Introduction
Liver cancer currently ranks as the third most
common cause of mortality associated cancer worldwide, with
>600,000 deaths reported annually (1,2). Liver
cancer commonly occurs in patients with a history of chronic liver
conditions, including hepatitis B and C viral infections, alcoholic
or non-alcoholic liver disease, fatty liver disease and chronic
liver disease that is caused by aflatoxin poisoning (3), in a vicious cycle of liver injury,
regeneration and inflammation (1).
Since effective clinical diagnosis and treatment of liver cancer is
typically hindered by high rates of recurrence and metastasis
(2,3), it is of importance to develop
innovative therapeutic strategies for the diagnosis and treatment
of liver cancer.
TP53 is an important human tumor suppressor
gene that is present on chromosome 17p13.1(4), which contains 11 exons that encodes
393 amino acid residues and is one of the most commonly mutated
genes in African-Asian populations (5). The p53 protein is a transcriptional
product of TP53 that is associated with the inhibition of
tumor cell division, induction of tumor cell apoptosis and the
repair of DNA damage (6). In
different types of tumors, mutations in the TP53 gene
directly result the inactivation of p53 protein (4,6,7).
TP53 mutations have been identified in malignant tumors of
the lung, gastric, liver, breast and bladder, where p53 was found
to be inactivated or dysfunctional (8,9).
Therefore, it would be of great significance to utilize the tumor
suppressive properties of functional p53 for use in cancer
treatment.
Recombinant human adenovirus p53 (rAd-p53; also
known as Gendicine®) is a replication-incompetent
recombinant human serotype 5 adenovirus, where the virulent E1
region has been replaced by the human wild-type p53 expression
cassette (10). It is the first
commercially available product used for gene therapy and has been
applied in the treatment of a number of cancer types, including
head and neck cancer, epithelial ovarian carcinoma and liver cancer
(11,12). rAd-p53 has been previously reported
to enhance the sensitivity of gastric cancer cells to chemotherapy
by regulating the expression of proteins that are associated with
apoptosis (13). Clinical research
has also demonstrated that rAd-p53-based transarterial
chemoembolization is an effective and safe strategy for the
treatment of unresectable liver cancer (14). However, treatment with rAd-p53 alone
has proven to be insufficient for improving the survival of
patients with cancer (15-17).
In some cancer malignancies, including in liver
cancer, the aberrant tumor microenvironment may lead to subsequent
mutation of the TP53 gene. In liver cancer cells, DNA damage
and TP53 mutation have been previously associated with
certain stimuli, including chronic inflammation and intracellular
oxygen or nitrogen metabolites (18). Therefore, the introduction of
exogenous wild-type TP53 in combination with
anti-inflammatory and anti-oxidant agents that interfere with the
tumor microenvironment but do not effect normal healthy cells, may
produce improved therapeutic outcomes. Curcumin, a naturally
occurring active compound extracted from the rhizome and root of
the Curcuma longa plant, possesses antioxidant and
anti-inflammatory properties (19)
and has been demonstrated to be safe under clinical settings
(20). As a result, curcumin has
been considered as a promising therapeutic and preventative agent
against liver cancer (21-23).
However, the use of curcumin remains limited by its low
bioavailability (21). In the
present study, the treatment strategy of rAd-p53 combined with
curcumin was investigated on HepG2 cells. Cell proliferation,
apoptosis, expression of proteins targeted by the TP53 gene
and the activation of mitogen-activated protein kinase (MAPK)
signaling pathways were evaluated following treatment with rAd-p53
or curcumin individually and in combination. The results from the
present study may assist in the development of therapeutic
strategies involving rAd-p53 for use in the treatment of liver
cancer.
Materials and methods
Cell culture and treatment
The human hepatocyte cell line HHL-5 and liver
cancer cell lines HepG2, Hep3B and Huh-7 were supplied by the Type
Culture Collection of the Chinese Academy of Sciences.
Authentication of the cell lines was performed by STR profiling.
Cells were maintained in RPMI 1640 medium (Hyclone; GE Healthcare
Life Sciences) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C under 5% CO2. All cells were
subjected to treatment once ~90% confluence was achieved. Curcumin
(Sigma-Aldrich; Merck KGaA) was dissolved in DMSO and administered
at 37˚C to the cells at 10 µM. rAd-p53 (Sibiono GeneTech Co. Ltd.)
was stored at -20˚C at a density of 1x1012 virus
particles/ml. Prior to each experiment, cells were infected at 37˚C
with rAd-p53 particles at a multiplicity of infection of 100 using
a previously reported protocol (10). HHL-5 cells either remained untreated
(control/Con) or were treated with curcumin (Cur) to evaluate the
toxicity of curcumin on normal hepatocytes. HepG2, Hep3B and Huh-7
cells were divided into four groups in accordance with the
treatments they Received: Con (control), rAd-p53 alone, Cur alone
and Cur + rAd-p53.
Cell counting Kit-8 (CCK-8) assay
HHL-5 (untreated or treated with curcumin), HepG2,
Hep3B and Huh-7 cells (either treated with/without rAD-p3 and/or
curcumin) were seeded at the density of 3x103 cells/well
into 96-well plates and subsequently cultured for 24, 48 and 72 h.
Subsequently, a total of 10 µl CCK-8 solution (Bioswamp; Wuhan
Beinglay Biotech Co., Ltd.) was added to each well, followed by
further incubation at 37˚C for 4 h. Subsequently, a
SpectraMax® 190 Microplate Reader (Molecular Devices,
LLC) was used to measure the absorbance in each well at 450 nm.
Following treatment with or without rAD-p53 and/or
curcumin for 72 h, HepG2 cells were incubated in RPMI-1640 medium
containing 10% FBS without rAD-p53 and/or curcumin at 37˚C for 0,
24 and 48 h. Cell viability was detected using a CCK-8 assay as
aforementioned. All experiments were performed in triplicate.
Wound healing assay
HepG2 cells were first seeded into six-well plates
at 1x106 cells/well and incubated in DMEM (Hyclone; GE
Healthcare Life Sciences) supplemented with 10% FBS. When ~90%
confluence was reached, the cell monolayers were wounded by
scratching with a sterile 200 μl plastic pipette tip, following
which the cells were cultured in serum-free medium (DMEM) at 37˚C
under 5% CO2 for 24, 48 or 72 h. The wounds were imaged
using an inverted fluorescence microscope equipped with a camera
(Nikon Corporation).
Flow cytometry analysis
Flow cytometry was performed to evaluate the
apoptosis and cell cycle progression in HepG2 cells. For apoptosis,
the Annexin V/PI staining method was performed according to the
manufacturer's protocol (Bioswamp; Wuhan Beinglay Biotech Co.,
Ltd.). Following treatment, the cells (2x106 cells/ml)
were digested using ethylenediaminetetraacetic acid-trypsin
(Bioswamp; Wuhan Beinglay Biological Technology Co., Ltd.), washed
with pre-cooled PBS and resuspended in binding buffer. Annexin
V-fluorescein isothiocyanate and PI (10 µl each) were subsequently
added to the cells, following which they were incubated for 30 min
at 4˚C in the dark and subjected to the flow cytometry (Beckman
Corporation) and the data were analyzed using CXP Analysis 2.0
software (Beckman Corporation).
For analyzing cell cycle progression, the harvested
cells (2x107 cells/ml) were washed twice with pre-cooled
PBS and incubated with a mixture of 100 µl 1 mg/ml RNase A (Takara
Biotechnology Co., Ltd.) and 400 µl 50 µg/ml PI in the dark at room
temperature for 10 min. The treated cells were then subjected to
flow cytometry (Beckman Coulter, Inc.) and analyzed using ModFit LT
2.0 (Verity Software House).
Western blot analysis
The expression of proteins associated with cell
cycle progression, apoptosis, TP53 targets p53 and p21, MAPK
signaling and epithelial-mesenchymal transition (EMT) was evaluated
using western blot analysis. Total protein content in HepG2 cells
was extracted using a radioimmunoprecipitation assay lysis buffer
(Bioswamp; Wuhan Beinglay Biotech Co., Ltd.) containing protease
and phosphatase inhibitors. Proteins were quantified using the BCA
kit (Bioswamp; Wuhan Beinglay Biotech Co., Ltd.). A total of 10 µg
proteins were separated using SDS-PAGE (12%) and transferred onto
PVDF (EMD Millipore). The membranes were then blocked with 5%
skimmed milk for 2 h at room temperature and incubated with primary
antibodies overnight at 4˚C. After washing, the membranes were
incubated with secondary antibody for 1 h at room temperature.
Immunoreactivity was visualized by colorimetric reaction using ECL
substrate buffer (EMD Millipore). The membranes were then detected
by an automatic chemiluminescence analyzer (Tanon-5200; Tanon
Science and Technology Co., Ltd.) and the band gray values were
read using TANON GIS 4.2 software (Tanon Science and Technology
Co., Ltd.). All experiments were performed in triplicate. Detailed
information of all antibodies used in the present study are
presented in Table I.
 | Table IAntibodies used in this study. |
Table I
Antibodies used in this study.
| Antibody | Species | Company | Product code | Dilution | Protein size
(kDa) |
|---|
| Primary
antibodies |
|
p53 | rabbit | Abcam | ab131442 | 1:1,000 | 53 |
|
p21 | rabbit | Abcam | ab109199 | 1:1,000 | 18 |
|
p-ERK1/2 | rabbit | Abcam | ab223500 | 1:400 | 42-44 |
|
ERK1/2 | rabbit | Abcam | ab17942 | 1:1,000 | 42-44 |
|
p-p38MAPK | rabbit | Abcam | ab47363 | 1:1,000 | 41 |
|
p38MAPK | rabbit | Abcam | ab27986 | 1:1,000 | 41 |
|
p-JNK | rabbit | Abcam | ab124956 | 1:5,000 | 46-54 |
|
JNK | rabbit | Abcam | ab179461 | 1:1,000 | 46-54 |
|
Caspase3 | rabbit | Abcam | ab90437 | 1:1,000 | 32 |
|
Caspase8 | rabbit | Abcam | ab227430 | 1:2,000 | 55 |
|
Caspase9 | rabbit | Abcam | ab2013 | 1:2,000 | 46 |
|
Bax | rabbit | Abcam | ab53154 | 1:1,000 | 21 |
|
Bcl-2 | rabbit | Abcam | ab196495 | 1:2,000 | 26 |
|
Cyclin
A | rabbit | Abcam | ab137769 | 1:2,000 | 49 |
|
Cyclin
E | rabbit | Abcam | ab33911 | 1:2,000 | 50 |
|
N-cadherin | rabbit | Abcam | ab18203 | 1:1,000 | 100 |
|
Snail | rabbit | Abcam | ab216347 | 1:1,000 | 29 |
|
Twist | rabbit | Abcam | ab49254 | 1:400 | 21 |
|
GAPDH | rabbit | Proteintech | 10494-1-AP | 1:5,000 | 36 |
| Secondary
antibody |
|
Goat
anti-rabbit IgG | goat | Bioswamp | SAB43658 | 1:20,000 | N/A |
Reverse transcription-quantitative PCR
(RT-qPCR)
p53 and p21 mRNA expression in HepG2 cells was
measured using RT-qPCR. Total RNA was extracted using
TRIzol® (Ambion; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol, and reverse-transcribed into
first-strand cDNA using the M-MLV kit (Takara Biotechnology Co.,
Ltd.) according to manufacturer's protocol. The temperature
protocol were as follows: 42˚C for 1 h; 70˚C for 15 min and hold at
16˚C. The cDNA was subsequently used for qPCR using the
SYBR® Green PCR kit (KAPA Biosystems; Roche diagnostics)
according to manufacturer's protocols. The following thermocycling
conditions were used for the PCR: 95˚C for 3 min; 39 cycles of
denaturation at 95˚C for 5 sec, annealing at 56˚C for 10 sec and
extension at 72˚C for 25 sec and final extension at 65˚C for 5 sec
and 95˚C for 50 sec. The primer sequences used are as follows: p53
forward, 5'-ATGTTTGTGCCTG CCT-3' and reverse,
5'-CAGTGGTTTCTTCTTTGG-3'; p21 forward, 5'-CGTGAGCGATGGAACTT-3' and
reverse, 5'-GCAGAGCAGGTGAGGTG-3' and GAPDH forward,
5'-CCACTCCTCCACCTTTG-3' and reverse, 5'-CACCAC CCTGTTGCTGT-3'. The
data were obtained using QuantStudio™ 6 Flex Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and analyzed
with the 2-ΔΔCq method (24). The expression of all mRNA was
normalized to that of GAPDH. All experiments were performed in
triplicate.
Statistical analysis
The data are presented as the mean ± standard
deviation and analyzed using SPSS 19 (IBM Corp.). Differences
between ≥2 groups were analyzed using one-way ANOVA followed by a
least significant difference whereas those between two groups were
analyzed using an unpaired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cytotoxicity effect of rAd-p53 and/or
curcumin on HepG2, Hep3B and Huh-7 cells
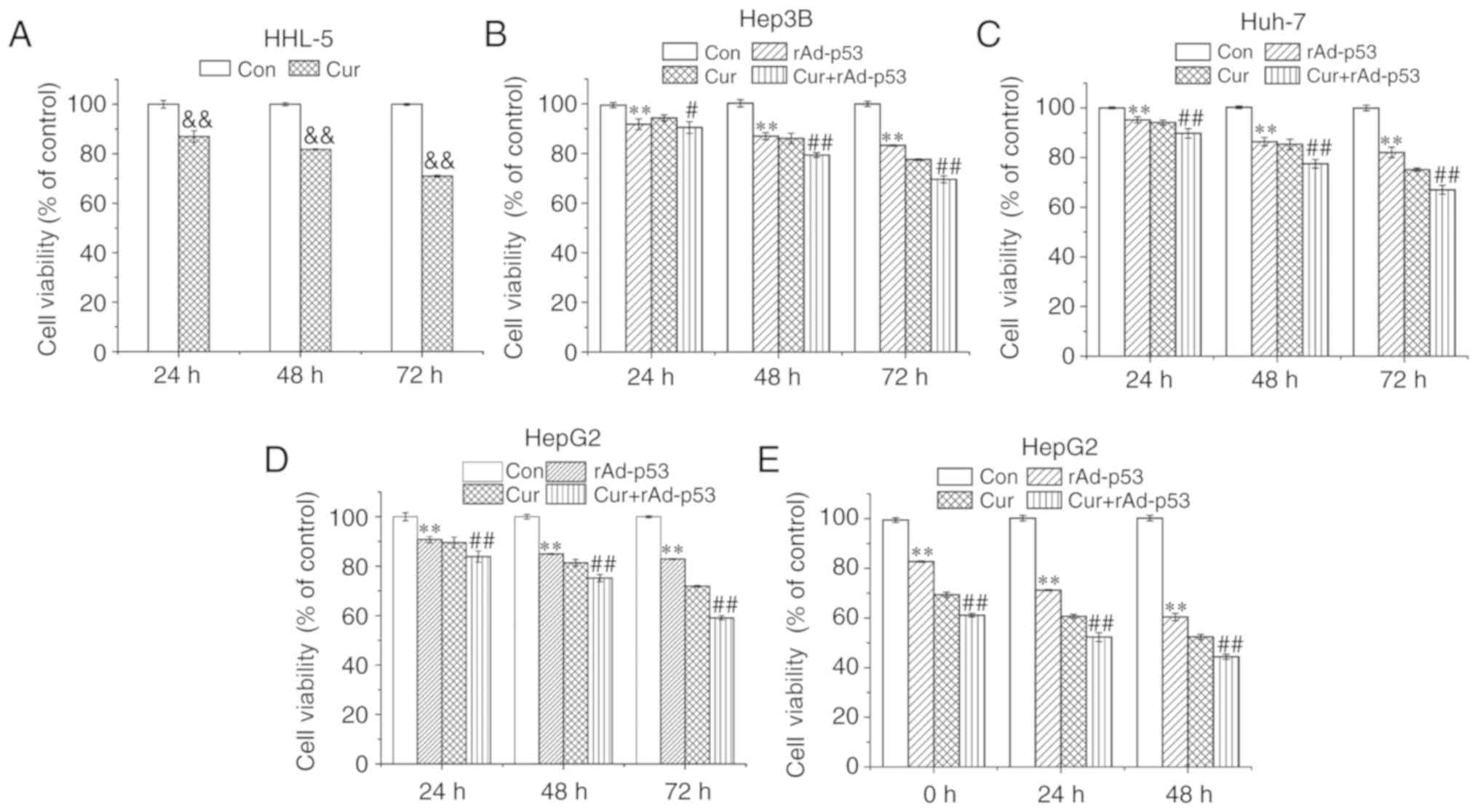
The extent of curcumin cytotoxicity was measured in
HHL-5 cells. Following 72 h curcumin treatment (10 µM), cell
viability was ~70% of that in control cells (Fig. 1A). rAd-p53 and curcumin treatments
alone reduced the cell viability of HepG2, Hep3B and Huh-7 cells,
whilst the combined administration of rAd-p53 and curcumin produced
additive inhibitory effects compared with Cur, in a time-dependent
manner (Fig. 1B-D). Since HepG2
cells appeared to exhibit the highest sensitivity to curcumin
and/or rAd-p53 among the liver cancer cell lines (Fig. 1D), it was selected for subsequent
experiments. HepG2 cell viability continued to decrease up to 48 h
after rAD-p53 and/or curcumin was removed (Fig. 1E). These results demonstrated that
the combined administration of curcumin and rAd-p53 synergistically
reduced HepG2, Hep3B and Huh-7 cell viability.
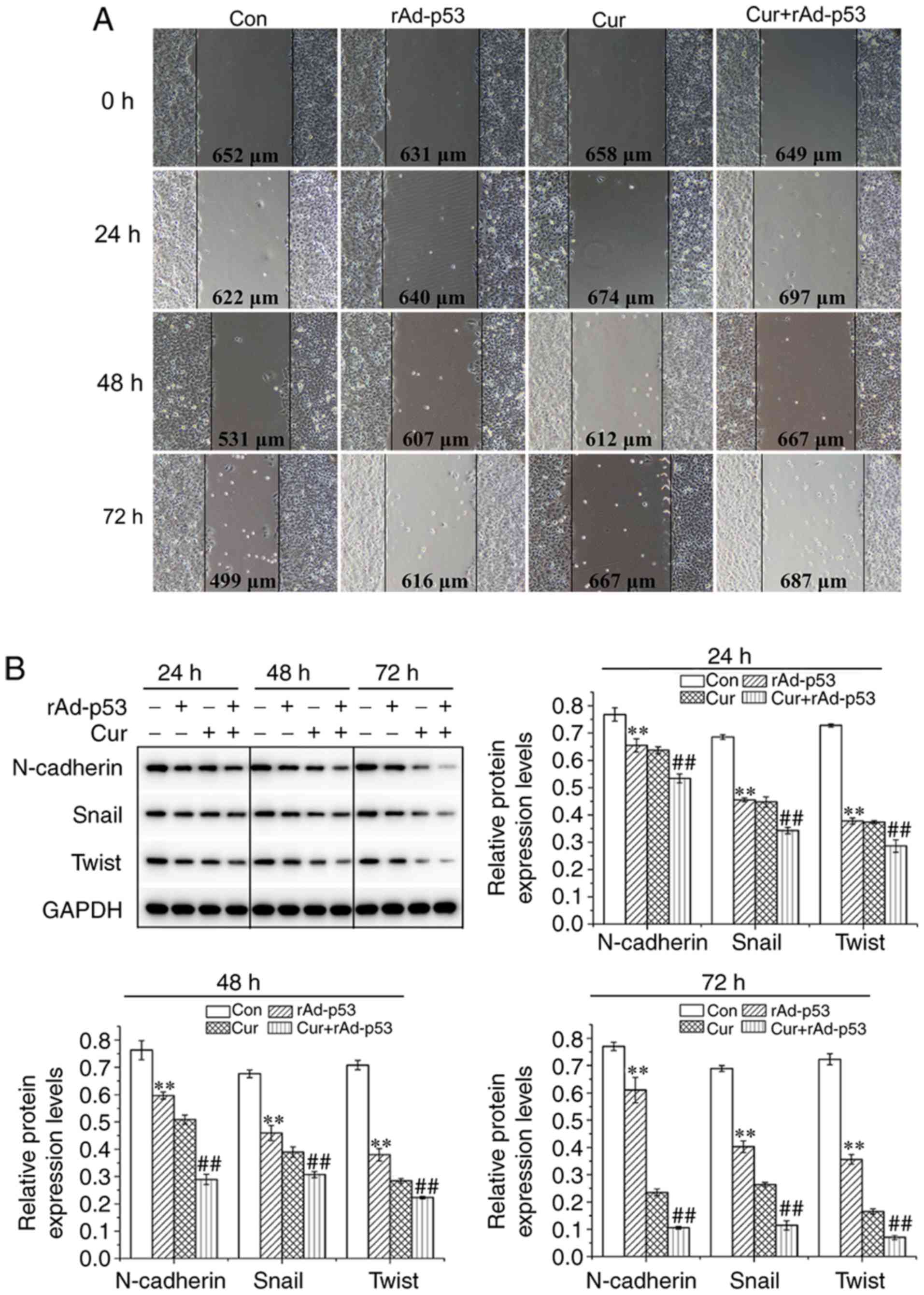
Combined effect of rAd-p53 and
curcumin treatment on HepG2 EMT
The wound healing ability of HepG2 cells treated
with either rAd-p53 or curcumin appeared to be inferior compared
with that observed for non-treated cells; with the combined
treatment of the two agents potentiating this inhibition further in
a time-dependent manner (Fig. 2A).
The expression of proteins associated with EMT were then evaluated
using western blot analysis. Compared with control cells, cells
treated with either rAd-p53 or Cur exhibited reduced N-cadherin,
snail and twist expression, which was reduced further following
combined rAd-p53 and curcumin treatment (Fig. 2B). These observations indicated that
the combined administration of curcumin and rAd-p53 additively
suppressed EMT in HepG2 cells in a time dependent manner.
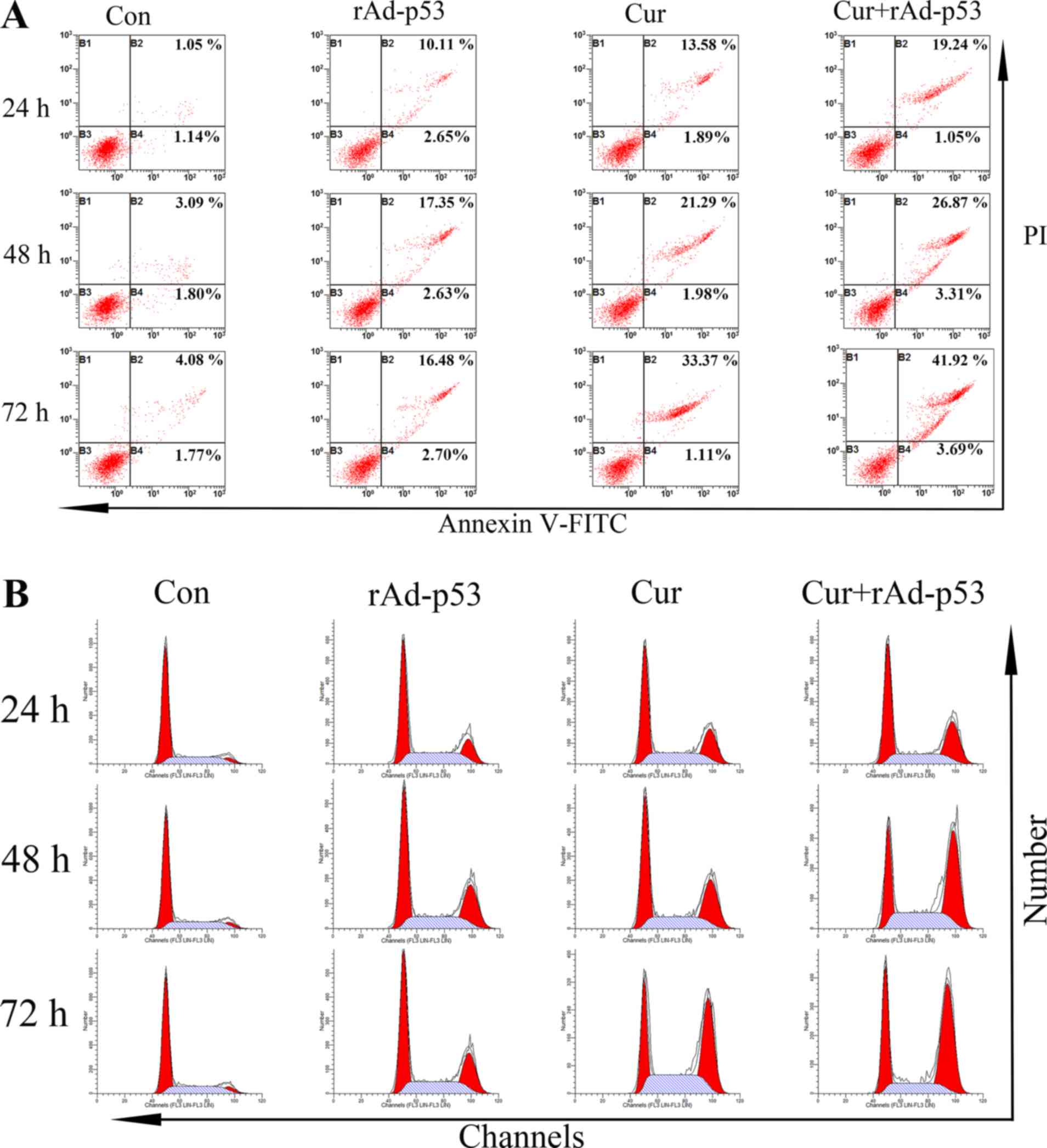
Combined effect of rAd-p53 and
curcumin administration on HepG2 apoptosis and intracellular
protein expression
The apoptosis of HepG2 cells following a number of
treatments is presented in Fig. 3A.
The percentage of apoptotic cells in the control group was revealed
to be ~2.19%, which were increased to 12.76 and 15.47% following
the individual treatment of either rAd-p53 or Cur alone after 24 h,
respectively (Fig. 3A). The
combined administration of rAd-p53 and curcumin resulted in a
further increase in the percentage of apoptotic cells to 20.29%.
After 72 h, whilst the percentage of apoptotic cells in the control
group increased slightly (5.85%), those in the treatment groups
were more prominent. A total of 45.61% of apoptotic cells were
observed in the Cur + rAd-p53 group (Fig. 3A).
The expression of proteins associated with apoptosis
in HepG2 cells was subsequently evaluated using western blot
analysis. Compared with control cells, cells treated with rAd-p53
alone demonstrated significantly higher expression of pro-apoptotic
proteins Bax and caspases 3, 8 and 9, which were potentiated
further in cells treated with rAd-p53 and curcumin together
(Fig. 4). Following the same
rAd-p53 and/or Cur treatment regimens, the expression of the
anti-apoptotic protein Bcl-2 exhibited the opposite trend compared
with that of the pro-apoptotic proteins (Fig. 4). These results were supported by
those obtained from the Annexin V/PI assay. The results
collectively indicated that the combined administration of curcumin
and rAd-p53 enhanced HepG2 apoptosis.
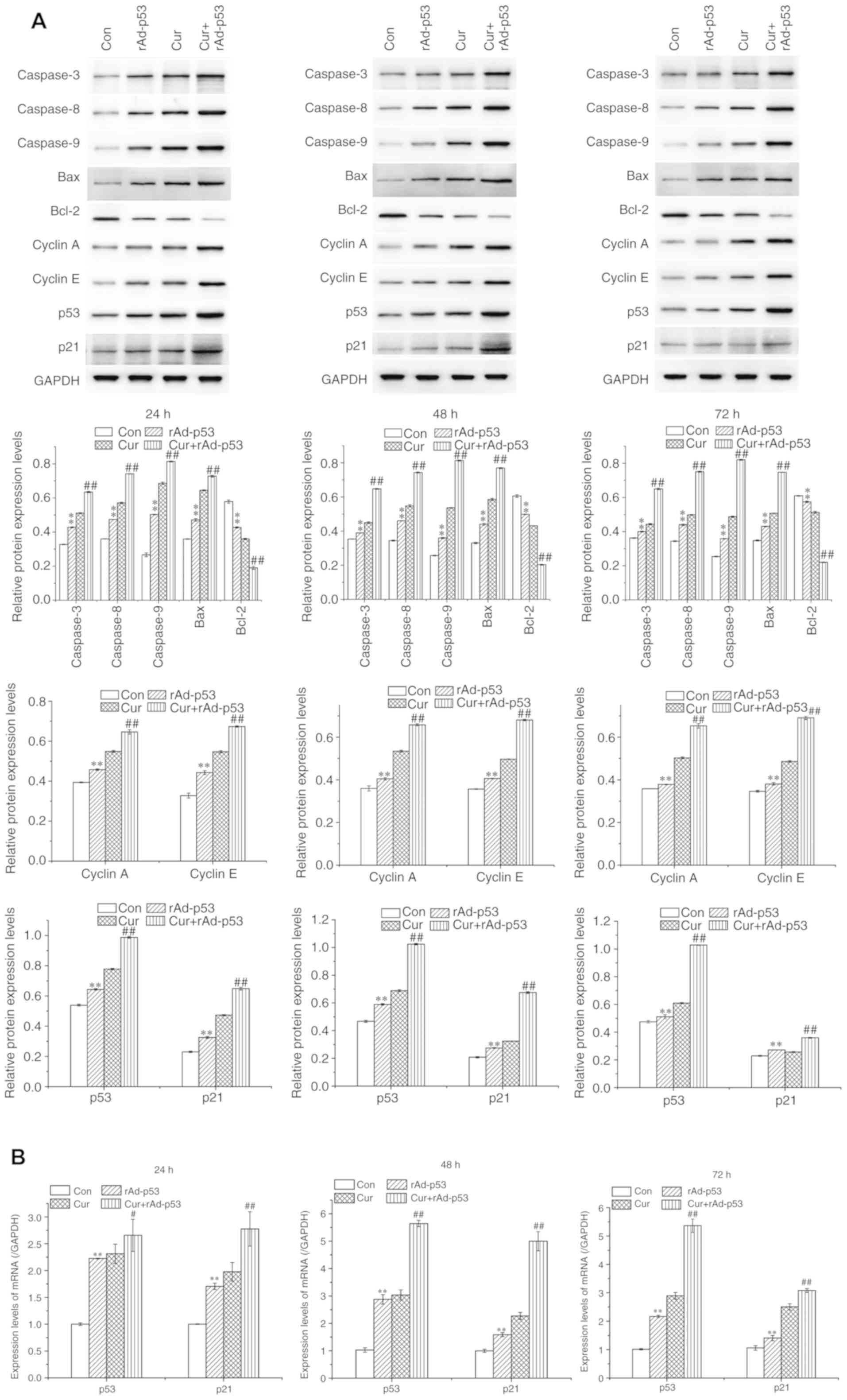 | Figure 4Effects of rAd-p53 and/or curcumin on
the expression of proteins associated with apoptosis and cell cycle
progression. (A) The proteins expression of Caspases 3, 8 and 9,
and Bax, Bcl-2, cell cycle regulators Cyclins A and E, p53, p21 and
(B) mRNA expression of p53 and p21 were measured following 24, 48
or 72 h rAd-p53 and/or curcumin treatment. Data are represented as
mean ± standard deviation (n =3). **P<0.01 vs. Con,
#P<0.05 vs. Cur and ##P<0.01 vs. Cur.
Cur, curcumin; rAd-p53, recombinant human adenovirus-p53; Con,
control |
Combined effect of rAd-p53 and
curcumin on HepG2 cell cycle progression and the expression of
associated proteins
Treatment with either Cur or rAd-p53 reduced the
proportion of cells in the G1/S phase whilst increasing
those in the G2/M phase compared with the control HepG2 cells. This
effect was potentiated further in cells treated with rAd-p53 and
curcumin combined (Fig. 3B). No
notable differences were observed in the proportion of cells in S
phase between all four treatment groups (Fig. 3B). Supporting this observation, the
expression of Cyclins A and E, which are proteins associated with
cell cycle progression, were significantly increased by either
rAd-p53 (Fig. 4). This increase was
potentiated further following the combined administration of
rAd-p53 and curcumin (Fig. 4).
These results indicated that the combined administration of
curcumin and rAd-p53 induced a stronger effect compared with Cur
treatment alone in altering cell cycle progression.
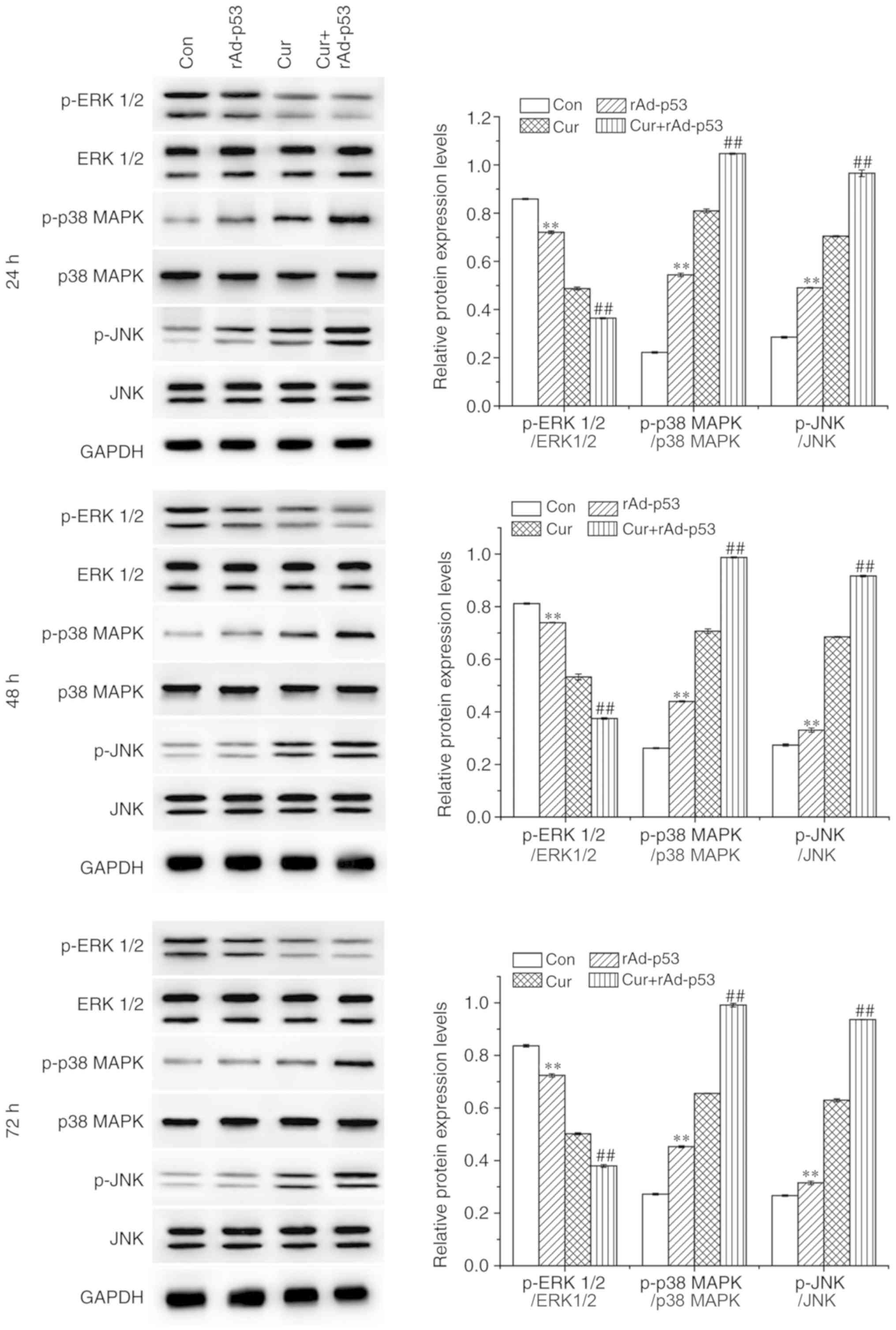
Combined effect of rAd-p53 and
curcumin on the expression of p53, p21 and MAPKs
In all time points tested, the administration of
rAd-p53 alone significantly upregulated p53 and p21 expression in
HepG2 cells, which was potentiated further by combined rAd-p53 and
curcumin treatment (Fig. 4).
Similar trends were observed for the phosphorylation levels of p38
MAPK (p-p38 MAPK) and c-Jun N-terminal kinase (p-JNK), whereas that
of extracellular signal-regulated kinases (p-ERK1/2) exhibited the
opposite trend (Fig. 5). These
results demonstrated that the combined administration of rAd-p53
and curcumin exerted additive regulatory effects on associated
signaling pathways compared with Cur treatment alone.
Discussion
TP53 is an important tumor suppressor gene in
the human body, the expression of which is reduced in cancer cells
(21). TP53 is found to be
functionally inactivated in 50% of all human cancer cases and 61%
of all liver cancer human cases (25,26),
and the downregulation of p53 protein expression promotes the
development of liver cancer (27).
Consequently, TP53 is a candidate gene for gene-targeted
therapy in human malignancies (28). rAd-p53 is the first commercially
available product for gene therapy that has been applied in the
treatment of head and neck cancer, epithelial ovarian carcinoma and
liver cancer (11,12). In most cases, liver cancer occurs
and develops as a result of inflammation and oxidative stress
(18,21). In the present study, the therapeutic
effects of combining rAd-p53 with curcumin, which is a compound
exhibiting excellent anti-inflammatory and antioxidant properties
(20,21), was explored in liver cancer, using
HepG2 cells as the model cell line. This combinatorial
administration was demonstrated to synergistically promote
apoptosis, inhibit G2/M phase progression and suppress
EMT in HepG2 cells.
rAd-p53 is a relatively effective and safe means of
treatment for liver cancer (29,30)
and is usually applied in combination with other therapies,
including transarterial chemoembolization (14) and 5-fluorouracil administration
(31). Previous clinical studies
have revealed that rAd-p53 injection may improve the survival rate
of patients with liver cancer (14,32,33).
Additionally, the combination of rAd-p53 with N-Myc
downstream-regulated gene 2 increased p53-mediated apoptosis of
HepG2 and Huh7 cells in a previous study (34). In the present study, rAd-p53 was
revealed to upregulate the expression of p53 in HepG2 cells
compared with non-treated cells. This observation is consistent
with a previous report, where the intratumoral injection of rAd-p53
resulted in increased p53 expression in prostate cancer (35,36).
Downstream, the expression of the p53-targeted gene CDKN1
followed a trend parallel to that of TP53, suggesting that
the p53 protein produced following rAd-p53 treatment is
physiologically active. The apoptosis rate was indicated to be
markedly increased, as demonstrated by the upregulation of the
pro-apoptotic proteins caspases 3, 8 and 9 in addition to Bax and
coupled with the downregulation of the anti-apoptotic protein
Bcl-2. EMT was also notably suppressed, as demonstrated by the
downregulation of N-cadherin, snail and twist expression, which are
well documented markers of EMT (37,38).
TP53 often serves as a ‘guardian of the
genome’, the deletion of which may result in the uncontrolled
proliferation of tumor cells (39).
The upregulation of TP53 expression that is induced by
rAd-p53 treatment promoted human cervical cancer cell apoptosis
through activation of the Bax gene and suppression of the
Bcl-x gene and resulted in cell cycle arrest at the G2/M
phase (40). The results of the
present study are consistent with those observed in previous
reports, which have demonstrated that TP53 activation is
associated with liver cancer cell apoptosis by regulating the
expression of Bcl-2 and caspases (41,42),
in addition to inhibiting cancer cell migration (43-45).
TP53 activation has also been previously reported to serve
an inhibitory role in the EMT process, in human oral mucosal
fibroblasts and oral submucous fibrosis by downregulating
N-cadherin expression (46) and in
colorectal cancer cells by downregulating Snail expression
(37), which are findings
consistent with the results of the present study.
Curcumin possesses anti-inflammatory and antioxidant
properties and has also been observed to upregulate TP53
expression in tumor cells to exert several therapeutic effects
(47,48). Curcumin induces apoptosis and cell
cycle arrest of cancer cells by targeting regulatory p53(49). Previous in vivo and in
vitro experiments have demonstrated that curcumin in
combination with metformin induces apoptosis and suppresses the
proliferation, invasion and metastasis of liver cancer cells by
upregulating TP53 (50). In
the present study, the combination of rAd-p53 and curcumin led to a
higher expression of p53 compared with Cur treatment alone,
synergistically promoting apoptosis, inhibiting cell proliferation
and migration by regulating TP53. In addition, curcumin
exerted anti-tumor effects by regulating the MAPK pathways. There
are three subfamilies of MAPKs, including p38MAPK, JNKs and ERKs,
all of which are related to apoptosis (51). A previous report has suggested that
curcumin treatment induced retinoblastoma cell apoptosis by
activating p38 MAPK and JNK (52).
Similarly, curcumin-induced p38 MAPK activation resulted in
FasL-associated apoptosis in human hepatocellular carcinoma Huh7
cells (53). Curcumin also induced
apoptosis in HepG2 cells by activating the ROS-ASK1-JNK pathway
(54). Consistent with previous
studies, the co-treatment of rAd-p53 with curcumin in the present
study resulted in the additive potentiation of p38MAPK and JNK
activation, potentially resulting in apoptosis in this manner.
In conclusion, rAd-p53 and curcumin were applied
individually or in combination to explore their influence on the
liver cancer cell line HepG2. Compared with Cur treatment alone,
the combined treatment synergistically promoted liver cancer
apoptosis and inhibited cell migration. Mechanistically, these
observed effects may be associated with TP53 expression and
subsequent MAPK signaling. Overall, the present study provides new
insights into possible targets for effective liver cancer
therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ and JY participated in the design of this work.
JQ, WL, MC, WG, CZ and BG performed the experiments and analyzed
data. JQ drafted the manuscript and JY revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang JF, Lao YC, Yuan BH, Yin J, Liu X,
Chen L and Zhong JH: Treatment of hepatocellular carcinoma with
portal vein tumor thrombus: Advances and challenges. Oncotarget.
8:33911–33921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bruix J, Han KH, Gores G, Llovet JM and
Mazzaferro V: Liver cancer: Approaching a personalized care. J
Hepatol. 62:S144–S156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poulain S, Roumier C, Bertrand E,
Renneville A, Caillault-Venet A, Doye E, Geffroy S, Sebda S,
Nibourel O, Nudel M, et al: TP53 mutation and its prognostic
significance in Waldenstrom's macroglobulinemia. Clin Cancer Res.
23:6325–6335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Friemel J, Rechsteiner M, Bawohl M, Frick
L, Müllhaupt B, Lesurtel M and Weber A: Liver cancer with
concomitant TP53 and CTNNB1 mutations: A case report. BMC Clin
Pathol. 16(7)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
McCubrey JA, Lertpiriyapong K, Fitzgerald
TL, Martelli AM, Cocco L, Rakus D, Gizak A, Libra M, Cervello M,
Montalto G, et al: Roles of TP53 in determining therapeutic
sensitivity, growth, cellular senescence, invasion and metastasis.
Adv Biol Regul. 63:32–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soussi T and Wiman KG: TP53: An oncogene
in disguise. Cell Death Differ. 22:1239–1249. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kashofer K and Regauer S: Analysis of full
coding sequence of the TP53 gene in invasive vulvar cancers:
Implications for therapy. Gynecol Oncol. 146:314–318.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li J, Pan J, Zhu X, Su Y, Bao L, Qiu S,
Zou C, Cai Y, Wu J and Tham IW: Recombinant adenovirus-p53
(Gendicine) sensitizes a pancreatic carcinoma cell line to
radiation. Chin J Cancer Res. 25:715–721. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Räty JK, Pikkarainen JT, Wirth T and
Ylä-Herttuala S: Gene therapy: The first approved gene-based
medicines, molecular mechanisms and clinical indications. Curr Mol
Pharmacol. 1:13–23. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Li B, Li CJ and Li LJ: Key points of
basic theories and clinical practice in rAd-p53 (Gendicine ™) gene
therapy for solid malignant tumors. Expert Opin Biol Ther.
15:437–454. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen GX, Zheng LH, Liu SY and He XH:
rAd-p53 enhances the sensitivity of human gastric cancer cells to
chemotherapy. World J Gastroenterol. 17:4289–4297. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen A, Liu S, Yu W, Deng H and Li Q: p53
gene therapy-based transarterial chemoembolization for unresectable
hepatocellular carcinoma: A prospective cohort study. J
Gastroenterol Hepatol. 30:1651–1656. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Li LJ, Wang LJ, Zhang Z, Gao N,
Liang CY, Huang YD and Han B: Selective intra-arterial infusion of
rAd-p53 with chemotherapy for advanced oral cancer: A randomized
clinical trial. BMC Med. 12(16)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guan YS, Liu Y, Zou Q, He Q, La Z, Yang L
and Hu Y: Adenovirus-mediated wild-type p53 gene transfer in
combination with bronchial arterial infusion for treatment of
advanced non-small-cell lung cancer, one year follow-up. J Zhejiang
Univ Sci B. 10:331–340. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Buller RE, Runnebaum IB, Karlan BY,
Horowitz JA, Shahin M, Buekers T, Petrauskas S, Kreienberg R,
Slamon D and Pegram M: A phase I/II trial of rAd/p53 (SCH 58500)
gene replacement in recurrent ovarian cancer. Cancer Gene Ther.
9:553–566. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Staib F, Hussain SP, Hofseth LJ, Wang XW
and Harris CC: TP53 and liver carcinogenesis. Hum Mutat.
21:201–216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chin KY: The spice for joint inflammation:
Anti-inflammatory role of curcumin in treating osteoarthritis. Drug
Des Devel Ther. 10:3029–3042. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lestari ML and Indrayanto G: Curcumin.
Profiles Drug Subst Excip Relat Methodol. 39:113–204.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: A review. Curr Pharm Biotechnol.
13:218–228. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
El-Houseini ME, El-Agoza IA, Sakr MM and
El-Malky GM: Novel protective role of curcumin and taurine
combination against experimental hepatocarcinogenesis. Exp Ther
Med. 13:29–36. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marquardt JU, Gomez-Quiroz L, Arreguin
Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D,
Breuhahn K, Conner EA, et al: Curcumin effectively inhibits
oncogenic NF-κB signaling and restrains stemness features in liver
cancer. J Hepatol. 63:661–669. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hsia CC, Nakashima Y, Thorgeirsson SS,
Harris CC, Minemura M, Momosaki S, Wang NJ and Tabor E: Correlation
of immunohistochemical staining and mutations of p53 in human
hepatocellular carcinoma. Oncol Rep. 7:353–356. 2000.PubMed/NCBI
|
|
26
|
Honda K, Sbisà E, Tullo A, Papeo PA,
Saccone C, Poole S, Pignatelli M, Mitry RR, Ding S, Isla A, et al:
p53 mutation is a poor prognostic indicator for survival in
patients with hepatocellular carcinoma undergoing surgical tumour
ablation. Br J Cancer. 77:776–782. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu W, Liu S, Liang Y, Zhou Z, Bian W and
Liu X: Stress hormone cortisol enhances Bcl2 like-12 expression to
inhibit p53 in hepatocellular carcinoma cells. Dig Dis Sci.
62:3495–3500. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li VD, Li KH and Li JT: TP53 mutations as
potential prognostic markers for specific cancers: Analysis of data
from The Cancer Genome Atlas and the International Agency for
Research on Cancer TP53 Database. J Cancer Res Clin Oncol.
145:625–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tu K, Zheng X, Zhou Z, Li C, Zhang J, Gao
J, Yao Y and Liu Q: Recombinant human adenovirus-p53 injection
induced apoptosis in hepatocellular carcinoma cell lines mediated
by p53-Fbxw7 pathway, which controls c-Myc and cyclin E. PLoS One.
8(e68574)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen SX, Xu WD, Yin GW, Xi W, Chen J, Xu
QY and Ma GJ: Clinical therapeutic effect and biological monitoring
of p53 gene in advanced hepatocellular carcinoma. Zhonghua Yi Xue
Za Zhi. 90:2182–2186. 2010.PubMed/NCBI(In Chinese).
|
|
31
|
Tian G, Liu J, Zhou JS and Chen W:
Multiple hepatic arterial injections of recombinant adenovirus p53
and 5-fluorouracil after transcatheter arterial chemoembolization
for unresectable hepatocellular carcinoma: A pilot phase II trial.
Anticancer Drugs. 20:389–395. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guan YS, Liu Y, He Q, Li X, Yang L, Hu Y
and La Z: p53 gene therapy in combination with transcatheter
arterial chemoembolization for HCC: One-year follow-up. World J
Gastroenterol. 17:2143–2149. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang ZX, Wang D, Wang G, Zhang QH, Liu JM,
Peng P and Liu XH: Clinical study of recombinant adenovirus-p53
combined with fractionated stereotactic radiotherapy for
hepatocellular carcinoma. J Cancer Res Clin Oncol. 136:625–630.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cao W, Zhang JL, Feng DY, Liu XW, Li Y,
Wang LF, Yao LB, Zhang H and Zhang J: The effect of
adenovirus-conjugated NDRG2 on p53-mediated apoptosis of
hepatocarcinoma cells through attenuation of nucleotide excision
repair capacity. Biomaterials. 35:993–1003. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sasaki R, Shirakawa T, Zhang ZJ, Tamekane
A, Matsumoto A, Sugimura K, Matsuo M, Kamidono S and Gotoh A:
Additional gene therapy with Ad5CMV-p53 enhanced the efficacy of
radiotherapy in human prostate cancer cells. Int J Radiat Oncol
Biol Phys. 51:1336–1345. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shirakawa T, Gotoh A, Gardner TA, Kao C,
Zhang ZJ, Matsubara S, Wada Y, Hinata N, Fujisawa M, Hanioka K, et
al: p53 adenoviral vector (Ad-CMV-p53) induced prostatic growth
inhibition of primary cultures of human prostate and an
experimental rat model. J Gene Med. 2:426–432. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bai Z, Wang J, Wang T, Li Y, Zhao X, Wu G,
Yang Y, Deng W and Zhang Z: The miR-495/Annexin A3/P53 axis
inhibits the invasion and EMT of colorectal cancer cells. Cell
Physiol Biochem. 44:1882–1895. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Angadi PV, Patil PV, Angadi V, Mane D,
Shekar S, Hallikerimath S, Kale AD and Kardesai SG:
Immunoexpression of epithelial mesenchymal transition proteins
E-Cadherin, β-Catenin, and N-Cadherin in oral squamous cell
carcinoma. Int J Surg Pathol. 24:696–703. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Curiel DT, Gerritsen WR and Krul MR:
Progress in cancer gene therapy. Cancer Gene Ther. 7:1197–1199.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu YG, Zheng XL and Liu FM: The mechanism
and inhibitory effect of recombinant human P53 adenovirus injection
combined with paclitaxel on human cervical cancer cell HeLa. Eur
Rev Med Pharmacol Sci. 19:1037–1042. 2015.PubMed/NCBI
|
|
41
|
Liao W, Liu J, Liu B, Huang X, Yin Y, Cai
D, Li M and Zhu R: JIB 04 induces cell apoptosis via activation of
the p53/Bcl 2/caspase pathway in MHCC97H and HepG2 cells. Oncol
Rep. 40:3812–3820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cao Y, Cao J, Yu B, Wang S, Liu L, Tao L
and Sun W: Berbamine induces SMMC-7721 cell apoptosis via
upregulating p53, downregulating survivin expression and activating
mitochondria signaling pathway. Exp Ther Med. 15:1894–1901.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tamura M, Sasaki Y, Koyama R, Takeda K,
Idogawa M and Tokino T: Forkhead transcription factor FOXF1 is a
novel target gene of the p53 family and regulates cancer cell
migration and invasiveness. Oncogene. 33:4837–4846. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Li L, Liu Y, Geng P, Li G, Yang Y
and Song H: RECK inhibits cervical cancer cell migration and
invasion by promoting p53 signaling pathway. J Cell Biochem.
119:3058–3066. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang B, Yin X and Sui S: Resveratrol
inhibited the progression of human hepatocellular carcinoma by
inducing autophagy via regulating p53 and the phosphoinositide 3
kinase/protein kinase B pathway. Oncol Rep. 40:2758–2765.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zheng L, Guan ZJ, Pan WT, Du TF, Zhai YJ
and Guo J: Tanshinone suppresses arecoline-induced
epithelial-mesenchymal transition in oral submucous fibrosis by
epigenetically reactivating the p53 pathway. Oncol Res. 26:483–494.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sidhar H and Giri RK: Induction of Bex
genes by curcumin is associated with apoptosis and activation of
p53 in N2a neuroblastoma cells. Sci Rep. 7(41420)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li W, Wang Y, Song Y, Xu L, Zhao J and
Fang B: A preliminary study of the effect of curcumin on the
expression of p53 protein in a human multiple myeloma cell line.
Oncol Lett. 9:1719–1724. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kasi PD, Tamilselvam R, Skalicka-Woźniak
K, Nabavi SF, Daglia M, Bishayee A, Pazoki-Toroudi H and Nabavi SM:
Molecular targets of curcumin for cancer therapy: An updated
review. Tumour Biol. 37:13017–13028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yu X, Zhong J, Yan L, Li J, Wang H, Wen Y
and Zhao Y: Curcumin exerts antitumor effects in retinoblastoma
cells by regulating the JNK and p38 MAPK pathways. Int J Mol Med.
38:861–868. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang WZ, Li L, Liu MY, Jin XB, Mao JW, Pu
QH, Meng MJ, Chen XG and Zhu JY: Curcumin induces FasL-related
apoptosis through p38 activation in human hepatocellular carcinoma
Huh7 cells. Life Sci. 92:352–358. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zheng R, You Z, Jia J, Lin S, Han S, Liu
A, Long H and Wang S: Curcumin enhances the antitumor effect of
ABT-737 via activation of the ROS-ASK1-JNK pathway in
hepatocellular carcinoma cells. Mol Med Rep. 13:1570–1576.
2016.PubMed/NCBI View Article : Google Scholar
|