Introduction
Stress-related mucosal disease (SRMD) is a common
complication in patients of the intensive care unit (ICU) (1). Moreover, endoscopic examination within
72 h of ICU admission shows that 75-100% of patients who are
critically ill display lesions and 1-6% patients in ICU have
bleeding in the upper gastrointestinal mucosa (2). The pathological stage of SRMD is
related to disease severity of patients in ICU (3), thus, the more severe the condition,
the higher the pathological damage score. Furthermore, disease
severity of patients in ICU is evaluated using the Acute Physiology
and Chronic Health Evaluation (APACHE) II score (4). As one of the primary applied scoring
systems in ICU, the APACHE II score is widely used in the
assessment of various critical illnesses, including disease
severity, treatment and prognosis (5).
Oxidative stress, ischemia-reperfusion injury,
endogenous nitric oxide (NO) and reduced mucosal blood flow are
causative factors of SRMD (6).
However, the underlying mechanisms of SRMD are not fully
understood. Oxidative stress is the state when there is an
imbalance between reactive oxygen species (ROS) and the activity
and availability of antioxidants (7). Moreover, assessment of oxidative
stress can be achieved mainly via the following two parameters: i)
level of malondialdehyde (MDA), which is used as an indicator of
lipid peroxidation (8); and ii)
activity of superoxide dismutase (SOD), which reflects antioxidant
capacity (9). The first stage of
ROS-mediated cell damage is peroxidation of the cell membrane
matrix; in particular, lipid peroxidation (9,10). In
the lipid peroxidation process, a polar group is inserted into the
lipid molecule and is settled in the lipid bilayer (9). Therefore, the cell membrane becomes
hydrophobic and permeable (9).
Furthermore, the products of lipid peroxidation, such as MDA, react
with the amines of the cell membrane proteins to produce a Schiff
base, so that the cell membrane becomes stiffer (9,11).
Therefore, the level of MDA is used to evaluate ROS-induced damage
in various organs and tissues (11).
Ischemic modified albumin (IMA), which can be
induced by oxidative stress, increases within and outside of the
heart when ischemia-reperfusion injury occurs (12). Therefore, it is a candidate marker
in the assessment of myocardial ischemia, and has also been
identified as a marker of oxidative stress (13,14).
NO plays an important role in the regulation of
various cellular functions, including those of the gastrointestinal
tract (15). In addition, NO is
synthesized from L-arginine via NO synthase (NOS), including
constitutive NOS (cNOS) and inducible NOS (iNOS) (16). cNOS, includes neuronal NOS and
endothelial NOS, which produce small amounts of NO that act as a
neurotransmitter and vasodilator, respectively (16). However, iNOS produces larger amounts
of NO and is only expressed during inflammation (17). It has been previously reported that
NO plays biphasic roles in the ulcerogenic response of the gastric
mucosa (18,19). Previous studies have also shown that
ischemia-reperfusion injury is often accompanied by inflammation,
and iNOS can be expressed by inflammatory cells (16,20,21).
The claudin family of tight junction proteins plays
an important role in regulating epithelial paracellular
permeability (22). In vitro
experiments by Hashimoto et al (23) revealed that the expression of
claudin-3 in the gastric epithelium is reduced by
H2O2, a type of ROS. However, the
relationship between oxidative stress and claudin-3 expression in
gastric mucosal cells of patients with SRMD has not been
reported.
Although the pathogenesis of SRMD has been examined
in animal models and in vitro, to the best of our knowledge
there have been no studies conducted in patients. Therefore, in the
present study, patients from ICU were enrolled and the roles of
oxidative stress, iNOS and claudin-3 in the pathogenesis of SRMD
were investigated.
Materials and methods
Ethical statement
The study was carried out in Central Hospital of
Tai'an (Tai'an, China), from January 2015 to May 2017, and was
approved by the Institutional Ethics Committee of Tai'an Central
Hospital. All subjects provided their informed consent. The study
was registered at www.clinicaltrials.gov (registration no.
NCT03200158).
Patients and volunteers
In total, 38 patients in ICU with SRMD were enrolled
within 72 h after hospitalization. These SRMD patients included 20
males and 18 females, with an average age of 47.61±6.28 years. All
patients were diagnosed by gastroscopy and had gastric mucosal
lesion under gastroscopy. Patients with gastric cancer, esophageal
cancer or history of taking Aspirin were excluded. In total, 15 age
and sex matched healthy volunteers (8 females and 7 males; mean
age, 47.0±5.58 years) were recruited as controls.
APACHE II score
The APACHE II score was completed within 24 h after
ICU admission. The APACHE II scoring system includes 12
physiological parameters (with each item 0-4 points and a total of
0-60 points), chronic disease health status (2-5 points) and age
(0-6 points); the total score is 0-71 points (5).
Sample collection
Gastric mucosa and blood samples of subjects were
collected within 72 h after hospitalization. Biopsy gastric mucosal
tissues of each individual were taken from four different areas of
the most prominent erosion area of gastric mucosa during
gastroscopy or the normal gastric antrum (for healthy controls).
Peripheral venous blood samples (6 ml) were collected from each
individual. The serum was collected after centrifugation at 1,000 x
g at 4˚C for 15 min.
Pathological damage score
Gastric mucosal tissues were fixed with 10% formalin
at room temperature for 24 h and routinely paraffin embedded,
sliced at 3-µm and stained with hematoxylin and eosin (H&E; 60
and 90 sec, respectively), at room temperature according to routine
procedure. The degree of pathological damage was scored according
to the Masuda criteria (24) with a
slight modification: i) 0, superficial epithelial cells were intact
and arranged well; ii) 1, surface epithelium was damaged and the
neutrophils were infiltrated; iii) 2, upper mucosa was congested or
edematous; iv) 3, cells were interrupted, the middle or lower layer
of the membrane was congested, with edema or bleeding; v) 4, upper
mucosal gland structure was disordered or necrotic; and vi) 5, deep
necrosis or ulceration. The mean score of five slides was
calculated as the pathological damage score of each patient.
Serum MDA, SOD and IMA
measurement
Serum MDA was detected using the MDA assay kit (cat.
no. A003-1; Nanjing Jiancheng Bioengineering Institute) as
previously described (25).
Serum IMA was measured by the ischemia modified
albumin detection kit (https://www.reebio.com/products/product-i328.html;
Ningbo Ruiyuan Biological Technology Co., Ltd.). The serum was
treated with 5.0 mmol/l cobalt oxide at 37˚C for 3-5 min, and serum
albumin combined with the cobalt ions and the absorbance value at
405 nm (A1) was measured using automatic biochemical
analyzer (Cobas® 8000; Roche Diagnostics). Subsequently,
2.8 mmol/l chromogenic agent (at 37˚C for 5 min) was added to the
residual cobalt ions to produce a reddish brown product and the
absorbance value at 510 nm (A2) were measured. The serum
IMA content was calculated using the following formula: IMA
concentration (U/ml)=(A2-A1)/ΔA calibration x
IMA calibrator concentration.
The activity of SOD was determined using a SOD
detection kit [http://www.co-health.com.cn/content/nr_fy.jsp?code=hyyksw_cpzx_cpml;
Co-Health (Beijing) Laboratories Co., Ltd.;]. After incubation with
10 mmol/l reagent 1 at 37˚C for 5 min, the absorbance value
(A1 405 nm) was measured before 20 mmol/l reagent 2 (at
37˚C for 5 min) was added and the absorbance value (A2
510 nm) were measured. SOD concentration in the serum sample was
calculated according to that in a standard substance, whose SOD
concentration was known, with the following formula: SOD
concentration (U/ml)=(A2-A1)/ΔA calibration x
SOD calibrator concentration.
Immunohistochemistry
Measurement of iNOS in gastric mucosa was carried
out by immunohistochemistry. The biopsy specimens were fixed with
10% formalin at room temperature for 24 h and routinely processed
in paraffin and cut into 5-µm sections. The paraffin sections were
then dewaxed and washed with PBS, and 50 µl 0.3%
H2O2 solution was added and incubated at room
temperature for 10 min to block endogenous peroxidase activity.
Subsequently, sections were incubated with 50 µl non-immunized
animal serum (OriGene Technologies, Inc.) for 10 min at room
temperature before 50 µl rabbit anti-human anti-iNOS polyclonal
antibody (cat. no. ab53769; 1:200; Abcam) was added and incubated
overnight at 4˚C. After washing with PBS, the sections were
incubated with 50 µl biotin-labeled goat anti-rabbit secondary
antibodies (1:1,000; cat. no. TA130016; OriGene Technologies, Inc.)
at room temperature for 15 min. After washing again, 50 µl
peroxidase-labeled streptavidin (cat. no. SP Kit-D1; Fuzhou Maixin
Biotechnology Development Co., Ltd.) was added and incubated at
room temperature for 15 mi, following which 100 µl freshly prepared
3'3' diaminobenzidine solution was added at room temperature and
color developed for 3-10 min. Sections were routinely stained with
hematoxylin at room temperature for 1 min, followed by regular
dehydration, made transparent and mounted with neutral gum.
Microscopic observation was performed with light microscopy at x400
magnification (Eclipse Ci-L; Nikon Corporation).
Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.) was used to select the brownish yellow color as a uniform
standard for determining the positive staining of all images. Each
image was analyzed to determine the average optical density.
Western blotting
Tissue was first lysed using RIPA buffer (cat. no.
R0010; Beijing Solarbio Science & Technology Co., Ltd.). The
protein concentration of the mucosal samples was detected by
bicinchoninic acid assay (Beijing Solarbio Science & Technology
Co., Ltd.). An equal amount of protein (40 µg) of each sample was
loaded onto 12% polyacrylamide gels for electrophoresis and then
transferred to a PVDF membrane. After blocking at 25˚C with 5%
fat-free milk for 1-2 h, the membranes were incubated with
anti-claudin-3 antibody (cat. no. ab15102; 1:200; Abcam) and
β-actin (1:5,000; cat. no. 66009-1-lg; ProteinTech Group, Inc.) at
4˚C overnight. After incubation at 25˚C with horseradish
peroxidase-conjugated secondary antibody (cat. no. SA00001-2,
dilution 1:2,000; ProteinTech Group, Inc.) for 2 h, the membranes
were visualized by Mini Chemi 610 electrochemiluminescence (Beijing
Sage Creation Science Co., Ltd.) and the gray value was analyzed
using Lane ID software 5.0 (Beijing Sage Creation Science Co,
Ltd.).
Statistical analysis
SPSS 16.0 software (IBM Corp.) was used for
statistical analysis. Data are presented as the mean ± SD. An
independent sample t-test was used for comparisons between two
groups. Measurement data were analyzed by linear correlation
analysis (Pearson's rank correlation test) after the normality
test. Bonferroni's correction test was used when analyzing the
correlation of multiple groups and the P-value was denoted as P
corrected (Pc). P<0.05 was considered to indicate a
statistically significant difference.
Results
APACHE II scores and pathological
damage scores
The APACHE score consists of four categories,
including APACHE I, II, III and IV, among which APACHE II provides
clinical values in prediction of hospital mortality, ICU, length of
stay, resource utilization, cost-effectiveness and necessities of
receiving positive life support treatment (5); the higher the score, the more severe
the illness will be (26). In the
present study, the average APACHE II score of the patients was
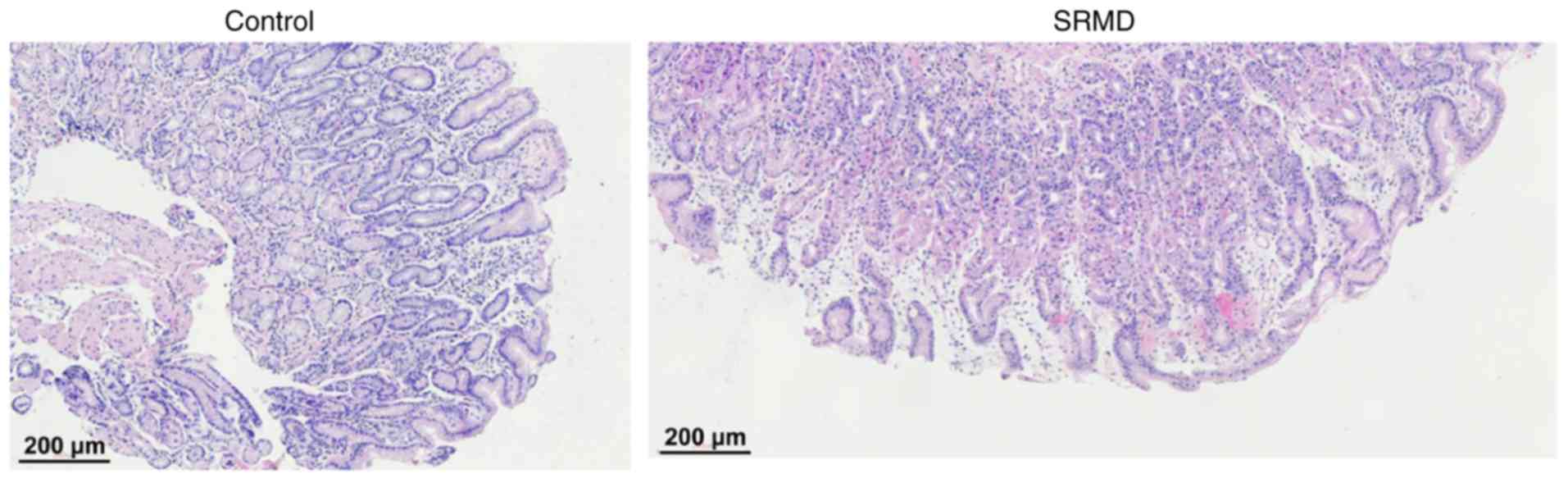
14.87±5.6 points. Furthermore, the average pathological damage
score of gastric mucosa was calculated by analyzing the H&E
staining (Fig. 1). The score was
2.92±1.08 points in patients with SRMD, which was significantly
higher compared with the control group (1.04±0.60; P<0.001; data
not shown).
Comparison of serum indexes between
patients and volunteers
Serum indexes of oxidative stress in patients with
SRMD were analyzed. It was found that the levels of MDA (4.74±2.89
nmol/ml) and IMA (93.61±10.78 U/ml) in patients with SRMD were
significantly higher compared with the healthy controls
(P<0.001; Table I). Furthermore,
the levels of SOD (89.66±12.85 U/ml) in patients with SRMD were
significantly lower than compared with the controls (P<0.001;
Table I). Thus, these results
suggest that the oxidative stress indexes of patients with SRMD
were significantly higher, while the antioxidant index was
significantly lower.
 | Table IComparison of serum index levels
between patients and healthy controls. |
Table I
Comparison of serum index levels
between patients and healthy controls.
| Parameter | Patients | Controls | t | P-value |
|---|
| MDA (nmol/ml) | 4.74±2.89 | 0.55±0.34 | 8.769 | <0.001 |
| SOD (U/ml) | 89.66±12.85 | 148.73±13.50 | -14.868 | <0.001 |
| IMA (U/ml) | 93.61±10.78 | 59.07±7.411 | 11.358 | <0.001 |
Comparison of mucosal indexes between
patients and volunteers
Next, the mucosal index was compared between
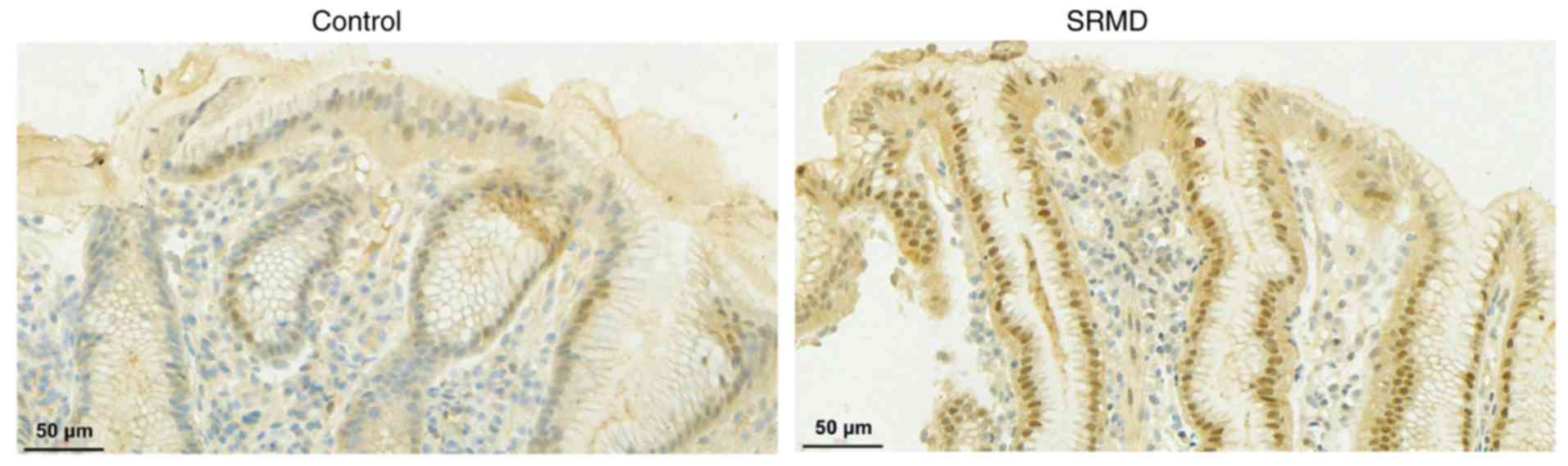
patients and healthy controls. More positive staining was
identified for iNOS in patients with SRMD compared with healthy
controls (Fig. 2). In addition, the
mean levels of iNOS in patients were significantly higher compared
with the controls (Table II).
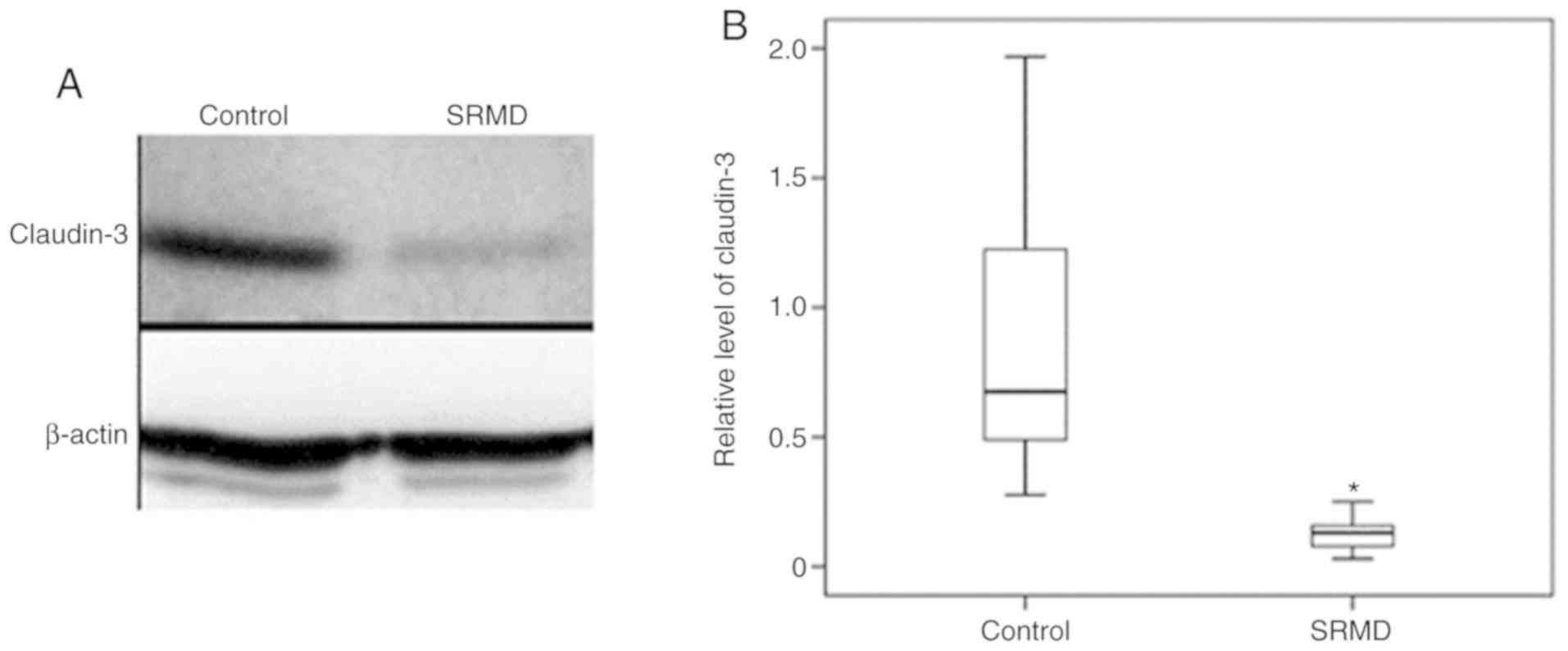
Furthermore, claudin-3 expression in mucosal epithelium was
measured by western blotting (Fig.
3A), and the mean expression of claudin-3 in patients was
significantly lower compared with the controls (P<0.001;
Fig. 3B; Table II).
 | Table IIComparison of gastric mucosal index
between patients and healthy controls. |
Table II
Comparison of gastric mucosal index
between patients and healthy controls.
| Protein | Patients | Controls | t | P-value |
|---|
| Claudin-3 (gray
value) | 0.12±0.050 | 0.95±0.615 | -5.195 | <0.001 |
| iNOS (average
optical density) | 0.1183±0.0173 | 0.0578±0.0031 | 2.180 | 0.034 |
Relationship of APACHE II score with
pathological damage score, MDA and SOD
To study the relationship between disease
progression and oxidative stress or mucosal damage, correlation
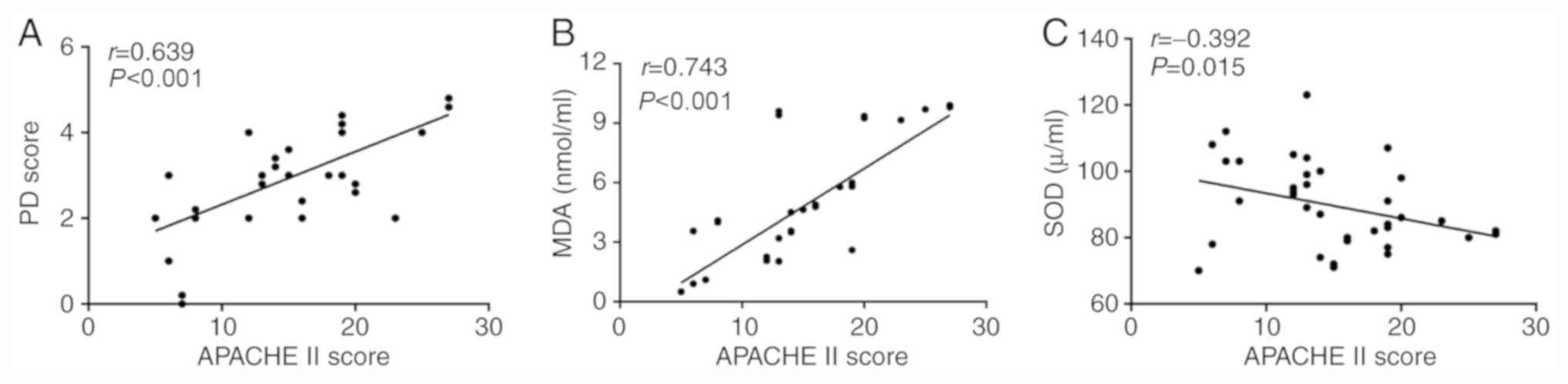
analysis was performed. It was demonstrated that the APACHE II
score was moderately positively correlated with pathological damage
score (r=0.639, P<0.001) and strongly positively correlated with
MDA (r=0.743, P<0.001; Fig. 4A
and B), and weakly negatively
correlated with SOD (r=-0.392, P=0.015; Fig. 4C; after Bonferroni's correction,
Pc=0.016). Therefore, it was speculated that oxidative stress was
increased with the severity of diseases.
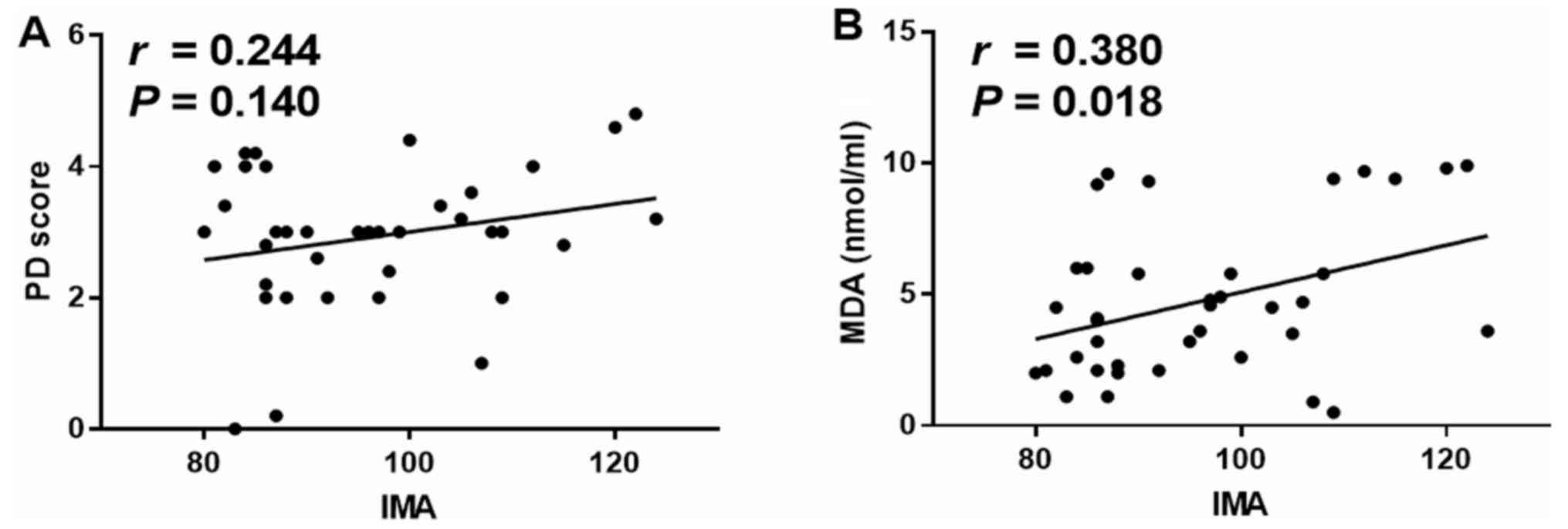
Relationship of IMA with pathological
damage score and MDA
To evaluate whether IMA can reflect the degree of
gastric mucosal lesions, the relationship of IMA with pathological
damage score and MDA was analyzed. It was demonstrated that IMA was
not significantly correlated with pathological damage score
(P=0.140; Fig. 5A). However, IMA
was weakly positively correlated with MDA (r=0.380, P=0.018;
Fig. 5B; after Bonferroni's
correction, Pc=0.05/2=0.025). Collectively, the results
indicated that IMA can reflect the degree of oxidative stress, but
not the degree of stress in gastric mucosal lesions.
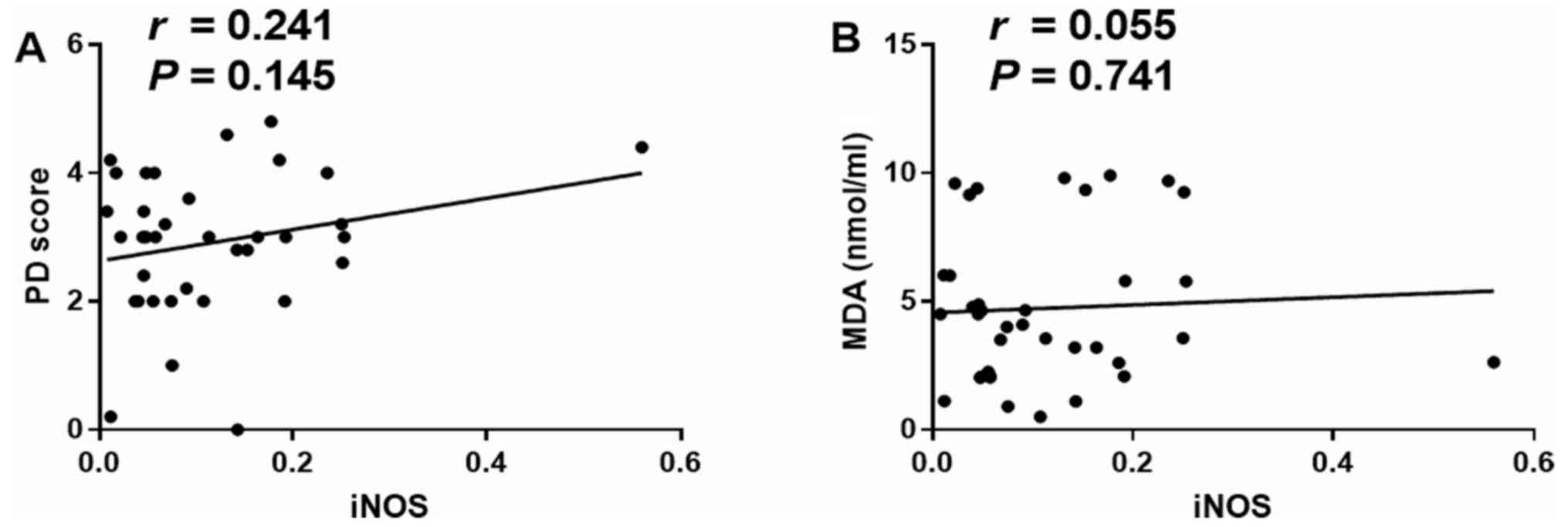
Relationship of iNOS with pathological
damage score and MDA
By analyzing the relationship between mucosal iNOS
and the mucosal damage or the oxidative stress index, it was
indicated that iNOS was not significantly correlated with
pathological damage score or MDA (P>0.05; Fig. 6A and B; after Bonferroni's correction,
Pc=0.05/2=0.025).
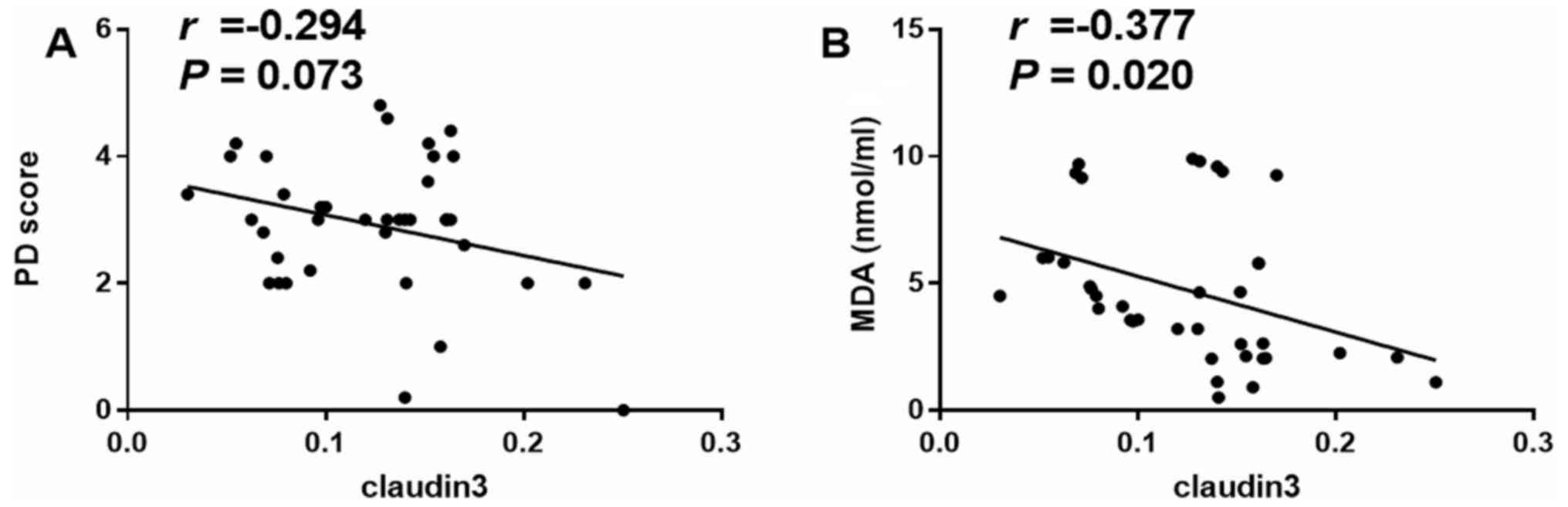
Relationship of claudin-3 with
pathological damage score and MDA
The relationship between mucosal permeability and
mucosal damage or oxidative stress was also investigated. It was
identified that claudin-3 was not significantly correlated with
pathological damage score (P>0.05; Fig. 7A). However, claudin-3 was weakly
negatively correlated with MDA (r=-0.377; P=0.020; Fig. 7B; after Bonferroni's correction,
Pc=0.05/2=0.025). Thus, it was speculated that mucosal
permeability may be related to the degree of oxidative stress.
Discussion
In clinical practice, SRMD is usually observed in
patients in ICU during gastroscopy (1). The present results suggested that the
levels of MDA and IMA in the serum of patients with SRMD were
significantly higher compared with the healthy controls. However,
the level of SOD in the serum of patients with SRMD was
significantly lower compared with the controls. Therefore, the
results indicated that the antioxidant system could not compete
with oxidative stress in patients with SRMD, and thus, it was
speculated that oxidative stress may be important in the
pathogenesis of SRMD.
MDA is currently the most widely used indicator for
the detection of lipid peroxidation products and is often used to
reflect the degree of oxidative stress (8). The present results identified
significantly elevated MDA levels in patients with SRMD, suggesting
that lipid peroxidation induced by ROS may be involved in the
formation of gastric mucosal damage in patients with SRMD.
The activity and level of SOD reflects the
antioxidant capacity (9). IMA is
also an indicator of oxidative stress. Moreover, IMA in the heart
and other organs or tissues increases when oxidative stress is
induced by ischemia-reperfusion injury (12,27,28).
In the hypoxic state, the production of hydroxyl radicals of ROS
leads to changes in the N-terminus of albumin and loss of transport
metal adhesion, which can produce IMA (29). In the present study, the lower mean
value of SOD, as well as higher mean IMA levels in patients with
SRMD collectively demonstrated that oxidative stress may be
involved in the pathogenesis of SRMD.
Previous studies have reported a correlation between
disease severity and oxidative stress state. For example, Lorente
et al (30) revealed that
the level of MDA in serum is associated with APACHE II score. In
addition, Satoh et al (31)
showed significantly positive correlations between IMA and APACHE
II score. The present results also suggested that APACHE II score
was positively correlated with MDA, and negatively correlated with
SOD, thus indicating that oxidative stress may be positively
correlated with disease severity. Furthermore, there was a positive
correlation between APACHE II score and mucosal pathological damage
score. Collectively, the results demonstrated that oxidative stress
may be an important aspect of the pathogenesis of SRMD.
NO vasodilatation, a basic gastric mucosal defense,
provides blood to the mucosa to resist damage from gastric acid and
pepsin (32,33). However, the role of NO in the
gastrointestinal tract is not just a defense mechanism, and
previous studies (18,19) have revealed that NO may play a dual
role in ischemia-reperfusion-induced mucosal damage (34). Furthermore, NO is increased in
ischemia myocardial tissue following ischemia-reperfusion (35). During ischemic tissue reperfusion,
Ca2+ flows into the cells and the formation of oxygen
free radicals may lead to ischemia-reperfusion injury (36). Moreover, NO can react with oxygen
radicals to yield peroxynitrite, which can cause tissue damage via
lipid peroxidation (37). The
present results suggested that the expression of iNOS in patients
with SRMD was significantly higher compared with healthy
volunteers. Therefore, it was speculated that ischemic hypoxic
conditions are more severe in patients who are critically ill and
that ischemia-reperfusion leads to higher iNOS expression in these
patients, which induces the production of large amounts of NO.
Thus, under oxidative stress, peroxynitrite produced by the
reaction of ROS with NO directly damages tissues and leads to SRMD
(17).
Based on the complexity of the pathogenesis of SRMD
and the changes of claudin-3 under oxidative stress (23), the present study also investigated
the effect of SRMD on claudin-3 expression. It was identified that
the expression of claudin-3 in patients with SRMD was significantly
lower compared with healthy controls, and claudin-3 expression in
patients was negatively correlated with MDA. As a type of ROS,
H2O2 can remain in cells for a long time and
act as a second messenger for a variety of physiological stimuli,
such as inflammatory cytokines and growth factors (38). It has also been reported that
H2O2 can activate cAMP-dependent protein
kinase, which can further phosphorylate claudin-3 (39,40).
Thus, it was speculated that H2O2
phosphorylates claudin-3 by activating the cAMP-dependent protein
kinase, resulting in a degradation of claudin-3. Collectively, it
was hypothesized that the pathogenesis of SRMD may be associated
with the decrease in tight junction proteins and an increase in
permeability due to the influence of ROS on claudin-3, but its
specific mechanism remains to be elucidated.
However, there were some limitations to the present
study. First, the sample size was small. Moreover, the interval
between the stress events and the sampling time was different,
which may have led to bias. Third, psychological stress may be an
important factor affecting the mucosal barrier in patients who are
critically ill (41), but this
factor was not included in this study due to practical
difficulties.
In conclusion, the present results suggested that
there was oxidative stress in patients who are critically ill with
SRMD, and that the degree of oxidative stress may be related to
disease severity of patients in ICU. Therefore, oxidative stress
may be an important aspect of the pathogenesis of SRMD. However,
oxidative stress may lead to SRMD via increased NO. Moreover,
oxidative stress may increase the permeability of cell membrane by
affecting claudin-3 expression. Thus, future studies should focus
on factors related to oxidative stress to identify sensitive
indicators of SRMD and to avoid over-medication for patients who
are low-risk.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW and XZ designed the study. XW, QZ, HS, FQ, NS, DB
and HY contributed to the data acquisition and conducted the
experiments. QZ, HS and XL performed the statistical analysis. XW
and DB prepared the manuscript. QZ, HS and NS conducted the
literature search. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Tai'an Central Hospital. All subjects provided their
informed consent. The study was registered at www.clinicaltrials.gov (study ID. NCT03200158).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kantorova I, Svoboda P, Scheer P, Doubek
J, Rehorkova D, Bosakova H and Ochmann J: Stress ulcer prophylaxis
in critically ill patients: A randomized controlled trial.
Hepatogastroenterology. 51:757–761. 2004.PubMed/NCBI
|
|
2
|
Lin PC, Chang CH, Hsu PI, Tseng PL and
Huang YB: The efficacy and safety of proton pump inhibitors vs.
histamine-2 receptor antagonists for stress ulcer bleeding
prophylaxis among critical care patients: A meta-analysis. Crit
Care Med. 38:1197–1205. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chu YF, Jiang Y, Meng M, Jiang JJ, Zhang
JC, Ren HS and Wang CT: Incidence and risk factors of
gastrointestinal bleeding in mechanically ventilated patients.
World J Emerg Med. 1:32–36. 2010.PubMed/NCBI
|
|
4
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985.PubMed/NCBI
|
|
5
|
Niewiński G, Starczewska M and Kański A:
Prognostic scoring systems for mortality in intensive care
units-the APACHE model. Anaesthesiol Intensive Ther. 46:46–49.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ali T and Harty RF: Stress-induced ulcer
bleeding in critically ill patients. Gastroenterol Clin North Am.
38:245–265. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Preiser JC: Oxidative stress. JPEN J
Parenter Enteral Nutr. 36:147–154. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Spirlandeli AL, Deminice R and Jordao AA:
Plasma malondialdehyde as biomarker of lipid peroxidation: Effects
of acute exercise. Int J Sports Med. 35:14–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kwiecień S, Brzozowski T and Konturek SJ:
Importance of aldehyde products of lipid peroxidation in the
formation of gastric lesions induced by aspirin,
ischemia-reperfusion and stress. Gastroenterol Polska. 9:273–280.
2002.
|
|
10
|
Matsuda T, Tao H, Goto M, Yamada H, Suzuki
M, Wu Y, Xiao N, He Q, Guo W, Cai Z, et al: Lipid
peroxidation-induced DNA adducts in human gastric mucosa.
Carcinogenesis. 34:121–127. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kwiecień S, Pawlik MW, Brzozowski T,
Pawlik WW and Konturek SJ: Reactive oxygen metabolite action in
experimental, stress model of gastric mucosa damage. Gastroenterol
Polska. 17:234–243. 2010.
|
|
12
|
Borderie D, Allanore Y, Meune C, Devaux
JY, Ekindjian OG and Kahan A: High ischemiamodifed albumin
concentration reflects oxidative stress but not myocardial
involvement in systemic sclerosis. Clin Chem. 50:2190–2193.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aydin O, Ellidag HY, Eren E, Kurtulus F,
Yaman A and Yilmaz N: Ischemia modified albumin is an indicator of
oxidative stress in multiple sclerosis. Biochem Med (Zagreb).
24:383–389. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gothe PR, Jose M, Pai VR, Harish S,
D'Souza J and Prabhu V: Investigation of the possibility of using
serum ischemia modified albumin (IMA) as a novel and early marker
of the extent of oxidative stress induced by various tobacco
products. J Clin Diagn Res. 9:ZC33–ZC35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martin MJ, Jimenez MD and Motilva V: New
issues about nitric oxide and its effects on the gastrointestinal
tract. Curr Pharm Des. 7:881–908. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stanek A, Gadowska-Cicha A, Gawron K,
Wielkoszyński T, Adamek B, Cieślar G, Wiczkowski A and Sieroń A:
Role of nitric oxide in physiology and pathology of the
gastrointestinal tract. Mini Rev Med Chem. 8:1549–1560.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kobata A, Kotani T, Komatsu Y, Amagase K,
Kato S and Takeuchi K: Dual action of nitric oxide in the
pathogenesis of ischemia/reperfusion-induced mucosal injury in
mouse stomach. Digestion. 75:188–197. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tanaka A, Kunikata T, Mizoguchi H, Kato S
and Takeuchi K: Dual action of nitric oxide in pathogenesis of
indomethacin-induced small intestinal ulceration in rats. J Physiol
Pharmacol. 50:405–417. 1999.PubMed/NCBI
|
|
19
|
Whittle BJ, Laszlo F, Evans SM and Moncada
S: Induction of nitric oxide synthase and microvascular injury in
the rat jejunum provoked by indomethacin. Br J Pharmacol.
116:2286–2290. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu J, Yang Y, Xun N, Zeng L, Li Z, Yang W,
Liang Y, Ma Z and Tang H: Osthole attenuates myocardial
ischemia/reperfusion injury in rats by inhibiting apoptosis and
inflammation. Am J Transl Res. 10:1109–1116. 2018.PubMed/NCBI
|
|
21
|
Zhu L, Wei T, Gao J, Chang X, He H, Luo F,
Zhou R, Ma C, Liu Y and Yan T: The cardioprotective effect of
salidroside against myocardial ischemia reperfusion injury in rats
by inhibiting apoptosis and inflammation. Apoptosis. 20:1433–1443.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel RM, Myers LS, Kurundkar AR,
Maheshwari A, Nusrat A and Lin PW: Probiotic bacteria induce
maturation of intestinal claudin 3 expression and barrier function.
Am J Pathol. 180:626–635. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashimoto K, Oshima T, Tomita T, Kim Y,
Matsumoto T, Joh T and Miwa H: Oxidative stress induces gastric
epithelial permeability through claudin-3. Biochem Biophys Res
Commun. 376:154–157. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Masuda E, Kawano S, Nagano K, Tsuji S,
Takei Y, Hayashi N, Tsujii M, Oshita M, Michida T and Kobayashi I:
Role of endogenous endothelin in pathogenesis of ethanol-induced
gastric mucosal injury in rats. Am J Physiol. 265:G474–G481.
1993.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yagi K: A simple fluorometric assay for
lipoperoxide in blood plasma. Biochem Med. 15:212–216.
1976.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Swann JW, Maunder CL, Roberts E,
McLauchlan G and Adamantos S: Prevalence and risk factors for
development of hemorrhagic gastro-intestinal disease in veterinary
intensive care units in the United Kingdom. J Vet Emerg Crit Care
(San Antonio). 26:419–427. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sbarouni E, Georgiadou P and Voudris V:
Ischemia modified albumin changes-review and clinical implications.
Clin Chem Lab Med. 49:177–184. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sharma R, Gaze DC, Pellerin D, Mehta RL,
Gregson H, Streather CP, Collinson PO and Brecker SJ:
Ischemia-modified albumin predicts mortality in ESRD. Am J Kidney
Dis. 47:493–502. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Apple FS, Quist HE, Otto AP, Mathews WE
and Murakami MM: Release characteristics of cardiac biomarkers and
ischemia-modified albumin as measured by the albumin cobalt-binding
test after a marathon race. Clin Chem. 48:1097–1100.
2002.PubMed/NCBI
|
|
30
|
Lorente L, Martín MM, Abreu-Gonzalez P,
Domínguez-Rodríguez A, Labarta L, Díaz C, Solé-Violán J, Ferreres
J, Borreguero-León JM, Jiménez A and Morera-Fumero A: Prognostic
value of malondialdehyde serum levels in severe sepsis: A
multicenter study. PLoS One. 8(e53741)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Satoh M, Kotani K, Gugliucci A, Horie H,
Caccavello R and Takeuchi M: Correlation of ischemia-modified
albumin with SOFA and APACHE II scores in preoperative patients
with colorectal cancer. ScientificWorldJournal.
2014(959075)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kotani T, Kobata A, Nakamura E, Amagase K
and Takeuchi K: Roles of cyclooxygenase-2 and prostacyclin/IP
receptors in mucosal defense against ischemia/reperfusion injury in
mouse stomach. J Pharmacol Exp Ther. 316:547–555. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Naito Y, Yoshikawa T, Matsuyama K, Yagi N,
Arai M, Nakamura Y, Kaneko T, Yoshida N and Kondo M: Neutrophils,
lipid peroxidation, and nitric oxide in gastric reperfusion injury
in rats. Free Radic Biol Med. 24:494–502. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lopez-Belmonte J, Whittle BJ and Moncada
S: The actions of nitric oxide donors in the prevention or
induction of injury to the rat gastric mucosa. Br J Pharmacol.
108:73–78. 1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu T, Yao Q, Wang W, Yao H and Chao J:
INOS induces vascular endothelial cell migration and apoptosis via
autophagy in ischemia/reperfusion injury. Cell Physiol Biochem.
38:1575–1588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Okuda M, Lee HC, Chance B and Kumar C:
Role of extracellular Ca2+ in ischemia-reperfusion injury in the
isolated perfused rat liver. Circ Shock. 37:209–219.
1992.PubMed/NCBI
|
|
37
|
Dijkstra G, Moshage H, van Dullemen HM, de
Jager-Krikken A, Tiebosch AT, Kleibeuker JH, Jansen PL and van Goor
H: Expression of nitric oxide synthases and formation of
nitrotyrosine and reactive oxygen species in inflammatory bowel
disease. J Pathol. 186:416–421. 1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bae YS, Kang SW, Seo MS, Baines IC, Tekle
E, Chock PB and Rhee SG: Epidermal growth factor (EGF)-induced
generation of hydrogen peroxide. Role in EGF receptor-mediated
tyrosine phosphorylation. J Biol Chem. 272:217–221. 1997.PubMed/NCBI
|
|
39
|
Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou
ZP, Zeng WS, Cheng BL and Luo SQ: Reactive oxygen species
stimulates receptor activator of NF-kappaB ligand expression in
osteoblast. J Biol Chem. 280:17497–17506. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
D'Souza T, Agarwal R and Morin PJ:
Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent
protein kinase regulates tight junction barrier function in ovarian
cancer cells. J Biol Chem. 280:26233–26240. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
De R, Mazumder S, Sarkar S, Debsharma S,
Siddiqui AA, Saha SJ, Banerjee C, Nag S, Saha D and Bandyopadhyay
U: Acute mental stress induces mitochondrial bioenergetic crisis
and hyper-fission along with aberrant mitophagy in the gut mucosa
in rodent model of stress-related mucosal disease. Free Radic Biol
Med. 113:424–438. 2017.PubMed/NCBI View Article : Google Scholar
|