLC, and the survival analysis indicated that decreased miR-4728
expression in patients with NSCLC predicted poor prognosis and
might serve as an independent prognostic biomarker. The results
indicated the considerable clinical value of miR-4728 in NSCLC and
the vital role during the progression of NSCLC, which was
consistent with previous studies (15,16).
The present results indicated the role of miR-4728
in the progression of NSCLC, and provided a potential therapeutic
target for NSCLC. Although the results indicated that miR-4728
inhibited NSCLC cell proliferation and regulated cell
apoptosis-related proteins, the effect of miR-4728 on cell
apoptosis was not examined in the present study, which may be a
limitation. In addition, the present study overlooked the mechanism
underlying the functional role miR-4728, which may aid with
understanding the function of miR-4728. Therefore, further studies
that comprehensively analyze the biological function of miR-4728 in
NSCLC progression and focus on the underlying mechanism should be
performed. Furthermore, the majority of the experiments conducted
in the present study were performed in vitro, therefore,
in vivo experiments need to be conducted to verify the
results.
In conclusion, the present study indicated that
patients with NSCLC displayed reduced expression levels of miR-4728
in serum and tissue samples compared with healthy controls.
Therefore, the results suggested that dysregulated miR-4728 may
serve as a candidate diagnostic and prognostic biomarker of NSCLC.
Compared with the mimic NC group, miR-4728 overexpression inhibited
NSCLC cell proliferation, migration and invasion, which indicated
that miR-4728 may be involved in tumor pathogenesis. Therefore,
elevating miR-4728 expression levels may serve as a therapeutic
strategy for NSCLC.
Acknowledgements
The authors would like to express gratitude to the
two experienced histopathologists Dr Jiansheng Rong and Dr Baohua
Zhang (Department of Pathology, Zibo Central Hospital, Zibo,
China), who contributed to the histopathologic diagnosis of the
tissue samples.
Funding
No funding was recieved.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and JL designed this study and analyzed the
expression of miR-4728 in serum and tissue samples, as well as the
clinical data. XZ and CG performed the cell experiments and
analyzed the corresponding data. YH wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qilu Hospital Huantai Branch (approval no.
HTh-200145). Written informed consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of lung cancer. Surg Oncol Clin N Am. 25:439–445.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dimitrakopoulos FD, Antonacopoulou AG,
Kottorou AE, Maroussi S, Panagopoulos N, Koukourikou I, Scopa C,
Kalofonou M, Koutras A, Makatsoris T, et al: NF-kB2 genetic
variations are significantly associated with non-small cell lung
cancer risk and overall survival. Sci Rep. 8(5259)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng S, Zhai J, Lu D, Lin J, Dong X, Liu
X, Wu H, Roden AC, Brandi G, Tavolari S, et al: TUSC3 accelerates
cancer growth and induces epithelial-mesenchymal transition by
upregulating claudin-1 in non-small-cell lung cancer cells. Exp
Cell Res. 373:44–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sahin S, Batur S, Aydin O, Ozturk T, Turna
A and Oz B: Programmed death-ligand-1 expression in non-small cell
lung cancer and prognosis. Balkan Med J. 36:184–189.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu KL, Tsai YM, Lien CT, Kuo PL and Hung
AJ: The roles of MicroRNA in lung cancer. Int J Mol Sci.
20(1611)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu S, Yang Y, Jiang S, Tang N, Tian J,
Ponnusamy M, Tariq MA, Lian Z, Xin H and Yu T: Understanding the
role of non-coding RNA (ncRNA) in stent restenosis.
Atherosclerosis. 272:153–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Palomer X, Pizarro-Delgado J and
Vazquez-Carrera M: Emerging actors in diabetic cardiomyopathy:
Heartbreaker biomarkers or therapeutic targets? Trends Pharmacol
Sci. 39:452–467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Liu H and Li M: Downregulation of
miR-505 promotes cell proliferation, migration and invasion, and
predicts poor prognosis in breast cancer. Oncol Lett. 18:247–254.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang S, Zhang R, Xu R, Shang J, He H and
Yang Q: MicroRNA-574-5p in gastric cancer cells promotes
angiogenesis by targeting protein tyrosine phosphatase non-receptor
type 3 (PTPN3). Gene. 733(144383)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vosa U, Vooder T, Kolde R, Fischer K, Valk
K, Tonisson N, Roosipuu R, Vilo J, Metspalu A and Annilo T:
Identification of miR-374a as a prognostic marker for survival in
patients with early-stage nonsmall cell lung cancer. Genes
Chromosomes Cancer. 50:812–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cai K, Li HX, Li PP, Guo ZJ and Yang Y:
MicroRNA-449b-3p inhibits epithelial-mesenchymal transition by
targeting IL-6 and through the JAK2/STAT3 signaling pathway in
non-small cell lung cancer. Exp Ther Med. 19:2527–2534.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cai T, Long J, Wang H, Liu W and Zhang Y:
Identification and characterization of miR-96, a potential
biomarker of NSCLC, through bioinformatic analysis. Oncol Rep.
38:1213–1223. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu J, Zeng Y, Xu C, Qin H, Lei Z, Shen D,
Liu Z and Huang JA: Expression profile analysis of microRNAs and
downregulated miR-486-5p and miR-30a-5p in non-small cell lung
cancer. Oncol Rep. 34:1779–1786. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Z, Zhang J, Gao J and Li Y:
MicroRNA-4728 mediated regulation of MAPK oncogenic signaling in
papillary thyroid carcinoma. Saudi J Biol Sci. 25:986–990.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li H, Zhou X, Zhu J, Cheng W, Zhu W, Shu Y
and Liu P: MiR-4728-3p could act as a marker of HER2 status. Cancer
Biomark. 15:807–814. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et
al: Staging system for breast cancer: Revisions for the 6th edition
of the AJCC cancer staging manual. Surg Clin North Am. 83:803–819.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hall RD, Gray JE and Chiappori AA: Beyond
the standard of care: A review of novel immunotherapy trials for
the treatment of lung cancer. Cancer Control. 20:22–31.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Walters S, Maringe C, Coleman MP, Peake
MD, Butler J, Young N, Bergstrom S, Hanna L, Jakobsen E, Kölbeck K,
et al: Lung cancer survival and stage at diagnosis in Australia,
Canada, Denmark, Norway, Sweden and the UK: A population-based
study, 2004-2007. Thorax. 68:551–564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei F, Wang M, Li Z, Wang Y and Zhou Y:
MiR593 inhibits proliferation and invasion and promotes apoptosis
in non-small cell lung cancer cells by targeting SLUG-associated
signaling pathways. Mol Med Rep. 20:5172–5182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang D, Li JS, Xu QY, Xia T and Xia JH:
Inhibitory Effect of MiR-449b on cancer cell growth and invasion
through LGR4 in non-small-cell lung carcinoma. Curr Med Sci.
38:582–589. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang X, Ding Y, Wang X, Zhao L and Bi H:
MiR-650 promotes non-small cell lung cancer cell proliferation and
invasion by targeting ING4 through Wnt-1/β-catenin pathway. Oncol
Lett. 18:4621–4628. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He S, Liao B, Deng Y, Su C, Tuo J, Liu J,
Yao S and Xu L: MiR-216b inhibits cell proliferation by targeting
FOXM1 in cervical cancer cells and is associated with better
prognosis. BMC Cancer. 17(673)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bi CW, Zhang GY, Bai Y, Zhao B and Yang H:
Increased expression of miR-153 predicts poor prognosis for
patients with prostate cancer. Medicine (Baltimore).
98(e16705)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schmitt DC, Madeira da Silva L, Zhang W,
Liu Z, Arora R, Lim S, Schuler AM, McClellan S, Andrews JF, Kahn
AG, et al: ErbB2-intronic microRNA-4728: A novel tumor suppressor
and antagonist of oncogenic MAPK signaling. Cell Death Dis.
6(e1742)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Newie I, Sokilde R, Persson H, Grabau D,
Rego N, Kvist A, von Stedingk K, Axelson H, Borg A,
Vallon-Christersson J and Rovira C: The HER2-encoded miR-4728-3p
regulates ESR1 through a non-canonical internal seed interaction.
PLoS One. 9(e97200)2014.PubMed/NCBI View Article : Google Scholar
|