Introduction
Anesthesia is a key process in the surgical
treatment of children considering their limited cooperation, and it
is also a factor that determines the success of the surgery without
endangering the children. For pediatric surgery, several options
for anesthesia are available. Among them, epidural anesthesia is
more extensively used based on its satisfactory muscle relaxation
and thorough pain-relieving effects (1).
A variety of anesthetics have been used to achieve
pediatric epidural anesthesia, including propofol and ketamine,
which have been indicated to be preferable in the clinical setting,
among which propofol has been reported to exhibit a good sedative
effect and a rapid working mechanism, and patients who receive
propofol have been indicated to exhibit a quick recovery from
anesthesia post surgery without significant adverse reactions
(2). Despite its satisfactory
performance in anesthesia, pain relief and sedation, ketamine
administration has been indicated to result in an increased release
of catechol and consequently in increased cardiovascular excitation
during the recovery period; therefore, children may experience
various side effects, such as nausea, vomiting, restlessness and
nightmares (3). Currently, an
auxiliary application of general anesthetics or sedative drugs is
required in epidural anesthesia. Procaine, a local anesthetic
commonly used in clinical practice, can stabilize the cell membrane
and reduce its permeability to ions, so that when the nerve impulse
reaches, sodium and potassium ions cannot move in and out of the
cell membrane to generate depolarization and action potentials,
thus producing a local anesthetic effect (4,5).
Procaine has poor penetration of mucous membrane and is not
suitable for surface anesthesia, but it is less toxic than morphine
and has a definite effect, which is also suitable for infiltration
anesthesia, block anesthesia and epidural anesthesia (6). Procaine can be hydrolyzed by esterase
in plasma and converted into para-aminobenzoic acid and
diethylaminoethanol, where the former can resist the antibacterial
effect of sulfonamides and should therefore be avoided to use with
sulfonamides (7).
The present study specifically analyzed the effect
of procaine in combination with ketamine and propofol in pediatric
epidural anesthesia by comparing patients receiving ketamine and
propofol with patients receiving procaine combined with ketamine
and propofol, aiming to identify more effective and safe options
for clinical pediatric epidural anesthesia.
Patients and methods
Patients
A total of 74 children with inguinal hernias, who
were subjected to herniorrhaphy under epidural anesthesia in the
Hong Hui Hospital (Xi'an, China) between June 2018 and September
2019 were included in the present study and divided into two groups
using a random number table. The control group included 20 males
and 17 females with a youngest age of 6 months and an oldest age of
18 years, who received ketamine and propofol for epidural
anesthesia. The observation group included 22 males and 15 females
with a youngest age of 4 months and an oldest age of 18 years, who
received procaine combined with ketamine and propofol for epidural
anesthesia. The inclusion criteria were as follows: Children with
i) American Society of Anesthesiologists (ASA) classification grade
I (8) who underwent no other
treatment prior to participation in the present study; ii) who
required surgery under epidural anesthesia; iii) whose general
condition was verified to be satisfactory and stable; and iv) whose
parents provided written informed consent. The study was approved
by the Ethics Committee of Hong Hui Hospital, Xi'an Jiaotong
University College of Medicine (Xi'an, China). Children with a
history of non-surgical treatment, anesthesia by means other than
epidural anesthesia, ASA grade II or above, and anesthesia
contraindications and concurrent cardiac, hepatic, pulmonary and
renal dysfunction were excluded from the study.
Anesthesia
According to the clinical experience of the hospital
and a previous study, epidural anesthesia was determined as the
preferred anesthesia method in the current study (9). Before surgery, all patients received
an intramuscular injection of atropine (0.02 mg/kg, approval no.
GYZ Zi H32020166; Jiangsu Lianshui Pharmaceutical Co., Ltd.),
diazepam (0.2 mg/kg, approval no. GYZ Zi H14022662, manufactured by
Shanxi Zhendong Pharmaceutical Co., Ltd.) and ketamine (4-5 mg/kg,
approval no. GYZ Zi H20054748; Xi'an Hanfeng Pharmaceutical Co.,
Ltd.) for basic anesthesia, and a venous access was established
when they entered a sleeping or quiescent state. Routine
disinfection was performed in a right lateral decubitus position.
Following the accurate placement of sterile towels, puncturing was
implemented between the L2 and L3 spinal segments until a sense of
penetration was perceived and positive return air test results were
obtained. Subsequently, local anesthesia using 1% lidocaine (5
mg/kg, approval no. GYZ Zi H31021071; Shanghai Zhaohui
Pharmaceutical Co., Ltd.), 0.375% bupivacaine (5 mg/kg, approval
no. GYZ Zi H20056442; Shanghai Zhaohui Pharmaceutical Co., Ltd.)
and 1/200,000 epinephrine (0.25-1 mg/kg, approval no. GYZ Zi
H14020817; Shanxi Zhendong Pharmaceutical Co., Ltd.) was
concomitantly administered before unplugging the epidural needle.
The ratio of lidocaine/bupivacaine was 1:1 and the anesthesia plane
did not exceed T8.
The control group was anesthetized using ketamine
and propofol (approval no. H20130535; AstraZeneca UK Ltd.). Prior
to skin incision, ketamine (1-2 mg/kg) was slowly injected
intravenously, and an intermittent intravenous injection of
propofol (1 mg/kg) combined with ketamine (1-2 mg/kg) using the
same approach at half the initial dose was maintained during
surgery, starting from 10-15 min after the initiation of surgery
until its completion. Ephedrine (total dose 10-12 mg/kg, approval
no. GYZ Zi H50020872, manufactured by Chongqing Dikang Changjiang
Pharmaceutical Co., Ltd.) was administered in case of a sharp
decrease in blood pressure (BP) and atropine (total dose 0.5-1.0
mg/kg) was intravenously injected to treat a decrease in heart rate
(HR). An oxygen mask was constantly used during the surgery, which
was supported by manually assisted respiration when required.
The observation group was anesthetized using
procaine (specification, 0.5 g; approval no. GYZ Zi H20020082;
Jincheng Haisi Pharmaceutical Co., Ltd.) combined with ketamine and
propofol. Prior to skin incision, ketamine (1-2 mg/kg) was slowly
injected intravenously, and an intermittent intravenous injection
of propofol (1 mg/kg) was maintained during surgery. In addition,
2.0% procaine (total dose 5 ml/kg) along with ketamine (1-2 mg/kg)
using the same approach at half the initial dose was intravenously
injected, starting from 10-15 min after the initiation of surgery
until its completion. Ephedrine (total dose 10-12 mg/kg) was
administered in case of a sharp decrease in BP and atropine (total
dose 0.5-1.0 mg/kg) was intravenously injected to treat a decrease
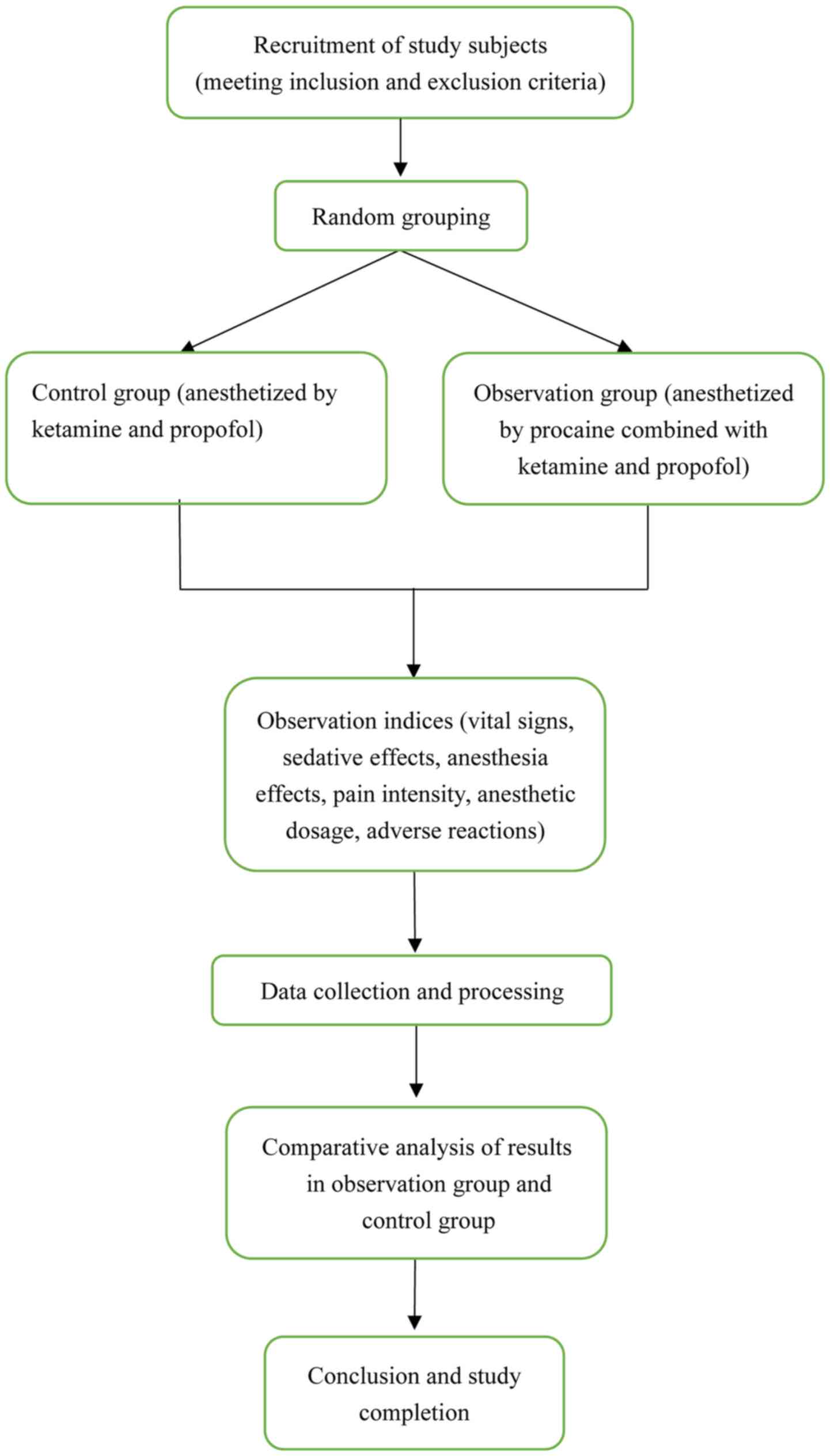
in HR. An oxygen mask was constantly used during surgery. Fig. 1 depicts a flow chart presenting the
research procedure of the current study.
Observation indices Vital signs
HR and mean arterial pressure (MAP) were measured in
the observation and control groups before anesthesia (T1), at 5 min
after anesthesia (T2), before epidural administration (T3) and at 5
min after epidural administration (T4).
Sedative effects
The sedative effects were assessed according to
Ramsay's criteria for myalgic encephalomyelitis (10), with six levels as follows: Level 1,
the patient is anxious and agitated or restless or both; level 2,
the patient is cooperative, oriented and tranquil; level 3, the
patient responds to commands only; level 4, the patient is asleep
and exhibits a rapid response to a light glabellar tap; level 5,
the patient is asleep and exhibits a slow response to a light
glabellar tap; and level 6, the patient is asleep and exhibits no
response to a light glabellar tap. Level 3 or above was considered
to indicate satisfactory sedative effects.
Anesthetic effects
Both groups were compared in terms of the latent
period for the anesthetic effect, disappearance of pain and
recovery of pain sensation, which were respectively defined as
follows: Time from epidural injection to perianal superficial
reflex or declined abdominal reflex, time from epidural injection
to no response when the surgical site was punctured and time from
epidural injection to the time when the patients regained
consciousness and complained of pain or when an evident writhing in
the limbs was observed despite the patients being unconscious.
Pain intensity
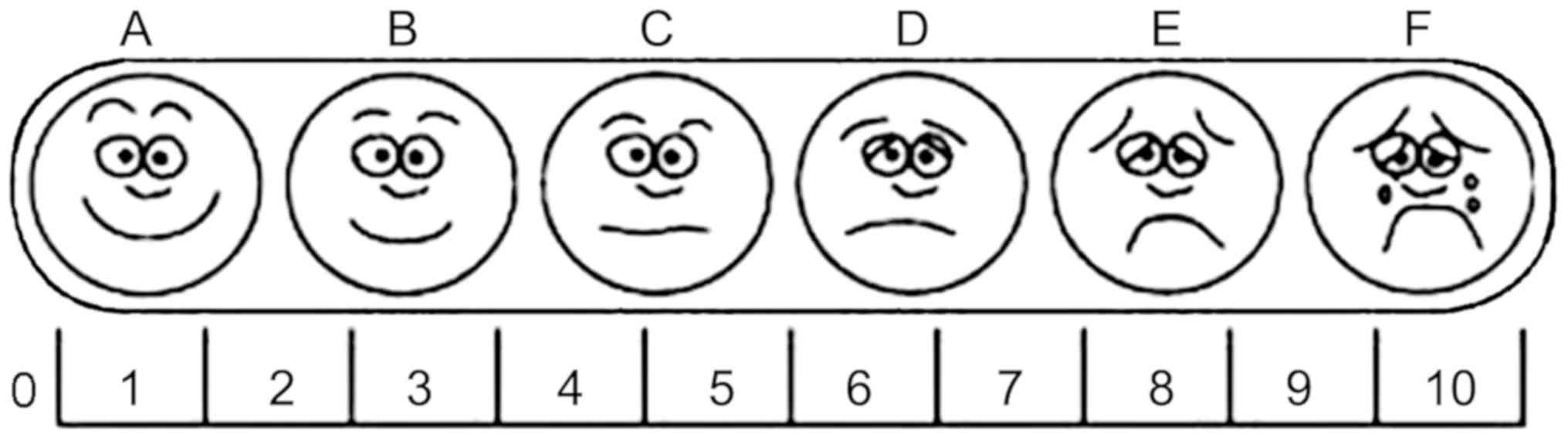
An 11-point visual analog scale (VAS; 0-10, no pain
to worst possible pain) (11) was
used to assess and represent the pain intensity at 6, 12, 18 and 24
h postsurgery (Fig. 2). The
patients were instructed to select a score that was specific to the
pain they were experiencing, where 0 indicates no pain, 1-3
indicates a moderate and tolerable pain, 4-6 indicates an evident
pain that affects sleep but is tolerable following a simple
intervention (distracting attention by reading, watching TVor
listening to music) and 7-10 indicates a progressively intense pain
that is intolerable and requires measures for relief.
Anesthetic dose
Both groups were compared regarding the doses of
ketamine and propofol.
Adverse reactions
Both groups were compared in terms of the incidence
of adverse reactions, such as restlessness, vomiting, bucking,
hypotension and bradycardia, during surgery and the anesthesia
recovery period.
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 (IBM Corp.). Numerical data are presented as the mean
± standard deviation, and were compared using independent samples
Student's t-test for data that were normally distributed and
Mann-Whitney U test for non-continuous variables. Nominal data are
presented as n (%), and were compared between groups using the
χ2 test or Fisher's exact test. Multipoint comparisons
within and among groups were performed using mixed ANOVA and the
F-test. The Tamhane test was used for post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of patient
characteristics
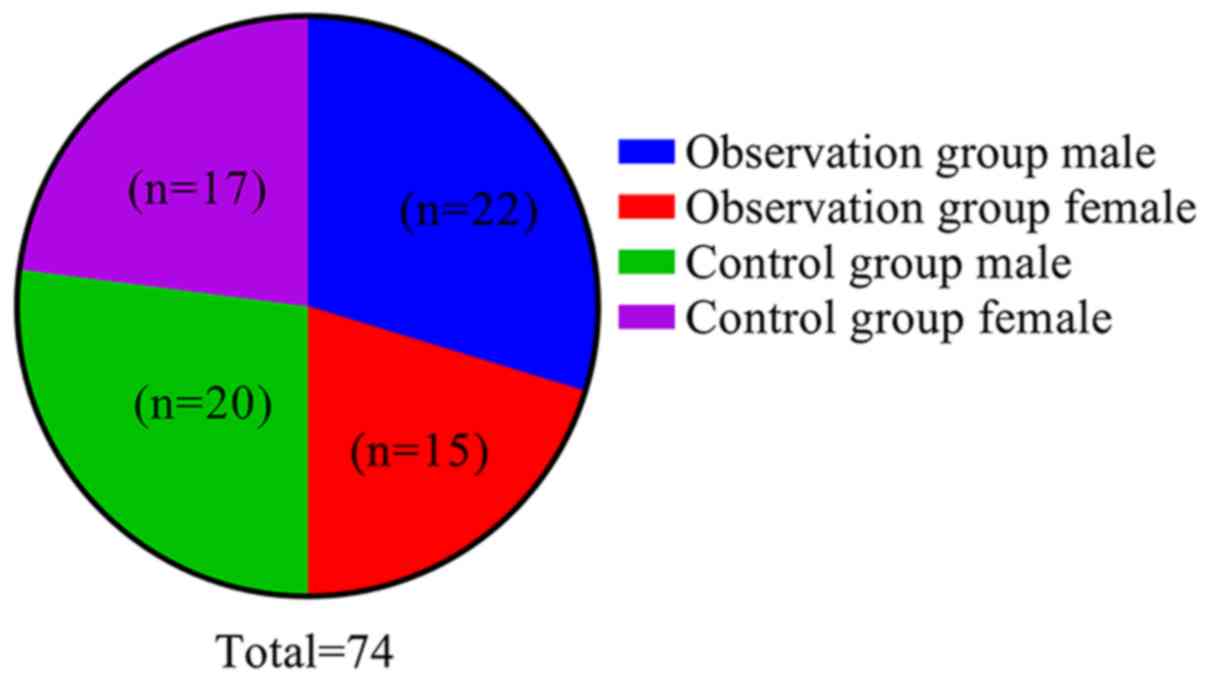
The observation and control groups exhibited no
significant differences in terms of sex (Fig. 3), mean age, height, body weight and
duration of surgery (P>0.05; Table
I).
 | Table IGeneral characteristics of the study
subjects. |
Table I
General characteristics of the study
subjects.
| Characteristics | Observation group
(n=37) | Control group
(n=37) | t/χ2
value | P-value |
|---|
| Sex, n (%) |
|
Male | 22 (59.46) | 20 (54.05) | 0.220 | 0.639 |
|
Female | 15 (40.54) | 17 (45.95) | | |
| Age, years | 7.26±3.64 | 7.84±3.91 | 0.294 | 0.162 |
| Height, cm | 112.64±10.27 | 114.75±11.43 | 0.835 | 0.406 |
| Body weight, kg | 29.86±2.49 | 30.44±2.61 | 0.978 | 0.331 |
| Duration of surgery,
min | 40.45±12.35 | 41.57±13.40 | 0.374 | 0.710 |
Comparison of vital signs
Both the observation and control groups showed no
significant difference in HR and MAP levels at time-points T1, T2,
T3 and T4 (P>0.05). There was no significant difference in HR
and MAP levels between the observation and control groups at
time-points T1, T2 and T3 (P>0.05), while the HR and MAP levels
in the observation group at T4 were lower than those in the control
group (P<0.05; Table II).
 | Table IIVital signs at different time-points
of anesthesia. |
Table II
Vital signs at different time-points
of anesthesia.
| Group | Time-point | HR (beats/min) | MAP (mmHg) | t-value (HR/MAP) | P-value (HR/MAP) |
|---|
| Observation group
(n=37) | T1 | 117.06±11.58 | 72.89±8.25 | 0.865/0.724 | 0.271/0.625 |
| | T2 | 112.34±12.85 | 71.03±6.34 | | |
| | T3 | 111.07±12.92 | 72.16±5.24 | | |
| | T4 | 105.54±9.83 | 69.31±6.25 | | |
| Control group
(n=37) | T1 | 113.57±12.16 | 73.46±5.19 | 0.859/0.234 | 0.152/0.321 |
| | T2 | 110.37±8.27 | 70.31±5.27 | | |
| | T3 | 109.33±10.16 | 73.29±6.15 | | |
| | T4 | 117.24±10.31 | 77.08±6.52 | | |
| t-value
1(intergroup T1) | | 0.594 | 0.421 | | |
| P-value
1(intergroup T1) | | 0.163 | 0.382 | | |
| t-value
2(intergroup T2) | | 0.958 | 0.758 | | |
| P-value
2(intergroup T2) | | 0.421 | 0.265 | | |
| t-value
3(intergroup T3) | | 0.286 | 0.362 | | |
| P-value
3(intergroup T3) | | 0.185 | 0.421 | | |
| t-value
4(intergroup T4) | | 5.829 | 6.382 | | |
| P-value
4(intergroup T4) | | <0.001 | <0.001 | | |
Comparison of sedative effects
No statistically significant difference was observed
in the proportion of patients with sedation at Ramsay levels 1-6
between the observation and the control group (P>0.05; Table III and Fig. 4).
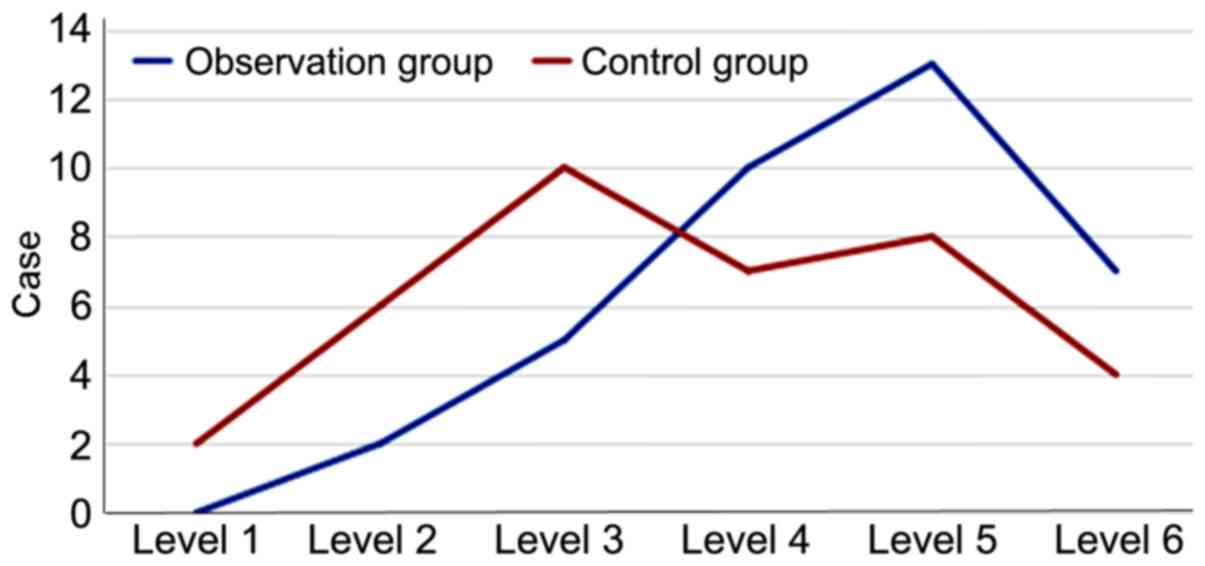 | Figure 4Levels of sedative effects in the
observation and control groups. In the observation group, patients
at levels 1, 2, 3, 4, 5 and 6 accounted for 0, 5.41, 13.51, 27.03,
35.14 and 18.92% of the cohort, respectively, whereas in the
control group they accounted for 5.41, 16.22, 27.03, 18.92, 21.62
and 10.81% of the cohort, respectively. The observation group
exhibited a lower proportion of patients at levels 1-3 and a higher
proportion of patients at levels 4-6 compared with the control
group, albeit with no significant difference (P>0.05). |
 | Table IIISedative effects of anesthesia. |
Table III
Sedative effects of anesthesia.
| | Ramsay levels, n
(%) | |
|---|
| Group | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 | χ2
value | P-value |
|---|
| Observation group
(n=37) | 0 (0.00) | 2 (5.41) | 5 (13.51) | 10 (27.03) | 13 (35.14) | 7 (18.92) | 7.638 | 0.156 |
| Control group
(n=37) | 2 (5.41) | 6 (16.22) | 10 (27.03) | 7 (18.92) | 8 (21.62) | 4 (10.81) | | |
Comparison of anesthetic effects
The use of procaine combined with ketamine and
propofol for epidural anesthesia resulted in a shorter time for the
onset of the anesthetic effect and for the disappearance of pain,
and a longer time for the recovery of pain sensation compared with
that in the control group, in which ketamine and propofol were
administered (all P<0.01; Table
IV).
 | Table IVTime effects of anesthesia. |
Table IV
Time effects of anesthesia.
| Group | n | Time for anesthesia
to take effect, min | Time for pain to
disappear, min | Time for pain sense
to recover, min |
|---|
| Observation
group | 37 | 1.98±0.45 | 4.22±0.69 | 38.25±3.67 |
| Control group | 37 | 4.32±0.73 | 6.95±0.82 | 23.42±2.73 |
| t-value | | 16.600 | 15.495 | 19.722 |
| P-value | | <0.001 | <0.001 | <0.001 |
Comparison of pain intensity
After epidural anesthesia using procaine combined
with ketamine and propofol, the observation group exhibited a lower
VAS score compared with the control group at 6, 12, 18 and 24 h
postsurgery (all P<0.01; Table
V).
 | Table VVisual analog scale scores at
different time-points. |
Table V
Visual analog scale scores at
different time-points.
| | Time after
surgery |
|---|
| Group | n | 6 h | 12 h | 18 h | 24 h |
|---|
| Observation
group | 37 | 2 (1-4) | 3 (1-6) | 4 (2-6) | 4 (2-7) |
| Control group | 37 | 3 (1-6) | 4 (2-7) | 5 (2-8) | 5 (2-9) |
| t-value | | 2.986 | 3.626 | 3.754 | 3.781 |
| P-value | | 0.025 | 0.012 | 0.009 | 0.011 |
Comparison of the anesthetic dose
The observation group received lower doses of
ketamine and propofol compared with the control group based on the
ketamine and propofol amounts that were required for anesthesia
(both P<0.01; Table VI).
 | Table VIDosages of ketamine and propofol used
for anesthesia. |
Table VI
Dosages of ketamine and propofol used
for anesthesia.
| Group | n | Ketamine, mg | Propofol, mg |
|---|
| Observation
group | 37 | 71.06±6.83 | 70.51±7.27 |
| Control group | 37 | 81.49±7.41 | 97.58±8.33 |
| t-value | | 6.296 | 14.893 |
| P-value | | <0.001 | <0.001 |
Comparison of the incidence of adverse
reactions
An incidence of adverse reactions of 8.11% was noted
in the observation group during surgery and the anesthesia recovery
period, which was lower compared with that in the control group
(29.73%; P<0.05; Table
VII).
 | Table VIIIncidence of adverse reactions during
surgery and anesthesia recovery period. |
Table VII
Incidence of adverse reactions during
surgery and anesthesia recovery period.
| | Adverse reactions,
n (%) |
|---|
| Group | Restlessness | Vomiting | Bucking | Hypotension | Bradycardia | Total
incidence |
|---|
| Observation group
(n=37) | 1 (2.70) | 1 (2.70) | 1 (2.70) | 0 (0.00) | 0 (0.00) | 3 (8.11) |
| Control group
(n=37) | 2 (5.41) | 4 (10.81) | 3 (8.11) | 1 (2.70) | 1 (2.70) | 11 (29.73) |
|
χ2-value | | | | | | 5.628 |
| P-value | | | | | | 0.018 |
Discussion
Children have been indicated to differ from adults
in terms of physiology and psychology, and to be more sensitive to
stress and pain, which results in a lower level of cooperation in
surgeries that produce pain and a high possibility of restlessness
(12). The differences between
children and adults also include a straighter spine, a narrower
epidural space and thinner neurosheaths, which have collectively
been reported to contribute to the rapid release and enhanced
effects of anesthetics observed in children (13). Although epidural anesthesia is
increasingly considered to be suitable for delivering anesthesia to
children during surgery, the varied anesthetic options available
offer a challenge to the safety of anesthesia, which should be
closely monitored.
Ketamine, which is a derivative of phencyclidine and
a dissociative anesthetic, is the only intravenous anesthetic that
has been reported to exert analgesic effects in safe doses
(14). In addition to causing
amnesia, ketamine has been indicated to efficiently and safely
alleviate pain in patients and shorten the time required to regain
consciousness; therefore, it is widely applied in pediatric
intravenous anesthesia (15).
However, ketamine has only been indicated to function at the body
surface rather than in the internal organs, and to only be adequate
for short-term surgeries, with no guarantee of success being
provided for long-term surgeries (16). Furthermore, Zeballos et al
(17) demonstrated that ketamine
alone was unable to adequately relax muscles and ease pain, and
that a higher dose was required for anesthesia; however, this may
result in increased oral secretion, glossocoma, prominent agitation
during the recovery period and even respiratory depression in
serious cases. Consequently, ketamine is clinically combined with
propofol for anesthesia to reduce the side effects and the dose of
ketamine required (18). Hayes
et al (19) reported that
children who were administered ketamine and propofol for anesthesia
had a lower likelihood of being restless during the recovery period
compared with those who received ketamine alone.
The present study focused on the addition of
procaine to the combination of ketamine and propofol for epidural
anesthesia. Compared with the control group, who were administered
ketamine and propofol, the HR and MAP levels following epidural
administration (T4) were lower in the observation group
(P<0.01), while there was no significant differences in HR and
MAP levels between the two groups before anesthesia (T1), following
anesthesia (T2) and before epidural administration (T3)
(P>0.05), indicating that throughout the entire anesthesia
process, HR and MAP in the observation group did not fluctuate
significantly, while the HR and MAP of the control group increased
following epidural administration. Moreover, the observation group
exhibited an incidence of adverse reactions of 8.11%, which was
lower compared with that of the control group (29.73%) (P<0.01),
suggesting that the combination of procaine with ketamine and
propofol can effectively control the fluctuations in vital signs
and adverse reactions during surgery, and the recovery period,
thereby guaranteeing the safety of the anesthesia. This observation
may be attributed to the ability of procaine to suppress
cardiovascular excitation, extend the recovery period that is
associated with ketamine and alleviate the inhibition of autonomic
nerves by propofol, thereby maintaining a stable circulation and
anesthetic effect (20). In the
current study, the observation group required a shorter time for
the onset of the anesthetic effect and the disappearance of pain,
and a longer time for the recovery of pain sensation (P<0.01).
The study by Giudici et al (21) indicated that following the combined
application of procaine in epidural anesthesia, the time for the
onset of the anesthetic effect and the disappearance of pain after
anesthesia were shortened, and the recovery of pain sensation
postsurgery was prolonged compared with the patients who were not
administrated with procaine, which were consistent with the results
of the present study. However, there are certain differences
between the specific data of the two studies, which may be
attributed to variation in the included subjects, the specific drug
dose and the pain evaluation criteria. In contrast to the study by
Giudici et al (21), the
present study defined the inclusion criteria more accurately, and
the patients were subjected to the same type of surgery, which may
considerably reduce the influence of the type of surgery on the
results.
Procaine is a local benzoate anesthetic (22). Yilbas et al (23) reported that chloroprocaine inhibited
the sensory and motor nerves for a shorter period compared with
lidocaine, ensuring that children regained consciousness and were
able to engage in activities at the earliest. By contrast, Jalili
and Saeedi (24) compared procaine
with lidocaine and demonstrated that as local anesthesia, procaine
reduced the incidence of transient neurological syndrome. In the
present study, no statistical difference was observed in the
proportion of patients with sedation at Ramsay levels 1-6 between
the observation and control groups (P>0.05), indicating that the
two methods of anesthesia resulted in a similar sedative effect.
This finding is in contrast with the study by Ying et al
(25), in which the combined use of
procaine for local anesthesia resulted in better sedative effects.
The present study also reported lower VAS scores in the observation
group at 6, 12, 18 and 24 h (P<0.01), which is consistent with
the findings of Wu et al (26), indicating that procaine in
combination with other local anesthetics can extend the analgesia
time and reduce the pain intensity post surgery.
Finally, in the present study, the observation group
received a lower dose of ketamine and propofol compared with the
control group (P<0.01), indicating that the combined use of
procaine for pediatric epidural anesthesia can exert better
sedative and pain relief effects and reduce the doses of ketamine
and propofol that are required. This may be attributed to the
characteristics of procaine, including the limited number of toxic
reactions, the efficient hydrolysis by pseudocholinesterase in
serum, and the reduced time for the anesthetic effect, the
achievement of analgesia and the recovery of movement (27,28).
In conclusion, the current study indicated that
procaine combined with ketamine and propofol in pediatric epidural
anesthesia may be more commonly used considering its advantages in
accelerating the anesthesia process, improving the anesthetic
effects and guaranteeing the safety of the anesthesia.
However, a small number of patients were included in
the present study, who were characterized by a narrow age range,
thereby potentially hampering the generalizability of the results.
Future long-term comprehensive analyses should prioritize the
inclusion of a larger number of patients to additionally explore
the advantages of the combined use of procaine with ketamine and
propofol in pediatric epidural anesthesia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH, YL and ZG conceived and designed the research,
and interpreted the experimental results. HH, YL, ZG and XW
performed the experiments, analyzed the data, prepared the figures
and drafted the manuscript. XW edited and revised the manuscript.
All authors read and approved the final version of manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hong Hui Hospital, Xi'an Jiaotong University College
of Medicine (approval no. ChiCTR1800013484). All patients and their
families agreed to participate in the experiment, and parents
provided written informed consent for all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moore AD and Anghelescu DL: Erratum to:
Emergence delirium in pediatric anesthesia. Paediatr Drugs.
19(267)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaniyil S, Smithamol PB, Joseph E,
Krishnadas A and Ramadas KT: A survey of current practice of
supraglottic airway devices in pediatric anesthesia from India.
Anesth Essays Res. 11:578–582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng D, Liu L and Hu Z: Prevention of
anesthesia-induced injection pain of propofol in pediatric
anesthesia. Pak J Med Sci. 33:752–756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Frawley G, Bell G, Disma N, Withington DE,
de Graaff JC, Morton NS, McCann ME, Arnup SJ, Bagshaw O, Wolfler A,
et al: Predictors of failure of awake regional anesthesia for
neonatal hernia repair: Data from the general anesthesia compared
to spinal anesthesia study-comparing apnea and neurodevelopmental
outcomes. Anesthesiology. 123:55–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Leemans B, Stout TA, Soom AV and Gadella
BM: pH-dependent effects of procaine on equine gamete activation.
Biol Reprod. 101:1056–1074. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Förster JG and Rosenberg PH: Revival of
old local anesthetics for spinal anesthesia in ambulatory surgery.
Curr Opin Anaesthesiol. 24:633–637. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wildsmith JA: Reactions to procaine after
caudal injection. Reg Anesth Pain Med. 43(446)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mak PH, Campbell RC and Irwin MG: American
Society of Anesthesiologists. The ASA physical status
classification: Inter-observer consistency. American society of
anesthesiologists. Anaesth Intensive Care. 30:633–640.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Korzh EA, Klymenko NA and Smolin SK:
Bioregeneration of the activated carbon layer spent in the dynamics
of procaine biofiltration. J Water Chem Technol. 39:103–107.
2017.
|
|
10
|
Mondello E, Siliotti R, Noto G, Cuzzocrea
E, Scollo G, Trimarchi G and Venuti FS: Bispectral Index in ICU:
Correlation with ramsay score on assessment of sedation level. J
Clin Monit Comput. 17:271–277. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Knop C, Oeser M, Bastian L, Lange U,
Zdichavsky M and Blauth M: Development and validation of the visual
analogue scale (VAS) spine score. Unfallchirurg. 104:488–497.
2001.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
12
|
Guo J, Jin X, Wang H, Yu J, Zhou X, Cheng
Y, Tao Q, Liu L and Zhang J: Emergence and recovery characteristics
of five common anesthetics in pediatric anesthesia: A network
meta-analysis. Mol Neurobiol. 54:4353–4364. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Walker BJ, Long JB, Sathyamoorthy M,
Birstler J, Wolf C, Bosenberg AT, Flack SH, Krane EJ, Sethna NF,
Suresh S, et al: Complications in pediatric regional anesthesia: An
analysis of more than 100,000 blocks from the pediatric regional
anesthesia network. Anesthesiology. 129:721–732. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lefevre A and Schnepper G: Development of
Harlequin Syndrome following placement of thoracic epidural
anesthesia in a pediatric patient undergoing Nuss procedure. Clin
Case Rep. 5:1523–1525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hambrecht-Wiedbusch VS, Li D and Mashour
GA: Paradoxical emergence: Administration of subanesthetic ketamine
during isoflurane anesthesia induces burst suppression but
accelerates recovery. Anesthesiology. 126(482)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tosh P, Rajan S, Puthenveettil N and Kumar
L: Oral clonidine premedication attenuates hemodynamic responses of
ketamine during total intravenous anesthesia. Anesth Essays Res.
11:617–620. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zeballos JL, Lirk P and Rathmell JP:
Low-dose ketamine for acute pain management: A timely nudge toward
multimodal analgesia. Reg Anesth Pain Med. 43:453–455.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Baradari AG, Alipour A, Habibi MR,
Rashidaei S and Emami Zeydi A: A randomized clinical trial
comparing hemodynamic responses to ketamine-propofol combination
(ketofol) versus etomidate during anesthesia induction in patients
with left ventricular dysfunction undergoing coronary artery bypass
graft surgery. Arch Med Sci. 13:1102–1110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hayes J, Matava C, Pehora C, El-Beheiry H,
Jarvis S and Finkelstein Y: Determination of the median effective
dose of propofol in combination with different doses of ketamine
during gastro-duodenoscopy in children: A randomised controlled
trial. Br J Anaesth. 121:453–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Teunkens A, Vermeulen K, Van Gerven E,
Fieuws S, Van de Velde M and Rex S: Comparison of 2-chloroprocaine,
bupivacaine, and lidocaine for spinal anesthesia in patients
undergoing knee arthroscopy in an outpatient setting: A
double-blind randomized controlled trial. Reg Anesth Pain Med.
41:576–583. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Giudici V, Baeza S, Douet JY and Regnier
A: Corneal anesthesia following application of 0.4% oxybuprocaine
hydrochloride ophthalmic solution to normal feline eyes. Vet
Ophthalmol. 18:141–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bodart JF, Flament S, Browaeys E, Bertout
M, Rousseau A, Gannon J and Vilain JP: MPF and procaine-induced
maturation of xenopus oocyte. Biol Cell. 88(71)1996.
|
|
23
|
Yilbas AA, Akca B, Buyukakkus B, Bahador
Zirh E, Zeybek D, Uzumcugil F and Saricaoglu F: Procaine and saline
have similar effects on articular cartilage and synovium in rat
knee. BMC Anesthesiol. 18(51)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jalili S and Saeedi M: Study of procaine
and tetracaine in the lipid bilayer using molecular dynamics
simulation. Eur Biophys J. 46:265–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ying B, Huang H, Li H, Song M, Wu S and
Ying H: Procaine inhibits proliferation and migration and promotes
cell apoptosis in osteosarcoma cells by upregulation of
MicroRNA-133b. Oncol Res. 25:1463–1470. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu F, Wang J, Sun J, Shen L, Liu M and
Zhao E: Procaine stimulates aquaporin-5 expression in human
salivary gland ductal cells via the suppression of DNA
methyltransferase-1. Mol Med Rep. 17:7996–8002. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hao K, Suryoprabowo S, Song S, Liu L,
Zheng Q and Kuang H: Development of an immunochromatographic test
strip for the detection of procaine in milk. Food Agric Immunol.
29:1150–1161. 2018.
|
|
28
|
Lin H, Wang Z, Shen J, Xu J and Li H:
Intravenous anesthetic ketamine attenuates complete Freund's
adjuvant-induced arthritis in rats via modulation of MAPKs/NF-κB.
Inflamm Res. 68:147–155. 2019.PubMed/NCBI View Article : Google Scholar
|