Introduction
In the 1950s, polymyxins were widely used as
antimicrobial agents in the clinical treatment of gram-negative
infections. Thereafter, polymyxins were replaced with new
antibiotics because of its narrow therapeutic index and the high
risk for nephrotoxicity and neurotoxicity (1). Over the past 30 years, few clinical
studies have been conducted regarding the antimicrobial spectrum,
pharmacokinetics, pharmacodynamics, toxicology and clinical use of
polymyxins, especially polymyxin B.
The outbreak of carbapenem-resistant
Enterobacteriaceae (CRE) is one of the main reasons for the
increased use of polymyxin B (2-4).
Most CRE outbreaks in China occur in the intensive care unit (ICU).
When the minimum inhibitory concentration (MIC) >4 mg/l,
enterobacteriaceae are resistant to carbapenem; the MIC of
carbapenem against CRE is usually >16 mg/l, and, therefore, the
MIC of carbapenem against CRE is very high (4,5). In
addition to CRE, the incidence of pan-drug resistant
Acinetobacter baumannii and Pseudomonas aeruginosa
(MIC values ≥16 mg/l for both) has also gradually increased in the
ICU of our hospital (The First Affiliated Hospital of Bengbu
Medical College; Bengbu, China) for the past 2 years. Data released
by the China Antimicrobial Surveillance Network (CHINET) in 2018
showed that the imipenem resistance rate of A. baumannii was
as high as 73.2%, whereas resistance rates of P. aeruginosa
and Klebsiella pneumoniae were 30.7 and 37.6%, respectively.
Polymyxin B is one of the main drugs used in combination therapy
for pan-drug resistant A. baumannii, P. aeruginosa
and K. pneumoniae, and has been considered as a treatment of
last resort for gram-negative infections (1,6-8).
Polymyxin B was first introduced to the Chinese
market in September 2017. Currently, limited clinical data is
available with respect to the rational use of polymyxin B in
Chinese patients. As a result, usage and dosage (including
calculation and administration of the loading dose) are mainly
based on international clinical literature, for example, 1.25-1.5
mg/kg every 12 h (2.5-3 mg/kg daily) by intravenous (i.v.) drip
(9-14).
Polymyxin B is mainly eliminated by non-renal pathways, and
continuous renal replacement therapy (CRRT) has limited effects in
the clearance of polymyxin B (14-16).
Therefore, the latest International Consensus Guidelines for the
Optimal Use of the Polymyxins does not recommend adjusting the
loading and maintenance doses for patients receiving CRRT (17). However, given that Chinese people
generally weigh significantly less compared with people of other
nationalities, additional investigations are required to determine
whether the currently recommended dosage of 1.25-1.5 mg/kg every 12
h is suitable for Chinese people, especially for those with renal
impairment.
In May 2018, polymyxin B was given to a patient with
renal impairment in the ICU of our hospital at a 100 mg daily dose
(50 mg/12 h, via i.v. drip; 1.25-1.5 mg/kg) for 24 days. The
infection was brought under control and the renal function improved
significantly before hospital discharge. Subsequently, all relevant
data on the use of polymyxin B in patients with renal impairment
treated in our hospital was collected for further analyses. In the
present study, clinical data of five patients with renal impairment
who received polymyxin B therapy in the ICU of the hospital between
February 2018 and May 2019 was analyzed to determine the optimal
polymyxin B dosage in patients with renal dysfunction.
Materials and methods
Patients
Clinical data was collected from patients with renal
impairment who received polymyxin B therapy in the ICU of our
hospital between February 2018 and May 2019. All relevant
information, including sex, age, weight were collected from medical
records (Table I). Body
temperature, procalcitonin (PCT) level, C-reactive protein (CRP)
level, white blood cell count, neutrophil count, cystatin C level,
creatinine level and urine volume before and after the use of
polymyxin B were also recorded. The specific time for the use of
CRRT during polymyxin B therapy was also documented. Creatinine
clearance was calculated by the following formula: Male, creatinine
clearance=(140-age) x body weight (kg)/0.818x creatinine level
(µmol/l); female, multiply the result of the male formula by
0.85.
 | Table IBasic information of the six
patients. |
Table I
Basic information of the six
patients.
| Patient number | Sex | Age (years) | Weight (kg) | Main clinical
diagnosis | Primary positive
results from culture tests |
|---|
| 1 | Male | 74 | 70 | 1. Septic shock | On February 20 and
25, blood culture analyses and on February 22 and 24, sputum
culture analyses detected Klebsiella pneumoniae (pan-drug
resistant bacteria; imipenem MIC ≥16 mg/l). |
| | | | | 2. Pulmonary
infection |
| | | | | 3. Bloodstream
infection |
| | | | | 4. Acute renal
failure |
| 2 | Male | 41 | 70 | 1. Multiple
injuries | |
| | | | | 2. Multiple organ
failure (acute respiratory distress syndrome; acute renal failure;
abnormal coagulation function) | On May 10, 16 and 17,
sputum culture detected Klebsiella pneumoniae (pan-drug
resistant bacteria; imipenem MIC ≥16 mg/l). |
| | | | | 3. Pulmonary
infection |
| | | | | 4. Pyemia |
| 3 | Male | 55 | 75 | 1. Severe acute
pancreatitis | On August 17,
ascitic fluid culture detected Klebsiella pneumoniae
(pan-drug resistant bacteria, imipenem MIC ≥16 mg/l). |
| | | | | 2. Multiple organ
failure (respiratory failure, acute hepatic injury, acute renal
failure) |
| | | | | 3. Spectic
shock |
| 4 | Female | 56 | 57.8 | 1. Chronic renal
disease stage V (on maintenance hemodialysis) | Multiple blood and
sputum cultures showed no bacterial growth. |
| | | | | 2. Pulmonary
infection |
| | | | | 3. Respiratory
failure |
| | | | | 4.
Chemotherapy-induced myelosuppression |
| | | | | 5. Multiple
myeloma |
| 5 | Male | 67 | 70 | 1. Gastrointestinal
bleeding | On March 31, and
April 7 and 9, sputum cultures detected Pseudomonas
aeruginosa (pan-drug resistant bacteria; imipenem MIC ≥16
mg/l). |
| | | | | 2. Pulmonary
infection |
| | | | | 3. Heart
failure |
| | | | | 4. Acute renal
failure |
| 6 | Female | 28 | 50 | 1. Fulminant
myocarditis | On April 28 and 30,
sputum culture detected multidrug-resistant Pseudomonas
aeruginosa (imipenem MIC=4 mg/l on April 28; imipenem MIC=8
mg/l on April 30). On 10 and 12 May, blood culture detected
Elizabethkingia meningoseptica (multi-drug resistant
bacteria; imipenem MIC ≥16 mg/l). |
| | | | | 2. Cardiogenic
shock |
| | | | | 3. Acute heart
failure |
| | | | | 4. Pulmonary
infection |
| | | | | 5. Sepsis |
| | | | | 6. Multiple organ
failure (acute respiratory failure; hepatic injury; acute renal
failure) |
| | | | | 7. Intensive care
unit-acquired muscle weakness |
X-ray or CT chest scans
Chest X-rays (AXIOM Luminos dRF; Siemens AG)
or CT scans (Revolution CT; GE Medical Systems, LLC) were used to
determine the status of pulmonary infection.
Muscle strength examination
Muscle strength was manually examined using the
6-grade scoring method, in which the subject takes a standard test
position and performs standard test movements. The muscle's ability
to complete the movements was observed and, if applicable, the
physician would palpate or passively move the patient's hand or
foot and judge contraction strength according to patient response.
Muscle strength was classified into grades of 0, I, II, III, IV or
v, with a total of 6 possible grades. Grade 0 indicated that there
was no muscle contraction. Representative symbols (zero, O), rated
as: Total paralysis, muscle strength 0% of normal muscle strength.
Grade I, there was muscle contraction; however, not enough to allow
the joints to move. Representative symbols (trace, T), rated as:
Slightly contracted, 10% of normal muscle strength. Grade II,
muscle contraction allowed the limb to do full range of joint
motion under conditions that remove gravity (the physician would
raise the patients' limb and then release to observe whether the
patient could move their limb freely). Representative symbol (poor,
P), rated as: Poor, 25% of normal muscle strength. Grade III,
muscle contraction enabled the limb to resist gravity for full
range of joint motion; however, not for added resistance.
Representative symbols (fair, F), rated as: Fair, 50% of normal
muscle strength. Grade IV, muscle contraction enabled the limb to
resist gravity and certain external resistance. Representative
symbols (good, G), rated as: Good, 75% of normal muscle strength.
Grade V, muscle contraction allowed limb movement to resist gravity
and added resistance. The representative symbols (normal, N), rated
as: normal, 100% of normal muscle strength.
Results
Basic information of patients
Between February 2018 and May 2019, a total of six
patients with renal impairment received polymyxin B combination
therapy (age range, 28-74 years; weight, 50-75 kg) in the ICU of
our hospital. Patients Nos. 2-6 exhibited infection control
following the use of polymyxin B (at 9-24 days post-treatment). Of
all six patients, five had acute renal failure caused by infection,
and only one patient (patient No. 4) had stage V chronic kidney
disease. Table I shows that
polymyxin B was used in patients nos. 1-3 primarily due the ascitic
fluid, sputum or blood cultures testing positive for
carbapenem-resistant K. pneumoniae. Polymyxin B was used in
patient No. 4 based on clinical experience with patients of
immunosuppression (18). Polymyxin
B was used in patient No. 5 because the sputum culture tested
positive for pan-resistant P. aeruginosa (imipenem MIC ≥16
mg/l). In patient No. 6, the sputum culture tested positive for
multidrug-resistant P. aeruginosa on April 28 and 30
(imipenem MICs were 4 and 8 mg/l, respectively). The patient also
had an increased body temperature and elevated levels of PCT and
CRP between April 28 and May 2, 2019. Because the patient had
typical clinical manifestations of systemic inflammatory response
syndrome and a high likelihood of a bloodstream infection,
polymyxin B combination therapy was initiated (19). Blood cultures on May 10 and 12 from
the same patient confirmed that the patient had Elizabethkingia
meningoseptica sepsis. The main infection-related diagnoses
observed in the six patients included septic shock, pulmonary
infections, bloodstream infections, pyemia, and sepsis (Table I).
A 100 mg daily dose of polymyxin B
effectively controls infection in five patients with renal
impairment
Infections were controlled in patients No. 2-6 with
a 100 mg daily dose of polymyxin B. The body weight of patient No.
1 was 70 kg. The patient received polymyxin B according to 1.25-1.5
mg/kg every 12 h (9-14)
and, therefore, 87.5-105 mg every 12 h should be given. However,
the patient had renal impairment and polymyxin B can induce
nephrotoxicity (20,21). Furthermore, the use of 1.25-1.5
mg/kg every 12 h in patients with renal impairment are not based on
Chinese data (9-14).
Therefore, the current study administered 50 mg every 12 h for
patient No. 1. However, family members of the patient decided to
discontinue treatment after 5 days of polymyxin B therapy. The body
weight of patient No. 2 was also 70 kg and 50 mg every 12 h of
polymyxin B for 24 days was administered and the infection was
controlled.
The patients had a notable decrease in body
temperature, white blood cell count, neutrophil count, PCT level
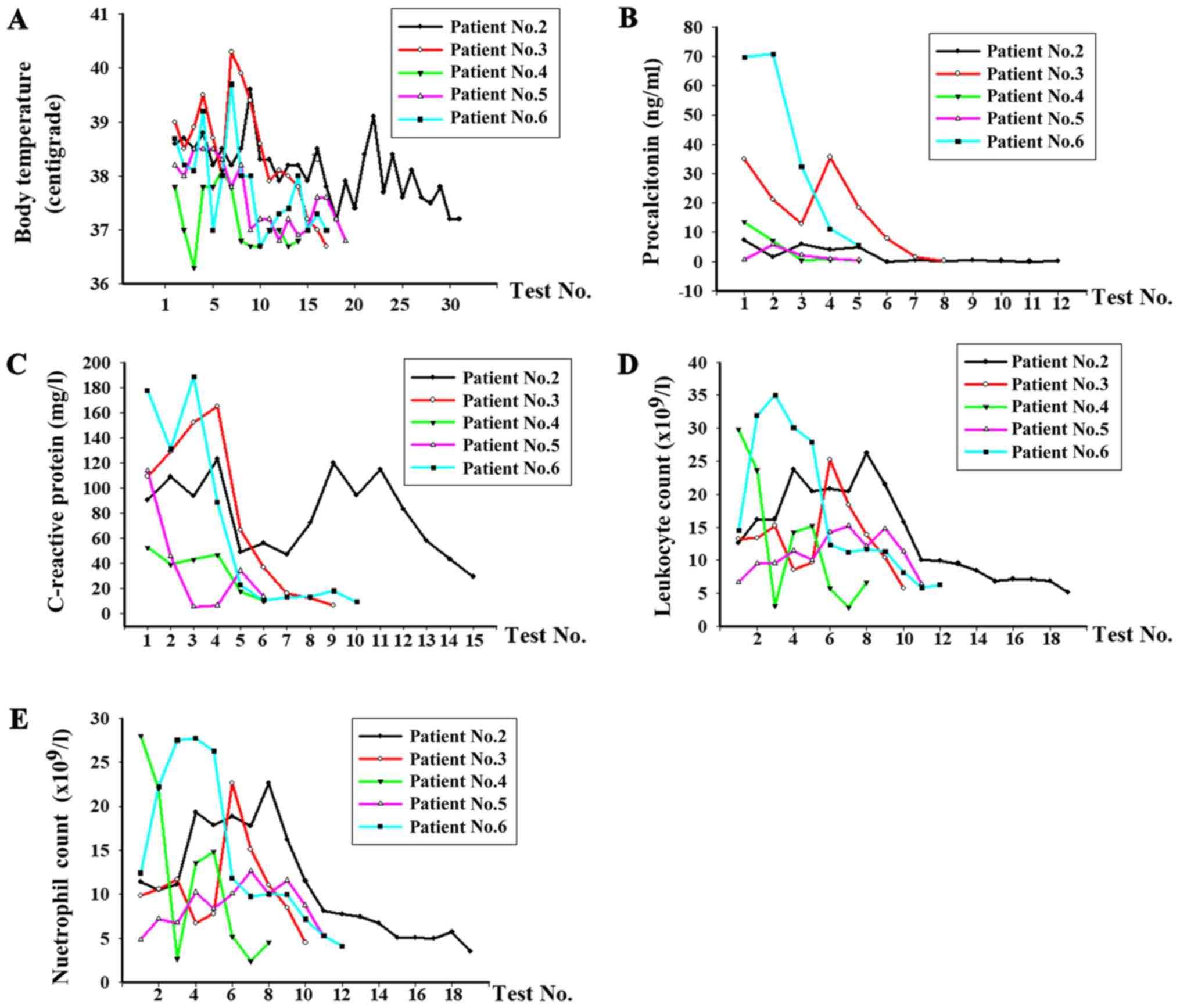
and CRP level during polymyxin B therapy (Fig. 1). Prior to polymyxin B treatment,
the average body temperature was 38.5˚C and decreased to 36.9˚C
(normal body temperature range, 36.1-37˚C) following polymyxin B
therapy. The average level of procalcitonin was 14.9 ng/ml prior to
treatment and decreased to 1.4 ng/ml (normal procalcitonin, <0.5
ng/ml). The average level of c-reactive protein of patients No. 2-6
was 112.5 mg/l prior to treatment and decreased to 15.3 mg/l
(normal c-reactive protein range, 0-10 mg/l) when the infections
were controlled. The average leukocyte count (normal leukocyte
count range, 4-10x109/l) were prior to treatment
21.7x109/l and decreased to 5.3x109/l.
Additionally, the average neutrophil count (normal neutrophil count
range, 2-7x109/l) decreased from 19.1x109/l
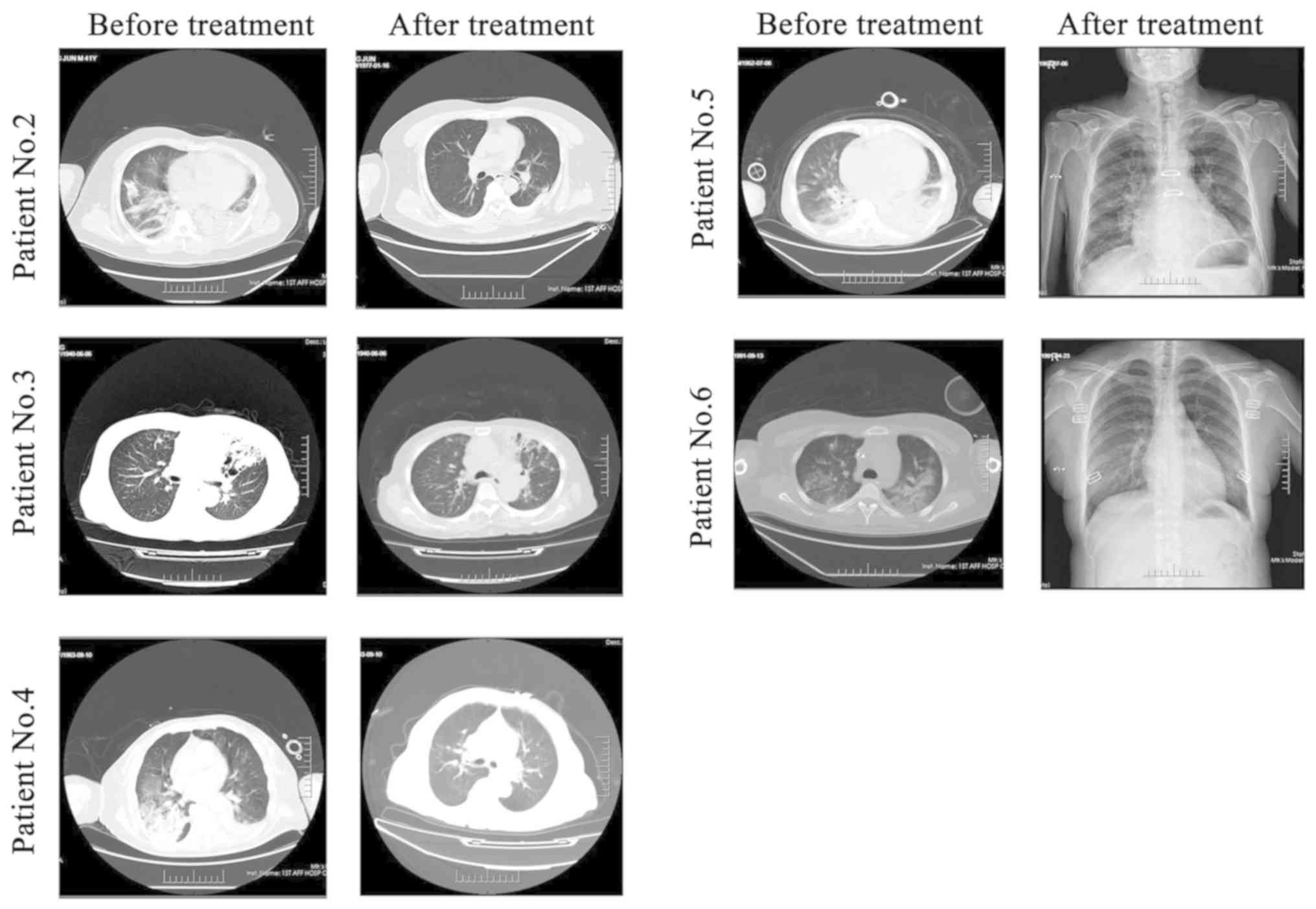
to 3.9x109/l. X-ray or CT chest scans of the five
patients before and after treatment also showed that pulmonary
infection was controlled (Fig.
2).
With the exception of patient No. 4, who had stage V
chronic renal disease, the renal function of the remaining
patients' improved when the infections were controlled and received
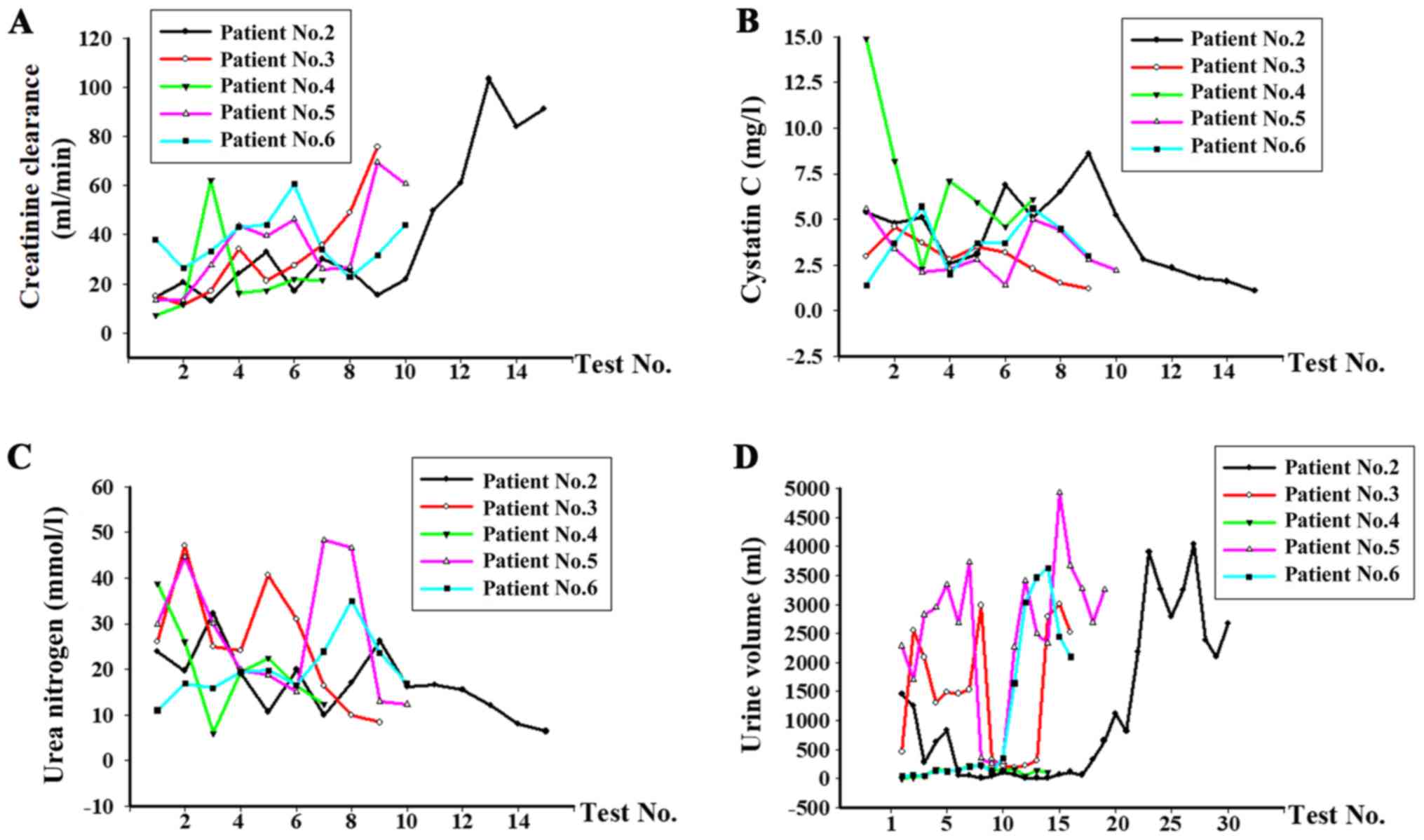
supportive treatment using CRRT, as demonstrated by elevated
creatinine clearance, urine volume and decreased levels of cystatin
C and urea (Fig. 3B and C). Prior to polymyxin B treatment, the
average creatinine clearance was 21.6 ml/min and increased to 58.7
ml/min (normal creatinine clearance range, 80-120 ml/min) following
polymyxin B therapy. The average level of cystatin C was 6.4 mg/l
before treatment and decreased to 2.4 mg/l (normal cystatin C
range, 0.6-1 mg/l). The average level of urea decreased from 30.1
to 12.1 mmol/l. Additionally, the urine volume of patient No. 2, 3,
5 and 6 improved following the infection control and were 2,675,
2,520, 3,260, 2,100 ml (normal urine volume range, 1,500-1,800
ml/24 h), respectively (Fig.
3D).
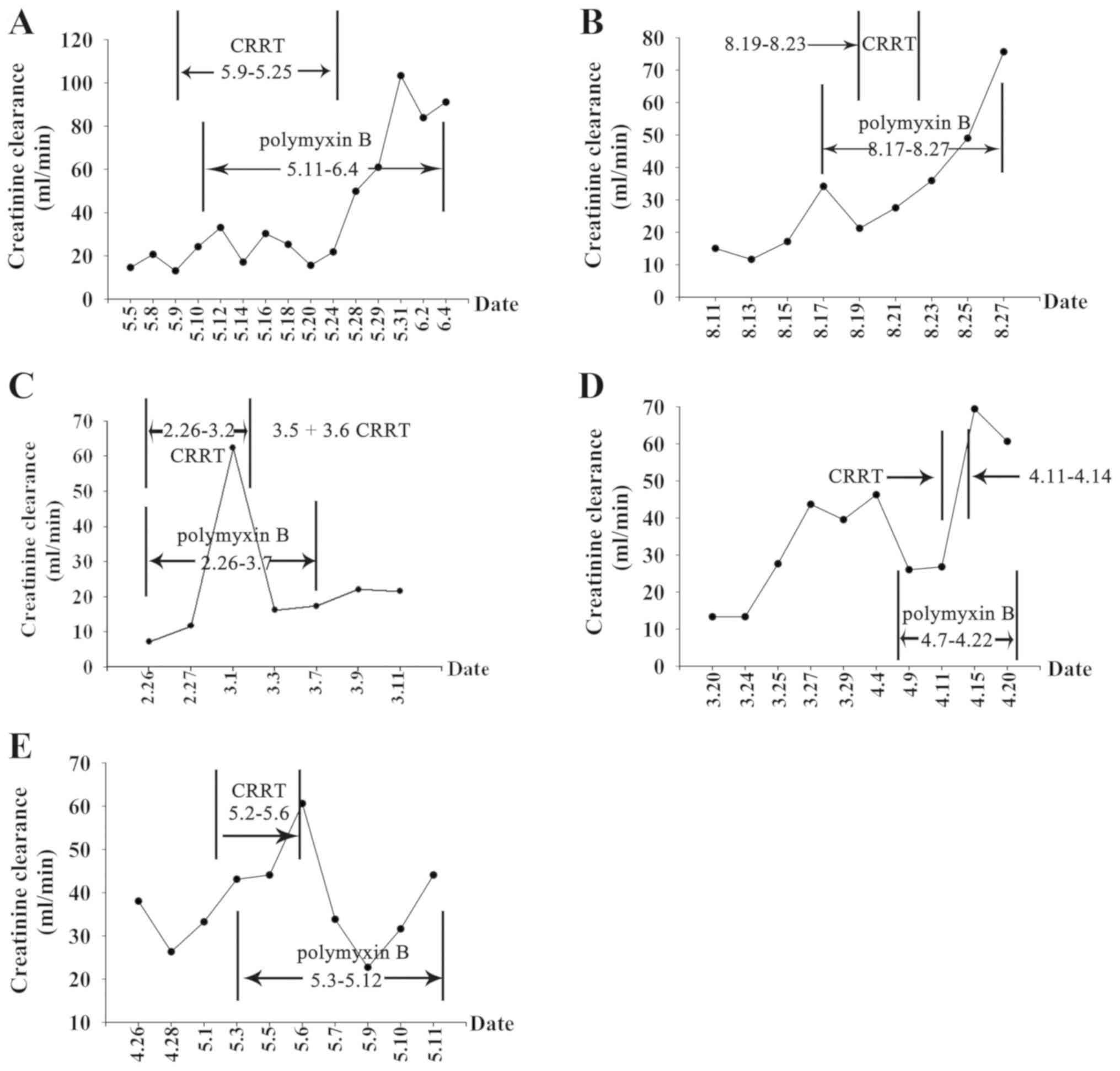
Changes in creatinine clearance and polymyxin B
treatment in the five patients (patients No. 2-6) while on
polymyxin B therapy were measured (Fig.
4). The creatinine clearance rate decreased in patient No. 2 on
May 13 and decreased again from May 17-24, however, the creatinine
clearance rate increased markedly after May 25. This increase was
observed following supportive treatment using CRRT was provided
between May 11 and 25. Creatinine clearance changes were similar
for the other three patients No. 3, 5 and 6. The creatinine
clearance rates increased to varying degrees after infection was
controlled and polymyxin B was discontinued. For patient No. 4 with
stage V chronic renal disease, and the creatinine clearance first
showed an extremely sharp drop, then increased following CRRT
supportive treatment. Additionally, the results demonstrated that,
during polymyxin B therapy, the five patients had similar trends in
creatinine clearance as in clearance first decreased, then
increased by varying degrees (Fig.
4).
Of the five patients in whom the infections were
controlled, four had normal renal function at the 1-month follow-up
evaluation and patient No. 4 remained with stage V chronic kidney
disease. These results suggested that a 100 mg daily dose of
polymyxin B was effective in controlling infections in patients
with renal impairment (weight range, 50-75 kg), and CRRT could be
used as a supportive treatment for deteriorating renal function
during polymyxin B therapy.
Neurotoxicity in patient No. 6 was
likely caused by polymyxin B
Patient No. 6, a 28-year-old female with fulminant
myocarditis, was administered polymyxin B combination therapy from
May 3-12, 2019. On May 5, the lower extremity muscle strength
decreased to grade III. On May 10, the lower extremity muscle
strength decreased to grade II. On 13 May, the lower extremity
muscle strength decreased to grade I and the upper extremity muscle
strength decreased to grade IV. In addition, on May 13, the patient
experienced transient cognitive impairment and could not recall her
name or her marital status. Her cognition returned to baseline
spontaneously later that day. On May 13, the patient underwent an
electromyogram (EMG) examination, which includes results of motor
nerve conduction, sensory nerve conduction and F-waves. The motor
nerve conduction study showed that the compound muscle action
potential amplitude of the left and right common peroneal nerves
decreased (Table SI). The results
of the sensory nerve conduction study were all normal (Table SI). The F-wave study showed that
the occurrence rate and latency of F-waves in the left median nerve
and left and right posterior tibial nerves were normal (Table SII). The EMG results (Table SIII) showed the presence of
spontaneous potentials in the left and right tibialis anterior
muscles, right extensor digitorum brevis muscle, left and right
biceps femoris muscles, and right L4 paraspinal muscle and their
inability to contract. The left and right gastrocnemius muscles,
right peroneus longus muscle and right vastus medialis muscle did
not show spontaneous potentials and were unable to contract. The
EMG findings concluded that the patient had neurogenic damage
involving both lower extremities.
It is widely accepted that decreased muscle strength
and cognitive impairment are manifestations of neurotoxicity
induced by polymyxin B (20,21).
Patient No. 6 had a total Naranjo adverse drug reactions
probability scale (Naranjo algorithm) score of 5, which indicated
that the adverse drug reactions in question were probably caused by
polymyxin B (22,23). Table
II shows the specific questions and the corresponding point
values of the Naranjo algorithm. Clinical data suggest that some
risk factors, such as drug exposure (the use of polymyxin B in this
case), hypoxia, female sex and renal injury, as well as the
concomitant use of muscle relaxants, sedatives, anesthetics and
corticosteroids, can increase risk for neurotoxicity after use of
polymyxin B (20,21). This female patient with renal
impairment was treated with polymyxin B from May 3-12, during which
time injections of sufentanil citrate, an anesthetic, and
methylprednisolone sodium succinate, a class of corticosteroid,
were also used (Table III).
Therefore, it is important that clinicians closely monitor and
assess the risk factors for adverse reactions of polymyxin B,
especially in patients with renal injuries.
 | Table IINaranjo adverse drug reactions
probability scale for patient number 6. |
Table II
Naranjo adverse drug reactions
probability scale for patient number 6.
| | Question point
values | |
|---|
| Questions | Yes | No | Don't know | Reasons for the
specific point(s) given |
|---|
| 1. Are there
previous conclusive reports on this reaction? | +1 | 0 | 0 | There are already
reports of neurotoxicity caused by polymyxin B (+1). |
| 2. Did the adverse
event appear after the suspected drug was administered? | +2 | -1 | 0 | The patient showed
symptoms of decreased limb muscle strength and cognitive impairment
after the use of polymyxin B (+2). |
| 3. Did the adverse
event improve when the drug was discontinued, or a specific
antagonist was administered? | +1 | 0 | 0 | The patient
restarted to walk on June 10 and climbed stairs on June 19 during
follow-ups after polymyxin B was discontinued. On May 13, the
result of EMG showed that the patient had neurogenic damage
involving both lower extremities. On June 19, no EMG abnormalities
were noted during the follow-up (+1). |
| 4. Did the adverse
event reappear when the drug was re-administered? | +2 | -1 | 0 | Polymyxin B was not
re-administered to the patient (+0). |
| 5. Are there
alternative causes that could on their own have caused the
reaction? | -1 | +2 | 0 | No other causes
alone can induce the adverse drug reaction for this patient
(+0). |
| 6. Did the reaction
reappear when a placebo was given? | -1 | +1 | 0 | The patient did not
take any placebos (+0). |
| 7. Was the drug
detected in the blood or other fluids in concentrations known to be
toxic? | +1 | 0 | 0 | The concentration
of polymyxin B was not measured (+0). |
| 8. Was the reaction
more severe when the dose was increased or less severe when the
dose was decreased? | +1 | 0 | 0 | The patient
received consistent dose of polymyxin B during the course of the
treatment (+0). |
| 9. Did the patient
have a similar reaction to the same or similar drugs in any
previous exposure? | +1 | 0 | 0 | The patient had not
previous exposure to the same or similar drugs (+0). |
| 10. Was the adverse
event confirmed by any objective evidence? | +1 | 0 | 0 | Physical
examination during hospitalization showed progressive decrease in
muscle strength of both lower extremities. On May 3, the patient
was given the first dose of polymyxin B. On May 5, muscle strength
of both lower extremities decreased to grade III; on May 10, muscle
strength of both lower extremities decreased to grade II; on May
13, muscle strength of both lower extremities decreased to grade I,
and that of both upper extremities dropped to grade IV. Also, on
May 13, the result of EMG showed that the patient had neurogenic
damage involving both lower extremities (+1). |
| Total score | 5 | 0 | 0 | 5 |
 | Table IIIRisk factors for neurotoxicity in
patient No, 6. |
Table III
Risk factors for neurotoxicity in
patient No, 6.
| Risk factors | Details |
|---|
| Drug exposure | The patient was
given polymyxin B 3-12 May 2019. |
| Sex | The patient is
female. |
| Concomitant use of
anesthetics | Sufentanil citrate
injection was administered (300 mg i.v. pump per day) 1-5 May
2019. |
| Concomitant use of
glucocorticoids | Methylprednisolone
sodium succinate for injection was administered (80 mg i.v. drip
per day) 2-5 May 2019. |
| Renal injury | The patient had
renal injury during polymyxin B therapy. |
Discussion
Of the six patients with renal impairment reported
herein, one patient discontinued treatment and the infections of
the other five patients were controlled with polymyxin B therapy
(50 mg every 12 h, i.v. drip). Supportive CRRT during polymyxin B
therapy was also required. The clinical data from the present
report provided a reference for the use of polymyxin B in Chinese
patients with renal impairment (weight range, 50-75 kg), but
further confirmation is needed.
The International Consensus Guidelines for the
Optimal Use of the Polymyxins recommends a polymyxin B dose of
1.25-1.5 mg/kg every 12 h for patients with severe infections
(17), which is supported by many
studies (9-14).
Low dose polymyxin B is an independent risk factor for treatment
failure and death (9-11).
Rigatto et al (11), showed
that polymyxin B is primarily eliminated via non-renal pathways and
renal replacement therapy is unlikely to remove a large percentage
of polymyxin B from the body. Therefore, in accordance with the
International Consensus Guidelines for the Optimal Use of the
Polymyxins recommendations, daily doses of polymyxin B should not
be adjusted in patients with renal impairment (14-17).
Furthermore, a total daily dose >200 mg was associated with a
lower risk of mortality, suggesting that an adequate daily dose of
polymyxin B is needed to treat patients with severe infections
(14-17).
However, additional research is needed to define the safety and
efficacy of high dose polymyxin B regimens and ensure that an
appropriate balance between safety and efficacy is achieved, as
nephrotoxicity and neurotoxicity are frequent occurrences with
polymyxin B therapy, especially in patients with renal
impairment.
By analyzing the clinical data of the five patients
in this study, a common characteristic was identified among
patients. Four of the patients (except for the patient with chronic
kidney disease) had different degrees of deteriorating renal
function after the use of polymyxin B. There was reason to believe
that this worsening renal function was either infection-induced or
caused by polymyxin B. Tests have been performed to further measure
multiple organ failure scores in the five studied patients to judge
if the increased renal dysfunction was caused by the progress of
the multiple organ failure, or caused by the nephrotoxicity induced
by polymyxin B. However, a common association has not been found,
and therefore, a subsequent study will aim to collect more detailed
information from patients to understand if there is an association
between organ failure and worsening renal function, that is
independent from polymyxin B treatment.
It has been shown that for patients with renal
impairment, 36% will experience deteriorating renal function when
on polymyxin B therapy (21). The
main independent risk factor for renal function damage caused by
polymyxin B is the dosage, with a higher dose corresponding to a
higher risk of renal function damage (20,21,24).
However, infection was controlled for the four patients after CRRT
was given, renal function also improved after discontinuing
polymyxin B. The patient with chronic kidney disease (patient No.
4), who also received CRRT during polymyxin B therapy, presented
reduced levels of cystatin C and urea. The patient's creatinine
clearance first exhibited an extremely sharp drop and then
increased following CRRT supportive treatment. These clinical data
suggest that CRRT is warranted to ensure the safety of polymyxin B
therapy for patients with renal impairment.
Patient No. 6 developed limb muscle weakness and
temporary cognitive impairment during polymyxin B therapy. She also
had a total Naranjo algorithm sore of 5, indicating these adverse
reactions were probably caused by polymyxin B. The patient could
ambulate on June 10 (27 days after discontinuing polymyxin B) and
climb stairs on 19 June. She also exhibited normal limb muscle
strength during follow-up evaluations. These results are consistent
with clinical literature (18,19).
The adverse reactions induced by polymyxin B were reversible, as
evidenced by the recovery of cognitive function and limb muscle
strength. Therefore, as with vancomycin (25), amikacin (26) and linezolid (27), it is necessary to monitor the
concentration of polymyxin B in the blood to explore a safe and
effective plasma concentration range, which can lower the incidence
of adverse reactions while controlling infection.
In the present report, a 100 mg daily dose of
polymyxin B controlled the infections among the five patients
examined. This daily dose is relatively low compared with the
recommended daily dose by other clinical studies. The reasons for
the difference could be that Chinese are generally much thinner and
shorter than their western counterparts, with the five reported
patients weighting between 50 and 75 kg. However, further research
is needed to determine whether the 100 mg daily dose is suitable
for patients who weigh more. In addition, despite the relatively
low daily dose of polymyxin B, the clinical data of the five
patients showed that renal toxicity and neurotoxicity caused by
polymyxin B should be a focus on future clinical studies. The
current report was limited by the number of cases presented.
However, in future clinical work, the safe use of polymyxin B in
Chinese patients with renal impairment will continue to be explored
in an effort to offer guidance for the optimal clinical use of
polymyxin B.
Supplementary Material
Results of motor and sensory nerve
conduction studies performed on May 13 for patient number 6.
Results of F-wave study performed on
May 13 for patient number 6.
Results of electromyogram performed on
May 13 for patient number 6.
Acknowledgements
The authors thank Dr Weidong Qian (Department of
Neurology, The First Affiliated Hospital of Bengbu Medical College)
and Dr Xiaomeng Chen (Department of the Electromyography Room, The
First Affiliated Hospital of Bengbu Medical College) for their
technical assistance.
Funding
The current work was supported by The 512 Talent
Cultivation Plan Foundation of Bengbu Medical College, The Natural
Science Foundation of the Provincial Education Department of Anhui
(grant no. KJ2018A0244) and The Foundation of Bengbu Medical
College (grant no. BYTM2019036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY, QW and XH designed the current retrospective
study. HW, SYZ and JX were responsible for acquisition of data. XD,
SZ and CL analyzed data and constructed the graphs. MY and QW wrote
the manuscript, QW and XH revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of The First Affiliated Hospital of Bengbu Medical
College. All patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent and
approved the publication of data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zakuan ZD and Suresh K: Rational use of
intravenous polymyxin B and colistin: A review. Med J Malaysia.
73:351–359. 2018.PubMed/NCBI
|
|
2
|
Wang M, Wei H, Zhao Y, Shang L, Di L, Lyu
C and Liu J: Analysis of multidrug-resistant bacteria in 3223
patients with hospital-acquired infections (HAI) from a tertiary
general hospital in China. Bosn J Basic Med Sci. 19:86–93.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mao T, Zhai H, Duan G and Yang H: Patterns
of drug-resistant bacteria in a general hospital, China, 2011-2016.
Pol J Microbiol. 68:225–232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yue D, Song C, Zhang B, Liu Z, Chai J, Luo
Y and Wu H: Hospital-wide comparison of health care-associated
infection among 8 intensive care units: A retrospective analysis
for 2010-2015. Am J Infect Control. 45:e7–e13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu A, Zheng B, Xu YC, Huang ZG, Zhong NS
and Zhuo C: National epidemiology of carbapenem-resistant and
extensively drug-resistant Gram-negative bacteria isolated from
blood samples in China in 2013. Clin Microbiol Infect. 22 (Suppl
1):S1–S8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rigatto MH, Falci DR and Zavascki AP:
Clinical use of polymyxin B. Adv Exp Med Biol. 1145:197–218.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De León-Borrás R, Sánchez-Sergentón C,
Mayor-Becerra A and Laureano-Cuadrado AF: Polymyxin B for negative
multidrug resistant bacteria in a Hispanic population. P R Health
Sci J. 38:15–21. 2019.PubMed/NCBI
|
|
8
|
Liang Q, Huang M and Xu Z: Early use of
polymyxin B reduces the mortality of carbapenem-resistant
klebsiella pneumoniae bloodstream infection. Braz J Infect Dis.
23:60–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Onufrak NJ, Rao GG, Forrest A, Pogue JM,
Scheetz MH, Nation RL, Li J and Kaye KS: Critical need for clarity
in polymyxin B dosing. Antimicrob Agents Chemother.
61:e00208–e00217. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Elias LS, Konzen D, Krebs JM and Zavascki
AP: The impact of polymyxin B dosage on in-hospital mortality of
patients treated with this antibiotic. J Antimicrob Chemother.
65:2231–2237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rigatto MH, Falci DR, Lopes NT and
Zavascki AP: Clinical features and mortality of patients Clinical
features and mortality of patients on renal replacement therapy
receiving polymyxin B. Int J Antimicrob Agents. 47:146–150.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manchandani P, Thamlikitkul V, Dubrovskaya
Y, Babic JT, Lye DC, Lee LS and Tam VH: Population pharmacokinetics
of polymyxin B. Clin Pharmacol Ther. 104:534–538. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Sandri AM, Landersdorfer CB, Jacob J,
Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J,
Forrest A, et al: Population pharmacokinetics of intravenous
polymyxin B inpatients: Implications for selection of dosage
regimens. Clin Infect Dis. 57:524–531. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miglis C, Rhodes NJ, Avedissian SN, Kubin
CJ, Yin MT, Nelson BC, Pai MP and Scheetz MH: Population
pharmacokinetics of polymyxin B in acutely ill adult patients.
Antimicrob Agents Chemother. 62:e01475–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abdelraouf K, He J, Ledesma KR, Hu M and
Tam VH: Pharmacokinetics and renal disposition of polymyxin B in an
animal model. Antimicrob Agents Chemother. 56:5724–5727.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tran TB, Velkov T, Nation RL, Forrest A,
Tsuji BT, Bergen PJ and Li J: Pharmacokinetics/pharmacodynamics of
colistin and polymyxin B: Are we there yet? Int J Antimicrob
Agents. 48:592–597. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsuji BT, Pogue JM, Zavascki AP, Paul M,
Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H,
Karaiskos I, et al: International Consensus guidelines for the
optimal use of the polymyxins: Endorsed by the American College of
Clinical Pharmacy (ACCP), European Society of Clinical Microbiology
and Infectious Diseases (ESCMID), Infectious Diseases Society of
America (IDSA), International Society for Anti-infective
Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and
Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy.
39:10–39. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Satlin MJ, Jenkins SG and Walsh TJ: The
global challenge of carbapenem-resistant Enterobacteriaceae in
transplant recipients and patients with hematologic malignancies.
Clin Infect Dis. 58:1274–1283. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bassetti M, Peghin M and Pecori D: The
management of multidrug-resistant Enterobacteriaceae. Curr Opin
Infect Dis. 29:583–594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Justo JA and Bosso JA: Adverse reactions
associated with systemic polymyxin therapy. Pharmacotherapy.
35:28–33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Falagas ME and Kasiakou SK: Toxicity of
polymyxins: A systematic review of the evidence from old and recent
studies. Crit Care. 10(R27)2006.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Arulappen AL, Danial M and Sulaiman SAS:
Evaluation of reported adverse drug reactions in antibiotic usage:
A retrospective study from a tertiary care hospital, Malaysia.
Front Pharmacol. 9(809)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gupta SK and Kumar KD: An assessment of
reported adverse drug reactions in a Tertiary Care Hospital in
South India: A retrospective cross-sectional study. Int J Pharm
Investig. 7:193–197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tuon FF, Rigatto MH, Lopes CK, Kamei LK,
Rocha JL and Zavascki AP: Risk factors for acute kidney injury in
patients treated with polymyxin B or colistin methane sulfonate
sodium. Int J Antimicrob Agents. 43:349–352. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang H, Wang R, Shu W, Tang R, Liang X,
Zhang J and Wu J: Monitoring of vancomycin serum concentrations and
the evaluation of its safety and treatment outcomes in adult
patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 30:538–543.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Jenkins A, Thomson AH, Brown NM, Semple Y,
Sluman C, MacGowan A, Lovering AM and Wiffen PJ: (BSAC Working
Party on Therapeutic Drug Monitoring). Amikacin use and therapeutic
drug monitoring in adults: Do dose regimens and drug exposures
affect either outcome or adverse events? A systematic review. J
Antimicrob Chemother. 71:2754–2759. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu H, Guo SC, Liu ZQ, Wang B, Fu L, Chu
NH and Lu Y: Therapeutic drug monitoring of cycloserine and
linezolid during anti-tuberculosis treatment in Beijing, China. Int
J Tuberc Lung Dis. 22:931–936. 2018.PubMed/NCBI View Article : Google Scholar
|