Introduction
It has been speculated that the occurrence of
cerebral infarction (CI) caused by most heart diseases may be
associated with the reduction of cardiac output (1). Hooghiemstra et al (2) think that the hemodynamic balance of
the ‘heart-brain axis’ is the key factor in maintaining brain
structure and any node problem may lead to cerebrovascular disease
in the vascular pathway from the heart to the brain. Recent studies
have indicated that left ventricular diastolic (LVD) dysfunction
may occur in hypertensive patients when their heart structure is
still normal (3). Left atrium, left
ventricular configuration and functional changes have been
associated with acute CI in patients with hypertension or coronary
heart disease (4). According to Nam
et al (5), LVD function is
an independent risk factor for cerebral small vessel disease. There
has even been an increase in the incidence, prevalence and duration
of hypertension along with a significant rise in the incidence of
cerebrovascular atherosclerosis and small vessel disease (6), whose cumulative effects cause vascular
endothelial damage and ultimately lead to cerebral vascular
occlusion.
A study by Shimizu et al (7) determined a positive correlation
between LVD dysfunction and cerebral white matter lesions in
elderly patients. A multicenter prospective cohort study indicated
that the left atrial diameter index was significantly associated
with the risk of stroke recurrence (8). Youth hypertension is defined as
hypertension occurring in patients aged between 18 and 45 years.
Importantly, the proportion of young individuals among patients
with stroke is increasing and the risk of disease increases even
further with age (9). It should be
noted that lifestyle changes, early intervention in hypertension,
exercise, regular examination and other precautions are all
important for the prevention of CI in young individuals (10). In fact, a combination of several
effective approaches is the most effective way to restrict the
occurrence and development of CI (11). Although studies have suggested that
the decline of LVD function is a transfer from a physiological to a
pathological process and that an early manifestation of
hypertension in heart damage is the reduction of diastolic function
(12), there have been no clinical
reports on the association between LVD dysfunction and CI among
young patients with hypertension, to the best of our knowledge. The
present study aimed to identify the association between LVD and
acute CI in young patients with hypertension.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of the Medical Ethics Committee of Baoji Central Hospital
(Baoji, China). All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
1964 Helsinki Declaration and its later amendments. Written
informed consent for data collection was obtained. A retrospective
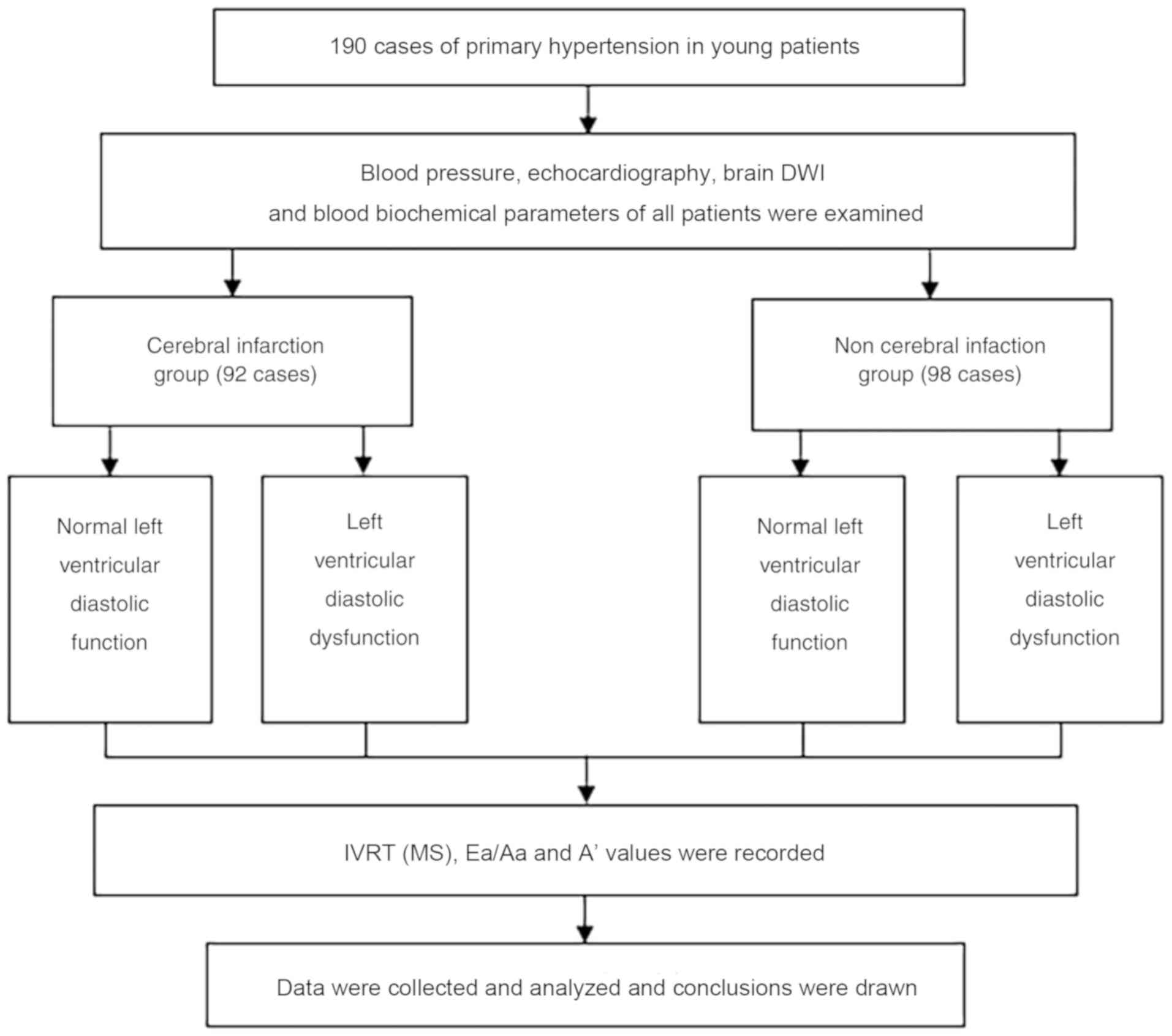
case-control study was performed. A total of 92 patients were
diagnosed as hypertensive with acute CI (CI group), comprising 55
males and 37 females aged 35-44 years (mean age, 35.26±8.36 years)
who were treated or hospitalized at the Department of Neurology at
Baoji Central Hospital. A total of 98 patients were diagnosed with
hypertension (non-CI group), comprising 65 males and 33 females
aged 36-45 years (mean age, 36.59±9.07 years); the patients were
retrospectively selected based on their general medical examination
records obtained between January 2014 and February 2018. Blood
pressure measurements, LVD functional assessment and brain MRI were
performed in all patients. According to the results of
echocardiography, patients were divided into the LVD function
reduction group and the normal LVD function group. A flow chart of
the scheme of the present study is provided in Fig. 1.
Major equipment
The following pieces of equipment were employed: i)
Echocardiography detector: Siemens s2000 echocardiograph instrument
(Siemens AG); ii) Magnetic resonance diffusion weighted imaging:
SIEMENS ESSENZA 1.5T magnetic resonance scanner (Siemens AG); iii)
Biochemical analyzer: AU2700 biochemical analyzer (Olympus).
Determination of LVD function
According to a system described in the international
literature (13), the degree of LVD
dysfunction was classified into grades numbered 0-3 with a higher
number indicating greater severity; grades 1 and above indicated
clinical diastolic dysfunction. The left ventricular isovolumic
relaxation time (IVRT) was measured by quantitative tissue velocity
imaging (QTVI) on tissue Doppler echocardiography (normal values:
Age of <40 years, 69±12 msec; age of >40 years, 76±13 msec).
The peak velocity between the early filling and late filling of the
left atrioventricular (AV) plane motion (Ea/Aa) (normal value:
>1) and the left atrial annulus systolic velocity (A'value)
(normal value: 4.89±0.67 cm/sec) are used to assess LVD function in
patients. The higher the IVRT and A'value or the lower the Ea/Aa,
the more severe is the LVD function damage (14-18).
Diagnosis of CI
The inclusion criteria were as follows: i) Stroke
onset had occurred within the past 7 days; ii) the case conformed
to the diagnostic criteria of the 2014 Chinese Acute Ischemic
Stroke Diagnosis and Treatment Guidelines (19), in that the symptoms and signs of
neurological deficits were well defined and persisted for 24 h
without any significant relief and the lesion was identified by
cranial MRI; iii) the patient consented to participate in the study
and was able to cooperate with the examination; iv) the patient had
primary CI. The exclusion criteria were as follows: i) The patient
was <18 years or >45 years of age at the time of the study;
ii) the stroke did not conform to the diagnostic criteria in the
2014 Chinese Acute Ischemic Stroke Diagnosis and Treatment
Guidelines; iii) the clinical data were incomplete; iv) due to
stroke, a patient suffering from aphasia, mental disorders or
dementia was not able to provide a complete medical history and the
family members were not able to provide the information either; v)
the patient had hemorrhagic stroke; vi) the patient had cardiogenic
CI or CI due to autoimmune or blood system disorders; vii) the
stroke was accompanied by severe heart, liver or kidney disease;
viii) the patient was using hormones, immunosuppressants or
non-steroidal anti-inflammatory drugs prior to and after the onset
of CI; ix) the patient had CI due to the onset of arteritis.
Lipid abnormalities
Blood was collected from the elbow vein of the
subject with a fasting time of >8 h and the serum was isolated
within 2 h. Blood lipids were determined with the Cobas-8000
automatic biochemical analyzer (Roche). In the present study, lipid
abnormality refers to abnormalities in the quantity and quality of
lipids in plasma. The 2013 American College of Cardiology/American
Heart Association American Adult Guidelines for the Reduction of
Atherosclerosis Lipids recommend low-density lipoprotein
cholesterol (LDL-C) <1.8 mmol/l (20).
Laboratory biochemical indicators
Fasting blood glucose and LDL-C were measured using
an Olympus AU2700 biochemical analyzer (Olympus Corp.).
Statistical analysis
The statistical software SPSS 20.0 (IBM Corp.) was
used for statistical analysis. Clinical data were manually
collected, and statistical software was used to organize and
analyze data to draw meaningful conclusions based on the
experimental results. The Wilcoxon rank-sum test was used in the
analysis of count data. Values of measurement data are expressed as
the mean ± standard deviation. The χ² test was used to compare
blood pressure and LVD function between patients in the CI group
and non-CI group. The χ² test was also used to compare
pre-infarction and post-infarction diastolic function in patients
of the CI group.
Results
Comparison of baseline data between
the two groups
Patients were excluded if they had lesions that may
affect the blood pressure, including secondary hypertension,
arrhythmia, coronary heart disease, valvular heart disease, anemia
or hyperthyroidism. The factors of gender, age, blood lipids, blood
glucose, smoking, alcohol consumption and family history of
vascular disease exhibited no statistically significant difference
between the CI group and the non-CI group (P>0.05), meaning that
the groups were comparable with respect to these factors (Table I). There was no significant
difference in blood pressure between the two groups, meaning that
they were comparable in this regard as well (Table II).
 | Table IComparison of baseline data between
the two groups. |
Table I
Comparison of baseline data between
the two groups.
| Group | CI (92) | Non-CI (98) |
|---|
| Age (years) | 35.26±8.36 | 36.59±9.07 |
| Male sex | 55 (28.95%) | 65 (34.21%) |
| Dyslipidemia | 48 (25.26%) | 42 (22.11%) |
| Abnormal fasting
blood glucose | 6 (3.16%) | 4 (2.11%) |
| Smoking | 36 (18.95%) | 32 (16.84%) |
| Alcohol
consumption | 28 (14.74%) | 23 (12.11%) |
| Family history of
vascular disease | 4 (2.11%) | 3 (1.58%) |
 | Table IIComparison of hypertension between the
two groups. |
Table II
Comparison of hypertension between the
two groups.
| Group | N | SBP (mmHg) | DBP (mmHg) |
|---|
| CI group | 92 | 167.18±12.75 | 103.36±7.03 |
| Non-CI group | 98 | 159.26±15.92 | 105.28±6.74 |
| χ² | | 25.436 | 16.125 |
| P-value | | 0.062 | 0.057 |
Grading comparison of left ventricular
diastolic function in all patients included in the study
According to the left heart function grading
standards reported in the international literature (3), the data of the present study indicated
the following: Among the 190 patients, 18 (9.47%) had grade 0
diastolic dysfunction (normal ventricular diastolic function) and
172 (90.53%) had diastolic dysfunction. Among the patients with
diastolic dysfunction, 117 cases (61.58%) had grade 1, 47 cases
(24.74%) had grade 2 and 8 cases (4.21%) had grade 3 diastolic
dysfunction (Table III).
 | Table IIIClassification of left ventricular
diastolic function in the patients of the present study
(n=190). |
Table III
Classification of left ventricular
diastolic function in the patients of the present study
(n=190).
| Classification | Grade | n (%) |
|---|
| Normal diastolic
function | 0 | 18 (9.47) |
| Early diastolic
dysfunction | 1 | 117 (61.58) |
| False
normalization | 2 | 47 (24.74) |
| Restrictive filling
disorder | 3 | 8 (4.21) |
Comparison of left ventricular
diastolic function before and after infarction in patients with
cerebral infarction
The LVD function indexes of the CI group prior to
infarction and after infarction were compared. The IVRT and
A'values of the CI group after infarction were higher than those of
prior to infarction, whereas the Ea/Aa value was lower. The
difference was statistically significant (P<0.05; Table IV).
 | Table IVComparison of left ventricular
diastolic function indexes prior to and after infarction in the
cerebral infarction group (n=92). |
Table IV
Comparison of left ventricular
diastolic function indexes prior to and after infarction in the
cerebral infarction group (n=92).
| Time-point | IVRT (msec) | Ea/Aa | A'value
(cm/sec) |
|---|
| Prior to
infarction | 81.37 | 1.15±0.12 | 5.10±0.92 |
| After
infarction | 114.97±10.16 | 0.79±0.11 | 5.21±1.03 |
| χ² | 19.146 | 16.541 | 15.019 |
| P-value | 0.048 | 0.040 | 0.046 |
Comparison of left ventricular
diastolic function between the cerebral infarction group after
infarction and the non-infarction group
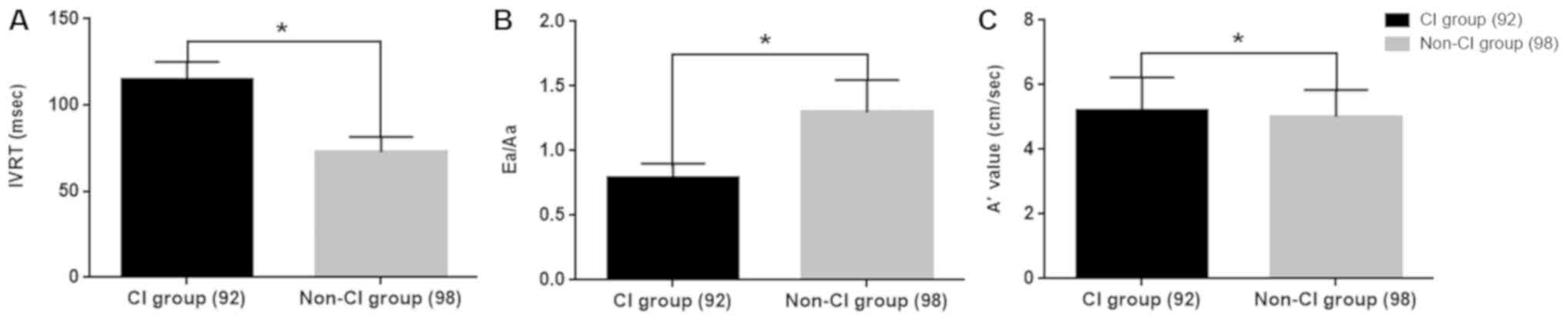
The LVD function indexes of the CI group after
infarction and the non-CI group were compared. The IVRT and
A'values of the CI group after infarction were higher than those of
the non-CI group, whereas the Ea/Aa value was lower. The difference
was statistically significant (P<0.05; Table V and Fig. 2).
 | Table VComparison of left ventricular
diastolic function indexes between the two groups. |
Table V
Comparison of left ventricular
diastolic function indexes between the two groups.
| Group | IVRT (msec) | Ea/Aa | A'value
(cm/sec) |
|---|
| CI group
(post-infarction, 92) | 114.97±10.16 | 0.79±0.11 | 5.21±1.03 |
| Non-CI group
(98) | 72.86±8.75 | 1.3±0.25 | 5.02±0.82 |
| χ² | 23.124 | 17.026 | 14.351 |
| P-value | 0.039 | 0.037 | 0.042 |
Discussion
Young ischemic stroke (YIS) refers to ischemic
stroke occurring in patients aged 45 or under and its incidence is
increasing annually (14). The
reason is probably its characteristics of early onset, early
disability and high recurrence. At present, YIS accounts for an
estimated 5-10% of all cases of CI and the most common pathogenetic
origin of the disease is atherosclerosis caused by hypertension
(21). The pathogenic effect of
hypertension has been indicated to be directly correlated with its
severity and duration, which indicates a connection between
hypertension and an increased risk of YIS (22).
Young patients pay insufficient attention to blood
pressure and frequently remain untreated (23). This long-term elevation of
peripheral blood pressure increases cardiac after-loading, while
increased peripheral vasoconstriction tension and retention of
sodium and water increase myocardial oxygen consumption,
deteriorate myocardial compliance and augment ventricular
end-diastolic pressure (24). As a
result, cardiac preload increases and left ventricular hypertrophy
occurs, whereas diastolic function is significantly reduced
(25). Cerebral hypoperfusion,
combined with intracranial arteriosclerosis and even occlusion
caused by high blood pressure, eventually leads to CI (26). It has been indicated that an early
onset of hypertension always triggers reduced LVD function
(3). The decrease precedes a change
in ventricular systolic function and leads to an altered cardiac
configuration (24,25). Cerrato et al (6) reported that heart problems accounted
for 24% of the causes of YIS. Another study reported that acute CI
may be associated with LVD dysfunction, which has an important role
in the pathogenesis and prognosis of acute CI (26). In the present study, 172 patients
with LVD dysfunction accounted for 90.53% of the 190 patients
enrolled, which suggests that hypertension may cause LVD
dysfunction in young patients. Diastolic function of grades I and
II accounted for the largest proportion, indicating that the
incidence of early diastolic dysfunction is high in young
hypertensive patients. These results suggest a requirement to
actively perform early diagnosis and early treatment to prevent the
occurrence of acute cerebrovascular events, which is essentially
consistent with the conclusions of previous studies.
The reason why LVD dysfunction induces abnormal
peripheral hemodynamics followed by cerebral hypoperfusion has yet
to be identified. First, we surmise that with decreasing diastolic
function, the left ventricle cannot effectively expand, causing the
returned blood volume and cerebral perfusion blood to decrease
(27). Second, the decreased LVD
function leads to a decrease in blood flow and friction between the
endothelia of blood vessels, atherosclerosis is promoted, acute
cerebrovascular occlusion is created and CI ultimately occurs
(28). Early detection, accurate
evaluation and timely prevention of LVD dysfunction are clinically
significant for the prevention of CI.
According to the American Society of
Echocardiography guidelines (13),
LVD function depends mainly on left ventricular flaccidity and left
ventricular compliance. The pressure indexes measured by left
cardiac catheterization, including left ventricular end-diastolic
pressure, maximum left ventricular pressure drop rate and the left
ventricular relaxation time constant are regarded as the gold
standard for assessing LVD dysfunction. The guideline recommends
the Ea/Aa ratio from non-invasive Doppler ultrasound measurements
as the indicator of LVD (13).
At present, the evaluation of LVD function is mainly
based on left ventricular filling (hemodynamic changes), myocardial
motion (particularly at the mitral annulus) and the structure of
the left atrium and left ventricle (29). Doppler tissue imaging is able to
reflect myocardial velocity in patients and the occurrence and
cessation of the motion waveforms near the diastolic and systolic
phases in the same cardiac cycle, based on which the LVD function
of patients is scientifically evaluated (30).
LVD function is currently evaluated by the ratio of
the early diastolic peak at the mitral valve (E) to the mitral
annulus velocity (Em) measured by echocardiographic pulses and
tissue Doppler imaging. An E/Em value of >15 suggests that the
left ventricular filling pressure is elevated and an E/Em of <8
is considered normal (13). This
indicator is not affected by ejection fraction, atrial fibrillation
or sinus tachycardia and is rarely influenced by left ventricular
filling pressure or the pressure gradient across the mitral valve.
Ishikawa et al (31)
reported LVD function and silent brain infarction in patients with
non-valvular atrial fibrillation by tissue Doppler measurements.
Olsen et al (32) recommend
the use of tissue Doppler imaging to predict diastolic myocardial
dysfunction in patients with CI. However, the feasibility of
predicting acute CI by examining LVD function with tissue Doppler
imaging in young hypertensive patients has not been previously
reported, to the best of our knowledge. The present clinical study
fills this research gap.
From the closure of the aortic valve, the LVD
process may be divided into the isovolumetric relaxation period,
the rapid filling period, the reduced filling period and the left
atrial systolic period. The contribution rates of these different
periods are 45-50, 35-40, 5 and 5-15%, respectively (33). QTVI by tissue Doppler imaging may,
in turn, reflect LVD function during the isovolumetric relaxation
period, rapid filling period and left atrial systolic period by
measuring IVRT, Ea/Aa and A' (34).
The higher the IVRT and A'values and the lower the Ea/Aa, the more
severe is the LVD function damage.
In the present study, the LVD function of CI
patients prior to infarction was lower than that of patients
post-infarction and the difference was statistically significant.
The LVD function of CI patients post-infarction was lower than that
of patients with non-CI patients and the difference was
statistically significant. These data indicate that LVD function
has a certain influence on the occurrence of CI in young
hypertensive patients. It was indicated that the LVD function of
patients with acute CI was lower than that of patients with
hypertension alone and the difference was statistically
significant. Conversely, the risk of acute CI is also significantly
higher in young hypertensive patients with lower left ventricular
function than in patients with normal left ventricular function.
This difference in risk further indicates that early monitoring of
left ventricular function has important clinical significance for
young hypertensive patients to prevent acute CI. Furthermore, the
probability of acute CI in young hypertensive patients with
decreased left heart function is significantly elevated and the
outcome indicated that the LVD function during the isovolumetric
relaxation period and rapid filling period was severely impaired in
patients with acute YIS. There are two probable reasons why
impaired LVD function tends to occur during these two phases and
trigger acute CI: i) In the majority of cases, CI occurs in these
two phases; diastolic filling is obviously limited when damage is
present and the blood volume return is reduced. In turn, the amount
of blood ejection in the left ventricular systolic phase is
insufficient and the peripheral circulatory blood volume is
decreased, causing the cerebral perfusion blood volume to decline
significantly. ii) The impairment of LVD function during these two
phases results in reduced fluid shear stress on the vessel wall. In
the long term, the conditions of reduced shear stress may induce
and promote atherosclerosis. Furthermore, head or neck vascular
atherosclerosis is well known as the most common cause of acute CI
(35). Together, these two causes
significantly increase the incidence of acute CI.
In conclusion, the present clinical study suggests
that hypertension in young individuals is associated with decreased
LVD function and is a risk factor for diastolic dysfunction in the
left ventricle. An association exists between acute YIS and LVD
dysfunction, which may help predict the onset of acute YIS. Hence,
LVD functional measurement is relevant to the individualized
treatment and prognostic evaluation of patients with YIS.
However, the present study had certain shortcomings.
First, the association between hypertension grading and LVD
function grading in young hypertensive patients remains to be fully
established. In addition, the LVD function of young patients with
acute CI is not regularly assessed. Furthermore, the effect of
anti-hypertensive drugs on LVD and CI was not assessed in the
present study, and this may be determined in future studies.
Therefore, the dynamics of these indicators and associated changes
were not observed. Finally, the literature on the association
between LVD function classification and the CI area was sparse and
further large-scale, multicenter clinical studies may be required
to assess this.
Acknowledgements
The authors would like to thank Ms. Xiao-Di Fu
(Department of Neurology, Baoji Municipal Central Hospital, China)
for her technical assistance in drawing charts in the present
study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJW and XYW conceived, designed and drafted the
manuscript, were responsible for the acquisition analysis, revision
and interpretation of data and read and approved of the final
version to be published. HJW and XYW agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Baoji Municipal Central Hospital (Baoji, China) and
the patients provided written informed consent.
Patient consent for publication
Written informed consents were obtained for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heckman GA, Patterson CJ, Demers C, St
Onge J, Turpie ID and McKelvie RS: Heart failure and cognitive
impairment: Challenges and opportunities. Clin Interv Aging.
2:209–218. 2007.PubMed/NCBI
|
|
2
|
Hooghiemstra AM, Bertens AS, Leeuwis AE,
Bron EE, Bots ML, Brunner-La Rocca HP, de Craen AJ, van der Geest
RJ, Greving JP, Kappelle LJ, et al: The missing link in the
pathophysiology of vascular cognitive impairment: Design of the
heart-brain study. Cerebrovasc Dis Extra. 7:140–152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Banecka-Majkutewicz Z, Sawula W, Kadzinski
L, Wegrzyn A and Banecki B: Homocysteine, heat shock proteins,
genistein and vitamins in ischemic stroke-pathogenic and
therapeutic implications. Acta Biochim Pol. 59:495–499.
2012.PubMed/NCBI
|
|
4
|
Pierdomenico SD, Pierdomenico AM, Di Carlo
S, Di Tommaso R and Cuccurullo F: Left atrial enlargement and risk
of ischemic stroke in elderly treated hypertensive patients. Am J
Hypertens. 27:1179–1184. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nam KW, Kwon HM, Kim HL and Lee YS: Left
ventricular ejection fraction is associated with small vessel
disease in ischaemic stroke patients. Eur J Neurol. 26:747–753.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cerrato P, Grasso M, Imperiale D, Priano
L, Baima C, Giraudo M, Rizzuto A, Azzaro C, Lentini A and
Bergamasco B: Stroke in young patients: Etiopathogenesis and risk
factors in different age classes. Cerebrovasc Dis. 18:154–159.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shimizu A, Kokubo M, Mitsui T, Miyagi M,
Nomoto K, Murohara T, Toba K and Sakurai T: Left ventricular
diastolic dysfunction is directly associated with cerebral white
matter lesions in elderly patients. Geriatr Gerontol Int. 15 (Suppl
1):S81–S82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ogata T, Matsuo R, Kiyuna F, Hata J, Ago
T, Tsuboi Y, Kitazono T and Kamouchi M: FSR Investigators. Left
atrial size and long-term risk of recurrent stroke after acute
ischemic stroke in patients with nonvalvular atrial fibrillation. J
Am Heart Assoc. 6(e006402)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smajlovic D: Strokes in young adults:
Epidemiology and prevention. Vasc Health Risk Manag. 11:157–164.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Allaoui A, Echchilali K, Moudatir M,
Alaoui FZ and Elkabli H: Causes of stroke among young people: Role
of the internist. Pan Afr Med J. 30(114)2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
11
|
Kim SY, Song CM, Bang W, Lim JS, Park B
and Choi HG: Nephrolithiasis predicts ischemic stroke: A
longitudinal follow-up study using a national sample cohort. Int J
Med Sci. 16:1050–1056. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Borlaug BA, Melenovsky V, Redfield MM,
Kessler K, Chang HJ, Abraham TP and Kass DA: Impact of arterial
load and loading sequence on left ventricular tissue velocities in
humans. J Am Coll Cardiol. 50:1570–1577. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Manouras A, Nyktari E, Sahlen A, Winter R,
Vardas P and Brodin LA: The value of E/Em ratio in the estimation
of left ventricular filling pressures: Impact of acute load
reduction: A comparative simultaneous echocardiographic and
catheterization study. Int J Cardiol. 166:589–595. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park CS, Kim YK, Song HC, Choi EJ, Ihm SH,
Kim HY, Youn HJ and Seung KB: Effect of preload on left atrial
function: Evaluated by tissue Doppler and strain imaging. Eur Heart
J Cardiovasc Imaging. 13:938–947. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rösner A, Avenarius D, Malm S, Iqbal A,
Baltabaeva A, Sutherland GR, Bijnens B and Myrmel T: Persistent
dysfunction of viable myocardium after revascularization in chronic
ischaemic heart disease: Implications for dobutamine stress
echocardiography with longitudinal systolic strain and strain rate
measurements. Eur Heart J Cardiovasc Imaging. 13:745–755.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chinese Medical Association C, CVD
editorial board. Chinese heart failure diagnosis and treatment
guide 2014. Chin J Cardiovascular Dis. 42:98–122. 2014.PubMed/NCBI
|
|
17
|
Correale M, Totaro A, Ieva R, Ferraretti
A, Musaico F and Di Biase M: Tissue Doppler imaging in coronary
artery diseases and heart failure. Curr Cardiol Rev. 8:43–53.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang M, Hu B, Zhang YL, Shen E and Pan XQ:
Effects of 3-aminobenzamide on ventricular function in infarct
heart assessed by quantitative tissue velocity imaging. J
Cardiovasc Med (Hagerstown). 17:793–802. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Neurology branch of the Chinese Medical
Association cvdg. Guidelines for the diagnosis and treatment of
acute ischemic stroke in China 2014. Chin J Neurol. 48:246–257.
2015.
|
|
20
|
Gunasekaran P, Jeevanantham V, Sharma S,
Thapa R and Gupta K: Implications of the 2013 ACC/AHA cholesterol
guidelines on contemporary clinical practice for patients with
atherosclerotic coronary and peripheral arterial disease. Indian
Heart J. 69:464–468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bersano A, Borellini L, Motto C,
Lanfranconi S, Pezzini A, Basilico P, Micieli G, Padovani A, Parati
E and Candelise L: Molecular basis of young ischemic stroke. Curr
Med Chem. 20:3818–3839. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han
MK, Park JM, Cho YJ, Hong KS, Kim DH, et al: Identifying target
risk factors using population attributable risks of ischemic stroke
by age and sex. J Stroke. 17:302–311. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cohen DL and Townsend RR: Approach to the
young patient with new-onset hypertension. Clin J Am Soc Nephrol.
13:929–932. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu Q, Dong H, Meng L, Cheng H, Yan Y, Liu
J and Mi J: Impacts of hypertension on early changes of
cardiovascular structure and function among children: A
case-control study. Zhonghua Liu Xing Bing Xue Za Zhi. 36:332–336.
2015.PubMed/NCBI(In Chinese).
|
|
25
|
Alpsoy S, Oran M, Topcu B, Akyuz A,
Akkoyun DC and Degirmenci H: Effect of lifestyle modifications on
diastolic functions and aortic stiffness in prehypertensive
subjects: A prospective cohort study. Anadolu Kardiyol Derg.
13:446–451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seo JY, Lee KB, Lee JG, Kim JS, Roh H, Ahn
MY, Park BW and Hyon MS: Implication of left ventricular diastolic
dysfunction in cryptogenic ischemic stroke. Stroke. 45:2757–2761.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gasiorek P, Sakowicz A, Banach M, von
Haehling S and Bielecka-Dabrowa A: Arterial stiffness and indices
of left ventricular diastolic dysfunction in patients with embolic
stroke of undetermined etiology. Dis Markers.
2019(9636197)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cherneva RV, Gospodinova MV, Denchev SV,
Petkov RB, Kostadinov DE and Cherneva ZV: Stress echocardiography
for left ventricular diastolic dysfunction detection in patients
with non-severe chronic obstructive pulmonary disease: A
cross-sectional study. Croat Med J. 60:449–457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schiebler ML, Bhalla S, Runo J, Jarjour N,
Roldan A, Chesler N and François CJ: Magnetic resonance and
computed tomography imaging of the structural and functional
changes of pulmonary arterial hypertension. J Thorac Imaging.
28:178–193. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zamojska J, Niewiadomska-Jarosik K, Wosiak
A, Lipiec P and Stanczyk J: Myocardial dysfunction measured by
tissue Doppler echocardiography in children with primary arterial
hypertension. Kardiol Pol. 73:194–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ishikawa S, Sugioka K, Sakamoto S, Fujita
S, Ito A, Norioka N, Iwata S, Nakagawa M, Takagi M, Miki Y, et al:
Relationship between tissue Doppler measurements of left
ventricular diastolic function and silent brain infarction in
patients with non-valvular atrial fibrillation. Eur Heart J
Cardiovasc Imaging. 18:1245–1252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Olsen FJ, Jørgensen PG, Møgelvang R,
Jensen JS, Fritz-Hansen T, Bech J, Sivertsen J and Biering-Sørensen
T: Diastolic myocardial dysfunction by tissue Doppler imaging
predicts mortality in patients with cerebral infarction. Int J
Cardiovasc Imaging. 31:1413–1422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Afolabi-Brown OO, Lynn Morris D and
Pressman GS: Systolic mitral valve opening and absent isovolumic
relaxation: Unusual hemodynamics of severe mitral regurgitation.
Echocardiography. 31:E189–E190. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Krzesiak-Lodyga A and Cwetsch A:
Echocardiographic methods for assessment of left ventricular
diastolic dysfunction-state of the art. Pol Merkur Lekarski.
35:63–66. 2013.PubMed/NCBI(In Polish).
|
|
35
|
Nacu A, Fromm A, Sand KM, Waje-Andreassen
U, Thomassen L and Naess H: Age dependency of ischaemic stroke
subtypes and vascular risk factors in western Norway: The Bergen
Norwegian stroke cooperation study. Acta Neurol Scand. 133:202–207.
2016.PubMed/NCBI View Article : Google Scholar
|