Introduction
Articular cartilage injury is a common and frequent
disease-related orthopedic condition (1). Inadequate and improper treatment can
lead to osteoarthritis, pain and dysfunctional walking, which
affect the quality of life of patients (2). Due to poor self-repairing ability of
the articular cartilage, the current clinical treatment of
articular cartilage defects is not satisfactory; in particular,
repair of the damage to the full-thickness articular cartilage is a
challenge for clinicians (3).
Tissue engineering can facilitate cartilage repair, with numerous
successful reports based on animal experiments (4,5).
Scaffold and seed cell selection are important factors affecting
cartilage repair.
Bone marrow mesenchymal stem cells (BMSCs) are
suitable cellular materials for articular cartilage repair.
Cartilage differentiation is an intrinsic property of mesenchymal
stem cells (6). Most in
vitro and in vivo studies suggest that BMSCs have the
potential to increase osteoinduction and osteogenesis (7-11).
A study has demonstrated that seeding BMSCs on biocompatible
scaffolds may be an effective method for treating nonunion
fractures (12). Mesenchymal stem
cells have the potential to home to damaged areas, which may
enhance repair in two respects: i) Differentiation of tissue cells,
specifically, the recovery of lost morphology and function; and ii)
secretion of various biologically active factors, which have
antiapoptotic effects and immunoregulatory functions, thereby
creating an environment that stimulates the proliferation of
endothelial progenitor cells and leads to subsequent repair
(13). A clinical study has
demonstrated the effectiveness of direct local BMSC delivery by
injection in promoting bone regeneration (14). However, in large bone defects where
a significant amount of bony tissue has been lost, the direct
injection method was found to be ineffective for BMSC delivery and
the commensurate acceleration of the bone healing process (15). Cartilage repair without a scaffold
or soft support does not provide sufficient mechanical support,
leading to insufficient cell enrichment in the repair area,
hindering early weight bearing and an environment with insufficient
pressure and nutritional support to induce cartilage repair
(16). The bioceramic hard scaffold
is prepared using a controlled microporous structure of
β-tricalcium phosphate (β-TCP) as the raw material (17). β-TCP is capable of triggering
proliferation, migration and differentiation of the bone cells
required for bone regeneration (18,19).
Porous ceramics have good biocompatibility and high mechanical
strength, and their porous structure and degradation rate can be
controlled according to the growth of the tissue (20). Physical microstructures, such as the
bore and internal junction, can be regulated to facilitate cell
adsorption and proliferation (15).
High-porosity scaffolds facilitate the migration and proliferation
of bone marrow cells in the scaffold, which is essential for the
repair of osteochondral injury (21).
Previous experiments were conducted to determine a
preliminary range of bioceramic scaffold microstructures suitable
for promoting chondrocyte adhesion and proliferation and for
maintaining the chondrocyte phenotype. The present study implanted
a three-dimensional culture of BMSC scaffolds into mice and used
RNA sequencing to explore the molecular mechanism of the bioceramic
scaffolds on the basis of their shape and duration of
implantation.
Materials and methods
Cell culture and flow cytometry
The 3rd generation BMSCs (CinoAsia Co., Ltd.) were
digested using trypsin, after which 1x105/ml cell
suspensions in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
were generated in an eppendorf tube. A total of 10 µl
fluorescently-labelled CD34 (cat. no. ab8158), CD45 (cat. no.
ab33923) and CD44 (cat. no. ab119348) antibodies (all, Abcam; all,
1:100) were added to 100 µl of the cell suspension and incubated
for 1 h at 37˚C. Flow cytometry was performed to detect individual
cell markers (FACSMelody; Becton, Dickinson and Company; software,
Flowjo, version 7.6; Becton, Dickinson and Company). BMSCs were
counted and resuspended in complete DMEM/F12 supplemented with 10%
fetal bovine serum and 1% senicillin-streptomycin solution (all,
Gibco; Thermo Fisher Scientific, Inc.). Samples were cultured at
37˚C and cell density was adjusted to 1x106
cells/ml.
Combined culture of BMSCs and
bioceramic scaffolds
The β-TCP bioceramic material (purchased from
Shanghai Bio-lu Biomaterials Co., Ltd.) was prepared and placed in
a 24-well plate. First, 200 µl of BMSCs suspended in DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc.) were added to one side of
the ceramic plate and incubated for 1 h at 37˚C. Subsequently, 200
µl of the cell suspension was added to the reverse side, where it
was incubated for another 1 h at 37˚C. DMEM/F12 was added to a
total volume of 1 ml/well and scaffolded cells were placed into
medium for routine culture.
Bioceramic scaffolds implanted in
mice
After 3 days of culture, the bioceramic scaffolds
were planted into the femoral trochlea of the mice, where they
remained for 3 or 6 days. The scaffolds were removed from the mice
and crushed with small tweezers. A total of 40, six-week-old male
mice (weight, 22-26g, Shanghai SLAC Laboratory Animal Co., Ltd.)
were randomly divided into 5 groups (each, n=8). Animals were
maintained at 25˚C, with a relative humidity of 40-70%, under
specific-pathogen free conditions. Animals were housed under a 12 h
light/dark cycle with free access to food and water. Mice
anaesthetized by intraperitoneal injection of 1% sodium
pentobarbital (50 mg/kg). Following implantation of the ceramic
material, the health and behavior of the mice were observed every
day. Following removal of the ceramic material after 3 or 6 days,
mice were intraperitoneally injected with an overdose (1%; 150
mg/kg) of sodium pentobarbital for euthanasia. Mice with no
breathing for 3 min and no corneal reflex were considered to be
successfully euthanized.
Sequencing
Several small particles were placed in cell lysates
(0.2% triton X-100+5% RNase inhibitor), and stored at -80˚C for
in vitro sequencing. The cultured ceramic material was
digested with trypsin, and the library was sequenced. The sequences
were grouped into controls (untreated mice; CONT); ceramic disc
in vitro (MAT1); ceramic rod in vitro (MAT2); ceramic
rod, 3 days in vivo (DAY3) and ceramic rod, 6 days in
vivo (DAY6). Experiments in each group were performed in
triplicate.
Ethical approval
All experiments were performed according to the
principles outlined in the Guide for the Care and Use of Laboratory
Animals and approved by the Ethics Committee of Huashan Hospital
Affiliated to Fudan University (approval no. 2018 Huashan Hospital
JS-098).
RNA database and sequencing
The cells cultured as described above were subjected
to RNA sequencing, and each group was replicated three times.
Cellular RNA was extracted and reverse transcribed into cDNA for
PCR amplification. The PCR amplification reaction product was
purified and subjected to quality control in a 96-well plate. The
samples described above were measured by molar conversion, and each
2 nM sample was diluted to 1:1. After dilution, 10 µl of the sample
was removed from the main tube and sent to the sequencing company
for quality control with a 2100 QC bioanalyzer (Agilent
Technologies, Inc.) before sequencing. The Hiseq2500
sequencer(Illumina, USA) was used, and the sequencing mode was set
to a 2x150 bp read length with a 250 M read number.
Raw data processing
The raw data were first processed using Trimmomatic
(http://www.usadellab.org/cms/) to remove
sequencing linker sequences and low quality read lengths. The
remaining high-quality reads were aligned with the 25,014 genes of
the Mus musculus genome using HiSat2 (http://daehwankimlab.github.io/hisat2/main/). The
expression levels of each gene were quantified using featureCounts
software, which produced raw count values for each sample. For raw
data analysis, the counts of the sample genes were defined as
>1, which was used as the base count value for the expressed
genes. The read count was then normalized to the transcripts per
kilobase million value for the log2 conversion with the ‘newSCESet’
function of the ‘scater’ package in R (https://www.r-project.org/). Principal component (PC)
analysis, one-way ANOVA and hierarchical cluster analysis (HCA)
using the ‘prcomp’, ‘cor’, ‘t.test’ and ‘cluster’ functions were
performed using the R ‘cluster stats’ package. Following analysis,
a heatmap was generated with the R ‘ComplexHeatmap’ package and the
PC analysis results were visualized using ‘ggplot’.
Analysis of differentially expressed
genes (DEGs)
DEGs were identified by calculating the fold-change
and P-values of the experimental and control groups. The multiples
were modified to be >two-fold; P<0.05 was used as the
standard for DEG selection and the ‘stat’ package in R was used.
DEG intersections were determined with a ‘Venn diagram’ online tool
(http://bioinformatics.psb.ugent.be/webtools/Venn/).
Pathway analysis was performed using and Gene Ontology (GO;
http://geneontology.org/) analysis was performed
using the Database for Annotation, Visualization and Integrated
Discovery (version 6.7; https://david.ncifcrf.gov/).
mRNA sequencing analysis
The quality of the sequencing data was evaluated
prior to data analysis using FastQC.
Results
Cell identification
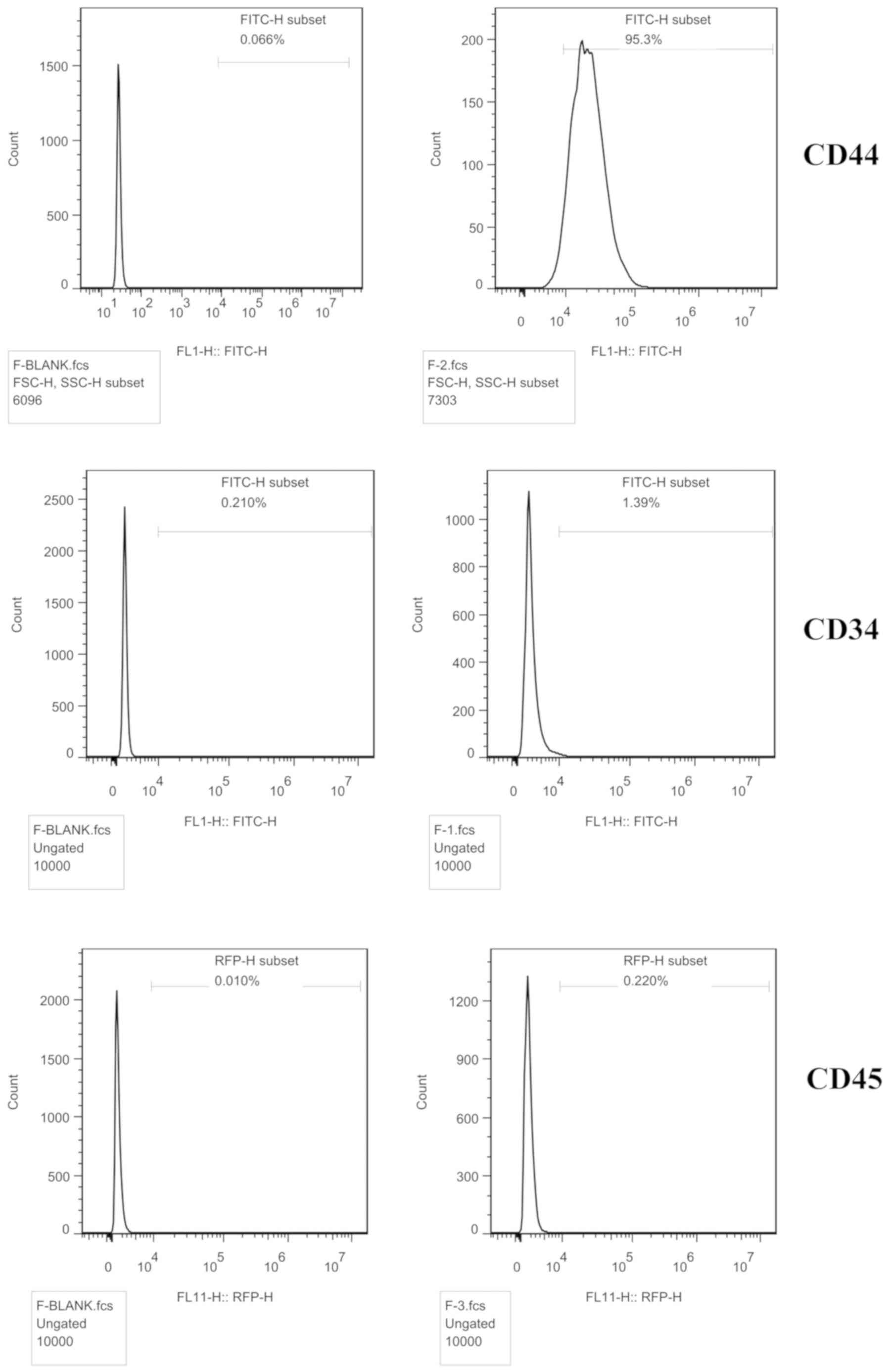
The cell surface antigens were detected by flow
cytometry. The BMSC marker CD44 (95.3%) was expressed, but not CD34
(1.39%) or CD45 (0.22%), indicating that the cells were BMSCs
(Fig. 1).
RNA sequencing analysis
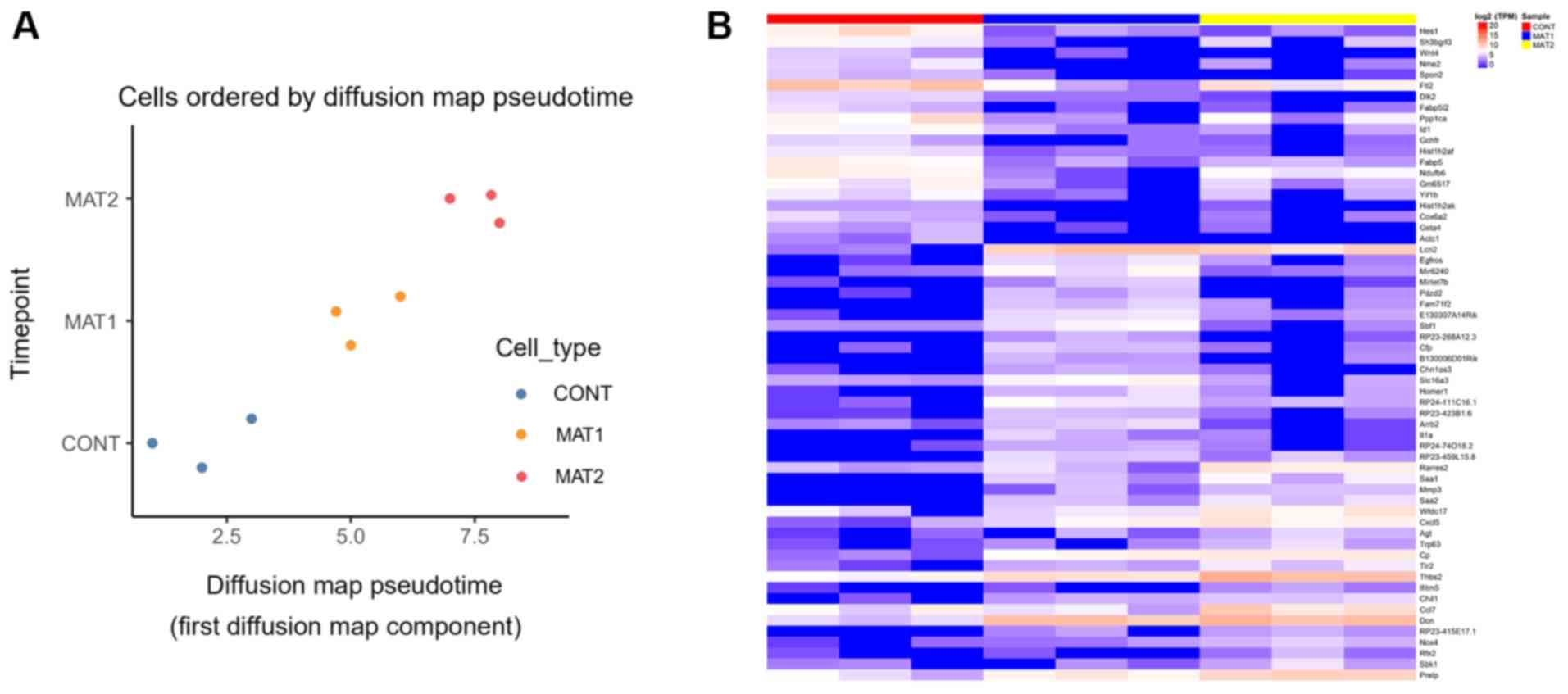
PC1 level analysis showed a good sample
repeatability within the group, and the ceramic scaffold culture
was superior compared with the CONT group. The degree of
differentiation in three groups was measured by diffusion
pseudotime analysis. Compared with MAT1 group, MAT2 group was
farther from the CONT group in terms of the evolutionary
trajectory, MAT2 was exhibited with a more differentiated state.
Compared with the CONT group, the MAT2 group was superior to the
MAT1 group (Fig. 2A). HCA revealed
the characteristic genes of each group (Fig. 2B). Characteristic genes of the CONT
group included transcription factor HES-1, ferritin light chain 2
and serine/threonine-protein phosphatase PP1-a catalytic subunit.
Characteristic genes of the MAT1 group included neutrophil
gelatinase-associated lipocalin (Lcn2), decorin (Dcn) and
thrombospondin 2 (Thbs2). Characteristic genes of the MAT2 group
included Lcn2, Dcn, Thbs2 and C-C motif chemokine ligand 7
(Ccl7).
Comparison between the top 10 upregulated and
downregulated genes in the MAT1 and MAT2 groups compared with the
CONT group found that the shared genes were serum amyloid A-3
protein (Saa3), Lcn2, nitric oxide synthase (Nos2) and nuclear
receptor subfamily 4 group A member 1 (Tables I and II).
 | Table ITop 10 differentially expressed genes
of the MAT1 group compared with the CONT group. |
Table I
Top 10 differentially expressed genes
of the MAT1 group compared with the CONT group.
| A, Upregulated
genes |
|---|
| Gene name | FC | P-value |
|---|
| Saa3 | 2847.6775 | 0.0012 |
| Lcn2 | 304.3738 | 0.0109 |
| RP23-459L15.8 | 47.7720 | 0.0012 |
| Egfros | 46.0328 | 0.0021 |
| RP23-380F8.2 | 44.2728 | 0.0194 |
| Nos2 | 43.0737 | 0.0023 |
| Fam71f2 | 40.7737 | 0.0026 |
| Gtf2a1l | 39.3699 | 0.0043 |
| RP24-111C16.1 | 37.2839 | 0.0125 |
| B, Downregulated
genes |
| Gene name | FC | P-value |
| Yif1b | 0.0348 | 0.0173 |
| Rpl23a-ps1 | 0.0295 | 0.0053 |
| Rps2-ps13 | 0.0284 | 0.0299 |
| RP24-574O8.7 | 0.0264 | 0.0023 |
| Hes1 | 0.0247 | 0.0043 |
| Ftl2 | 0.0199 | 0.0376 |
| Ppp1ca | 0.0193 | 0.0307 |
| Nme2 | 0.0165 | 0.0051 |
| Nr4a1 | 0.0246 | 0.0215 |
 | Table IITop 10 differentially expressed genes
of the MAT2 group compared with the CONT group. |
Table II
Top 10 differentially expressed genes
of the MAT2 group compared with the CONT group.
| A, Upregulated
genes |
|---|
| Gene name | FC | P-value |
|---|
| Saa3 | 2584.2307 | 0.0037 |
| Lcn2 | 158.9995 | 0.0130 |
| Saa1 | 66.5714 | 0.0167 |
| Saa2 | 62.8477 | 0.0043 |
| Cp | 48.7990 | 0.0084 |
| Nos2 | 44.6269 | 0.0083 |
| Cxcl5 | 41.3231 | 0.0220 |
| Dcn | 38.1207 | 0.0004 |
| Mmp3 | 37.9426 | 0.0004 |
| B, Downregulated
genes |
| Gene name | FC | P-value |
| Slc34a2 | 0.0675 | 0.0263 |
| Fos | 0.0660 | 0.0003 |
| Rps2 | 0.0643 | 0.0067 |
| Hist1h2af | 0.0479 | 0.0461 |
| Cdsn | 0.0439 | 0.0147 |
| Spon2 | 0.0368 | 0.0040 |
| Wnt4 | 0.0287 | 0.0097 |
| Dlk2 | 0.0234 | 0.0099 |
| Nr4a1 | 0.0221 | 0.0243 |
The DEGs between the MAT1 and MAT2 groups were
compared, and the top 10 upregulated and downregulated genes are
listed in Table III. Functional
and pathway analysis of the DEGs was further performed (Fig. 3A). Three bone-related genes, NADH:
Ubiquinone oxidoreductase subunit B6 (Ndufb6), ribosomal protein
L17, pseudogene 9 (Rpl17-ps9) and Ccl7, were selected for
comparison (Fig. 3B).
 | Table IIITop 10 differentially expressed genes
in the MAT1 and MAT2 groups. |
Table III
Top 10 differentially expressed genes
in the MAT1 and MAT2 groups.
| A, Upregulated
genes |
|---|
| Gene name | FC | P-value |
|---|
| Ndufb6 | 36.7160 | 0.0245 |
| RP24-574O8.7 | 22.5928 | 0.0039 |
| Rpl17-ps9 | 20.2378 | 0.0356 |
| Rpl23a-ps1 | 18.4759 | 0.0090 |
| Mrpl24 | 14.4720 | 0.0260 |
| Hspe1-ps2 | 14.2868 | 0.0333 |
| Ccl7 | 13.0649 | 0.0221 |
| Mrps36-ps2 | 12.4257 | 0.0297 |
| RP24-272N10.4 | 11.2402 | 0.0132 |
| Fth-ps2 | 10.6130 | 0.0475 |
| B, Downregulated
genes |
| Gene name | FC | P-value |
| Tmem74b | 0.0797 | 0.0383 |
| Cacna1g | 0.0792 | 0.0152 |
| Nr4a3 | 0.0691 | 0.0005 |
| Crlf2 | 0.0649 | 0.0065 |
| Nr4a1 | 0.0643 | 0.0427 |
| Rnu3b4 | 0.0625 | 0.0050 |
| Il3ra | 0.0545 | 0.0071 |
| Fosb | 0.0454 | 0.0198 |
| Arrb2 | 0.0427 | 0.0045 |
| Sbf1 | 0.0376 | 0.0353 |
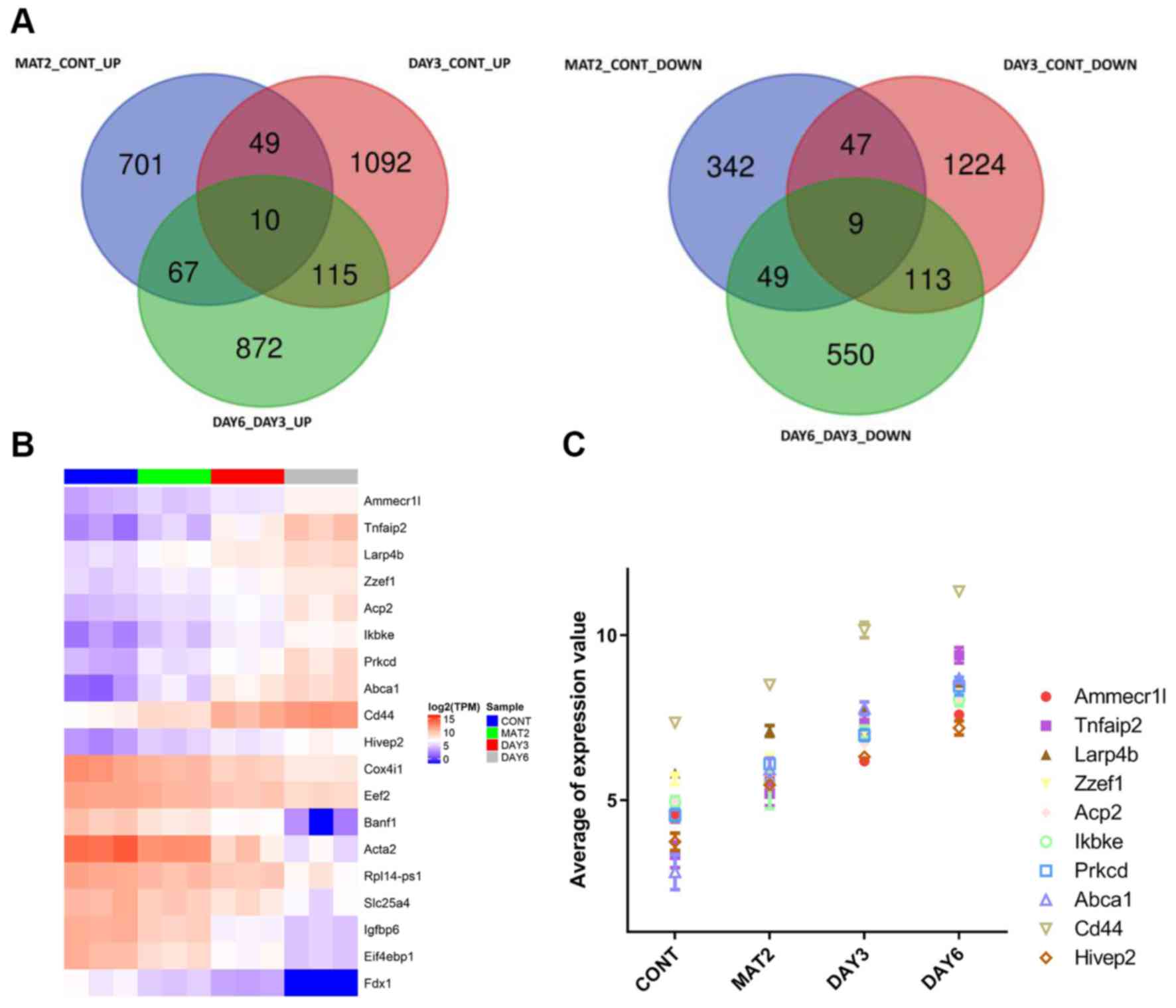
Based on the DEG analysis, there were 1,274 DEGs in
the MAT2 and CONT groups, 2,659 DEGs in the DAY3 and CONT groups
and 1,785 DEGs in the DAY6 and DAY3 groups. With additional
searches for genes with consistently changed expression compared
with the CONT, MAT2 and the DAY3 and DAY6 groups, 10 genes were
found to have increased expression levels: AMMECR1L-like protein
(Ammecr1l), tumor necrosis factor a-induced protein 2 (Tnfaip2), La
Ribonucleoprotein 4B, Zinc Finger ZZ-Type And EF-Hand Domain
Containing 1, Acid Phosphatase 2, inhibitor of nuclear factor κ-B
kinase subunit ε (Ikbke), protein kinase C δ type (Prkcd), ATP
Binding Cassette Subfamily A Member 1, CD44 Molecule and HIVEP Zinc
Finger 2. A total of nine genes were found to have decreased
expression levels:, including ferredoxin-1 (Fdx1), Eukaryotic
Translation Initiation Factor 4E Binding Protein 1 (Eif4ebp1),
insulin-like growth factor-binding protein 6 (Igfbp6), Solute
Carrier Family 25 Member 4 (Slc25a4), Ribosomal Protein L14
(Rpl14), Actin Alpha 2, Smooth Muscle (Acta2),
barrier-to-autointegration factor 1 (Banf1), Cytochrome C Oxidase
Subunit 4I1 (Cox4i1) and elongation factor 2 (Eef2) (Fig. 4A). The heatmaps of these 19 common
upregulated and downregulated genes were generated, with blue
indicating lower expression (TPM ≤5) and red indicating higher
expression (TPM >5) (Fig. 4B).
The top 10 continuously elevated genes were included in a trend
graph (Fig. 4C).
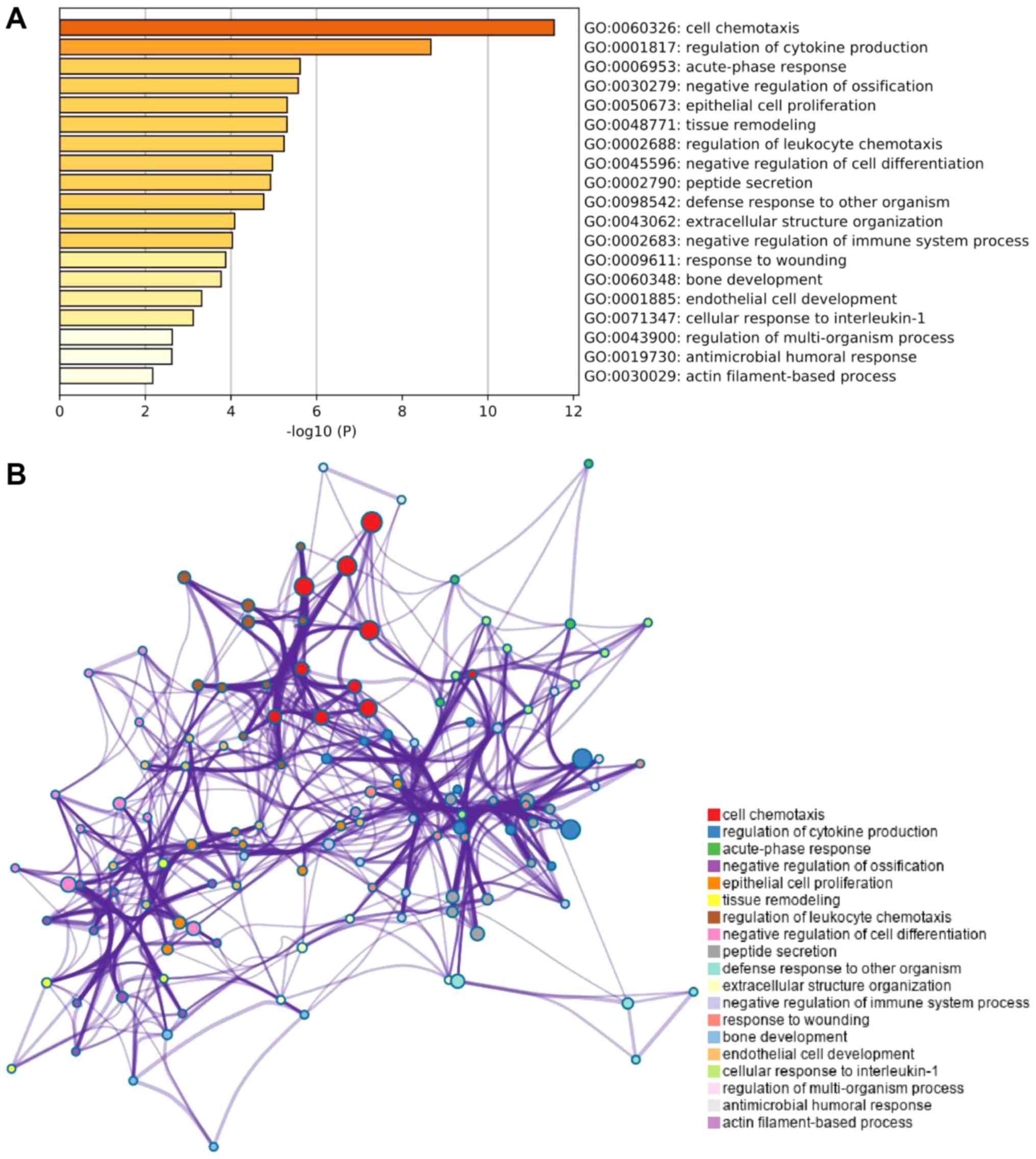
Further GO analysis of trend genes revealed that the
main enriched pathways were ‘cell chemotaxis’, ‘negative regulation
of ossification’ and ‘bone development pathway’ (Fig. 5).
Discussion
Articular cartilage repair is a prominent focus of
current research and represents a problem that needs to be resolved
in clinical practice. The current experiments involve seed cells
cultured in vitro and conventional soft scaffold structures
that cannot be regulated and have biomechanical problems, which
hinder the transformation of experimental results into clinical
applications. It is crucial to prepare biodegradable scaffolds with
osteoinductive properties; their application can provide a 3D
environment for BMSCs at the site of the defect, where they can
promote angiogenesis and thus contribute to the healing process
(22). The biological materials
currently used for bone repair include medical bioceramics, medical
polymer materials, medical composite materials and artificial
nanobone (23).
In the present study, based on the microstructure
characteristics of the hard bioceramic scaffold, the bioceramic
scaffold was used to implant into the articular cartilage defect in
mice. The scaffold suitable for microstructure absorbs autologous
bone marrow cells and proliferates in the scaffold to repair
articular cartilage. Seed cells do not need to be cultured in
vitro, and the experimental results may provide a basis for
clinical application. Since BMSCs and their differentiation during
remodeling processes have essential roles in bone regeneration, it
is thought that understanding the molecular signaling pathways
involved is crucial to the development of bone implants, bone
substitute materials and cell-based scaffolds for bone regeneration
(24). The present study cultured
and implanted BMSC scaffolds into mice and used RNA sequencing to
explore the molecular mechanism of the bioceramic scaffolds
according to the scaffold shape and duration of implantation.
PC1 level analysis showed that the measurements were
reproducible, and the ceramic scaffold culture was better compared
with the CONT group. The outcome for the MAT2 group was better
compared with the MAT1 group; HCA results revealed the DEGs of each
group. The MAT2 rod material is easier to implant compared with the
MAT1 disc and has a high degree of surface smoothness; this result
can be used to optimize the clinical choices of bioceramics. By
comparing the top 10 upregulated genes in the MAT1 group compared
with the CONT group and in the MAT2 group compared with the CONT
group, the intersection genes were found to be Saa3, Lcn2 and Nos2.
Saa3 is a secreted protein that is prominently expressed in bone
cells and affects bone metabolism by regulating genes expressed
during inflammation, apoptosis and bone matrix remodeling (25). Saa3 may have a number of different
biological functions related to extracellular matrix repair, bone
remodeling, bone resorption and bone development (25). LCN2 is secreted by osteoblasts and
is involved in bone metabolism, which is essential for bone health
(26). Nos2 is elevated in
osteoblasts and chondroblasts in bones during the early stages of
fracture healing (27). Therefore,
the bioinformatics analysis performed in the current study suggests
that ceramic materials may be beneficial for bone formation.
Further comparison of the DEGs in the MAT2 and MAT1
groups revealed that the expression levels of osteogenesis-related
genes, such as Ndufb6, Rpl17-ps9 and Ccl7, were upregulated. Ndufb6
is a component of the human skeletal muscle respiratory chain
(28). Rpl17 can be used in the
early stages of osteogenic differentiation in mouse bone marrow
mesenchymal stem cells (29). CCL7
increases the mRNA levels of a number of genes involved in
metastasis and osteolysis (30).
Therefore, it was hypothesized that the material used in the MAT2
group may be superior to that used in the MAT1 group. Therefore,
rod-shaped ceramic implants used in the MAT2 group were selected
for subsequent in vivo experiments.
The ceramic rods were implanted into mice for 3 or 6
days. By comparing the DEGs of each group, it was found that there
were 10 genes with increased expression levels, such as Ammecr1l,
Tnfaip2, Ikbke and Prkcd. Nine genes, including Fdx1, Igfbp6, Banf1
and Eef2 showed decreased expression levels. Further GO analysis of
the 19 DEG expression trends revealed that the main pathways
involved were ‘cell chemotaxis’, ‘negative regulation of
ossification’ pathway and ‘bone development’. The involvement of
the important signaling pathways associated with the bone
development during embryogenesis, as well as during fracture
healing and repair has been demonstrated by various studies. These
include the Wnt/β-catenin, Notch, bone morphogenic
protein/transforming growth factor-β, PI3K/Akt/mTOR,
mitogen-activated protein kinase, platelet-derived growth factor,
insulin-like growth factor, fibroblast growth factor and
Ca2+ pathways (24). It
has been suggested that BMSCs and their differentiation during the
remodeling processes, as well as specific signaling pathways and
their activated downstream networks, are implicated in bone
regeneration (31). The present
study indicated that the BMSC ceramic rod was successfully
implanted into the mouse and played a role in repairing bone
damage. The bone repair effect was more obvious as the implantation
time was increased. Numerous treatment strategies can improve bone
repair, which opens up new avenues for bone regeneration therapies.
One of the strategies is based on the study of molecular signaling
pathways. Changing gene expression levels to increase the number of
osteoblasts or promote their maturity is expected to stimulate bone
regeneration. The experimental limitation of the present study is
the lack of quantitative PCR and western blot validation data,
which will be performed in subsequent studies.
The present study successfully performed
three-dimensional culture of mesenchymal stem cell composite
scaffolds in mice and used RNA sequencing to explore its molecular
mechanism based on the shape of bioceramic scaffold and the
duration of implantation in vivo. Molecular studies found
that rod-shaped ceramic materials were superior to the disc shape.
This conclusion may be used to optimize clinical choices for
bioceramics. By comparing the DEGs of each group, it was further
confirmed that BMSCs were suitable as seed cells for cartilage
tissue engineering, and the β-TCP scaffold may be ideal cartilage
tissue engineering scaffold material. The present research provided
new insights into the molecular mechanism of cartilage repair by
BMSCs and bioceramic scaffolds.
Acknowledgements
The authors would like to acknowledge Dr Xunxia Bao
and Dr Sibo Zhu of (CinoAsia Co., Ltd.) for their technical
assistance and editing of the text.
Funding
The current study was supported by The National
Natural Science Foundation of China (grant no. 81572128). Shanghai
Municipal Commission of Health and Family Planning (grant no.
201940170).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and ZC conceptualized the study, designed the
research and performed the experiments. GZ and JS performed the
experiments. GH, FC, YW and JX analyzed and interpreted the data.
JC and SW designed the experiments, analyzed and interpreted the
data, wrote and edited the manuscript, and supervised the project.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
principles outlined in the Guide for the Care and Use of Laboratory
Animals and the current study was approved by the Ethics Committee
of Huashan Hospital Affiliated to Fudan University (approval no.
2018 Huashan Hospital JS-098).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang Z, Ding C, Li T and Yu SPC: Current
status and future prospects for disease modification in
osteoarthritis. Rheumatology (Oxford). 57 (Suppl 4):iv108–iv123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abramson SB, Attur M and Yazici Y:
Prospects for disease modification in osteoarthritis. Nat Clin
Pract Rheumatol. 2:304–312. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karsdal MA, Leeming DJ, Dam EB, Henriksen
K, Alexandersen P, Pastoureau P, Altman RD and Christiansen C:
Should subchondral bone turnover be targeted when treating
osteoarthritis? Osteoarthritis Cartilage. 16:638–646.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ito Y, Ochi M, Adachi N, Sugawara K,
Yanada S, Ikada Y and Ronakorn P: Repair of osteochondral defect
with tissue-engineered chondral plug in a rabbit model.
Arthroscopy. 21:1155–1163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ebihara G, Sato M, Yamato M, Mitani G,
Kutsuna T, Nagai T, Ito S, Ukai T, Kobayashi M, Kokubo M, et al:
Cartilage repair in transplanted scaffold-free chondrocyte sheets
using a minipig model. Biomaterials. 33:3846–3851. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sivasubramaniyan K, Ilas DC, Harichandan
A, Bos PK, Santos DL, de Zwart P, Koevoet WJLM, Owston H, Bühring
HJ, Jones E and van Osch GJVM: Bone marrow-harvesting technique
influences functional heterogeneity of mesenchymal stem/stromal
cells and cartilage regeneration. Am J Sports Med. 46:3521–3531.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Otsuru S, Tamai K, Yamazaki T, Yoshikawa H
and Kaneda Y: Bone marrow-derived osteoblast progenitor cells in
circulating blood contribute to ectopic bone formation in mice.
Biochem Biophys Res Commun. 354:453–458. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li H, Dai K, Tang T, Zhang X, Yan M and
Lou J: Bone regeneration by implantation of adipose-derived stromal
cells expressing BMP-2. Biochem Biophys Res Commun. 356:836–842.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hayashi O, Katsube Y, Hirose M, Ohgushi H
and Ito H: Comparison of osteogenic ability of rat mesenchymal stem
cells from bone marrow, periosteum, and adipose tissue. Calcif
Tissue Int. 82:238–247. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pitchford SC, Furze RC, Jones CP, Wengner
AM and Rankin SM: Differential mobilization of subsets of
progenitor cells from the bone marrow. Cell Stem Cell. 4:62–72.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Girolamo L, Lucarelli E, Alessandri G,
Avanzini MA, Bernardo ME, Biagi E, Brini AT, D'Amico G, Fagioli F,
Ferrero I, et al: Mesenchymal stem/stromal cells: A new ‘cells as
drugs’ paradigm. Efficacy and critical aspects in cell therapy.
Curr Pharm Des. 19:2459–2473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies,
and future directions. J Orthop Surg Res. 9(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tuli R, Tuli S, Nandi S, Wang ML,
Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG
and Tuan RS: Characterization of multipotential mesenchymal
progenitor cells derived from human trabecular bone. Stem Cells.
21:681–693. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hernigou P and Beaujean F: Treatment of
osteonecrosis with autologous bone marrow grafting. Clin Orthop
Relat Res 14-23, 2002.
|
|
15
|
Qin X, Raj RM, Liao XF, Shi W, Ma B, Gong
SQ, Chen WM and Zhou B: Using rigidly fixed autogenous tooth graft
to repair bone defect: An animal model. Dent Traumatol. 30:380–384.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Korhonen RK and Jurvelin JS: Compressive
and tensile properties of articular cartilage in axial loading are
modulated differently by osmotic environment. Med Eng Phys.
32:155–160. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Siqueira L, Passador FR, Costa MM, Lobo AO
and Sousa E: Influence of the addition of β-TCP on the morphology,
thermal properties and cell viability of poly (lactic acid) fibers
obtained by electrospinning. Mater Sci Eng C Mater Biol Appl.
52:135–143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am. 84:454–464.
2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36 (Suppl 3):S20–S27.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu JX, Flautre B, Anselme K, Hardouin P,
Gallur A, Descamps M and Thierry B: Role of interconnections in
porous bioceramics on bone recolonization in vitro and in vivo. J
Mater Sci Mater Med. 10:111–120. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ikeda R, Fujioka H, Nagura I, Kokubu T,
Toyokawa N, Inui A, Makino T, Kaneko H, Doita M and Kurosaka M: The
effect of porosity and mechanical property of a synthetic polymer
scaffold on repair of osteochondral defects. Int Orthop.
33:821–828. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oryan A, Kamali A, Moshiri A and Baghaban
Eslaminejad M: Role of mesenchymal stem cells in bone regenerative
medicine: What is the evidence? Cells Tissues Organs. 204:59–83.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kashte S, Jaiswal AK and Kadam S:
Artificial bone via bone tissue engineering: Current scenario and
challenges. Tissue Eng Regen Med. 14:1–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Majidinia M, Sadeghpour A and Yousefi B:
The roles of signaling pathways in bone repair and regeneration. J
Cell Physiol. 233:2937–2948. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thaler R, Sturmlechner I, Spitzer S,
Riester SM, Rumpler M, Zwerina J, Klaushofer K, van Wijnen AJ and
Varga F: Acute-phase protein serum amyloid A3 is a novel paracrine
coupling factor that controls bone homeostasis. FASEB J.
29:1344–1359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Capulli M, Ponzetti M, Maurizi A,
Gemini-Piperni S, Berger T, Mak TW, Teti A and Rucci N: A complex
role for lipocalin 2 in bone metabolism: Global ablation in mice
induces osteopenia caused by an altered energy metabolism. J Bone
Miner Res. 33:1141–1153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang W, Zhang K, Zhu Y, Wang Z, Li Z and
Zhang J: Genetic polymorphisms of NOS2 and predisposition to
fracture non-union: A case control study based on han Chinese
population. PLoS One. 13(e0193673)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kacerovsky-Bielesz G, Chmelik M, Ling C,
Pokan R, Szendroedi J, Farukuoye M, Kacerovsky M, Schmid AI, Gruber
S, Wolzt M, et al: Short-term exercise training does not stimulate
skeletal muscle ATP synthesis in relatives of humans with type 2
diabetes. Diabetes. 58:1333–1341. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hermann-Kleiter N, Ghaffari-Tabrizi N,
Blumer MJ, Schwarzer C, Mazur MA and Artner I: Lasp1 misexpression
influences chondrocyte differentiation in the vertebral column. Int
J Dev Biol. 53:983–991. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Han S, Wang T, Chen Y, Han Z, Guo L, Wu Z,
Yan W, Wei H, Liu T, Zhao J, et al: High CCL7 expression is
associated with migration, invasion and bone metastasis of
non-small cell lung cancer cells. Am J Transl Res. 11:442–452.
2019.PubMed/NCBI
|
|
31
|
Knight MN and Hankenson KD: Mesenchymal
stem cells in bone regeneration. Adv Wound Care (New Rochelle).
2:306–316. 2013.PubMed/NCBI View Article : Google Scholar
|