Introduction
Low-back pain is a worldwide socio-economic problem
that affects more than half the population during their whole life
(1,2), and intervertebral disc degeneration
(IDD) has been extensively recognized as the most common source of
this disorder. Currently, advances in our understanding of the IDD
pathogenesis have contributed to the emerging novel therapeutic
strategies for retarding the progression or deterioration of it,
ranging from pain palliation at early stage to invasive operations
at the late stage (3).
Unfortunately, these treatment options are not capable of
effectively restoring the normal physiological functions of
intervertebral disc because of the non-renewable characteristic of
nucleus pulposus (NP), one of the major components of
intervertebral disc. In addition, surgical interventions for IDD
such as spinal arthroplasty and discectomy are usually susceptible
to infection and many other complications, hence it is a
predominant challenge for spinal surgeons.
IDD is generally characterized by an increase
release of pro-inflammatory cytokines, including TNF-α and IL-1β,
which will recruit the activated immune cells into degenerated
tissues and further trigger the inflammatory cascade (4). On the other hand, the inflammatory
milieu is also responsible for the aberrant activity of MMPs,
ADAMTSs and disintegrins, leading to the interruption of ECM
homeostasis (5,6) and thus exacerbating the degenerative
process. There are increasing number of studies addressing the
utilization of mesenchymal stem cells (MSCs) in the treatment of
IDD. Previous studies have proved that MSCs differentiate into
NP-like cells with elevated expression of NP-related marker genes
when co-cultured with nucleus pulposus cells (NPCs) (7). It was also demonstrated that the MSCs
could restore the molecular environments of matrix (8), in addition to its capacities of
immunomodulation and multilineage differentiation (9). Hitherto, the mechanisms underlying the
effect of MSCs on the degenerative process in NPCs and the involved
pathophysiological signaling pathways have not been fully
described.
Herein, the effect of MSCs on inflammatory response
within NPCs was evaluated after exposure to pro-inflammatory
cytokine stimulation, and the pathophysiological pathways that may
be involved were also investigated. Our results provide insights
into the mechanisms underlying the effects of MSCs on TNF-α-induced
inflammatory response in NPCs, which will further facilitate the
application of stem cells in the therapy for the degenerative
process.
Materials and methods
NPCs isolation and culture
The nucleus pulposus samples were collected from
patients undergoing surgery from Jan 2016 to May 2018. The magnetic
resonance classification of IDD was performed according to a
previous report (10). Written
informed consents were obtained from patients and counterparts and
all the experimental protocols were approved by the Ethic Committee
of Honghui Hospital affiliated to Xi'an Jiaotong University
(approval no. 2019-12).
The NP tissues were dissected into pieces of 1.0
mm3 and digested with 0.25% protease at 37˚C for 1 h,
followed by 0.2 mg/ml of collagenase type II for 4 h, prior to
filtration through a sterilized mesh with 70-µm pore size and
centrifugation at 300 x g, at 4˚C for 5 min. Cells were resuspended
and maintained in DMEM/F12 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) at 37˚C in 5%
CO2. The medium was replaced every 2-3 days and the
primary culture was sub-cultured when cells grew to ~80˚C
confluence. NPCs were incubated with 10 ng/ml TNF-α (Sigma-Aldrich;
Merck KGaA) or equal amount of DMEM medium as a negative control
for 24 h before further assessment.
Isolation and culture of Wharton's
Jelly-derived MSCs (WJ-MSCs)
The fresh umbilical cord was obtained from in a full
term neonate, followed by being washed with pre-cold PBS to remove
the blood clot. Afterwards, the Wharton's jelly was isolated from
umbilical cord and cut into 1 mm3 fragments, which were
incubated with type II collagenase (0.2 mg/ml, Sigma-Aldrich; Merck
KGaA) at 37˚C for 18 h and then digested using 2.5% trypsin for 30
min with agitation. Finally, the cells were fixed with 2 ml FBS for
2 h and then maintained in 8 ml of L-DMEM medium (HyClone; GE
Healthcare Life Sciences) containing 1% penicillin-streptomycin, 50
µg/ml L-ascorbic acid and 2 mM glutamine at 37˚C in a humidified
atmosphere of 5% CO2. The culture medium was replaced
every 2 days. The cells were passaged when the confluence reached
80-90%. WJ-MSCs from passage 3 were used for determination of cell
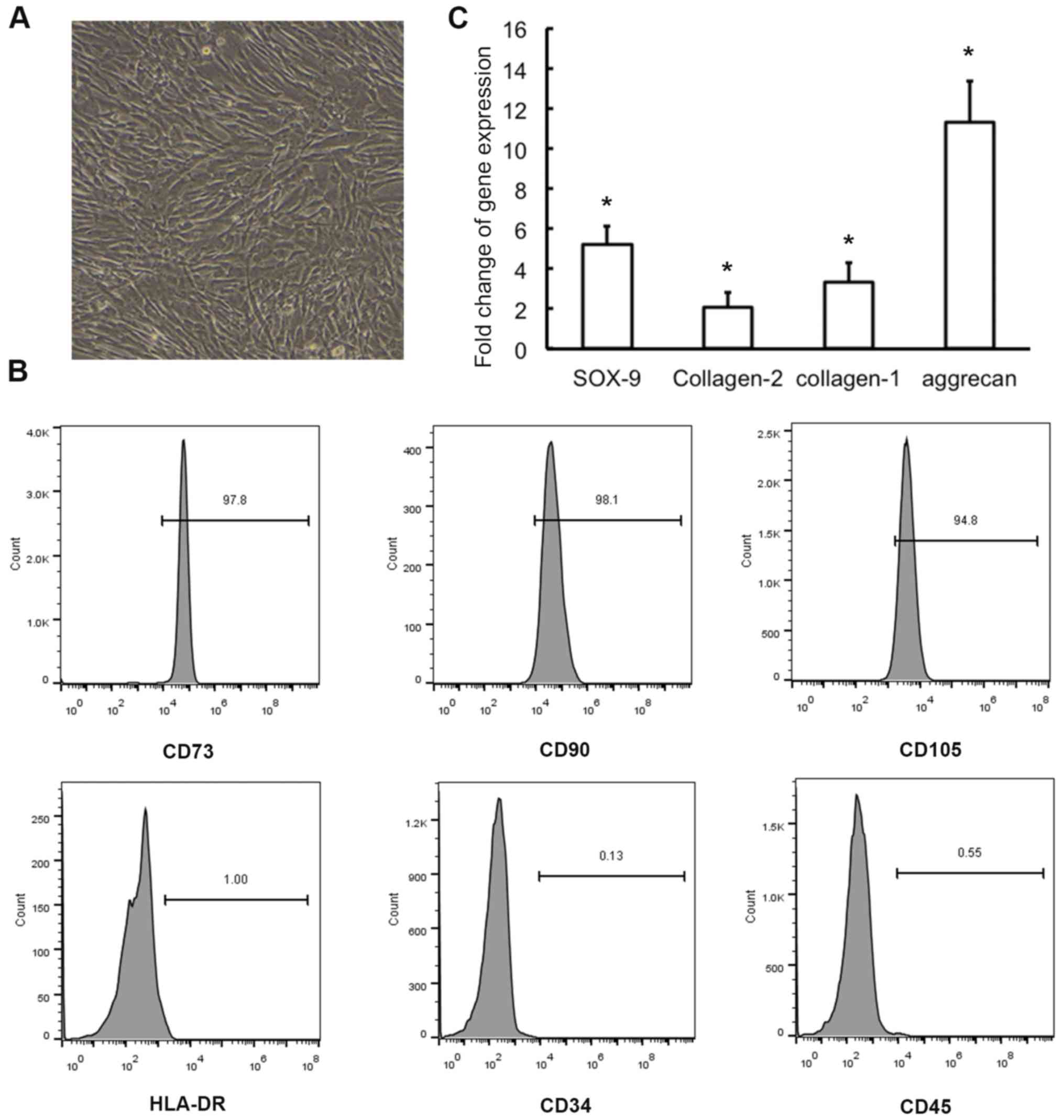
surface antigens. The identification of MSCs is illustrated in
Fig. 1 and MSCs differentiate into
NP-like cells, with elevated expression of NP-related marker genes,
when co-cultured with NPCs.
Enzyme-linked immunosorbent assay
(ELISA)
The content of released proteins in cell culture
supernatant was measured using specific ELISA kits (R&D
Systems). Briefly, the wells of a high protein binding 96-well
plate (Corning Costar, Inc.) were coated overnight with 2 ng of
monoclonal antibody against IL-1β, IL-6 or IL-8. Wells were then
washed 4 times with PBS and blocked for at least 1 h with PBS
containing 10% FBS, followed by the addition of clear cell
supernatants and standard dilutions of recombinant human IL-1β,
IL-6 or IL-8, prior to 2-h incubation at 37˚C. After washing
thoroughly with a buffer containing PBS and 0.05% Tween-20, each
well was incubated with biotinylated antibody for 60 min and then
washed 3 times. After staining with streptavidin-HRP for 30 min,
substrate working solution was added to each well and incubated for
15-20 min in the dark. The reaction was then stopped by the
addition of 100 µl of 2 M H2SO4, and the
OD450 was measured with an ELISA plate reader (Model
550; Bio-Rad Laboratories, Inc.). Each experiment was independently
repeated at least three times.
Western blotting
Primary polyclonal antibodies against human p-p38,
p38, ASK-1, MKK-3, MKK-6, MMP-3, MMP-13, aggrecan, and actin were
purchased from Abcam. Proteins were extracted from NP tissues or
cell lysates with RIPA lysis buffer supplemented with protease
inhibitors and phosphorylase inhibitor. The protein concentration
was measured using the BCA assay (Pierce) following the
manufacturer's instructions. The total proteins were resolved by
10% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) before being transferred to polyvinylidene fluoride
(PVDF) membrane, which were blocked with 5% silk milk for at least
1 h and incubated overnight at 4˚C with specific primary antibodies
(1:1,000 diluted). After rinsing with TBST, the membrane was
incubated for 1 h at room temperature with HRP-conjugated goat
anti-rabbit IgG (1:3,000 diluted). Finally, the labeled proteins
were visualized with ECL substrates (EMD Millipore) before
quantitative analysis using AlphaView software (ProteinSimple).
Each experiment was independently repeated at least three
times.
Co-culture of WJ-MSCs and NPCs
For co-culture without direct cell-cell contact,
NPCs (1x106 cells/well) were seeded on the bottom of
transwell 6-well plates, and WJ-MSC cells were placed onto the
apical compartment of a 6-well polyester track-etched cell culture
insert with a membrane pore size of 0.4-µm (Thermo Fisher
Scientific, Inc). Sequentially, cells were grown in DMEM/F12 medium
at 37˚C in a humidified atmosphere of 5% CO2. NPCs were
cultured on common plates as a control and subjected to further
analysis.
Statistical analysis
Data are presented as means ± standard deviation
(SD). All in vitro experiments included at least 3
replicates per group. Groups were compared using the two-tailed
Student's t-test for parametric data. All statistical analyses were
carried out using SPSS 19.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
WJ-MSCs suppress TNF-α-induced
inflammation response in NPCs
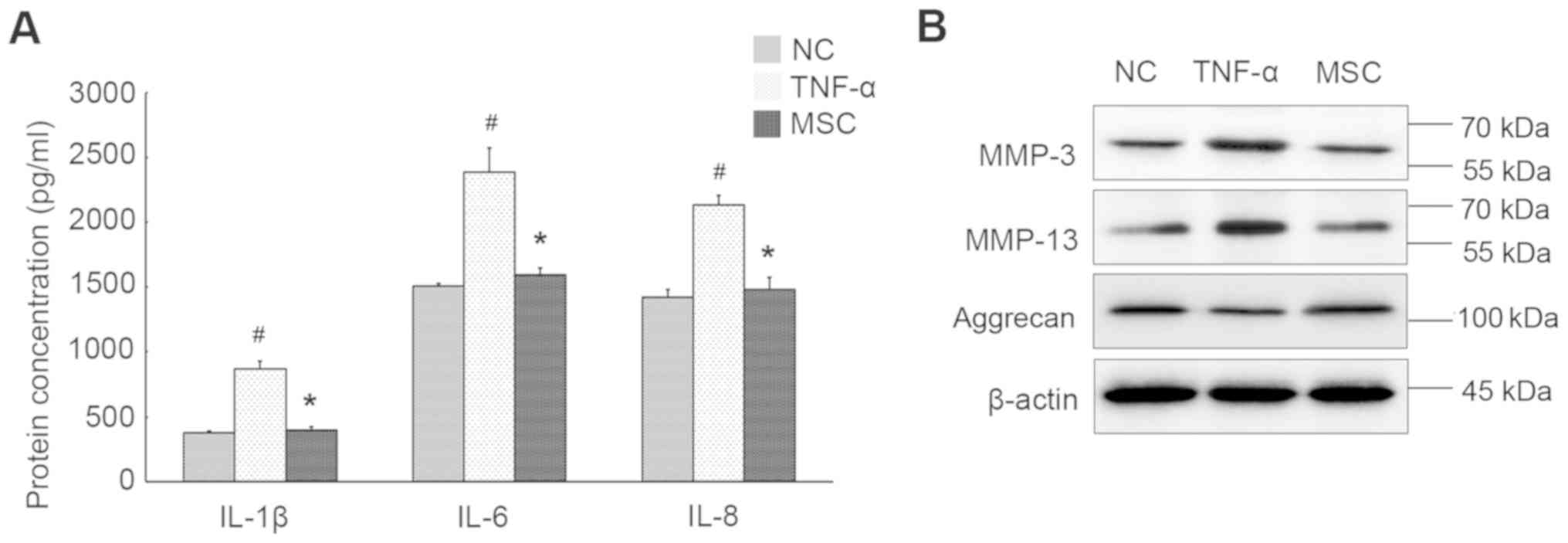
Pro-inflammatory cytokine TNF-α was used to mimic
the inflammatory milieu of IDD, and the expression profiles of
several inflammation markers in NPCs were evaluated. As shown in
Fig. 2A, the levels of IL-1β, IL-6
and IL-8 were dramatically increased upon TNF-α stimulation. We
also observed remarkable augment in the expression of MMP-3 and
MMP-13. Inversely, aggrecan level synthesized by NPCs was
significantly declined after cytokine treatment (Fig. 2B), suggestive of aggrecan
fragmentation and breakdown of hydrated ECM within the nucleus.
TNF-α-treated NPCs were co-cultured with WJ-MSCs to
determine the effect of WJ-MSCs on the intracellular inflammatory
response and the involved signaling transduction. As displayed in
Fig. 2A, WJ-MSCs evidently
compromised the increased production of IL-1β, IL-6 and IL-8 by
NPCs. In fact, there was no significant difference in the
expression of these markers within MSCs, in the presence and
absence of TNF-α stimulation (data not shown). Additionally,
expression levels of both MMP-3 and MMP-13 were suppressed in NPCs
that co-cultured with WJ-MSCs in comparison to cells exposed to
TNF-α alone, whereas aggrecan production was elevated in the
presence of WJ-MSCs (Fig. 2B).
Therefore, WJ-MSCs can ameliorate inflammatory status within the
co-cultured NPCs without direct contact.
WJ-MSCs inhibit p38 MAPK signaling
transduction activated by the inflammatory milieu
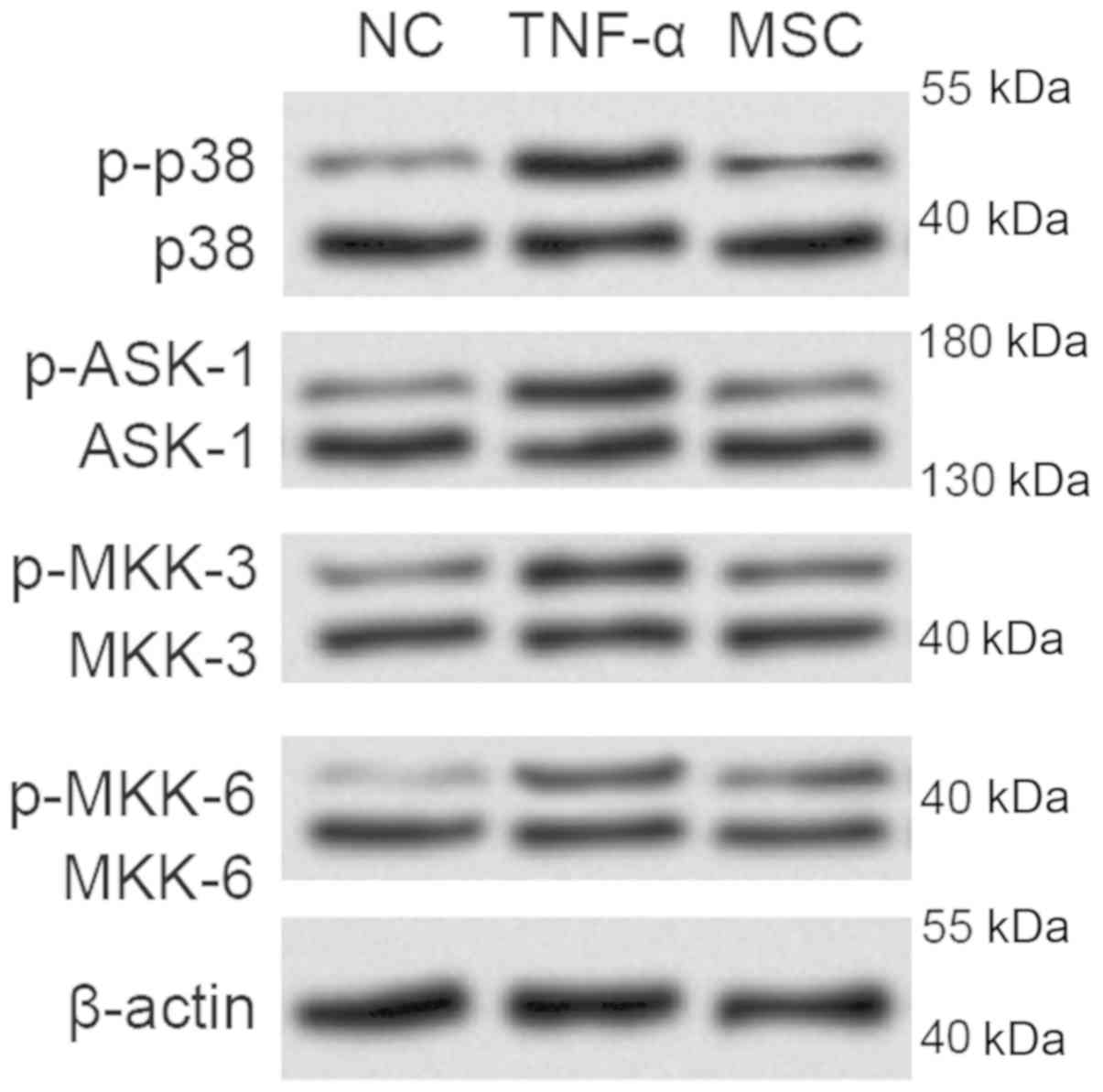
Following TNF-α stimulation, the phosphorylation of
apoptosis signal regulating kinase-1 (ASK-1) was significantly
enhanced (Fig. 3). Moreover, the
MAPK kinases including MKK-3 and MKK-6 were also activated after
incitement by the pro-inflammatory cytokine TNF-α, hence promotion
of p38 phosphorylation. The above indicate that the p38 MAPK
signaling was activated, which might contribute to the promotion of
inflammatory responses during the degenerative process.
Notably, the phosphorylated forms of the
above-mentioned molecules implicated in p38 MAPK pathway were found
decreased due to indirect co-culture between NPCs and WJ-MSCs,
suggesting that WJ-MSCs may exert the protective role by virtue of
the activation of p38 MAPK pathway.
WJ-MSCs suppress TNF-α-induced
inflammation by inhibiting p38 MAPK signaling
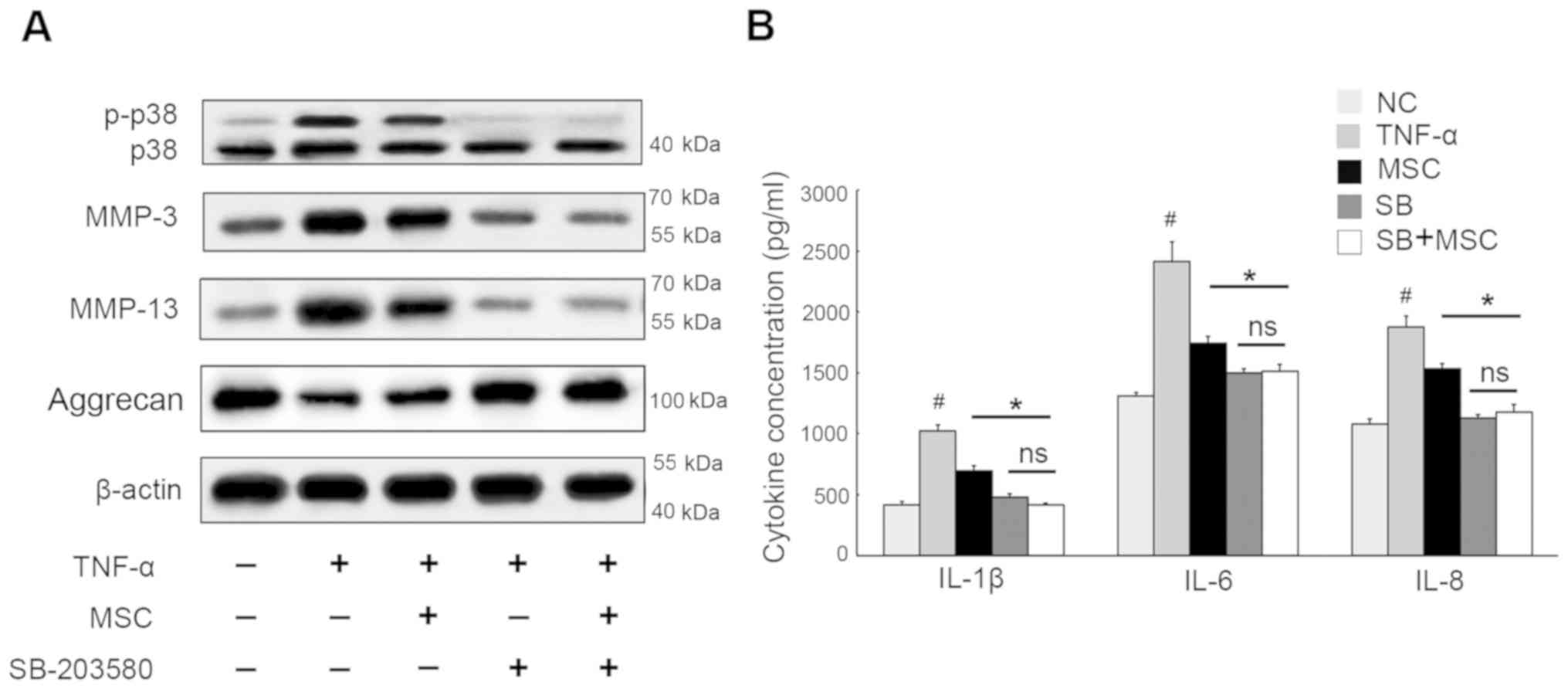
To verify this observation, p38 MAPK signaling
inhibitor SB-203580 was utilized and, as expected, it was found
that this led to an obvious decrease in the phosphorylation forms
of p38 (Fig. 4). Although
TNF-α-induced MMP-3 and MMP-13 was decreased due to WJ-MSC
co-culture, their levels synthesized by NPCs that were co-cultured
with WJ-MSCs were further reduced in the presence of SB-203580,
whereas aggrecan showed the opposite results. Furthermore, when
cells were treated with SB-203580, NPCs co-cultured with WJ-MSCs
showed no significant difference from those cultured alone, which
suggested that blockage of p38/MAPK pathway protected NPCs against
ECM breakdown, regardless of the analogous function of WJ-MSCs.
Considering IL-1β, IL-6 and IL-8 protection, NPCs exposed to
SB-203580 displayed values lower than those observed in the
co-cultured cells devoid of SB-203580. Also there was barely
detectable difference between NPCs cultured alone and the
co-cultured cells when they were subjected to SB-203580 treatment.
Thus, we concluded that WJ-MSCs might suppress TNF-α-mediated
inflammation in NPCs by partially inactivating p38/MAPK
signaling.
Discussion
Not until recently have MSCs been introduced into
the therapeutic managements of IDD, emerging as a promising
strategy from bench (11-13)
to bedside (14). MCSs are probably
implicated in the degenerative disc repair in the following
aspects: i) complement damaged cells through regeneration of
disc-specific cells that have the ability to produce ECM
components; ii) control the inflammatory response and (III) promote
tissue regeneration by virtue of paracrine signaling factors
(15).
Multiple types of tissues are currently proposed as
source of MSCs, and among them adipose tissue and bone marrow are
predominantly used in IDD-related research. Owing to limited
stemness properties and invasive harvesting procedure, MSCs derived
from both tissues are restricted in practice (16-19).
Herein, WJ-MSCs were introduced in the present study which
attempted to provide novel insight into the molecular mechanisms
underlying how MSCs play their role in the therapies of IDD
disease.
The mitogen-activated protein kinase (MAPK) plays a
pivotal role in mediating many cellular processes including
responses to inflammation, damage or stress, and cell
differentiation. In particular, the implication of p38 MAPK
signaling cascades in the pathogenesis of IDD is gradually
revealed. By blocking the p38 MAPK pathway, Studer et al
(20) found that p38 MAPK was
linked to the increased synthesis of inflammation- and pain-related
prostaglandin E2 (PGE2) and IL-6, as well as the aggrandized ratio
of MMP-3 to TIMP-1. Various stimuli accelerate the breakdown of ECM
or inflammation through the activation of p38 MAPK signaling
(20-22).
In addition, p38 MAPK participate in the regulation of cell
senescence of NPCs within the degenerative disc tissue (23). Our results demonstrated that p38
MAPK was initiated upon TNF-α stimulation mimicking the
inflammatory microenvironment within IDD, but p38 MAPK and the
upstream signaling in NPCs were suppressed when co-cultured with
WJ-MSCs. This indicated that WJ-MSCs might exert the effects on
inflammatory response through inhibition of p38 MAPK pathway.
Given that MSCs and NPCs were co-cultured without
cell-cell interaction, it was suggested that MSCs modulate local
inflammatory milieu via paracrine signaling transduction.
Afterwards, p38/MAPK inhibitor SB-203580 was utilized and it
resulted in an obvious decrease in the TNF-α-induced synthesis of
inflammatory markers. Considering MMP-3 and MMP-13, NPCs exposed to
SB-203580 displayed values lower, whilst a higher amount of
aggrecan, than those observed in absence of SB-203580. Inactivation
of p38 MAPK pathway can protect NPCs against the detrimental
inflammatory response, which is strictly consistent with previous
studies. Intriguingly, once cells were pre-treated with SB-203580,
levels of all these proteins expressed in NPCs co-cultured with
WJ-MSCs were analogous to those NPCs cultured alone, suggesting
that blocking p38 MAPK enhanced the protective actions of WJ-MSCs
in suppressing inflammatory response. Co-culture with WJ-MSCs might
have impact on other signaling pathways that interplay with p38
MAPK during this process. Once p38 MAPK signaling was blocked,
there was barely detectable difference between NPCs co-cultured
with WJ-MSCs and the cells cultured alone. That is, p38 MAPK
signaling plays a predominant role in this process, and WJ-MSCs
potentially suppress the TNF-α-mediated inflammation within NPCs in
a p38 MAPK-dependent manner.
In conclusion, our data demonstrate that after
co-culture with WJ-MSCs, TNF-α-induced inflammatory response in
NPCs was significantly diminished, and the activities of p38 MAPK
and the related upstream pathways were decreased, indicating an
inhibitory effect of WJ-MSCs on inflammation as well as ECM
degeneration by suppressing p38 MAPK activation. Furthermore,
blocking p38 MAPK pathway markedly further enhanced the applaudable
performance of WJ-MSCs in anti-inflammation, which suggested that
WJ-MSCs mitigated the degenerative process within disc tissue by
predominantly inactivating p38 MAPK signaling pathway. These
findings highlight a possible mechanism underlying the role of MSCs
in meliorating the inflammatory milieu within degenerated NPCs,
which may be applicable to MSCs-based therapy for disc
degeneration.
Acknowledgements
Not applicable.
Funding
This study was supported by China Postdcotoral
Science Foundation (no. 2016M602943XB), Shaanxi Postdoctoral
Research Fund (no. 2017BSHQYXMZZ19), The Fundamental Research Funds
for the Central Universities.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ made substantial contributions to the design of
the study and the experiments. YQ and SW performed ELISA. DHu and
HH were responsible for immunoblotting. XZ and DHa contributed to
the analysis of the observation indexes. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Honghui Hospital, Xi'an Jiaotong University (Xi'an, China).
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients
and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McBeth J and Jones K: Epidemiology of
chronic musculoskeletal pain. Best Pract Res Clin Rheumatol.
21:403–425. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mathew J, Singh SB, Garis S and Diwan AD:
Backing up the stories: The psychological and social costs of
chronic low-back pain. Int J Spine Surg. 7:e29–e38. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zeng Y, Chen C, Liu W, Fu Q, Han Z, Li Y,
Feng S, Li X, Qi C, Wu J, et al: Injectable microcryogels
reinforced alginate encapsulation of mesenchymal stromal cells for
leak-proof delivery and alleviation of canine disc degeneration.
Biomaterials. 59:53–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Phillips KL, Chiverton N, Michael AL, Cole
AA, Breakwell LM, Haddock G, Bunning RA, Cross AK and Le Maitre CL:
The cytokine and chemokine expression profile of nucleus pulposus
cells: Implications for degeneration and regeneration of the
intervertebral disc. Arthritis Res Ther. 15(R213)2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Loreto C, Musumeci G, Castorina A, Loreto
C and Martinez G: Degenerative disc disease of herniated
intervertebral discs is associated with extracellular matrix
remodeling, vimentin-positive cells and cell death. Ann Anat.
193:156–162. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang SH, Yang KC, Chen CW, Huang TC, Sun
YH and Hu MH: Comparison of transforming hrowth factor-beta1 and
lovastatin on differentiating mesenchymal stem cells toward nucleus
pulposus-like phenotype: An in vitro cell culture study. Asian
Spine J. 13:705–712. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vadalà G, Russo F, Ambrosio L, Loppini M
and Denaro V: Stem cells sources for intervertebral disc
regeneration. World J Stem Cells. 8:185–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei A, Shen B, Williams L and Diwan A:
Mesenchymal stem cells: Potential application in intervertebral
disc regeneration. Transl Pediatr. 3:71–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine. 26:1873–1878.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yan HS, Hang C, Chen SW, Wang KK and Bo P:
Salvianolic acid B combined with mesenchymal stem cells contributes
to nucleus pulposus regeneration. Connect Tissue Res: Apr 26, 2019
(Epub ahead of print). doi: 10.1080/03008207.2019.1611794.
|
|
12
|
Cao C, Zou J, Liu X, Shapiro A, Moral M,
Luo Z, Shi Q, Liu J, Yang H and Ebraheim N: Bone marrow mesenchymal
stem cells slow intervertebral disc degeneration through the NF-κB
pathway. Spine J. 15:530–538. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lan WR, Pan S, Li HY, Sun C, Chang X, Lu
K, Jiang CQ, Zuo R, Zhou Y and Li CQ: Inhibition of the Notch1
pathway promotes the effects of nucleus pulposus cell-derived
exosomes on the differentiation of mesenchymal stem cells into
nucleus pulposus-like cells in rats. Stem Cells Int.
2019(8404168)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arutyunyan I, Elchaninov A, Makarov A and
Fatkhudinov T: Umbilical cord as prospective source for mesenchymal
stem cell-based therapy. Stem Cells Int.
2016(6901286)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fierabracci A, Del Fattore A, Luciano R
and Muraca M, Teti A and Muraca M: Recent advances in mesenchymal
stem cell immunomodulation: The role of microvesicles. Cell
Transplant. 24:133–149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Szychlinska MA, Stoddart MJ, D'Amora U,
Ambrosio L, Alini M and Musumeci G: Mesenchymal stem cell-based
cartilage regeneration approach and cell senescence: Can we
manipulate cell aging and function? Tissue Eng Part B Rev.
23:529–539. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gardner OFW, Musumeci G, Neumann AJ, Eglin
D, Archer CW, Alini M and Stoddart MJ: Asymmetrical seeding of MSCs
into fibrin-poly(ester-urethane) scaffolds and its effect on
mechanically induced chondrogenesis. J Tissue Eng Regen Med.
11:2912–2921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Szychlinska MA, Castrogiovanni P, Nsir H,
Di Rosa M, Guglielmino C, Parenti R, Calabrese G, Pricoco E,
Salvatorelli L, Magro G, et al: Engineered cartilage regeneration
from adipose tissue derived-mesenchymal stem cells: A
morphomolecular study on osteoblast, chondrocyte and apoptosis
evaluation. Exp Cell Res. 357:222–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Musumeci G, Mobasheri A, Trovato FM,
Szychlinska MA, Graziano ACE, Lo Furno D, Avola R, Mangano S,
Giuffrida R and Cardile V: Biosynthesis of collagen I, II, RUNX2
and lubricin at different time points of chondrogenic
differentiation in a 3D in vitro model of human mesenchymal stem
cells derived from adipose tissue. Acta Histochem. 116:1407–1417.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Studer RK, Aboka AM, Gilbertson LG,
Georgescu H, Sowa G, Vo N and Kang JD: p38 MAPK inhibition in
nucleus pulposus cells: A potential target for treating
intervertebral disc degeneration. Spine. 32:2827–2833.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Krupkova O, Sadowska A, Kameda T, Hitzl W,
Hausmann ON, Klasen J and Wuertz-Kozak K: p38 MAPK facilitates
crosstalk between endoplasmic reticulum stress and IL-6 release in
the intervertebral disc. Front Immunol. 9(1706)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu C, Yang H, Gao F, Li X, An Y, Wang J
and Jin A: Resistin promotes intervertebral disc degeneration by
upregulation of ADAMTS-5 through p38 MAPK signaling pathway. Spine.
41:1414–1420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pang L, Li P, Zhang R, Xu Y, Song L and
Zhou Q: Role of p38-MAPK pathway in the effects of high-magnitude
compression on nucleus pulposus cell senescence in a disc perfusion
culture. Biosci Rep. 37(BSR20170718)2017.PubMed/NCBI View Article : Google Scholar
|