Introduction
Bullous keratopathy (BK) is amongst the most common
causes of corneal decompensation, frequently requiring corneal
transplantation (1). Endothelial
keratoplasty (EK) has become an effective alternative to
penetrating keratoplasty (PKP) for treating BK caused by a
dysfunctional endothelium (2).
Compared to PKP, the application of EK provides more rapid recovery
of vision, minimized induction of astigmatism, and, more
importantly, better maintenance of the integrity of the globe
(3,4).
At present, the major techniques of EK include
Descemet's stripping endothelial keratoplasty (DSEK)/Descemet's
stripping with automated endothelial keratoplasty (DSAEK) and
Descemet's membrane endothelial keratoplasty (DMEK) (5). Due to the inapplicability of DMEK in
patients with serious edematous stroma or aphakic eyes, which have
previously undergone vitrectomy, DSAEK remains in use as the
primary procedure for EK (2). In
DSAEK, creating thinner grafts is thought to improve visual
outcomes and anatomic success; however, the creation of thinner
donor tissue is technically challenging (3).
A major problem with the use of DSEK is the
intricate manual preparation required for the lamellar graft,
frequently resulting in irreparable damage to the material intended
for transplantation (6,7). The implantation method used at our
department has been simplified. For the simplified DSEK, the graft
may be successfully implanted through a transparent limbal incision
without the use of special instruments and the surgical incision is
small with rapid recovery. The present study aimed to evaluate the
difference in the clinical efficacy between the simplified DSEK
method and PKP for BK.
Materials and methods
Patients
A total of 65 patients (65 eyes) with BK between
December 2002 and June 2018 from the General Hospital of Northern
Theater Command (Shenyang, China) were retrospectively included in
the present study. Patients who received modified simplified DSEK
were assigned to the modified simplified DSEK group (n=38) and
patients who received PKP were designated as the PKP group (n=27).
The present study was approved by the Ethics Committee of the
General Hospital of Shenyang Military Region and all patients
provided written informed consent.
The inclusion criteria were as follows: i) Patients
with BK; ii) underwent their first corneal transplantation; iii)
≥18 years and ≤90 years of age and iv) a follow-up period of ≥1
year. The following exclusion criteria were the presence of fundus
lesions that affected postoperative vision, including macular
degeneration, fundus hemorrhage or optic nerve atrophy.
The donor corneas were all from healthy and fresh
eyes of deceased domestic donors. Preoperative corneal examination
of the donor was completed as follows: Slit-lamp microscope
examination was performed to confirm the transparency and
smoothness; corneal endothelium was used to observe the morphology
and clarity of endothelial cells, and the density of endothelial
cells was required to be >2,000/mm2.
Surgical methods
The combined surgical methods of the simplified DSEK
and PKP groups are presented in Tables I and II, respectively. All
surgeries were performed by the same corneal transplant surgeon.
Pilocarpine eye drops (5 min/administration) were given 6 times to
patients undergoing corneal transplantation. For patients
undergoing combined surgery, pupil dilatation was performed
according to the surgical conditions, and then, 0.1 ml of carbachol
injection was given to shrink the pupil for subsequent
keratoplasty.
Prior to the operation, the patients were given
routine treatments, including cleaning the eyelid margin, eyelash
trimming, conjunctival sac irrigation and lacrimal tract
irrigation. All patients were given topical anesthesia with
promevacaine hydrochloride eye drops (Erkeine); 2% lidocaine
injection was used for sufficient retrobulbar and peribulal block
anesthesia. Oorbicularis oculi muscle anesthesia was also performed
for the PKP group.
Simplified DSEK
The graft bed was prepared. First, a mark on the
corneal epithelium was made with a suitable trephine. A
paracentesis knife was used to make a 5-mm-wide transparent limbal
main incision at the clock position of 12:00 to enter the anterior
chamber and the viscoelastic agent was injected. Two transparent
limbal auxiliary incisions at the clock positions of 3:00 and 9:00
were made. After removing the hook and scraping the elastic layer
and endothelial cell layer along the marking ring, the viscoelastic
agent in the anterior chamber was then rinsed and sterile air was
injected to press the graft bed to form the anterior chamber.
The corneal endothelial graft was then prepared as
follows: A vacuum negative pressure trephine with different
diameters was rotated perpendicular to the donor cornea surface to
make the anterior deep lamellar corneal slice, with a thickness of
400-450 µm, and the anterior deep lamellar corneal slice was
removed. The donor posterior lamellar corneal slice was cut in a
1-2 mm ring along the outer edge of the sclera, the inner skin was
placed on the special corneal incision pillow, and then the
endothelial graft with partial posterior stroma was drilled with a
trephine used for the graft bed.
The graft was implanted using the slide method
(8) as follows: A plastic slide
with a width of ~4 mm was placed into the anterior chamber through
the upper main incision. After dripping an appropriate quantity of
viscoelastic agent on the surface of the slide outside the
incision, the graft endothelium was laid on the slide remaining
outside the incision and the graft was then moved into the anterior
chamber with a self-made 1-ml syringe crochet needle. A small
quantity of lactated Ringer's solution was injected into the
anterior chamber to make the graft expand appropriately. The main
incision was intermittently sutured with 10/0 suture for 3
stitches. Ringer's lactate solution was re-injected from the side
incision into the anterior chamber to fully expand the graft. The
position of the graft was adjusted and sterile air was injected
into the anterior chamber to make the graft closely attach to the
graft bed. The surface gas injection was successful when a ‘double
ring sign’ was observed. Finally, iris restorer was placed firmly
on the corneal surface to drain the excess liquid and gas between
the graft and the graft bed, which was conducive to the close
attachment of the graft and the graft bed. After surgery, all
patients were treated with tobramycin and dexamethasone eye
ointment (Dianshu).
The patient was instructed to lie in the supine
position within postoperative 24 h and the gas was placed directly
under the implant, which was conducive for better adhesion of the
corneal endothelium and increased the success rate of implant
transplantation.
Phacoemulsification + intraocular lens
(IOL) implantation + simplified DSEK
First, a transparent limbal tunnel incision was made
at the clock position of 12:00 with a stab knife, followed by
routine phacoemulsification + IOL implantation. The main incision
was expanded to 5 mm and simplified DSEK was then performed.
Separation of the chamber angle from
the anterior synechia + coreoplasty + phacoemulsification + IOL
implantation + simplified DSEK
A stab knife was used to make a 5-mm-wide
transparent limbal main incision at the clock position of 12:00 and
the viscoelastic agent was injected. A transparent auxiliary
incision was made near the anterior synechia. Capsular scissors
were used to cut the adhesive iris and the iris restorer was used
to separate the adhesive chamber angle. The iris was cut and
sutured to make the pupil circular. Routine phacoemulsification +
IOL implantation and simplified DSEK were then performed.
IOL reduction + coreoplasty +
simplified DSEK
The main and auxiliary corneal limbal incisions were
made at the 10:00 and 2:00 clock positions, respectively, and then,
the iris restorer was used to enter the anterior chamber through
the main incision to restore the intraocular lens to the normal
position and restore the iris shape. Simplified DSEK was then
performed.
Phacoemulsification + IOL implantation
+ iris root resection + simplified DSEK
The transparent limbal main incision was made at the
clock position of 12:00 to enter the anterior chamber and the
viscoelastic agent was injected. The iris restorer was used to
separate the adhesive iris and the bulbar conjunctiva was cut open
to make the scleral flap. The 8/0 suture was inserted into the
anterior chamber from the clock position of 12:00 and was
perforated from the detached iris root under the scleral flap. The
detached iris root was sutured using mattress suture, the scleral
flap was reset and the scleral flap and conjunctiva were sutured
with 8/0 absorbable suture.
PKP
The operational procedure was performed as described
previously (9). The graft bed was
prepared as follows: A suitable trephine was selected perpendicular
to the surface of the cornea and rotated clockwise into the cornea
to drill the graft bed.
Corneal scissors were used to cut 2 mm along the
limbus sclera to obtain the donor cornea and the inner skin was
placed on the special corneal incision pillow. A trephine with a
diameter of >0.25 mm was used to drill the donor cornea to
obtain the graft. The 10/0 suture was first performed
intermittently at the 3, 6, 9 and 12 o'clock positions to fix the
graft and suturing was then performed for a total of 12-16 stitches
to bury the knot. A total of 50 µl of balanced saline solution was
injected into the anterior chamber. All patients were treated with
tobramycin dexamethasone eye ointment and monocular compression
bandaging, and the eye condition and the intraocular pressure were
closely monitored.
If the cataracts were combined, extracapsular
cataract extraction + IOL or phacoemulsification + IOL implantation
was performed, and the graft and graft bed were then sutured
intermittently.
Postoperative management
For the simplified DSEK group, all patients were
placed in a supine position for 24 h after surgery. Patients were
given systemic ocular hypotensive drugs (20% mannitol, 250 ml
b.i.d.) the day after surgery. Eyes were opened on the day after
surgery and anti-infection and anti-rejection treatment were
administered. During the postoperative follow-up, the medication
regimen was adjusted according to the recovery of the operated eye.
The sutures were removed at 3-6 months after the operation.
For the PKP group, a monocular compression bandage
was applied for 3 days following surgery. If the anterior chamber
was well formed, the eyes were opened and anti-infection and
anti-rejection treatment were given. During the postoperative
follow-up, the medication regimen was adjusted according to the
recovery of the operative eye. The changes in the intraocular
pressure of the surgical eye were observed. The eye sutures were
removed 6-12 months after surgery.
Postoperative medication
All patients were given levofloxacin eye drops
(t.i.d.) for 1 year, sodium hyaluronate eye drops (b.i.d.) for 1
year, tobramycin dexamethasone eye ointment (q.d.) for 6 months,
tacrolimus (b.i.d.) for 1 year and prednisolone acetate eye drops
(6 times a day) for 6 months. The combined anti-rejection therapy
of Belit was administered after epithelialization was complete.
Follow-up
The patients were all followed up on postoperative
weeks 1 and 2, and after 1, 3, 6, 12 and 24 months. At each
follow-up point, slit-lamp microscopy was used to observe the
attachment of eye grafts to graft beds. Intraocular pressure (IOP),
average best-corrected visual acuity (BCVA), corneal opacity,
postoperative complications and corneal graft survival rates were
determined. When the BCVA was measured in Snellen, it was converted
to a logarithm of the minimum angle of resolution (logMAR) to
facilitate statistical analysis. Counting fingers, hand movements
and perception of light were assigned the arbitrary values of 1.60,
1.90 and 2.20 logMAR, respectively. The same assumptions were made
previously (10).
Secondary glaucoma was defined as follows: IOP or
estimated IOP ≥24 mmHg and requires intervention with IOP-lowering
medication, postoperative IOP is 10 mmHg higher than the
preoperative IOP, or the IOP cannot be controlled by drugs and
surgical treatment is required (11-14).
Primary transplant failure refers to transparent persistent
irreversible corneal edema after surgery (15).
Postoperative implant displacement and
detachment
Displacement refers to the partial separation of the
graft from the graft bed. Detachment refers to the complete
separation of the graft from the graft bed, or even dislocation
into the anterior chamber (16).
Statistical analysis
Statistical analysis was performed using SPSS
version 24.0 (IBM Corp.). Values are expressed as the mean ±
standard deviation or n (%). Age and preoperative BCVA were
compared using Student's t-test. Preoperative diagnosis, lens
state, history of glaucoma, gender and incidence of postoperative
complications were compared using χ2tests. Postoperative
visual acuity was compared using the Mann-Whitney U-test.
Kaplan-Meier curves were generated to analyze the graft survival
rate of the two groups. Log-rank test was used for comparison
between the two Kaplan-Meier curves. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics
A total of 65 patients (65 eyes) with bullae
keratopathy were included in this study, including 36 male eyes and
29 female eyes. The mean age was 67.3±10.4 (45-84) years. There
were no significant differences in the preoperative basic
characteristics including age, sex, preoperative diagnosis, history
of glaucoma, lens state and BCVA between the two groups (all
P>0.05; Table III).
 | Table IIIBaseline information of the
patients. |
Table III
Baseline information of the
patients.
| Item | Simplified DSEK
(n=38) | PKP (n=27) | χ²/t | P-value |
|---|
| Age (years) | 68.03±10.82 | 66.26±9.80 | 0.674 | 0.503 |
| Male, n (%) | 22 (57.89) | 14 (51.85) | 0.233 | 0.629 |
| Preoperative
diagnosis, n (%) | | | 3.944 | 0.414 |
|
PBK | 29 (76.32) | 23 (85.19) | | |
|
Fuchs | 3 (7.89) | 3 (11.11) | | |
|
Viral
endocorneal dermatitis | 4 (10.53) | 0 | | |
|
ICE
syndrome | 1 (2.63) | 1 (3.70) | | |
|
Trauma | 1 (2.63) | 0 | | |
| History of
glaucoma, n (%) | 15 (39.47) | 6 (22.22) | 2.148 | 0.143 |
| Lens state, n
(%) | | | 0.375 | 0.829 |
|
Artificial
lens | 27 (71.05) | 21 (77.78) | | |
|
Lens | 9 (23.69) | 5 (18.52) | | |
|
Absence of
lens | 2 (5.26) | 1 (3.70) | | |
| BCVA (logMAR) | 1.81±0.24 | 1.83±0.23 | -0.351 | 0.727 |
BCVA
As presented in Table
IV, the average BCVA logMAR at 1, 3, 6 and 12 months in the
simplified DSEK group was significantly lower than that in the PKP
group (P<0.05). The BCVA interval distribution at the last
follow-up is presented in Table V
and the results indicated that the visual acuity of the simplified
DSEK group was significantly better than that in the PKP group.
 | Table IVPostoperative mean best-corrected
visual acuity (logarithm of the minimum angle of resolution)
compared between the two groups. |
Table IV
Postoperative mean best-corrected
visual acuity (logarithm of the minimum angle of resolution)
compared between the two groups.
| Time-point | Simplified
DSEK | PKP | Z-value | P-value |
|---|
| Following surgery
(months) |
| 1 | 0.95±0.41
(n=38) | 1.10±0.34
(n=27) | -2.421 | 0.015 |
| 3 | 0.81±0.54
(n=38) | 1.04±0.38
(n=27) | -3.499 | <0.001 |
| 6 | 0.67±0.63
(n=37) | 0.99±0.52
(n=26) | -3.196 | 0.001 |
| 12 | 0.62±0.72
(n=35) | 1.02±0.65
(n=25) | -3.403 | 0.001 |
 | Table VBCVA interval distribution of the
simplified DSEK group and the PKP group at 1-year follow-up. |
Table V
BCVA interval distribution of the
simplified DSEK group and the PKP group at 1-year follow-up.
| BCVA (logMAR) | Simplified DSEK
(%) | PKP (%) |
|---|
| ≥1.0 | 17.14 | 28.00 |
| 0.7-1.0 | 8.75 | 12.00 |
| >0.4, ≤0.7 | 11.43 | 56.00 |
| ≤0.4 | 62.86 | 4.00 |
Graft survival rate at the last
follow-up
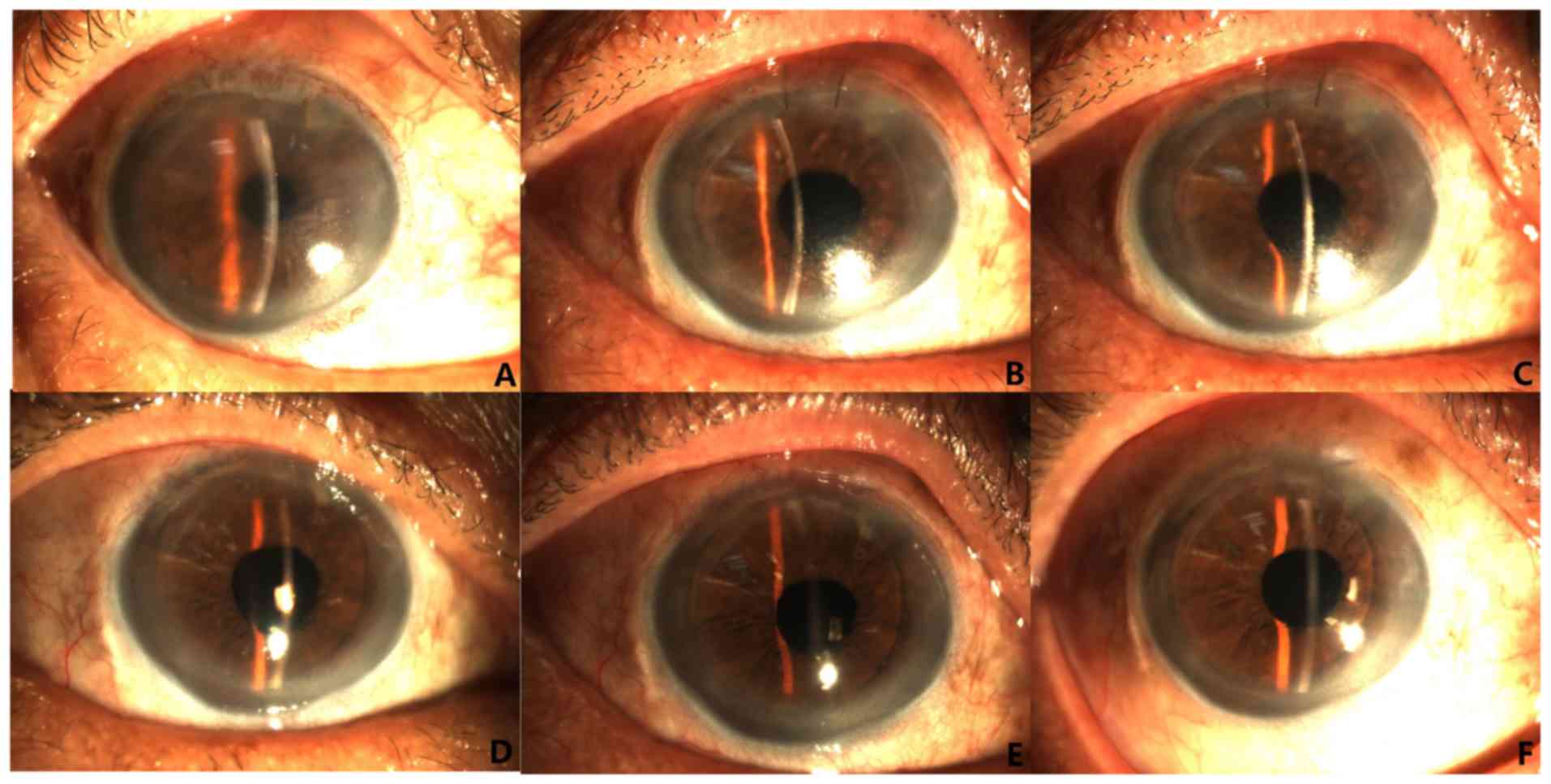
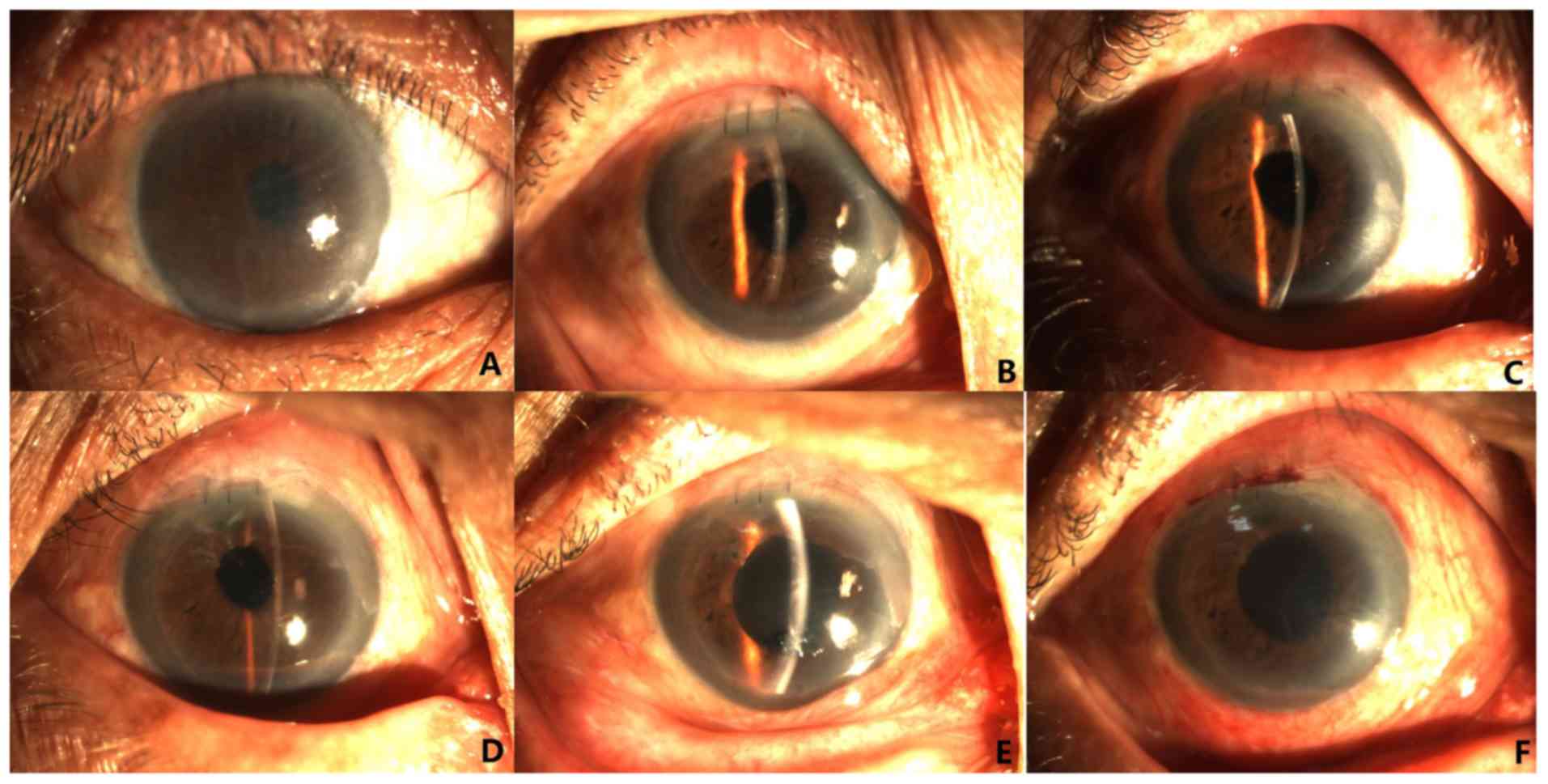
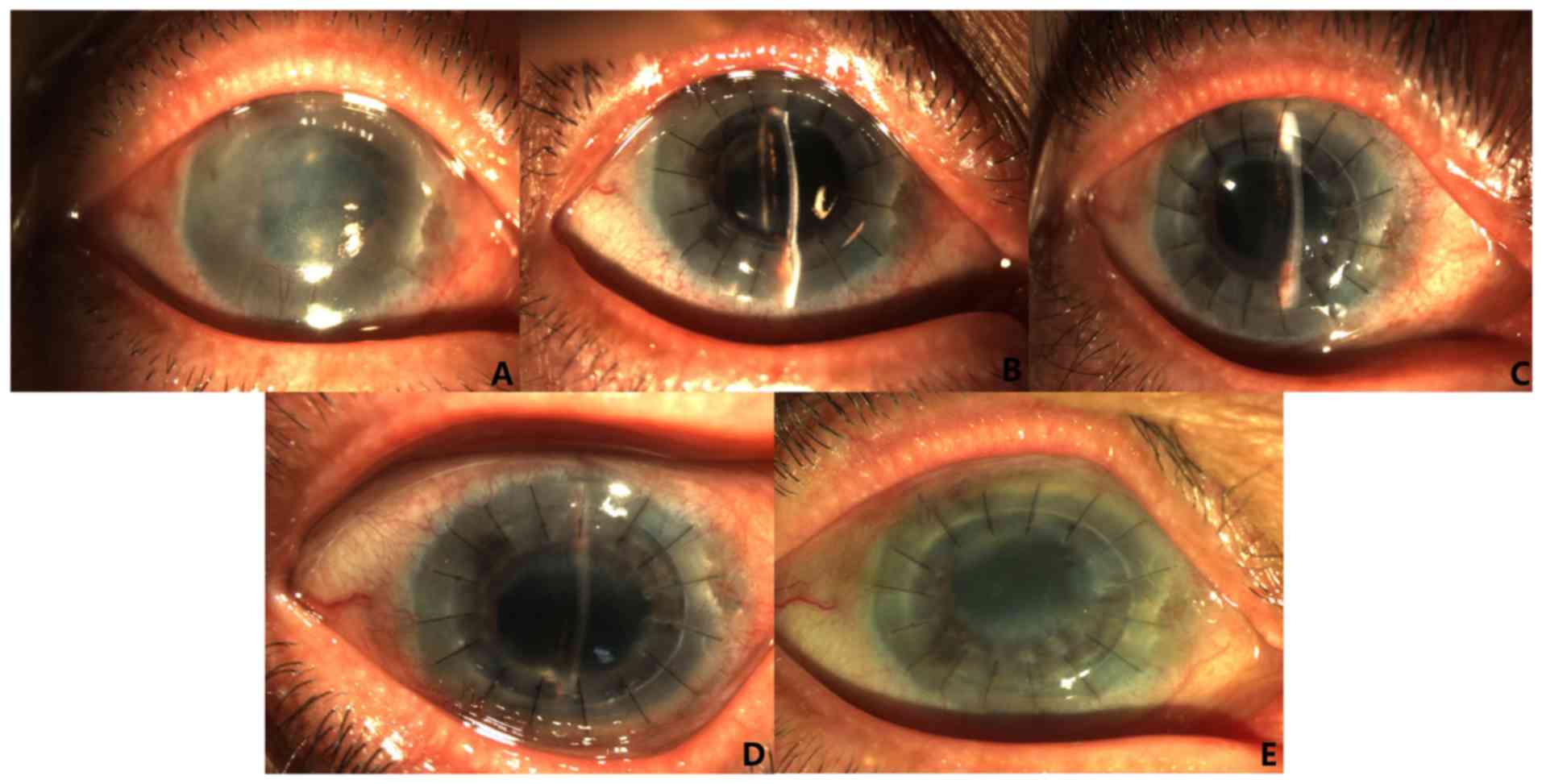
Corneal graft changes within 1 year after simplified
DSEK and PKP in representative cases are presented in Figs. 1 and 2, respectively. At the last follow-up at 1
year after surgery, the graft survival rate in the simplified DSEK
group was significantly higher compared with that in the PKP group
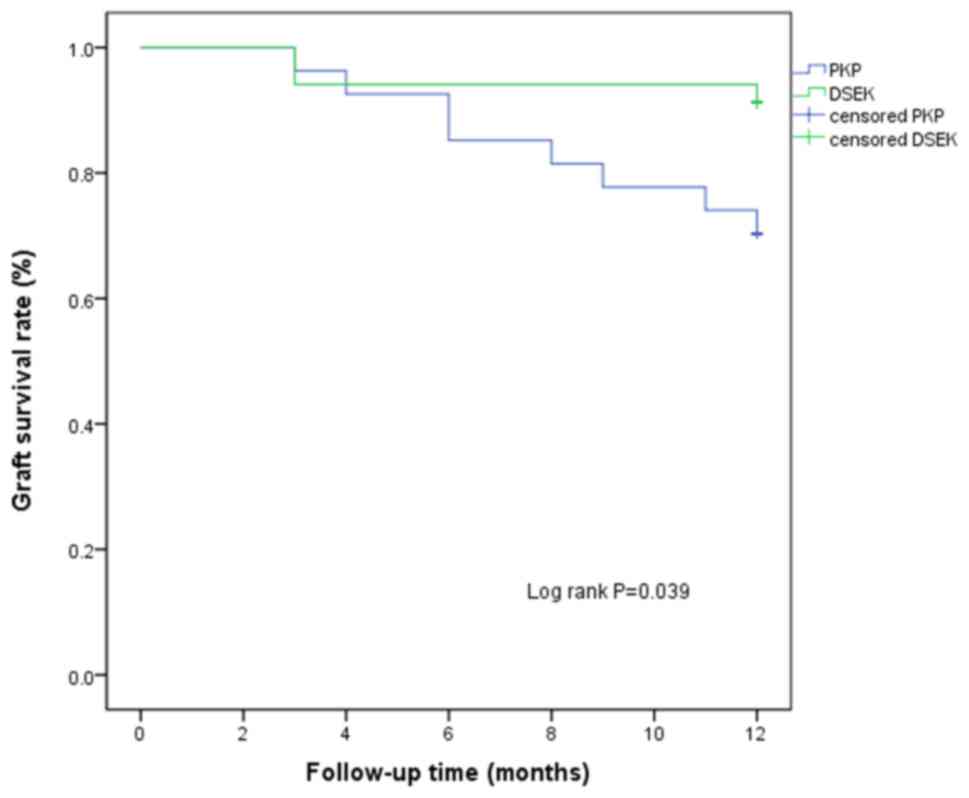
(91.17 vs. 70.37%, P=0.039). Kaplan-Meier curves for graft survival
in the two groups are provided in Fig.
3.
Postoperative complications and
management
As presented in Table
VI, 13 eyes (34.21%) in the simplified DSEK group and 11 eyes
(40.74%) in the PKP group developed secondary glaucoma; this
difference was not statistically significant (P=0.591). A total of
8 eyes (61.54%) in the simplified DSEK group were treated with
antihypertensive drugs and the IOP dropped to within the normal
range. A total of 3 eyes (23.08%) developed drug dependence. In
addition, 2 eyes (15.38%) received anti- glaucoma surgery, 1 for
glaucoma drainage implant surgery and 1 for glaucoma valve implant
surgery. In the PKP group, 4 eyes (36.36%) were treated with
antihypertensive drugs and the IOP dropped within the normal range.
In addition, 5 eyes (45.45%) developed drug dependence and 2 eyes
(18.18%) received anti-glaucoma surgery, 1 for glaucoma
trabeculectomy and 1 for glaucoma valve implant surgery.
 | Table VIPostoperative complications compared
between the two groups. |
Table VI
Postoperative complications compared
between the two groups.
| Complication | Simplified DSEK
(n=38) | PKP (n=27) | χ² | P-value |
|---|
| Secondary
glaucoma | 13 (34.21) | 11 (40.74) | 0.289 | 0.591 |
| Graft
rejection | 5 (13.16) | 8 (29.63) | 1.171 | 0.279 |
| Graft
infection | 1 (2.63) | 6 (22.22) | 6.304 | 0.012 |
As presented in Table
VI, graft rejection was observed in 5 eyes (13.16%) from the
simplified DSEK group and 8 eyes (29.63%) from the PKP group; this
difference was not statistically significant (P=0.279).
Immunological rejection occurred in 5 eyes (13.16%) of the
simplified DSEK group and 8 eyes (29.63%) of the PKP group. A total
of 3 eyes from the simplified DSEK group were subjected to
anti-rejection treatment and 2 eyes had progressive
development-induced transplantation failure, 1 of which was treated
with PKP 12 months after the operation. In addition, 4 eyes from
the PKP group were subjected to anti-rejection treatment and 4 eyes
exhibited progressive development-induced transplantation failure.
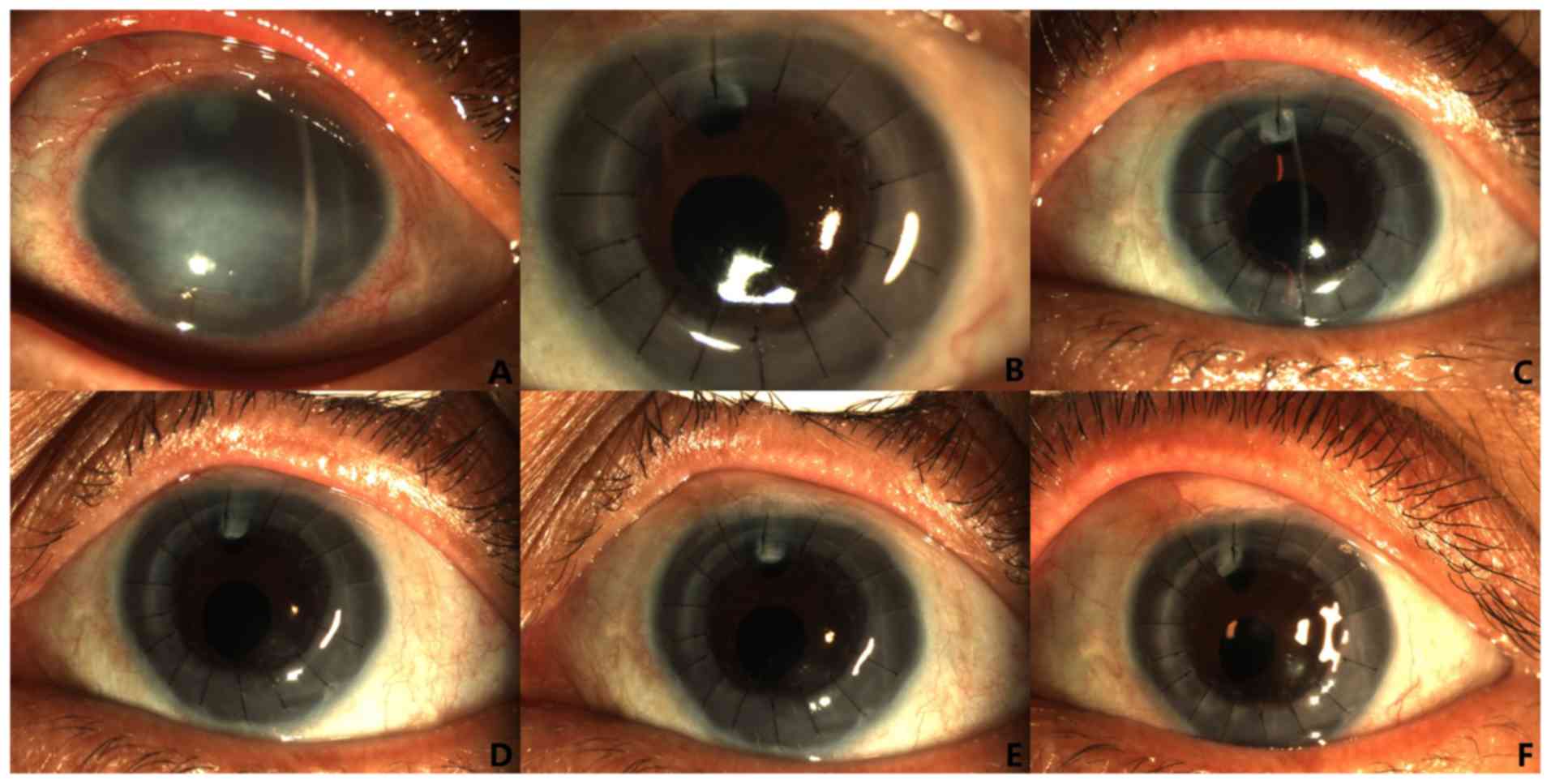
Graft rejection changes of two representative cases after
simplified DSEK and PKP are presented in Figs. 4 and 5.
As indicated in Table
VI, the graft infection rate in the simplified DSEK group was
significantly lower than that in the PKP group (P=0.012). Only 1
eye (2.63%) in the simplified DSEK group had a secondary bacterial
infection at 1 year after the operation. The infection was under
control after anti-infection treatment, but the visual function was
lost. A total of 6 eyes (22.22%) from the PKP group had secondary
graft infection after surgery, including 4 eyes with bacterial
infection, 1 eye with fungal infection and 1 eye with viral
infection. In the 4 eyes with bacterial infection, the infection
was controlled after anti-infective treatment in 2 eyes, but the
corneal graft lost its transparency. The other 2 eyes received PKP
and eyeball removal, respectively. In 1 eye with a fungal
infection, the infection was controlled and the cornea recovered
its transparency after the lesion was scraped and antifungal
treatment was applied. In 1 eye with viral infection, the infection
was also controlled following antiviral treatment.
Discussion
The advantages of the simplified DSEK operation
implemented at our department compared with conventional DSEK are
as follows: i) The implantation incision was a transparent limbal
incision with a width of ~5.0 mm, which resulted in smaller
surgical trauma and faster recovery; ii) the graft may be implanted
into the anterior chamber using a special 1-ml syringe without
suture traction and special traction instruments; and iii) a
plastic slide we used was a flexible soft material, which caused
little injury to the endothelial cells during implantation.
In the present study, the overall average BCVA in
the simplified DSEK group was significantly improved compared with
that in the PKP group. Kosker et al (15) indicated that the visual acuity of
patients in the simplified DSEK group remained stable after 3
months, while the visual acuity of the patients in the PKP group
fluctuated throughout the follow-up. The authors assumed that the
visual acuity fluctuations after PKP were primarily due to non-stop
treatment of corneal astigmatism. Similar results were obtained in
the present study. In the simplified DSEK group, the BCVA had
gradually stabilized at 3 months after the operation with a small
fluctuation range, while the average postoperative BCVA of the PKP
group were unstable. For these reasons, it was speculated that
corneal graft sutures require constant removal of the sutures,
resulting in large fluctuations in corneal astigmatism and
curvature. In addition, long-term use of local immunosuppressants
and glucocorticoid eye drops cause ocular surface immunity to
decrease, and therefore, grafts are prone to secondary infections,
which results in failure.
In the present study, 35.48% of patients in the
simplified DSEK group and no patients in the PKP group had an
average BCVA of >0.5, which was lower than that in a previous
study by Kosker et al (15),
where the average BCVA was >0.5 in 80% of the patients treated
with simplified DSEK and 46% of the patients in the PKP group. It
was speculated that this was primarily associated with the
preoperative diagnosis, as the patients in the study performed by
Kosker et al (15) had Fuchs
corneal dystrophy; however, the majority of the patients in the
present study had pseudophakic BK. In the present study, the large
difference in the postoperative visual acuity between the two
groups was considered to be associated with the large astigmatism
of the cornea caused by sutures after PKP, while the overall visual
acuity after simplified DSEK was better because there were only a
few sutures in the clear corneal margin after the surgery and the
anterior corneal surface curvature was not damaged during the
surgery.
The graft survival rate of the simplified DSEK group
in the present study (91.2%) was similar to that in a previous
study (95.5%); however, in the PKP group, the graft survival rate
was significantly lower (70.4%) compared with that in the previous
study (91.4%) (17). This may be
due to the increased incidence of corneal transplantation failure
caused by delayed treatment of postoperative complications, or
instead, it may be due to the small number of cases in the PKP
group. In addition, the incidence of graft rejection and graft
infection in the PKP group was significantly higher than that in
the simplified DSEK group and these two complications tend to lead
to secondary graft failure (18).
Glaucoma is one of the most common complications
after corneal transplantation and the degree of damage is generally
linked to the surgical method, particularly PKP, DSEK and Boston
artificial corneal transplantation (19). The postoperative incidence was
33.33%, which was consistent with that of previous studies
(13,19,20).
The incidence of postoperative glaucoma in the DSEK group of the
present study was 34.21%, which was higher than that in previous
studies, which reported incidences of 0-15% (21,22),
but was similar to that in other studies that indicated that the
incidence of glaucoma after DSEK may be as high as 35-39% (23,24).
The high incidence of glaucoma in the present study may have been
due to the requirement to inject sterile air during DSEK to press
the graft onto the graft bed. Relevant studies also suggested that
the incidence of postoperative glaucoma is closely linked to the
history of glaucoma disease prior to surgery (25,26).
In the present study, 54.55% of patients with postoperative
glaucoma in the PKP group had glaucoma prior to surgery, while
84.62% of the patients with postoperative glaucoma in simplified
DSEK group had glaucoma prior to surgery.
During the 1-year follow-up, the incidence of graft
rejection in the PKP group was higher than that in the simplified
DSEK group, consistent with a previous study (27). The reasons for the lower incidence
of postoperative rejection in the DSEK group as compared with that
in the PKP group are as follows (28): i) Preservation of the integrity of
the host corneal epithelium may reduce the antibody response of the
host's immune system to donor alloantigens; ii) the donor tissue
does not include the shallow stroma of the cornea, while the donor
stroma contains dendritic cells, which serve an important role in
host immune activation. Associated studies have indicated that poor
postoperative compliance and unauthorized withdrawal or reduction
of glucocorticoid eye drops is one of the most important causes of
rejection following DSEK (29,30).
Glucocorticoid eye drops are currently the major treatment for
rejection after corneal transplantation and its effective rate in
reversing rejection was reported to be between 39 and 92% (31).
In the present study, the incidence of postoperative
graft infection in the PKP group was 22.22% (6/27) and the
treatment failure rate was 66.67% (4/6). In the simplified DSEK
group, 1 eye had secondary corneal ulcers caused by bacterial
infection at 1 year after the operation, which was controlled
following anti-infective treatment. The incidence of graft
infection after PKP was higher than that of simplified DSEK. The
possible reasons are as follows: i) PKP reduces the integrity of
the cornea and destroys the ocular surface barrier; ii) the
innervation of the corneal surface was cut during surgery and
postoperative hypoesthesia of the corneal graft became a
susceptibility factor; iii) there are numerous sutures of corneal
grafts after surgery and loosening or rupture of sutures may easily
induce corneal graft infection. It was reported that if
interstitial infection and endophthalmitis or advanced keratitis
occur early after DSEK (within 3 months after surgery), the
consequences may be severe and certain patients require treatment
with PKP (21). Therefore, although
the incidence of postoperative infection after DSEK is low, the
occurrence of infection should also be taken into account and early
intervention should be provided.
Patients in the DSEK group were more frequently
subjected to combined procedures compared with the PKP group, which
certainly impacted the BCVA results. However, corneal endothelial
transplantation combined with cataract extraction surgery has
gradually become a routine procedure, particularly for elderly
patients. After removal of the lens and the placement of the donor
IOL, the anterior chamber is deepened, which is conducive to the
insertion of endothelial implants and also reduces the risk of
postoperative high IOP. Although the number of combined operations
in the two groups was not balanced, the degree of BK was equivalent
and did not affect the results, therefore allowing for comparison.
Furthermore, most patients without combined surgery underwent
phacoemulsification surgery or IOL implantation. Therefore, this
does not affect the overall results and comparison is possible.
There were certain limitations to the present study.
First, selective suture removal was not performed in the present
study, which should be performed to achieve optimal vision. In
addition, endothelial cell density was not assessed in the study.
The small sample size and short follow-up were further limitations
of the present study. Therefore, a large randomized controlled
study should be performed in the future.
In conclusion, the simplified DSEK technique for the
treatment of BK achieved good postoperative outcomes and the
complications were similar to those of the conventional DSEK
technique. Compared with that in the PKP group, the visual acuity
in the simplified DSEK group was significantly better than that of
the PKP group during and at the end of the postoperative follow-up.
The incidence of postoperative secondary glaucoma, graft rejection
and secondary infection was lower in the simplified DSEK than in
the PKP group. However, only the difference in the incidence of
secondary infection was statistically significant, which may have
been due to the small number of cases included and the short
follow-up time.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YC and MG conceived the study. YC and MG performed
the literature search and writing of the manuscript. SS and QL
analyzed and interpreted the data. SW and ZW collected and
assembled the data. MG submitted the manuscript and is the
corresponding author. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Institutional Review Board of The General Hospital of Shenyang
Military Area Command k(2017)25 (Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cardascia N, Pastore V, Bini V, Lategola
MG and Alessio G: Graft detachment after Descemet's stripping
automated endothelial keratoplasty in bullous keratopathy and fuchs
dystrophy. Med Hypothesis Discov Innov Ophthalmol. 9:15–22.
2020.PubMed/NCBI
|
|
2
|
Zhang T, Li SW, Chen TH, He JL, Kang YW,
Lyu FQ, Ning JH and Liu C: Clinical results of non-Descemet
stripping endothelial keratoplasty. Int J Ophthalmol. 10:223–227.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Price MO, Gorovoy M, Price FW Jr, Benetz
BA, Menegay HJ and Lass JH: Descemet's stripping automated
endothelial keratoplasty: Three-year graft and endothelial cell
survival compared with penetrating keratoplasty. Ophthalmology.
120:246–251. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Acar BT, Akdemir MO and Acar S: Visual
acuity and endothelial cell density with respect to the graft
thickness in Descemet's stripping automated endothelial
keratoplasty: One year results. Int J Ophthalmol. 7:974–979.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Phillips PM, Terry MA, Shamie N, Chen ES,
Hoar K, Dhoot D, Shah AK, Friend DJ, Rao NK and Davis-Boozer DD:
Descemet stripping automated endothelial keratoplasty in eyes with
previous trabeculectomy and tube shunt procedures: Intraoperative
and early postoperative complications. Cornea. 29:534–540.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maier P, Reinhard T and Cursiefen C:
Descemet stripping endothelial keratoplasty-rapid recovery of
visual acuity. Dtsch Arztebl Int. 110:365–371. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Price MO and Price FW Jr: Descemet's
stripping with endothelial keratoplasty: Comparative outcomes with
microkeratome-dissected and manually dissected donor tissue.
Ophthalmology. 113:1936–1942. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mehta JS, Por YM, Beuerman RW and Tan DT:
Glide insertion technique for donor cornea lenticule during
Descemet's stripping automated endothelial keratoplasty. J Cataract
Refract Surg. 33:1846–1850. 2007.
|
|
9
|
Ang M, Mehta JS, Lim F, Bose S, Htoon HM
and Tan D: Endothelial cell loss and graft survival after
Descemet's stripping automated endothelial keratoplasty and
penetrating keratoplasty. Ophthalmology. 119:2239–2244.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shinton AJ, Tsatsos M, Konstantopoulos A,
Goverdhan S, Elsahn AF, Anderson DF and Hossain P: Impact of graft
thickness on visual acuity after Descemet's stripping endothelial
keratoplasty. Br J Ophthalmol. 96:246–249. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Price MO, Price FW Jr, Kruse FE, Bachmann
BO and Tourtas T: Randomized comparison of topical prednisolone
acetate 1% versus fluorometholone 0.1% in the first year after
descemet membrane endothelial keratoplasty. Cornea. 33:880–886.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Borderie VM, Loriaut P, Bouheraoua N and
Nordmann JP: Incidence of intraocular pressure elevation and
glaucoma after lamellar versus full-thickness penetrating
keratoplasty. Ophthalmology. 123:1428–1434. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sandhu S, Petsoglou C, Grigg J and
Veillard AS: Elevated intraocular pressure in patients undergoing
penetrating keratoplasty and descemet stripping endothelial
keratoplasty. J Glaucoma. 25:390–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kaleem M, Ridha F, Shwani Z, Swenor B,
Goshe J and Singh A: Rates of intraocular pressure elevation and
use of topical antihypertensive medication after descemet stripping
automated endothelial keratoplasty. Cornea. 36:669–674.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kosker M, Suri K, Duman F, Hammersmith KM,
Nagra PK and Rapuano CJ: Long-term outcomes of penetrating
keratoplasty and Descemet stripping endothelial keratoplasty for
fuchs endothelial dystrophy: Fellow eye comparison. Cornea.
32:1083–1088. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aldave AJ, Chen JL, Zaman AS, Deng SX and
Yu F: Outcomes after DSEK in 101 eyes with previous trabeculectomy
and tube shunt implantation. Cornea. 33:223–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ang M, Soh Y, Htoon HM, Mehta JS and Tan
D: Five-year graft survival comparing descemet stripping automated
endothelial keratoplasty and penetrating keratoplasty.
Ophthalmology. 123:1646–1652. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Woo JH, Ang M, Htoon HM and Tan D:
Descemet membrane endothelial keratoplasty versus descemet
stripping automated endothelial keratoplasty and penetrating
keratoplasty. Am J Ophthalmol. 207:288–303. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kornmann HL and Gedde SJ: Glaucoma
management after corneal transplantation surgeries. Curr Opin
Ophthalmol. 27:132–139. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huber KK, Maier AK, Klamann MK, Rottler J,
Özlügedik S, Rosenbaum K, Gonnermann J, Winterhalter S and Joussen
AM: Glaucoma in penetrating keratoplasty: Risk factors, management
and outcome. Graefes Arch Clin Exp Ophthalmol. 251:105–116.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Basak SK and Basak S: Complications and
management in Descemet's stripping endothelial keratoplasty:
Analysis of consecutive 430 cases. Indian J Ophthalmol. 62:209–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee WB, Jacobs DS, Musch DC, Kaufman SC,
Reinhart WJ and Shtein RM: Descemet's stripping endothelial
keratoplasty: Safety and outcomes: A report by the American academy
of ophthalmology. Ophthalmology. 116:1818–1830. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Espana EM, Robertson ZM and Huang B:
Intraocular pressure changes following Descemet's stripping with
endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol.
248:237–242. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maier AK, Klamann MK, Torun N, Gonnermann
J, Schroeter J, Joussen AM and Rieck P: Intraocular pressure
elevation and post-DSEK glaucoma after Descemets stripping
endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol.
251:1191–1198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Karadag O, Kugu S, Erdogan G, Kandemir B,
Eraslan Ozdil S and Dogan OK: Incidence of and risk factors for
increased intraocular pressure after penetrating keratoplasty.
Cornea. 29:278–282. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Allen MB, Lieu P, Mootha VV, Bowman RW,
Petroll WM, Tong L, Kooner KS, Cavanagh HD, Whitson JT and Aggarwal
NK: Risk factors for intraocular pressure elevation after descemet
stripping automated endothelial keratoplasty. Eye Contact Lens.
36:223–227. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hjortdal J, Pedersen IB, Bak-Nielsen S and
Ivarsen A: Graft rejection and graft failure after penetrating
keratoplasty or posterior lamellar keratoplasty for fuchs
endothelial dystrophy. Cornea. 32:e60–e63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jordan CS, Price MO, Trespalacios R and
Price FW Jr: Graft rejection episodes after Descemet stripping with
endothelial keratoplasty: Part one: Clinical signs and symptoms. Br
J Ophthalmol. 93:387–390. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li JY, Terry MA, Goshe J, Shamie N and
Davis-Boozer D: Graft rejection after Descemet's stripping
automated endothelial keratoplasty: Graft survival and endothelial
cell loss. Ophthalmology. 119:90–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Allan BD, Terry MA, Price FW Jr, Price MO,
Griffin NB and Claesson M: Corneal transplant rejection rate and
severity after endothelial keratoplasty. Cornea. 26:1039–1042.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hashemian MN, Latifi G, Ghaffari R,
Ghassemi H, Zarei-Ghanavati M, Mohammadi SF, Yasseri M, Fallah
Tafti MR and Tafti ZF: Topical tacrolimus as adjuvant therapy to
corticosteroids in acute endothelial graft rejection after
penetrating keratoplasty: A randomized controlled trial. Cornea.
37:307–312. 2018.PubMed/NCBI View Article : Google Scholar
|