Introduction
Peripheral nerve injury (PNI) is a common injury
that can impact the quality of life of individuals (1). After an injury, peripheral axons
activate the intrinsic growth capacity in the neuronal cell body
and promote axonal regeneration (2). Previous studies have suggested that
Schwann cells are important in the regeneration of peripheral axons
and functional recovery (3,4). After the events of a PNI, mature
Schwann cells dedifferentiate into progenitor-like Schwann cells,
known as Schwann cell precursors, promote myelin debris clearance
and tubular reconstruction, and also form a cellular bridge
spanning the gap between ends of damaged nerves (5,6).
Subsequently, Schwann cell precursors secrete neurotrophic factors
to enhance Schwann cell proliferation and can lead to the formation
of Bunger bands (6-8).
The Ras/Raf/ERK signaling pathway plays an important
role in Schwann cell dedifferentiation (9). Previous studies have demonstrated that
Ras/Raf/ERK activation results in cell cycle arrest of primary
neurons and promotes differentiation (9,10).
Additionally, ERK has a role in regulating Wallerian degeneration
and regeneration; sustained ERK activation is known to initiate
cell proliferation (7). Glial
cell-derived neurotropic factor (GDNF) is a survival factor that
stimulates both peripheral regeneration and the functional recovery
of several types of neurons (11-13).
Previous studies have shown that GDNF transcription can be
increased via the activation of the Schwann cell purinergic
receptor, followed by the activation of protein kinase C (PKC) and
protein kinase D (PKD) (14,15).
PKD, originally characterized as an isoform of PKC, regulates
proliferation, migration and differentiation of numerous cell
types, and promotes neuron regeneration (14,15).
Although microsurgical techniques have been
introduced to repair PNI, in the past two decades only a small
number of patients have regained full functional recovery (16). Therefore, it is important to search
for new approaches that could promote nerve regeneration after
PNI.
Melatonin is a hormone secreted by the pineal gland.
Previous studies have demonstrated that melatonin stimulates the
proliferation of avian astrocytes, dentate neurons and hippocampal
neurons (17,18). The action of melatonin is mediated
through a receptor-dependent signaling pathway (19). Additionally, melatonin has free
radical scavenging and antioxidant properties, which can decrease
malondialdehyde levels and may reverse the ischemia-reperfusion
injury of the sciatic nerve (12).
In the present study, the effects of melatonin and the molecular
mechanism involved in promoting the proliferation of Schwann cells
were investigated. A better understanding of the molecular
mechanism of melatonin-induced Schwann cell proliferation may
provide important insight regarding the potential use of melatonin
in improving nerve regeneration.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, EDTA were purchased
from Gibco; Thermo Fisher Scientific, Inc. MTT, dimethyl sulfoxide,
Triton X-100, Tergitol NP-40, Tris-HCl, PBS, dithiothreitol, SDS,
ammonium acetate, Tris-borate-EDTA buffer, Bradford reagent and
phenylmethyl sulfonyl fluoride were purchased from Sigma-Aldrich;
Merck KGaA. Primary antibodies against SAPK-JNK (cat. no. 9926T),
p38 (cat. no. 9926T), ERK (cat. no. 9926T), p-SAPK-JNK (cat. no.
9910T), p-p38 (cat. no. 9910T), p-ERK (cat. no. 9910T), Ras (cat.
no. 18070T), c-Raf (cat. no. 9422T), p-c-Raf (cat. no. 9427S),
b-Raf (cat. no. 9433S) and p-b-Raf (cat. no. 2696S), in addition to
horseradish peroxidase-conjugated goat anti-rabbit (cat. no. 7074S)
and anti-mouse (cat. no. 7076S) secondary antibodies were purchased
from Cell Signaling Technology, Inc. Primary antibodies against MT1
(cat. no. sc-13177), MT2 (cat. no. sc-13186), PKC (cat. no.
sc-10800), GDNF (cat. no. sc328) and α-tubulin (cat. no. sc-8035)
were purchased from Santa Cruz Biotechnology, Inc. Primary antibody
against Sox2 (cat. no. ab79351) and Alexa-Fluor®
488-conjugated goat anti-mouse secondary antibody (cat. no.
ab150117) were purchased from Abcam. Amersham ECL-Plus Western
Blotting Reagents and PVDF membranes were obtained from GE
Healthcare Life Sciences. RNeasy Mini kit and QuantiNova SYBR Green
PCR kit were purchased from Qiagen, GmbH.
Cell culture and treatment
RT4-D6P2T (RT4), a rat neural Schwann cell line, was
purchased from The American Type Culture Collection and cultured in
DMEM supplemented with 10% heat-inactivated FBS. The cells were
maintained in incubator at 37˚C with 5% CO2. Melatonin
stock concentration of 50 mg/ml (215.26 µM) was freshly prepared
with ethanol.
Cell morphology
Schwann cells (3x105) were seeded into
35-mm tissue culture dishes overnight. The cells were then treated
with various concentrations of melatonin (0.5-10 µM) dissolved in
serum-free medium at 37˚C. After 24 h treatment, the morphological
changes in the Schwann cells were observed using light microscopy
(magnification, x200).
MTT assay
Cell proliferation was determined by an MTT assay.
In total, 1x105 Schwann cells were seeded in a 96-well
plate overnight. The cells were treated with melatonin
concentrations of 0.5-10 µM in serum-free medium. After 24 h of
treatment, Schwann cells were added with MTT to a final
concentration of 1 mg/ml. After 3 h incubation, formazan crystals
were dissolved using 100 µl of DMSO and absorbance was measured
using a microplate reader (Infinite 200 PRO; Tecan Group, Ltd.) at
a wavelength of 570 nm.
Immunofluorescence staining
Melatonin-induced Schwann cell dedifferentiation was
demonstrated by Sox2 immunofluorescence staining. Schwann cells
were treated with melatonin (1 and 10 µM) in serum-free medium.
After 24 h treatment, Schwann cells were fixed with 4%
paraformaldehyde in PBS at 37˚C for 30 min and blocked with 1% BSA
(Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C. The cells were then
incubated with anti-Sox2 antibody (1:50) overnight at 4˚C followed
by incubation with the AlexaFluor® 488-conjugated
antibodies (1:200 dilution) for 1 h at 37˚C. Primary and secondary
antibodies were diluted in PBS. Cells were counterstained with 0.5
µg/ml DAPI (Invitrogen; Thermo Fisher Scientific, Inc.) diluted in
PBS (1:500) for 15 min at 37˚C and observed under a fluorescent
microscope (magnification, x200).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of melatonin-treated cells was extracted
using the RNeasy Mini kit. The cells were homogenised with the
RNeasy lysis buffer provided in the kit. RNA was collected with the
RNeasy Mini spin column, according to the manufacturer's
instruction. RNA (1 µg) was quantified at absorbance of 260/280 nm
with a plate reader (Infinite® 200 PRO NanoQuant; Tecan
Group, Ltd.).
cDNA was synthesized using the Maxima First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. The temperature protocol for reverse
transcription was as follows: 2 min at 37˚C, 10 min at 25˚C, 15 min
at 50˚C and 85˚C for 5 min. RT-qPCR was performed using QuantiNova
SYBR Green PCR kit. The procedures followed the manufacturer's
recommended amplification conditions with iQ5 real-time PCR
detection system. The sequences of Rattus norvegicus primers
used for the PCR were as follows: GAPDH-forward,
5'-TGCACCACCAACTGCTTAG-3' and reverse, 5'-GGATGCAGGGATGATGTTC-3';
MT1-forward, 5'-TCATCTTTACTATCGTGGTGG-3' and reverse,
5'ACCACAAATATATTCCCTGCG-3'; MT2-forward, 5'-ACCTGTTACTGAATGTTGCC-3'
and reverse, 5'-AACGAAGTCTCACTTCAACAC-3'; GDNF-forward,
5'-TGACCAGTGACTCCAATATG-3' and reverse, 5'-TACCTTGTCACTTGTTAGCC-3';
and PKC-forward, 5'-CCAAAAGCTAGAGACAAGCG-3' and reverse,
5'-TGGAATCTGCATTCACCTAC-3'. The thermocycling conditions were as
follows: Initial denaturation 95˚C for 2 min, followed by 40 cycles
of 95˚C for 5 sec and 60˚C for 10 sec. Relative mRNA expression of
each gene to GAPDH was calculated based on the 2-ΔΔCq
method (20).
Western blotting assay
Schwann cells treated with melatonin were lysed with
10X SDS sample buffer [62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10%
glycerol and 100 mM DTT]. Protein concentration was determined
using the Quick Start™ Bradford Protein Assay kit
(Bio-Rad Laboratories, Inc.). Protein samples (25 µg) were applied
to 10% gels and separated by SDS-PAGE and transferred to PVDF
membranes. The membranes were blocked with 5% BSA for 1 h at room
temperature and then incubated with primary antibodies against MT1,
MT2, GDNF, PKC, Ras, B-raf, p-B-Raf, C-raf, p-C-Raf, ERK1/2,
p-ERK1/2, p39, p-p38, SAPK-JNK, p-SAPK-JNK and α-tubulin at 4˚C
overnight. All primary antibodies were prepared in 1:500 dilutions
in blocking buffer. The blots were then incubated with horseradish
peroxidase-conjugated anti-rabbit/mouse secondary antibodies at
1:10,000 dilution for 1 h at room temperature. The blots were
subsequently developed with SuperSignal™ West Femto
Maximum Sensitivity (Thermo Fisher Scientific, Inc.) substrate and
visualised using ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc.). Quantitative blot data were analysed and
calculated using Image Lab Software (version 4.0; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times and data
were presented as the mean ± standard deviation. Statistical
analysis was performed using one-way ANOVA followed by Dunnett's
test (SPSS 21.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Melatonin enhances Schwann cell
proliferation
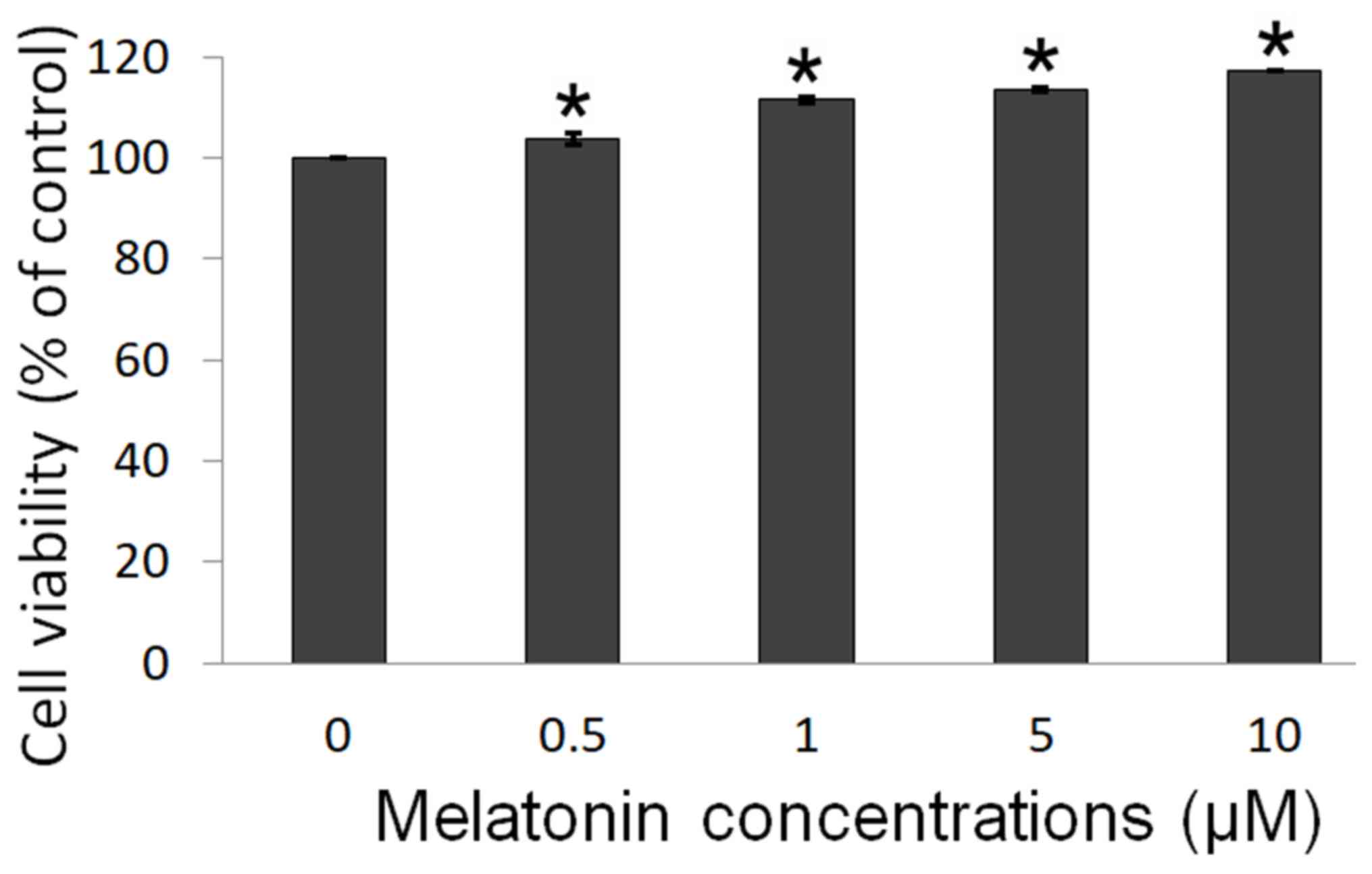
An MTT assay was performed to determine the
melatonin-induced proliferation of Schwann cells. The results
showed that melatonin increases Schwann cell proliferation, after
treatment with 0.5, 1, 5 and 10 µM melatonin (Fig. 1). The cell viability was
significantly increased from 3.8 to 17.2% across the different
treatment groups, suggesting that melatonin promoted Schwann cell
proliferation in a dose-dependent manner.
Melatonin increases the gene
expression of MT1 and MT2 receptors in Schwann cells
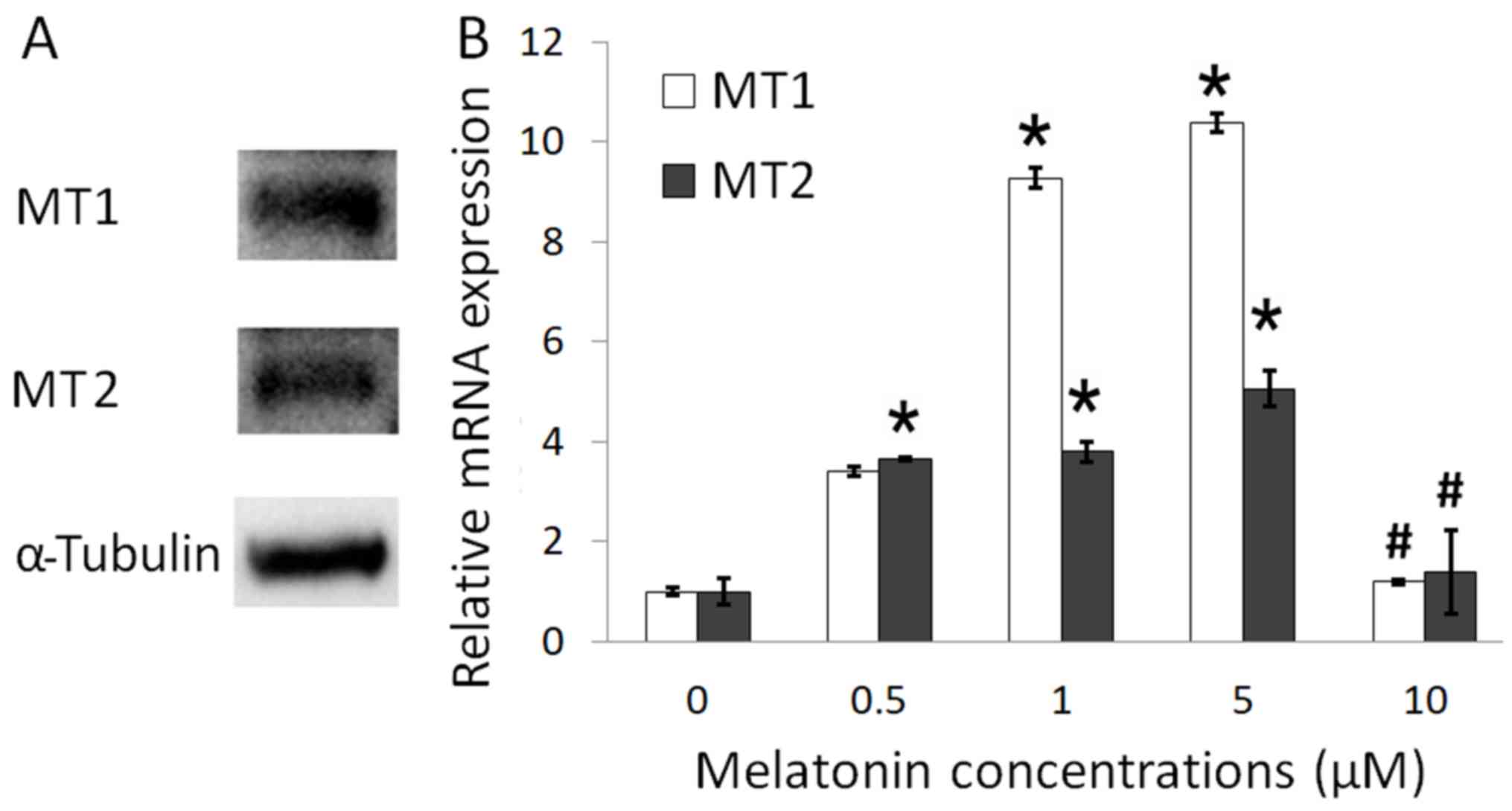
To determine the presence of melatonin receptors on
Schwann cells, western blotting (Fig.
2A) and RT-qPCR (Fig. 2B) were
performed. The present study identified the presence of both MT1
and MT2 receptors in Schwann cells. Following treatment with 1 and
5 µM melatonin, the expression of MT1 and MT2 receptor mRNA were
significantly increased compared with control. However, after
treatment with 10 µM melatonin, the expression of MT1 and MT2
receptor mRNA was significantly decreased compared with 0.5, 1 and
5 µM melatonin treatment (Fig. 2B).
MT1 gene expression was higher compared with MT2 following
treatment with 1 and 5 µM melatonin, suggesting that MT1 was more
responsive to the melatonin treatment and may serve a role in
inducing proliferation in Schwann cells.
Melatonin-induced Schwann cell
dedifferentiation
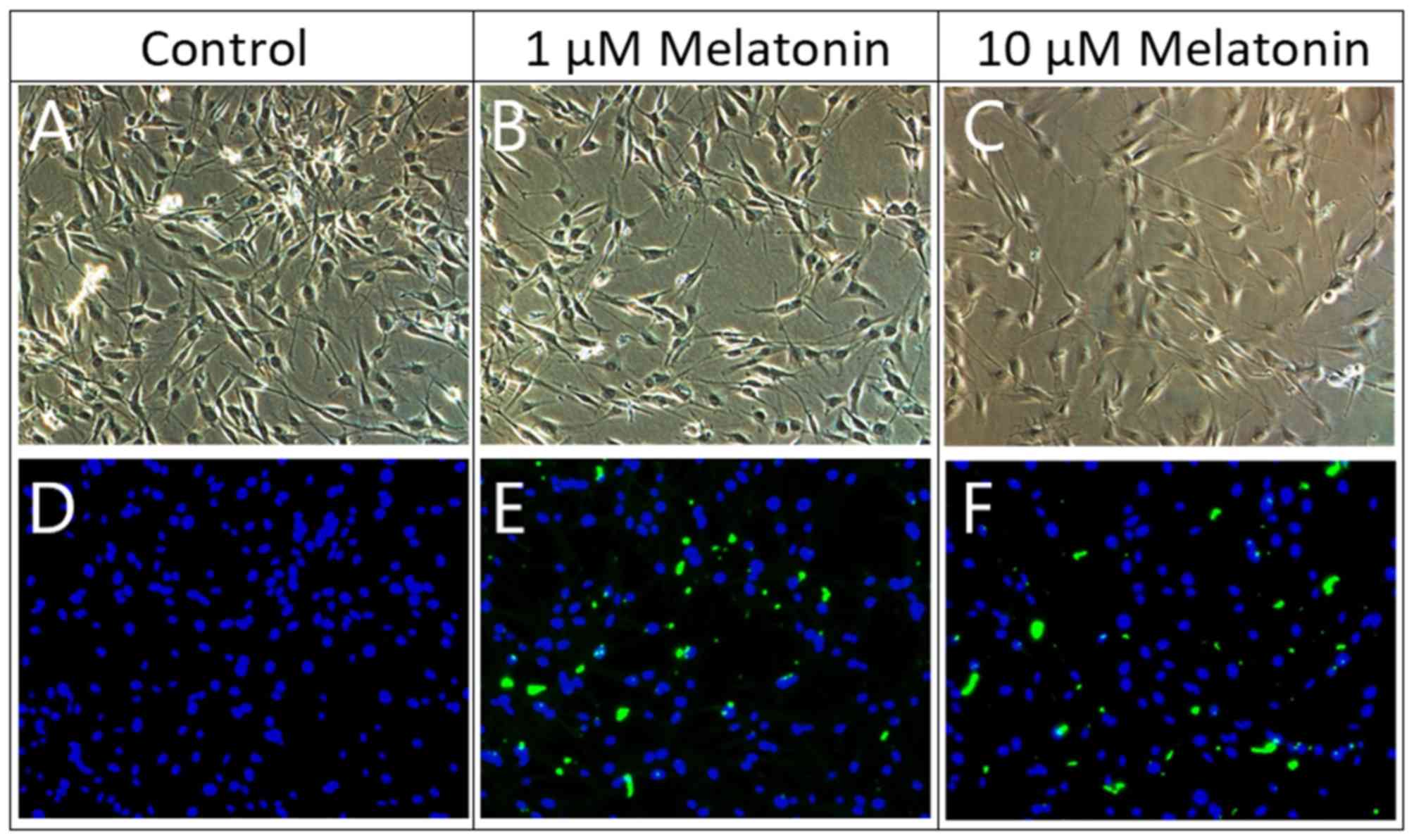
To determine if dedifferentiation of Schwann cells
was induced by melatonin, immunofluorescence staining and a western
blotting assay were conducted. Under light microscope observation,
Schwann cells have oval, spindle-shape or bipolar-like cell
morphology. The cell morphology between control cells and
melatonin-treated cells did not differ much (Fig. 3). Under fluorescent microscope
observation, the cell nucleus was stained with DAPI (blue
fluorescence). However, only melatonin-treated cells expressed the
Sox2 marker and showed green fluorescence (Fig. 3), which indicated a positive cell
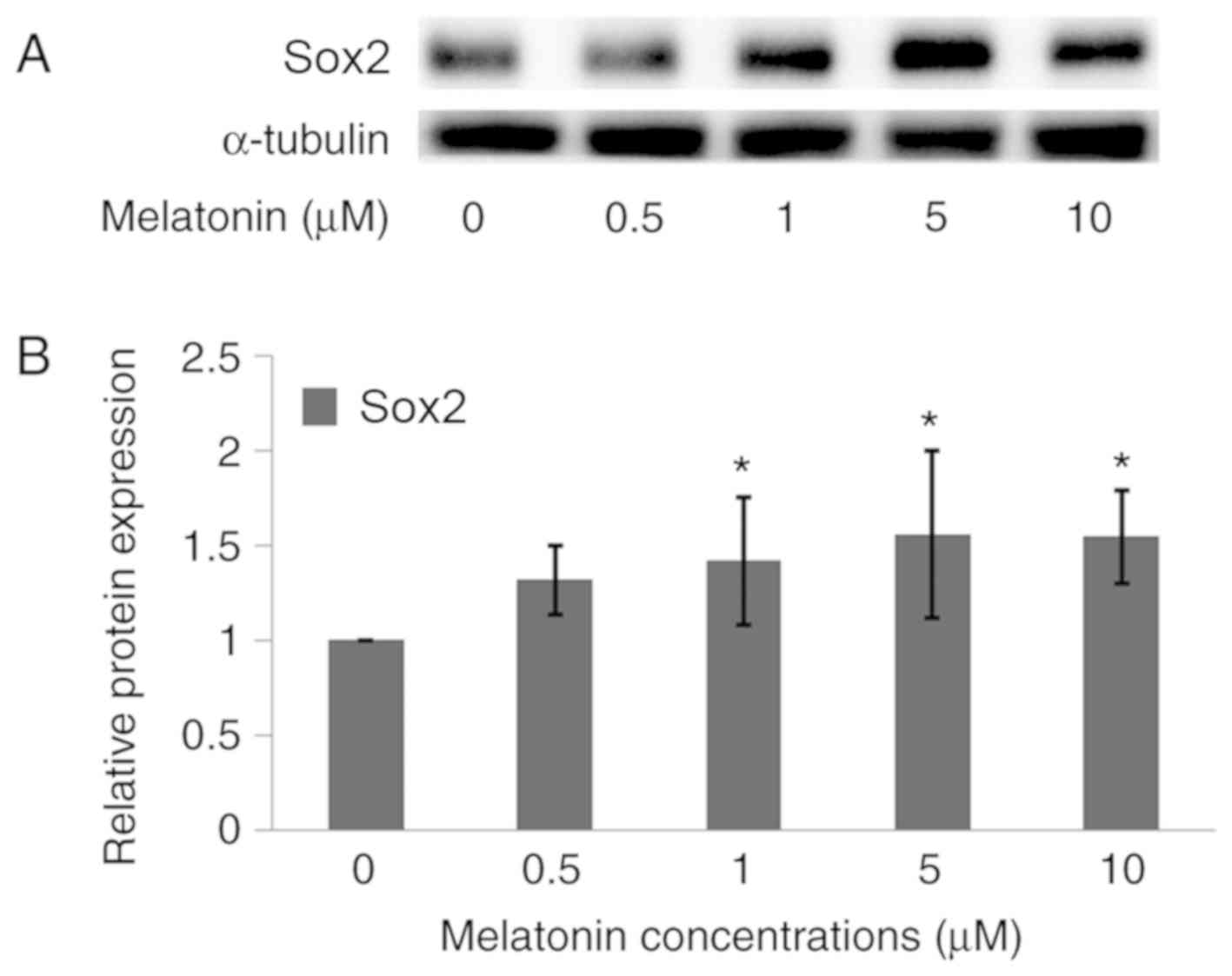
dedifferentiation process. Moreover, Sox2 protein expression
increased in a dose-dependent manner as shown by the western
blotting assay (Fig. 4).
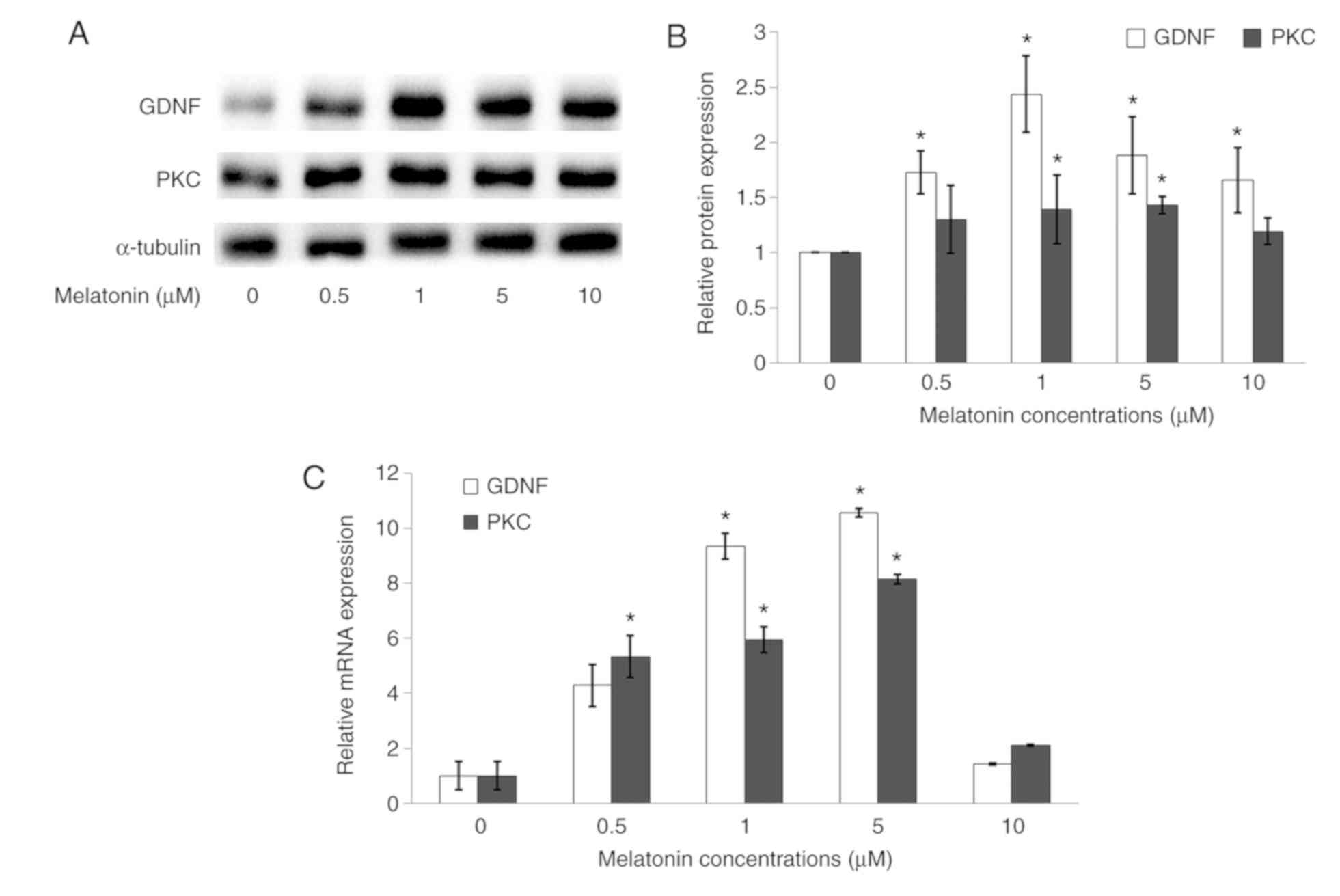
Melatonin increases GDNF and PKC
protein expressions in Schwann cells
To determine GDNF and PKC gene and protein
expressions in Schwann cells, RT-qPCR and western blotting assays
were conducted. The GDNF protein expression was significantly
upregulated when the cells were treated with 1, 5 and 10 µM
melatonin as compared with the control, whilst PKC protein
expression was significantly upregulated following treatment with 1
and 5 µM melatonin compared with control (Fig. 5A and B). RT-qPCR analysis showed that GDNF and
PKC gene expressions were significantly upregulated in cells
treated with 1 and 5 µM melatonin as compared with the control
(Fig. 5C).
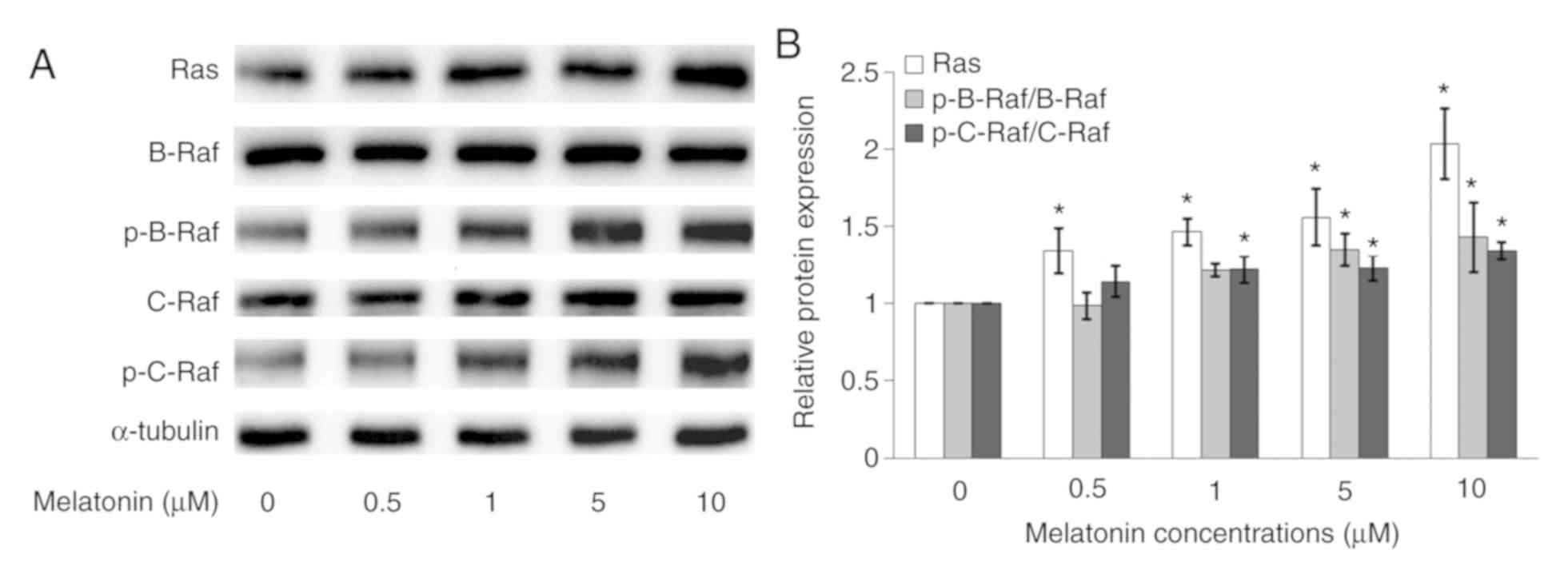
Melatonin increases Ras and p-Raf
protein expression in Schwann cells
The Ras, B-Raf, p-B-Raf C-Raf and p-C-Raf protein
expressions in Schwann cells were measured by western blotting. In
Fig. 6A, after melatonin treatment,
the Ras, p-B-Raf and p-C-Raf protein expressions increased in a
dose-dependent manner. However, there were no protein expressions
changes for B-Raf and C-Raf. In Fig.
6B, the normalized phosphorylated protein was compared with the
respective total protein, which showed that p-B-Raf/B-Raf and
p-C-Raf/C-Raf protein expressions increased in a dose-dependent
manner, especially at higher concentrations of melatonin (5 and 10
µM).
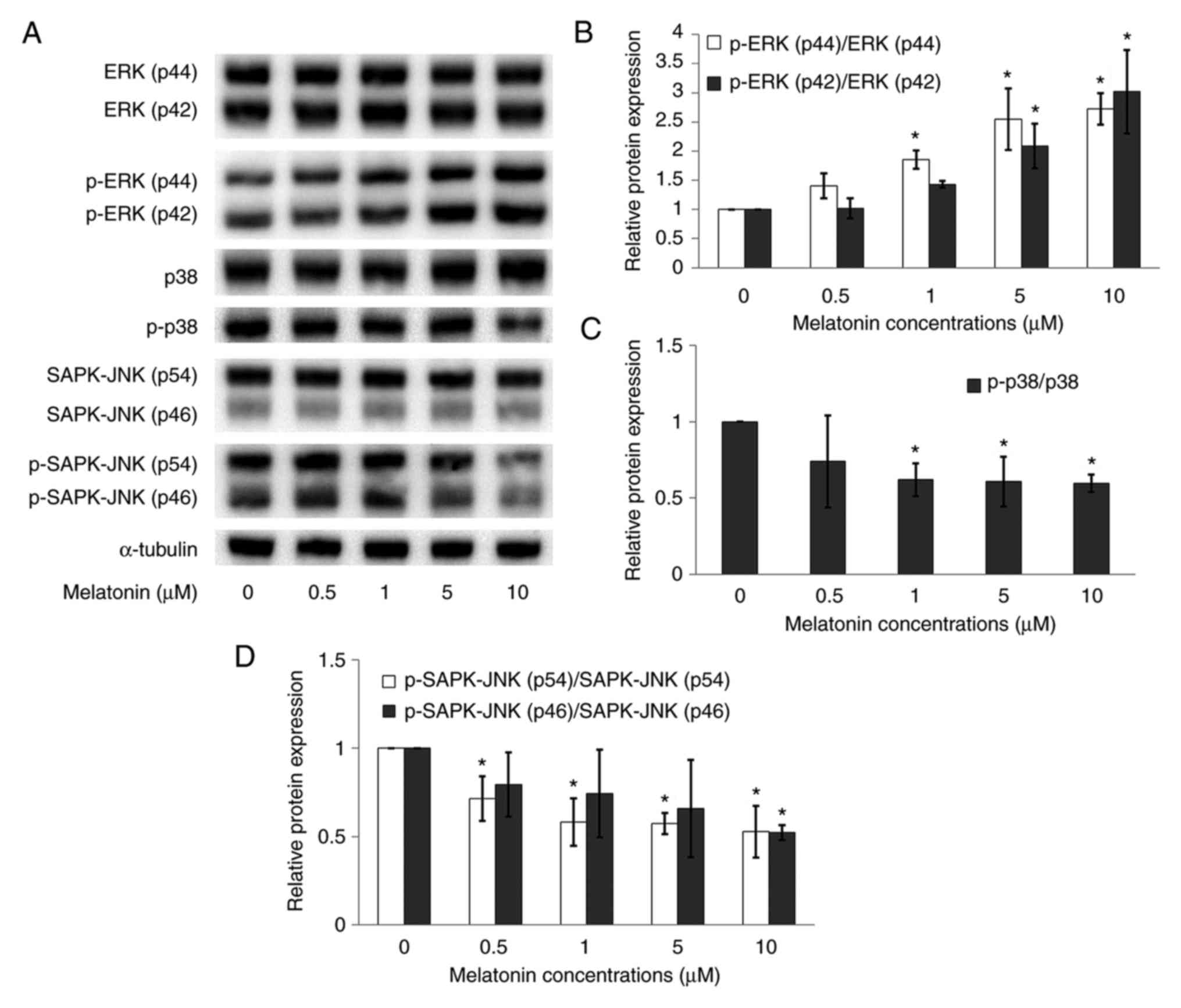
Melatonin regulates ERK, SAPK-JNK and
p38 protein expressions in Schwann cells
ERK, p-ERK, SAPK-JNK, p-SAPK-JNK, p38 and p-p38
protein expressions were measured in Schwann cells using a western
blotting assay. The present study showed no significant difference
in the expression of total protein levels of ERK, SAPK/JNK and p38
between the control and the melatonin treated groups (Fig. 7A). However, while normalized
phosphorylated proteins with the respective total proteins, the
results showed that in the melatonin treated groups,
p-ERK(p44)/ERK(p44) and p-ERK(p42)/ERK(p42) increased in a
dose-dependent manner (Fig. 7B),
especially at higher concentrations of melatonin (5 and 10 µM).
Furthermore, melatonin downregulated the expressions of
p-SAPK-JNK(p54)/SAPK-JNK(p54), p-SAPK-JNK(p46)/SAPK-JNK(p46)
(Fig. 7D) and p-p38/p38 (Fig. 7C) proteins as compared with the
control.
Discussion
Melatonin has been shown to promote glial cell
survival, axonal regeneration and neuronal stem cell proliferation
(21,22). Turgut et al (23) demonstrated that melatonin inhibited
both collagen production and neuroma formation, resulting in axonal
regeneration of the transected sciatic nerve in rats. In
pinealectomized rats, melatonin suppressed transforming growth
factor-β and basic fibroblast growth factor protein expressions
(24). The findings from a previous
study suggested that melatonin could suppress collagen scar
formation after PNI (23).
Melatonin also increased the number of axons and the thickness of
the myelin sheath after PNI (4).
Stavisky et al (25)
demonstrated that melatonin enhanced the repair of sciatic nerve
crush injuries in rats. However, the exact molecular mechanism
involved is not yet known.
Schwann cells play an important role in the
regeneration of damaged nerves. Upon PNI, Schwann cells lose their
connection to axons. This phenomenon triggers the continuous
proliferation of Schwann cells and the formation of Bunger bands
that provide guidance for axonal regeneration (5). The present study indicated that
melatonin induced RT4 Schwann cell proliferation at concentrations
of 0.5, 1, 5 and 10 µM, while Chang et al (26) identified that the optimal melatonin
concentrations to enhance RSC 96 Schwann cell proliferation were
0.1, 1 and 10 nM. This difference may be due to different cell
lines being used in the previous study and melatonin having
different efficacies in various cell lines. Moreover, Chang et
al (26) also demonstrated that
melatonin enhanced RSC 96 Schwann cell proliferation mainly via the
MT1 receptor as well as phosphorylation of ERK1/2 protein
expression. However, the present study not only suggested that
melatonin is able to dedifferentiate RT4 Schwann cells, the present
study also demonstrated that melatonin may enhance cell
proliferation through three different pathways, which are the
Ras/Raf/ERK, MAPK and GDNF/PKC pathways. The identification of
these proliferation pathways could have important implications for
future development of therapeutic approaches. The present results
demonstrated that melatonin induces Schwann cell proliferation via
MT1 and MT2 receptors, and the MT1 receptor expression was higher
than MT2. Previous studies demonstrated that MT1 activation is
involved in proliferation, differentiation and firing in neuronal
cells (27,28). Chern et al (29) observed that MT2 activation enhanced
endogenous neurogenesis in rats. MT2 also played an important role
in axonogenesis in in vivo and in vitro models
(30). However, a previous study
demonstrated that melatonin can induce cell proliferation in rat
pancreatic stellate cells, independent of MT1/MT2 receptor
activations via the MAPK pathway (31). In the present study, following
treatment with 1 and 5 µM melatonin, the expression of MT1 and MT2
receptor mRNA were significantly increased compared with control.
However, after treatment with 10 µM melatonin, the expression of
MT1 and MT2 receptor mRNA was reduced compared with 0.5, 1 and 5 µM
melatonin treatment. This means high concentrations of melatonin
may not result in a high responses due to negative feedback effects
that altered melatonin receptor or the saturation of melatonin
receptors (32).
After PNI, Schwann cells upregulate inflammatory
cytokines [tumour necrosis factor-α, interleukin (IL)-1α, IL-6,
IL-1β, leukaemia inhibitory factor and monocyte chemotactic protein
1] production in the distal stump (33,34).
IL-6 and leukaemia inhibitory factor attract macrophages to the
injured nerve and act on neurons to promote axonal regeneration
(35,36). Macrophages co-operate with the
Schwann cells to degrade myelin debris that potentially inhibit
axon growth (37). Macrophages also
produce cytokines to promote vascularisation of the distal nerve
(38,39). Specifically, upregulation of
neurotrophic factors (GDNF, artemin, brain-derived neurotrophic
factor, neurotrophin-3, nerve growth factor and vascular
endothelial growth factor) promote axonal elongation and the
survival of injured neurons (40,41).
The present study identified the involvement of GDNF and PKC in the
melatonin-mediated Schwann cell proliferation. Loss of GNDF
signaling is one of the features of PNI, especially at the distal
stump of injured sciatic nerves (42). Previous studies demonstrated that
GDNF is a survival factor for several types of neurons (43-45).
The addition of exogenous GDNF has been shown to improve peripheral
nerve regeneration and functional recovery (46). The present study showed that GDNF
was highly expressed in the melatonin-treated Schwann cells. A
previous study has reported that Schwann cells secrete a range of
neurotrophic factors, including GDNF, which have been shown to be
involved in promoting the development and maintenance of a subset
of dorsal root ganglion sensory neurons (11). Increased PKC protein expression was
also observed in the present study. PKC phosphorylation is involved
in the formation of the growth cone after neuronal injury, which is
an important step for neuronal regeneration (47,48).
Therefore, the present data supported the hypothesis that melatonin
promotes Schwann cell proliferation through the activation of the
GDNF/PKC pathway.
Activation of the Ras/Raf/ERK signaling pathway is
an important process in the development of Schwann cell-derived
tumours (9,49). Harrisingh et al (9) identified that the continuous
activation of the Ras/Raf/ERK signaling pathway is able to induce
Schwann cell dedifferentiation and proliferation. The present
results demonstrated that melatonin induced increases in Ras,
p-C-Raf/C-Raf, p-B-Raf/B-Raf, p-ERK (p44)/ERK(p44) and
p-ERK(p42)/ERK(p42) protein expressions in a dose-dependent manner.
There are three different Raf isoforms (Raf-1/C-Raf, B-Raf and
A-Raf), which consist of three conserved regions (CR; CR1, CR2 and
CR3) (50). CR1 consists of a Ras
binding domain and a cysteine rich domain (51). CR2 consists of activating
phosphorylation and inhibitory sites, which regulate Ras binding
and Raf activation (52). CR3
consists of a kinase activation domain (53). The major differences between the
three Raf isoforms are dependent on the number and location of
activating phosphorylation, inhibitory and autophosphorylation
sites (54,55). All Raf proteins share MEK1/2 kinases
as substrates (56). MEK1/2 in turn
activates ERK1/2, and this pathway regulates cell proliferation and
differentiation (50,57). p-ERK (p44) and p-ERK (p42) proteins
are rapidly phosphorylated in response to all mitogens and are
ubiquitously expressed; there are no obvious regulatory differences
inferred from their protein sequences, their regulation or their
sub-cellular localization (49).
With Schwann cells being dedifferentiated to Schwann cell
precursors, the proliferation rate was enhanced in the presence of
cAMP (10). Other previous studies
have demonstrated that Ras/Raf/ERK signaling activation also drives
the demyelination of peripheral nerves, and the Schwann cells
remain in dedifferentiated states, which ultimately leads to axon
regeneration and tubular reconstruction (9,58).
MAPKs, consisting of ERK, SAPK-JNK, and p38 MAPKs,
are all activated in Schwann cells after PNI (50). Previous studies suggested that the
ERK pathway seems to play a more distinct role in Schwann cell
dedifferentiation than the SAPK-JNK and p38 MAPK pathways (59,60).
The present results demonstrated that after melatonin treatment,
p-ERK(44)/ERK(44) and p-ERK(42)/ERK(42) protein expressions increased in a
dose-dependent manner. However, p-SAPK-JNK(p54)/SAPK-JNK(p54),
p-SAPK-JNK(p46)/SAPK-JNK(p46), p-p38/p38 ratio decreased in manner
that is negatively associated with the dose of melatonin
concentration used. In conclusion, the ERK pathway plays a more
important role than the SAPK-JNK and p38 MAP kinase pathways in
Schwann cell dedifferentiation. In summary, the present study
provided evidence that melatonin promotes Schwann cell
dedifferentiation and proliferation through the GDNF/PKC,
Ras/Raf/ERK and MAPK pathways. Schwann cell proliferation plays a
crucial role in PNI; the present study suggested the use of
melatonin as a therapeutic agent to enhance recovery from PNI. In
the future, the present results (melatonin concentrations and
mechanisms involved) could be applied to treat sciatic nerve injury
and pinealectomized rats to determine optimal melatonin
concentrations and parameters for progress in functional
recovery.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Malaysia Toray
Science Foundation (grant no. 14/G72).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
YLT performed the experiments, data analysis and
interpretation. GP performed the RT-qPCR, western blotting, data
analysis and critical revision of the manuscript. RYK and KYN
conceived and designed the study. SMC designed the study, wrote the
original draft, edited and critically revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ciaramitaro P, Mondelli M, Logullo F,
Grimaldi S, Battiston B, Sard A, Scarinzi C, Migliaretti G, Faccani
G and Cocito D: Italian Network for Traumatic Neuropathies.
Traumatic peripheral nerve injuries: Epidemiological findings,
neuropathic pain and quality of life in 158 patients. J Peripher
Nerv Syst. 15:120–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Richardson PM and Issa VM: Peripheral
injury enhances central regeneration of primary sensory neurones.
Nature. 309:791–793. 1984.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Fex Svennigsen A and Dahlin LB: Repair of
the peripheral nerve-remyelination that works. Brain Sci.
3:1182–1197. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aktas A, Turgut M, Kaplan S, Ulkay B,
Odacı E, Akyüz O, Çolakoğlu S, Yazıcı AC and İnce O: The effect of
intrauterine acute ethanol exposure on developing sciatic nerves
and their myelination: A stereological study. J Exp Clin Med.
26:35–41. 2009.
|
|
5
|
Mirsky R, Jessen KR, Brennan A, Parkinson
D, Dong Z, Meier C, Parmantier E and Lawson D: Schwann cells as
regulators of nerve development. J Physiol Paris. 96:17–24.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Naidu M and David P: Major cellular events
in peripheral nerve regeneration. A brief overview. Int Med J.
8:69–72. 2009.
|
|
7
|
Agthong S, Kaewsema A, Tanomsridejchai N
and Chentanez V: Activation of MAPK ERK in peripheral nerve after
injury. BMC Neurosci. 7(45)2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barras FM, Pasche P, Bouche N, Aebischer P
and Zurn AD: Glial cell line-derived neurotrophic factor released
by synthetic guidance channels promotes facial nerve regeneration
in the rat. J Neurosci Res. 70:746–755. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Harrisingh MC, Perez-Nadales E, Parkinson
DB, Malcolm DS, Mudge AW and Lloyd AC: The Ras/Raf/ERK signalling
pathway drives Schwann cell dedifferentiation. EMBO J.
23:3061–3071. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ridley AJ, Paterson HF, Noble M and Land
H: Ras-mediated cell cycle arrest is altered by nuclear oncogenes
to induce Schwann cell transformation. EMBO J. 7:1635–1645.
1988.PubMed/NCBI
|
|
11
|
Hὃke A, Ho T, Crawford TO, LeBel C, Hilt D
and Griffin JW: Glial cell line-derived neurotrophic factor alters
axon Schwann cell units and promotes myelination in unmyelinated
nerve fibers. J Neurosci. 23:561–567. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sayan H, Ozacmak VH, Ozen OA, Coskun O,
Arslan SO, Sezen SC and Aktas RG: Beneficial effects of melatonin
on reperfusion injury in rat sciatic nerve. J Pineal Res.
37:143–148. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Piirsoo M, Kaljas A, Tamm K and Timmusk T:
Expression of NGF and GDNF family members and their receptors
during peripheral nerve development and differentiation of Schwann
cells in vitro. Neurosci Lett. 469:135–140. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu P, Rosen KM, Hedstrom K, Rey O, Guha S,
Hart C and Corfas G: Nerve injury induces glial cell line-derived
neurotrophic factor (GDNF) expression in Schwann cells through
purinergic signaling and the PKC-PKD pathway. Glia. 61:1029–1040.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Svensson K, Zeidman R, Trollér U, Schultz
A and Larsson C: Protein Kinase C beta1 is implicated in the
regulation of neuroblastoma cell growth and proliferation. Cell
Growth Differ. 11:641–648. 2000.PubMed/NCBI
|
|
16
|
Martins RS, Bastos D, Siqueira MG, Heise
CO and Teixeira MJ: Traumatic injuries of peripheral nerves: A
review with emphasis on surgical indication. Arq Neuropsiquiatr.
71:811–814. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramirez-Rodriguez G, Klempin F, Babu H,
Benítez-King G and Kempermann G: Melatonin modulates cell survival
of new neurons in the hippocampus of adult mice.
Neuropsychopharmacology. 34:2180–2191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rennie K, De Butte M and Pappas BA:
Melatonin promotes neurogenesis in dentate gyrus in the
pinealectomized rat. J Pineal Res. 47:313–317. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fredrich M, Christ E and Korf HW:
Differential regulation of cell proliferation and apoptosis by
melatonin receptor subtype-signaling in the adult murine brain.
Neuroendocrinology. 107:158–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Borlongan CV, Yamamoto M, Takei N,
Kumazaki M, Ungsuparkorn C, Hida H, Sanberg PR and Nishino H: Glial
cell survival is enhanced during melatonin-induced neuroprotection
against cerebral ischemia. FASEB J. 4:1307–1317. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kong PJ, Byun JS, Lim SY, Lee JJ, Hong SJ,
Kwon KJ and Kim SS: Melatonin induces AKt phosphorylation through
melatonin receptor- and PI3K-dependent pathways in primary
astrocytes. Korean J Physiol Pharmacol. 12:37–41. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Turgut M, Uyanikgil Y, Baka M, Tunc AT,
Yavapodlu A, Yurtseven ME and Kaplan S: Pinealectomy exaggerates
and melatonin treatment suppresses neuroma formation of transected
sciatic nerve in rats: Gross morphological, histological and
stereoligical analysis. J Pineal Res. 38:284–291. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Turgut M, Oktem G, Uysal A and Yurtseven
ME: Immunohistochemical profile of transforming growth factor-beta1
and basic fibroblast growth factor in sciatic nerve anastomosis
following pinealectomy and exogenous melatonin administration in
rats. J Clin Neurosci. 13:753–758. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stavisky RC, Britt JM, Zuzek A, Truong E
and Bittner GD: Melatonin enhances the in vitro and in vivo repair
of severed rat sciatic axons. Neurosci Lett. 376:98–101.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chang HM, Liu CH, Hsu WM, Chen LY, Wang
HP, Wu TH, Chen KY, Ho WH and Liao WC: Proliferative effects of
melatonin on Schwann cells: Implication for nerve regeneration
following peripheral nerve injury. J Pineal Res. 56:322–332.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dubocovich ML and Markowska M: Functional
MT1 and MT2 melatonin receptors in mammals. Endocrine. 27:101–110.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kaneko Y, Hayashi T, Yu S, Tajiri N, Bae
EC, Solomita MA, Chheda SH, Weinbren NL, Parolini O and Borlongan
CV: Human amniotic epithelial cells express melatonin receptor MT1,
but not melatonin receptor MT2: A new perspective to
neuroprotection. J Pineal Res. 50:272–280. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chern CM, Liao JF, Wang YH and Shen YC:
Melatonin ameliorates neural function by promoting endogenous
neurogenesis through the MT2 melatonin receptor in ischemic-stroke
mice. Free Radic Biol Med. 52:1634–1647. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu D, Wei N, Man HY, Lu Y, Zhu LQ and
Wang JZ: The MT2 receptor stimulates axonogenesis and enhances
synaptic transmission by activating Akt signalling. Cell Death
Differ. 22:583–596. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Santofimia-Castaño P, Garcia-Sanchez L,
Ruy DC, Sanchez-Correa B, Fernandez-Bermejo M, Tarazona R, Salido
GM and Gonzalez A: Melatonin induces calcium mobilization and
influences cell proliferation independently of MT1/MT2 receptor
activation in rat pancreatic stellate cells. Cell Biol Toxicol.
31:95–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu GD, Rusak B and Piggins HD: Regulation
of melatonin-sensitivity and firing-rate rhythms of hamster
suprachiasmatic nucleus neurons: Constant light effects. Brain Res.
602:191–199. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Martini R, Fischer S, López-Vales R and
David S: Interactions between Schwann cells and macrophages in
injury and inherited demyelinating disease. Glia. 56:566–1577.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rotshenker S: Wallerian degeneration: The
innate-immune response to traumatic nerve injury. J
Neuroinflammation. 8(109)2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hirota H, Kiyama H, Kishimoto T and Taga
T: Accelerated nerve regeneration in mice by upregulated expression
of interleukin (IL) 6 and IL-6 receptor after trauma. J ExpMed.
183:2627–2634. 1996.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cafferty WB, Gardiner NJ, Gavazzi I,
Powell J, McMahon SB, Heath JK, Munson J, Cohen J and Thompson SW:
Leukemia inhibitory factor determines the growth status of injured
adult sensory neurons. J Neurosci. 21:7161–7170. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hirata K and Kawabuchi M: Myelin
phagocytosis by macrophages and nonmacrophages during Wallerian
degeneration. Microsc Res Tech. 57:541–547. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Barrette B, Hébert MA, Filali M, Lafortune
K, Valli'eres N, Gowing G, Julien JP and Lacroix S: Requirement of
myeloid cells for axon regeneration. J Neurosci. 28:9363–9376.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cattin AL, Burden JJ, Van Emmenis L,
Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M,
Rosenberg LH, Quereda V, et al: Macrophage-induced blood vessels
guide Schwann cell-mediated regeneration of peripheral nerves.
Cell. 162:1127–1139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wood MD and Mackinnon SE: Pathways
regulating modality-specific axonal regeneration in peripheral
nerve. Exp Neurol. 265:171–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Boyd JG and Gordon T: Neurotrophic factors
and their receptors in axonal regeneration and functional recovery
after peripheral nerve injury. Mol Neurobiol. 27:277–324.
2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shi JY, Liu GS, Liu LF, Kuo SM, Ton CH,
Wen ZH, Tee R, Chen CH, Huang HT, Chen CL, et al: Glial cell
line-derived neurotrophic factor gene transfer exerts protective
effect on axons in sciatic nerve following constriction-induced
peripheral nerve injury. Hum Gene Ther. 22:721–731. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pascual A, Hidalgo-Figueroa M, Piruat JI,
Pintado CO, Gómez-Díaz R and López-Barneo J: Absolute requirement
of GDNF for adult catecholaminergic neuron survival. Nat Neurosci.
11:755–761. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ortiz-Ortiz MA, Morán JM, Ruiz-Mesa LM,
Bonmatty RG and Fuentes JM: Protective effect of the glial cell
line-derived neurotrophic factor (GDNF) on human mesencephalic
neuron-derived cells against neurotoxicity induced by paraquat.
Environ Toxicol Pharmacol. 31:129–136. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Meka DP, Müller-Rischart AK, Nidadavolu P,
Mohammadi B, Motori E, Ponna SK, Aboutalebi H, Bassal M, Annamneedi
A, Finckh B, et al: Parkin cooperates with GDNF/RET signaling to
prevent dopaminergic neuron degeneration. J Clin Invest.
125:1873–1885. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen ZY, Cao L, Lu CL, He C and Bao X:
Protective effect of exogenous glial cell line derived neurotrophic
factor on neurons after sciatic nerve injury in rats. Sheng Li Xue
Bao. 52:295–300. 2000.PubMed/NCBI(In Chinese).
|
|
47
|
Kawakami T, Kawakami Y and Kitaura J:
Protein kinase C beta (PKC beta): Normal functions and diseases. J
Biochem. 132:677–682. 2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Spinsanti P, De Vita T, Caruso A,
Melchiorri D, Misasi R, Caricasole A and Nicoletti F: Differential
activation of the calcium/protein kinase C and the canonical
beta-catenin pathway by Wnt1 and Wnt7a produces opposite effects on
cell proliferation in PC12 cells. J Neurochem. 104:1588–1598.
2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Buscà R, Pouysségur J and Lenormand P:
ERK1 and ERK2 map kinases: Specific roles or functional redundancy?
Front Cell Dev Biol. 4(53)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Leicht DT, Balan V, Kaplun A, Singh-Gupta
V, Kaplun L, Dobson M and Tzivion G: Raf kinases: Function,
regulation and role in human cancer. Biochim Biophys Acta.
1773:1196–1212. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tran NH, Wu X and Frost JA: B-Raf and
Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol
Chem. 280:16244–16253. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dhillon AS, Meikle S, Yazici Z, Eulitz M
and Kolch W: Regulation of Raf-1 activation and signaling by
dephosphorylation. EMBO J. 21:64–71. 2002.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chong H, Lee J and Guan KL: Positive and
negative regulation of Raf kinase activity and function by
phosphorylation. EMBO J. 20:3716–3727. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Morrison DK, Heidecker G, Rapp UR and
Copeland TD: Identification of the major phosphorylation sites of
the Raf-1 kinase. J Biol Chem. 268:17309–17316. 1993.PubMed/NCBI
|
|
55
|
Stephens RM, Sithanandam G, Copeland TD,
Kaplan DR, Rapp UR and Morrison DK: 95-kilodalton B-Raf
serine/threonine kinase: Identification of the protein and its
major autophosphorylation site. Mol Cell Biol. 12:3733–3742.
1992.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Matallanas D, Birtwistle M, Romano D,
Zebisch A, Rauch J, von Kriegsheim A and Kolch W: Raf family
kinases: Old dogs have learned new tricks. Genes Cancer. 2:232–260.
2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wellbrock C, Karasarides M and Marais R:
The RAF proteins take centre stage. Nat Rev Mol Cell Biol.
5:875–885. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Napoli I, Noon LA, Ribeiro S, Kerai AP,
Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ,
Woodhoo A and Lloyd AC: A central role for the ERK-signaling
pathway in controlling Schwann cell plasticity and peripheral nerve
regeneration in vivo. Neuron. 73:729–742. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lee HJ, Shin YK and Park HT: Mitogen
activated protein kinase family proteins and c-jun signaling in
injury-induced Schwann cell plasticity. Exp Neurobiol. 23:130–137.
2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Shin YK, Jang SY, Park JY, Park SY, Lee
HJ, Suh DJ and Park HT: The Neuregulin-Rac-MKK7 pathway regulates
antagonistic c-jun/Krox20 expression in Schwann cell
dedifferentiation. Glia. 61:892–904. 2013.PubMed/NCBI View Article : Google Scholar
|