Introduction
Spontaneous hypertension (SH) is one of the most
critical factors in leading to cardiovascular diseases (1,2).
Persistently elevated blood pressure in patients who are
hypertensive results in vascular tone increasement and vascular
smooth muscle systolic dysfunction, leading to myocardial ischemia
and ventricular and vascular remodeling (3,4).
Furthermore, left ventricular and aorta remodeling is a frequent
pathological change in hypertension, which contributes to
arrhythmia, heart failure and cardiovascular mortality (5-7).
Caduet is a single pill containing a combination of
amlodipine besylate and atorvastatin calcium tablet (AM + AT),
which is widely used in the clinical treatment of cardiovascular
diseases, such as hypertension and coronary heart disease (8). In addition, previous experimental and
clinical studies have reported that AM + AT can prevent cardiac
hypertrophy and remodeling (9,10).
The gap junction (GJ) channel is the structural
basis for cellular electric coupling and signal transmission, which
mainly functions though regulating the GJ protein connexin
(11,12). Connexin 43 (Cx43)-associated GJ
protein in ventricular cardiomyocytes and vascular smooth muscle
cells is involved in cardiac and vascular remodeling (13). Moreover, left ventricular remodeling
is closely associated with the expression and distribution of Cx43
in cardiomyocytes (9,14), and our previous study revealed that
the expression of Cx43 was enhanced in the thoracic aorta of SH
model rats (SHR) (15). Previous
studies have also reported that Cx43 phosphorylation contributes to
ischemia-associated remodeling of Cx43 channels in cardiomyocytes
(16,17), and Cx43 dephosphorylation
contributes to arrhythmias and cardiomyocyte apoptosis in
ischemia/reperfusion hearts (18).
Based on these clinical data and laboratory reports, it was
hypothesized that Cx43 phosphorylation may be involved in the
process of hypertensive left ventricular and thoracic aorta
remodeling, and AM + AT may improve this remodeling by enhancing
Cx43 phosphorylation.
Therefore, the aim of the present study was to
investigate the effect of Cx43 phosphorylation in the process of
hypertensive left ventricular and thoracic aorta remodeling, and to
examine whether AM + AT improved this remodeling by regulating Cx43
phosphorylation.
Materials and methods
Experimental reagents
A ProteoPrep Total Extraction Sample kit was
obtained from Sigma-Aldrich (Merck KGaA), the BCA protein assay kit
was from Beyotime Institute of Biotechnology and nitrocellulose
membrane was purchased from Thermo Fisher Scientific, Inc. Primary
antibody against rat total (T)-Cx43 was obtained from Thermo Fisher
Scientific, Inc. (1:1,000 for western blotting; 1:100 for
immunofluorescence; cat. no. 3D8A5). Primary antibody against rat
phosphorylated (P)-Cx43 was obtained from Cell Signaling Technology
(1:1,000 for western blotting; 1:100 for immunofluorescence; cat.
no. 52559). Horseradish peroxidase (HRP)-conjugated goat anti-mouse
(1:1,000; cat. no. G-21040) and goat anti-rabbit polyclonal
(1:1,000; cat. no. 31466) secondary antibodies were obtained from
Thermo Fisher Scientific, Inc. ECL reagents were purchased from
Thermo Fisher Scientific, Inc. (cat. no. 35055). The
FITC-conjugated goat anti-mouse secondary antibody was obtained
from Thermo Fisher Scientific, Inc. (1:50; cat. no. 62-6511), and
the tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat
anti-rabbit secondary antibody was purchased from Thermo Fisher
Scientific, Inc. (1:100; cat. no. A16101). Bovine serum albumin
(BSA) was from Thermo Fisher Scientific, Inc. (cat. no. 37520).
Drugs and animals
The Chinese drug administration license nos. of AM,
AT and AM + AT were H10950224, J20070061 and J20171045,
respectively, which were from Betriebsstätte Freiburg, Inc.. All
procedures and ethics associated with animal use were reviewed and
approved by Medical School of Xi'an Jiaotong University (approval
no. 2018-898). In total, 32 male SHR (age, 8 weeks; weight, 210-265
g) were obtained from Weitong Lihua Experimental Animal Technology
Co., Ltd, with clean grade certificate [SCXK (Beijing) 2007-0001],
and eight Wistar-Kyoto (WKY) rats were purchased from Skerries
Laboratory Animal Center, Ltd. as the WKY control group, with clean
grade certificate [SCXK (Shanghai) 2007-0005]. The AM group
(SHR-AM; n=8), AT group (SHR-AT; n=8) and AM + AT group (SHR-AM +
AT; n=8) were orally administered 10 mg/kg/day of AM, AT or AM + AT
as treatment groups for 8 weeks (19,20),
respectively. The WKY control group (n=8) and SHR control group
(n=8) were orally administrated with the same volume of water as
the treatment group. All rats received humane care and were raised
in the same clean environment, with ambient temperature at 22±1˚C,
humidity of 50±5%, 14/10-h light/dark cycle, free access to food
and water.
Measurement of body weight, heart rate
(HR), left ventricular mass index (LVMI), blood pressure and plasma
lipid levels in each group
The systolic blood pressure (SBP) and diastolic
blood pressure (DBP) of the rats were measured at 0, 2, 4, 6 and 8
weeks after treatment using the Non-Invasive Blood Pressure system
(LE-5001 HX-II tail-cuff small animal blood pressure meter; Panlab
S.L.U). After 8 weeks, HR was measured and anal temperature were
measured by thermometer. Then the rats were weighed and
anesthetized with pentobarbital (40 mg/kg) via intraperitoneal
injection. A total of 2 ml blood was collected from heart of every
rat, and the plasma lipid levels of each group were measured using
a Hitachi 7170 biochemical analyzer (Hitachi, Inc.); these included
total cholesterol (TC), high-density lipoprotein cholesterol (HDL),
low-density lipoprotein cholesterol (LDL) and triglycerides (TG).
Then the chest of the rat was opened, the heart was quickly removed
and the left ventricular free wall was cut along the
atrioventricular ring and weighed as the LVMI (left ventricular
mass/body weight; mg/g).
Comparison of pathological changes in
cardiac tissues
The free wall of the left ventricle was fixed in 10%
formaldehyde for 2 h at room, dehydrated in an ethanol gradient
(80, 90, 95 and 100%), then embedded in paraffin. Sections (3 µm
thick) were cut and conventionally dewaxed to water in a series,
including xylene I, xylene II, 100% alcohol I and 100% alcohol II
(10 min each), then a 95, 90, 80, 70% ethanol series (10 min each)
and finally distilled water. Sections were stained with hematoxylin
for 1 min, rinsed with water once, stained with eosin for 2 min,
then dehydrated with conventional gradient alcohol (70, 80, 90 and
95% ethanol, 2 min each; 100% ethanol I, 100% alcohol II, 10 min
each), cleared with xylene, and finally mounted with neutral gum
and observed with Olympus BX41 fluorescent microscope
(magnification, x400; Olympus Corporation). Images were captures
and used to quantitatively analyze the myocardial cell
cross-sectional area using Image-Pro Plus analysis software 6.0
(Media Cybernetics, Inc.).
Analysis of T-Cx43 and P-Cx43 protein
expression levels in the left ventricular and thoracic aortic
tissue using western blotting
Western blotting was performed as reported
previously (21). Proteins of the
left ventricular free wall and thoracic aortic ascending tissue
were extracted using ProteoPrep Total Extraction Sample kit and
measured using a BCA protein assay kit. Each sample was mixed with
40 µl 1X SDS electrophoresis sample buffer and boiled for 2-3 min.
Protein samples (30 µg) were separated by 12% SDS-PAGE gel and
transferred onto a nitrocellulose membrane. The membranes were
blocked with 5% skimmed milk for 2 h at room temperature, and
washed twice with Tris-buffered saline with 0.1% Tween-20.
Membranes were incubated with T-Cx43 and P-Cx43 primary antibodies
overnight at 4˚C, then probed with HRP-conjugated secondary
antibody for 2 h at room temperature. Protein bands were visualized
using ECL reagents and imaged using an Alpha Innotech FluorChem FC2
Imaging System (Alpha Innotech Inc.), the densitometric analysis
was used ImageJ software v1.46 (National Institutes of Health), and
β-actin expression was used to normalize the data.
Expression and distribution of T-Cx43
and P-Cx43 in the left ventricular and thoracic aortic tissue using
immunofluorescence double labeling
The frozen tissue embedded with optimum cutting
temperature compound was cut into 8-µm thick tissue sections and
fixed for 15 min with pre-cooled acetone at 4˚C. Then, the samples
were blocked with 1% BSA for 2 h at room temperature, and incubated
with the primary antibodies against rat T-Cx43 and P-Cx43 at 4˚C
overnight, followed by incubation with the FITC-conjugated
secondary antibody at 37˚C for 1 h. Then, the sections were
incubated with a TRITC-conjugated secondary antibody in 1% fetal
bovine serum at 37˚C for 1 h in the dark. After washing three times
with PBS (5 min each), five random fields of each section were
examined under an Olympus BX41 fluorescent microscope
(magnification, x400; Olympus Corporation), and the fluorescence
intensity was determined using ImageJ software.
Statistical analysis
The data were analyzed with SPSS 21.0 statistical
software (IBM Corp.). Data are presented as the mean ± SD; the
experiments were repeated three times. Comparisons between groups
were performed using one-way ANOVA followed Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of body weight, HR and LVMI
in each group
As presented in Table
I, no significant differences were identified in the body
weight in all groups after 8 weeks of drug treatment (P>0.05).
It was demonstrated that HR and LVMI were significantly lower in
treatment groups compared with the respective SHR group
(P<0.05); and LVMI was especially lower in the SHR-AM + AT
group.
 | Table IEffects of the different drugs on body
weight, HR and LVMI (n=8). |
Table I
Effects of the different drugs on body
weight, HR and LVMI (n=8).
| | Group |
|---|
| Parameter | WKY | SHR | SHR-AM | SHR-AT | SHR-AM + AT |
|---|
| Body weight, g | 299.91±9.90 | 298.33±8.52 | 292.00±10.58 | 288.72±10.68 | 288.72±10.68 |
| HR, bpm |
346.61±6.92a | 384.88±8.26 | 372.44±8.79 | 376.50±5.96 | 360.77±6.56 |
| LVMI, mg/g |
2.06±0.43a,b | 2.92±0.24 |
2.55±0.28a,b |
2.65±0.38a,b |
2.31±0.49a |
Comparison of blood pressure, plasma
lipid level and myocardial tissue morphology
After treatment for 8 weeks, SBP and DBP in the
SHR-AM and SHR-AM + AT groups were significantly decreased compared
with the SHR group (P<0.05; Fig.
1A). However, there was no difference between SHR-AM and SHR-AM
+ AT group (P>0.05; Fig.
1A).
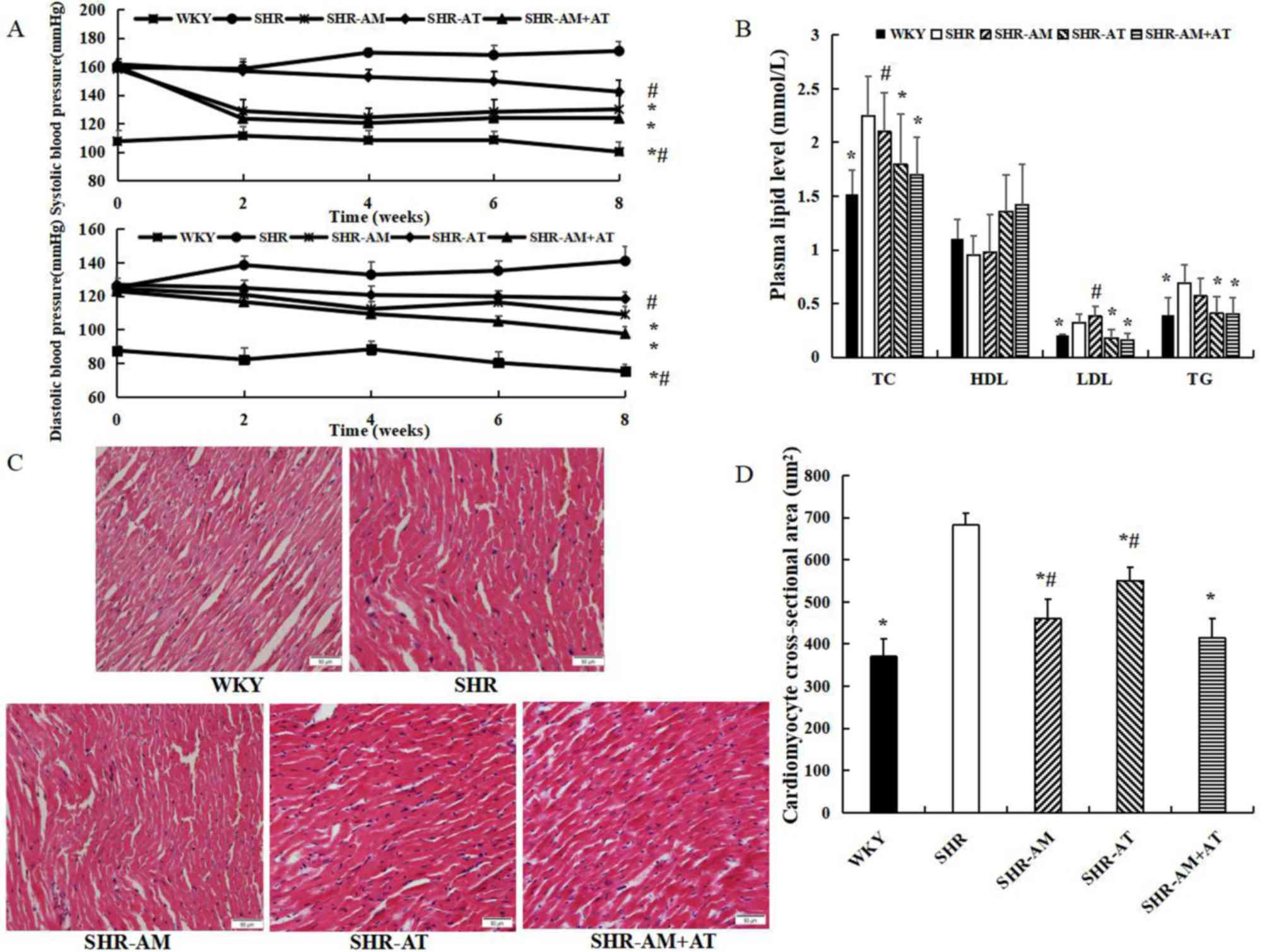 | Figure 1Comparison of blood pressure, plasma
lipid level and myocardial tissue morphology in each group. (A)
Comparison of systolic blood pressure and diastolic blood pressure
in each group. (B) Comparison of plasma lipid levels. (C)
Representative micrographs of myocardial tissue using hematoxylin
and eosin staining in each group; magnification, x400. (D)
Cardiomyocyte cross-sectional area in each group. Data are
presented as the mean ± SD; n=8; *P<0.05 vs. SHR;
#P<0.05 vs. SHR-AM + AT. AM, amlodipine; AT,
atorvastatin; HDL, high-density lipoprotein cholesterol; LDL,
low-density lipoprotein cholesterol; SHR, spontaneous hypertension
model rat; TC, total cholesterol; TG, triglycerides; WKY,
Wistar-Kyoto. |
The plasma lipid results identified that TC, LDL and
TG in the SHR-AT + AM group were significantly lower compared with
the SHR group (P<0.05), and TC and LDL was lower in the SHR-AM +
AT compared with levels in the SHR-AM group (P<0.05; Fig. 1B). Furthermore, there was no
significant difference in HDL in all groups (P>0.05).
Compared with the WKY group, hematoxylin and eosin
staining demonstrated that the myocardiocytes in the SHR group were
hypertrophied, swelled and arranged disorderly, and the
cross-sectional area of cardiomyocytes were significantly increased
(Fig. 1C). Furthermore, compared
with SHR group, the cardiomyocyte was arranged regularly and the
cross-sectional area was reduced in the treatment groups
(P<0.05), with an enhanced reduction in the SHR-AM + AT group
(P<0.05; Fig. 1D).
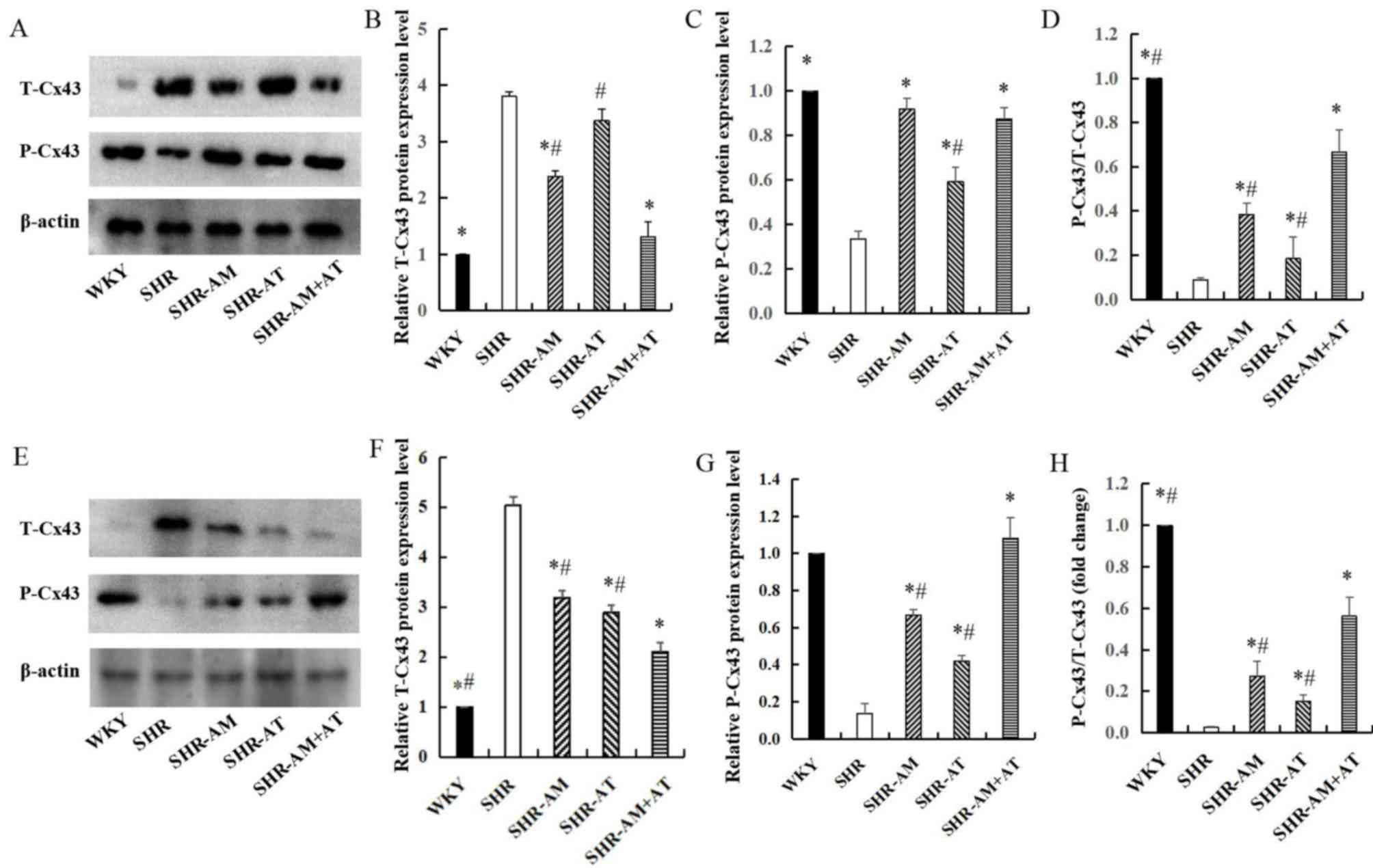
Comparison of T-Cx43 and P-Cx43
protein expression levels in the left ventricular and thoracic
aortic tissue using western blotting in each group
Compared with the WKY group, the expression of
T-Cx43 in the free wall of the left ventricle was significantly
increased in the SHR group (P<0.05). After treatment, compared
with the SHR group, the expression of T-Cx43 was significantly
decreased in SHR-AM and SHR-AM+AT (P<0.05), especially in SHR-AM
+ AT group (Fig. 2A and B). Moreover, compared with the SHR group,
the expression of P-Cx43 was significantly increased in treatment
groups (Fig. 2A and C). And the P-Cx43/T-Cx43 ratio
demonstrated significantly increased in treatment groups compared
with the ratio in the SHR group, (P<0.05), especially in
SHR-AM+AT (Fig. 2A and D). The results suggested that drug
treatment could enhance P-Cx43 protein expression, and that AM + AT
was superior to either AM or AT alone.
In thoracic aortic tissue, the expression of T-Cx43
was decreased in the treatment groups compared with the SHR group
(P<0.05), especially in SHR-AM + AT group (Fig. 2E and F). In addition, compared with SHR group,
P-Cx43 expression was significantly increased in the treatment
groups, with the highest upregulation in the SHR-AM + AT group
(P<0.05; Fig. 2E and G). The P-Cx43/T-Cx43 ratio was also
increased to a greater extent in the SHR-AM + AT group compared
with the SHR-AM and SHR-AT groups (P<0.05; Fig. 2E and H).
Expression of T-Cx43 and P-Cx43 in
myocardial tissue using immunofluorescence double labeling
Compared with the SHR group, the expression of
T-Cx43 in the left ventricular myocardium was significantly
decreased in other groups (P<0.05; Fig. 3A and B), whereas the expression of P-Cx43 was
significantly increased (P<0.05), particularly in the SHR-AM +
AT group (P<0.05; Fig. 3A and
C).
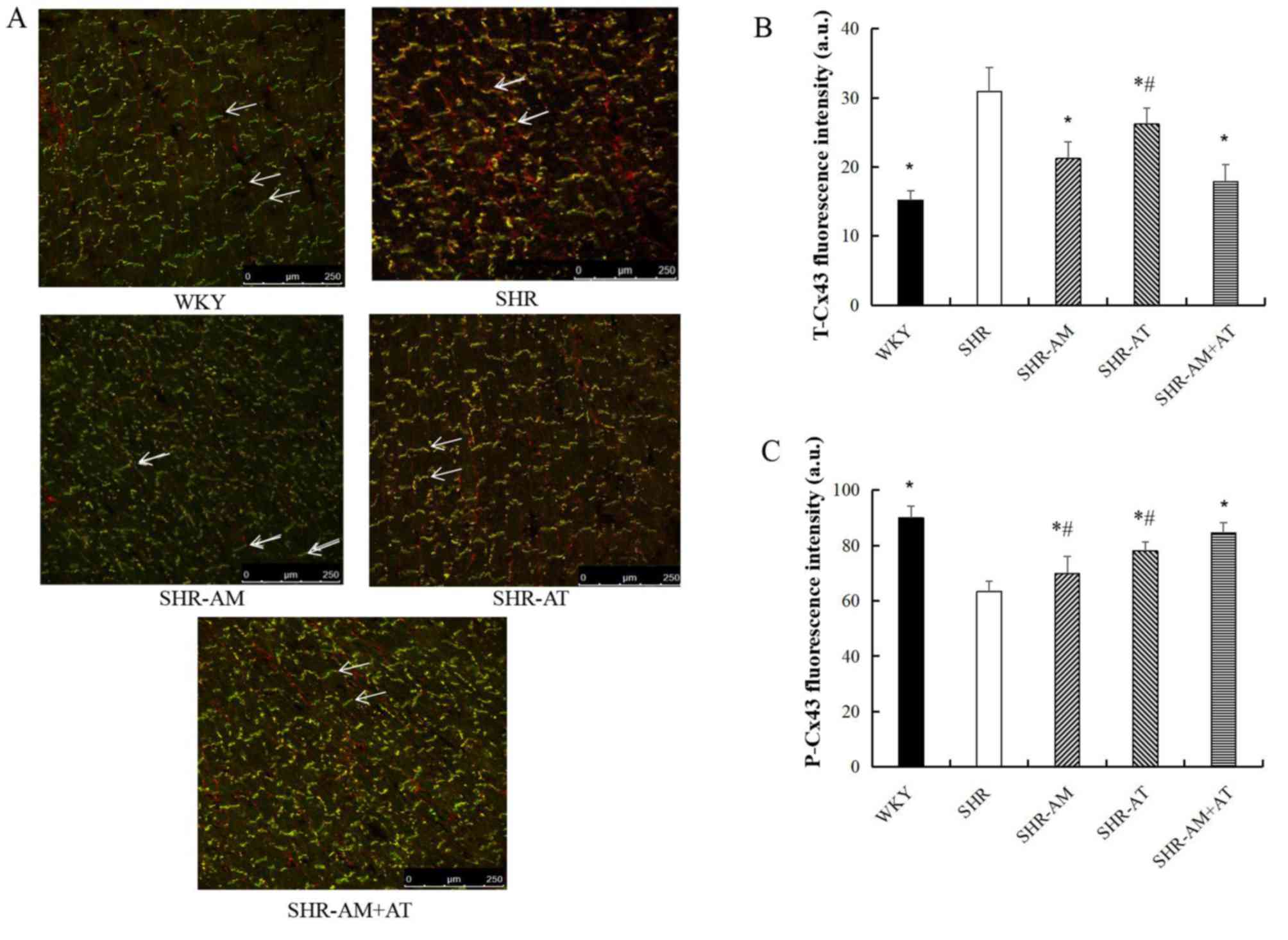 | Figure 3Comparison of T-Cx43 and P-Cx43
protein expression levels in left ventricular tissue using
immunofluorescence double labeling. (A) Results of
immunofluorescence double labeling analysis in left ventricular
tissue. Green fluorescence, T-Cx43; red fluorescence, P-Cx43.
Arrows indicate the intercalated disc. Magnification, x400.
Fluorescence intensity for (B) T-Cx43 and (C) P-Cx43. Data are
expressed as the mean ± SD; n=8; *P<0.05 vs. SHR;
#P<0.05 vs. SHR-AM + AT. AM, amlodipine; AT,
atorvastatin; a.u., arbitrary unit; Cx43, connexin 43; P-,
phosphorylated; SHR, spontaneous hypertension model rat; T-, total;
WKY, Wistar-Kyoto. |
Expression and distribution of T-Cx43
and P-Cx43 in the thoracic aorta using immunofluorescence double
labeling
Compared with WKY group, T-Cx43 expression was
increased and mainly distributed at the inner and medial membrane
of the thoracic aorta. Moreover, P-Cx43 expression was decreased
and mainly distributed at the medial and outer membrane in the SHR
group. Compared with the SHR group, the expression of T-Cx43 was
significantly decreased in the treatment groups, especially in the
SHR-AM+AT group (all P<0.05; Fig.
4A and B), and the expression
of P-Cx43 was significantly increased in treatment groups
(P<0.05), especially in the SHR-AM+AT group (P<0.05; Fig. 4A and C).
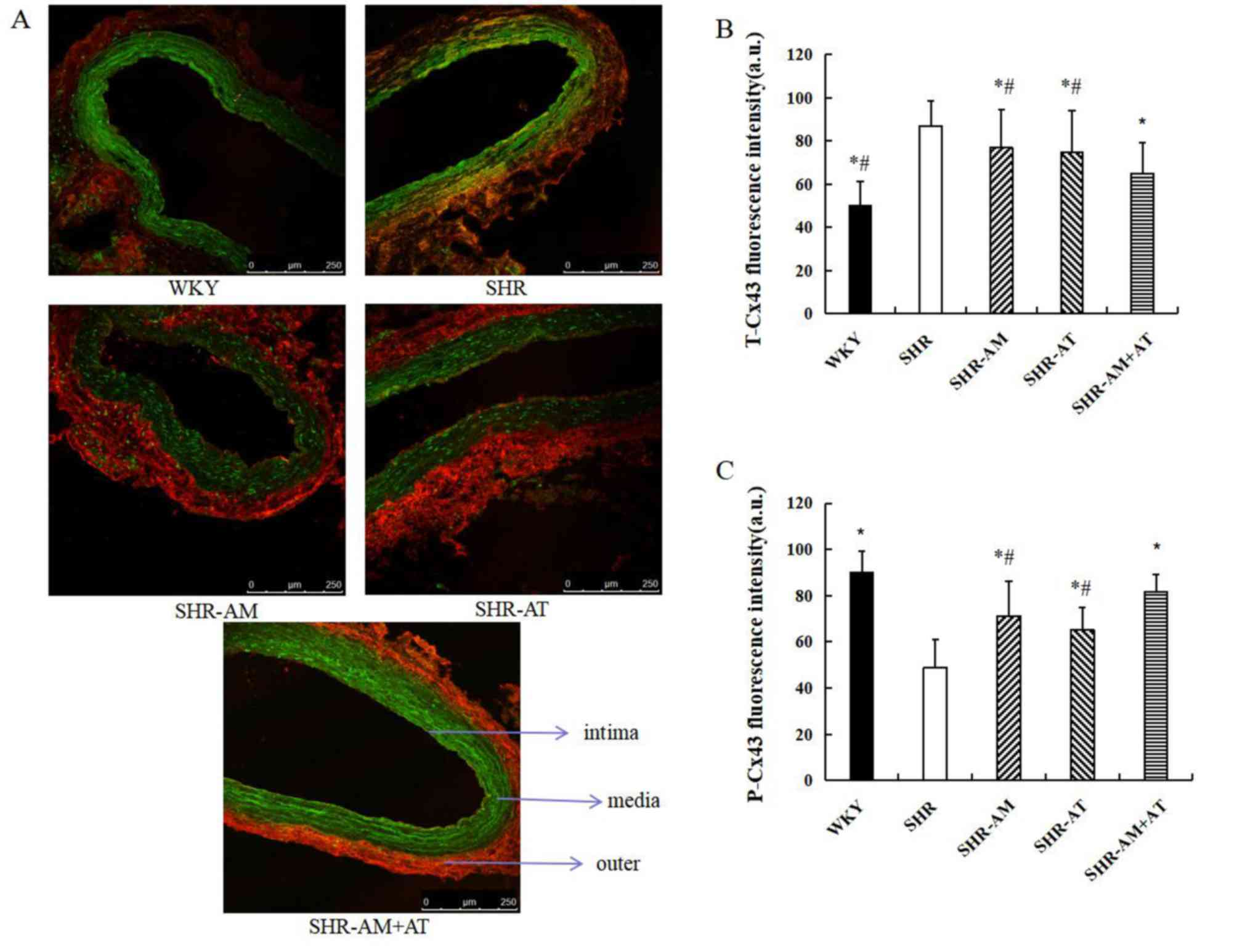 | Figure 4Comparison of T-Cx43 and P-Cx43
protein expression in the thoracic aorta using immunofluorescence
double labeling. (A) Results of immunofluorescence double labeling
analysis in thoracic aortic tissue. Green fluorescence, T-Cx43; red
fluorescence, P-Cx43. Magnification, x400. Fluorescence intensity
for (B) T-Cx43 and (C) P-Cx43 of thoracic aorta. Data are expressed
as the mean ± SD; n=8; *P<0.05 vs. SHR;
#P<0.05 vs. SHR-AM + AT. AM, amlodipine; AT,
atorvastatin; a.u., arbitrary unit; Cx43, connexin 43; P-,
phosphorylated; SHR, spontaneous hypertension model rat; T-, total;
WKY, Wistar-Kyoto. |
Discussion
Hypertension is one of the most critical factors to
cause left ventricular and vascular remodeling (22,23).
Furthermore, ventricular remodeling is a risk factor of various
severe arrhythmias, as well as sudden mortality (24). Owing to improved compliance, AM + AT
have been used for treating hypertension and hyperlipidemia, and
can protect vascular smooth muscle and myocardium from remodeling
(25,26). Caduet is a single pill containing AM
and AT, which blocks Ca2+ transmembrane influx, inhibits
β-Hydroxy β-methylglutaryl-coenzyme A reductase, decreases vascular
resistance and reverses ventricular remodeling (27). In addition, our previous study
revealed that the expression of Cx43 was enhanced in the
ventricular and thoracic aorta of SHR (15). However, the exact mechanism of AM +
AT improving ventricular and thoracic aorta remodeling by
regulating Cx43 phosphorylation it yet to be fully elucidated.
The present results indicated that AM + AT enhanced
Cx43 phosphorylation in the left ventricular and thoracic aorta of
SHR, and that AM + AT was superior to AM and AT treatment
alone.
GJs serve an important role in vascular tone and
blood pressure regulation (28).
The GJ protein Cx43 is a sensitive pressure receptor that is
closely related to the development of hypertension (29). Cardiovascular diseases have been
reported to affect the expression and localization of Cx43, and
dysregulated Cx43 is related to the loss of cardioprotection
(30,31). Furthermore, ischemic preconditioning
may reduce the incidence of arrhythmias by increasing Cx43
expression and altering GJ channel remodeling (32). In the current study, it was
suggested that the upregulation of T-Cx43 and downregulation of
P-Cx43 in myocardial tissue of SHR may be an adaptive response for
increasing blood pressure and cardiac load. Moreover, the
expression of P-Cx43 was increased and T-Cx43 was decreased after
AM + AT treatment, which could contribute to enhancing GJ
communication among vascular smooth muscle cells in resistant
arteries of SHR group, and causing an increased response to
vasoconstrictors and hypertension (33). Thus, it was concluded that AM + AT
may mitigate ventricular and vascular remodeling via increased
phosphorylation of Cx43.
In summary, AM + AT could improve ventricular and
vascular remodeling by inhibiting T-Cx43 expression and enhancing
Cx43 phosphorylation in SHR, indicating that AM + AT is superior to
AM and AT alone.
Acknowledgements
Not applicable.
Funding
This research was supported by The National Key
Research and Development Program of China (grant no.
2016YFD0500700), The National Natural Science Foundation of China
(grant no. 81573823) and The Science and Technology Incubation Fund
Project of Shaanxi Provincial People's Hospital, China (grant no.
2019YXQ-11).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XH, JY and GC conceived and designed the
experiments; XH, JY, BS, MM, HW, SW and GC performed the
experiments; XH, NW and GC analyzed the data; XH, SH and NW made
data interpretation and critical manuscript revisions; XH and JY
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures and ethics associated with animals
were reviewed and approved by Medical School of Xi'an Jiaotong
University (approval no. 2018-898).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Olsen MH, Angell SY, Asma S, Boutouyrie P,
Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP,
Hering D, et al: A call to action and a lifecourse strategy to
address the global burden of raised blood pressure on current and
future generations: The lancet commission on hypertension. Lancet.
388:2665–2712. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ettehad D, Emdin CA, Kiran A, Anderson SG,
Callender T, Emberson J, Chalmers J, Rodgers A and Rahimi K: Blood
pressure lowering for prevention of cardiovascular disease and
death: A systematic review and meta-analysis. Lancet. 387:957–967.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vriz O, Magne J, Jarosh J, Bossone E,
Aboyans V and Palatini P: Local carotid arterial stiffness is an
independent determinant of left ventricular remodeling in
never-treated hypertensive patients. Blood Press. 28:23–33.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Johnson RD and Camelliti P: Role of
non-myocyte gap junctions and connexin hemichannels in
cardiovascular health and disease: Novel therapeutic targets? Int J
Mol Sci. 19(866)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Desai CS, Ning H and Lloyd-Jones DM:
Competing cardiovascular outcomes associated with
electrocardiographic left ventricular hypertrophy: The
atherosclerosis risk in communities study. Heart. 98:330–334.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Opie LH, Commerford PJ, Gersh BJ and
Pfeffer MA: Controversies in ventricular remodeling. Lancet.
367:356–367. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Camargo LL, Harvey AP, Rios FJ,
Tsiropoulou S, Da Silva RNO, Cao Z, Graham D, McMaster C, Burchmore
RJ, Hartley RC, et al: Vascular Nox (NADPH oxidase)
compartmentalization, protein hyperoxidation, and endoplasmic
reticulum stress response in hypertension. Hypertension.
72:235–246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schaffer AL, Buckley NA and Pearson SA:
Who benefits from fixed-dose combinations? Two-year statin
adherence trajectories in initiators of combined
amlodipine/atorvastatin therapy. Pharmacoepidemiol Drug Saf.
26:1465–1473. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Chen HJ, Yao L, Chen TG, Yu M, Wang LH and
Chen JZ: Atorvastatin prevents connexin43 remodeling in
hypertrophied left ventricular myocardium of spontaneously
hypertensive rats. Chin Med J (Engl). 120:1902–1907.
2007.PubMed/NCBI
|
|
10
|
Waters D, Higginson L, Gladstone P,
Kimball B, Le May M, Boccuzzi SJ and Lespérance J: Effects of
monotherapy with an HMG-CoA reductase inhibitor on the progression
of coronary atherosclerosis as assessed by serial quantitative
arteriography. The Canadian coronary atherosclerosis treatment
trial. Circulation. 3:959–968. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salameh A: Life cycle of connexins:
Regulation of connexin synthesis and degradation. Adv Cardiol.
42:57–70. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodriguez-Sinovas A: Cx43 phosphorylation
and cardioprotection. Cardiovasc Res. 83:613–614. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Egan Benova T, Szeiffova Bacova B,
Viczenczova C, Diez E, Barancik M and Tribulova N: Protection of
cardiac cell-to-cell coupling attenuate myocardial remodeling and
proarrhythmia induced by hypertension. Physiol Res. 65 (Suppl
1):S29–S42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng G, Chen CY, Shou XL and Han XY:
Effects of captopril on the expression of connexin 43 in thoracic
aorta from spontaneous hypertensive rats. J Shaanxi Med.
44:1571–1573. 2015.
|
|
16
|
Martins-Marques T, Catarino S, Marques C,
Matafome P, Ribeiro-Rodrigues T, Baptista R, Pereira P and Girão H:
Heart ischemia results in connexin43 ubiquitination localized at
the intercalated discs. Biochimie. 112:196–201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martins-Marques T, Catarino S, Zuzarte M,
Marques C, Matafome P, Pereira P and Girão H: Ischaemia-induced
autophagy leads to degradation of gap junction protein connexin43
in cardiomyocytes. Biochem J. 467:231–245. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xue J, Yan X, Yang Y, Chen M, Wu L, Gou Z,
Sun Z, Talabieke S, Zheng Y and Luo D: Connexin 43
dephosphorylation contributes to arrhythmias and cardiomyocyte
apoptosis in ischemia/reperfusion hearts. Basic Res Cardiol.
114(40)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hradec J, Zamorano J and Sutradhar S: Post
hoc analysis of the cluster randomized usual care versus caduet
investigation assessing long-term risk (CRUCIAL) trial. Curr Med
Res Opin. 29:589–596. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu J, Liu F, Chen F, Jin Y, Chen H, Liu D
and Cui W: Amlodipine and atorvastatin improve ventricular
hypertrophy and diastolic function via inhibiting TNF-α, IL-1β and
NF-κB inflammatory cytokine networks in elderly spontaneously
hypertensive rats. Biomed Pharmacother. 83:330–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang ZY, Li Y, Li R, Zhang AA, Shang B,
Yu J and Xie XD: Tetrahydrobiopterin protects against
radiation-induced growth inhibition in H9c2 cardiomyocytes. Chin
Med J (Engl). 129:2733–2740. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin Y, Jing M, Zhang L, Song S and Ma X:
Internet access and hypertension management among the elderly
population: A nationally representative cross-sectional survey in
China. J Med Internet Res. 31(e11280)2019.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Delvaeye T, Vandenabeele P, Bultynck G,
Leybaert L and Krysko DV: Therapeutic targeting of connexin
channels: New views and challenges. Trends Mol Med. 24:1036–1053.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nagasawa K, Takahashi K, Matsuura N,
Takatsu M, Hattori T, Watanabe S, Harada E, Niinuma K, Murohara T
and Nagata K: Comparative effects of valsartan combination with
cilnidipine or amlodipine on cardiac remodeling and diastolic
dysfunction in Dahl salt-sensitive rats. Hypertens Res. 38:39–47.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen Y, Chang Y, Zhang N, Guo X, Sun G and
Sun Y: Atorvastatin attenuates myocardial hypertrophy in
spontaneously hypertensive rats via the C/EBPβ/PGC-1α/UCP pathway.
Cell Physiol Biochem. 46:1009–1018. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Naydenov Naydenov S, Margaritov Runev N,
Ivanov Manov E and Georgieva Torbova-Gigova S: Efficacy and safety
of a single-pill combination of atorvastatin/amlodipine in patients
with arterial hypertension and dyslipidemia. Acta Clin Croat.
57:464–472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song D, Liu X, Liu R, Yang L, Zuo J and
Liu W: Connexin 43 hemichannel regulates H9c2 cell proliferation by
modulating intracellular ATP and [Ca2+]. Acta Biochim Biophys Sin
(Shanghai). 42:472–482. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seki A, Nishii K and Hagiwara N: Gap
junctional regulation of pressure, fluid force, and electrical
fields in the epigenetics of cardiac morphogenesis and remodeling.
Life Sci. 129:27–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhai H, Dai W and Wang Y: Metoprolol
protects cardiomyocytes in rabbit model of heart failure by
regulating Cx43. Exp Ther Med. 15:1902–1905. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nao T, Ohkusa T, Hisamatsu Y, Inoue N,
Matsumoto T, Yamada J, Shimizu A, Yoshiga Y, Yamagata T, Kobayashi
S, et al: Comparison of expression of connexin in right atrial
myocardium in patients with chronic atrial fibrillation versus
those in sinus rhythm. Am J Cardiol. 91:678–683. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xing D, Kjølbye AL, Nielsen MS, Petersen
JS, Harlow KW, Holstein-Rathlou NH and Martins JB: ZP123 increases
gap junctional conductance and prevents reentrant ventricular
tachycardia during myocardial ischemia in open chest dogs. J
Cardiovasc Electrophysiol. 14:510–520. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang LJ, Ma KT, Shi WY, Wang YZ, Zhao L,
Chen XY, Li XZ, Jiang XW, Zhang ZS, Li L and Si JQ: Enhance gap
junction channel activity between vascular smooth muscle cells in
cerebral artery of spontaneously hypertensive rats. Clin Exp
Hypertens. 39:295–305. 2017.PubMed/NCBI View Article : Google Scholar
|