Introduction
Insulin autoimmune syndrome (IAS) is a rare type of
hypoglycemia, which was first reported in Japan by Hiram in 1970,
which is why this disease is known as Hiram disease (1). Subsequently, IAS was also reported in
China by Xiang et al (2).
This disease is more common in individuals of Asian descent and
less common in non-Asian populations (3). In Japan, IAS is the third leading
cause of hypoglycemia, after insulinoma and extra pancreatic
neoplasias (4). Hypoglycemia of IAS
is characterized by spontaneous and irregular occurrence, and its
severity can vary (5,6). Although clinical manifestations are
usually mild and transient, very severe cases have also been
described, with prolonged hypoglycemia and life-threatening
consequences (5). IAS is
characterized by: i) Hyperinsulinemic hypoglycemia, ii) elevated
insulin autoantibody (IAA) titers, iii) no prior exposure to
exogenous insulin; and iv) no pathological abnormalities of the
pancreatic islets (6). While the
cause of IAS is not completely understood, previous exposure to
environmental triggers such as medication and certain Human
Leukocyte Antigen (HLA) types have been associated with the
pathogenesis of this syndrome (5).
IAS is a clinically rare disease, and is prone to misdiagnosis and
missed diagnosis. The clinical data, diagnosis and treatment of IAS
induced by methimazole in a patient with Graves' disease
complicated with hypokalemia are described in the present case
report.
Case report
The patient, who was male and 27-years-old, was
referred to the People's Hospital of Jizhou District (Tianjin,
China), due to palpitation, fatigue, fear of heat, sweating and
weight loss of 10 kg within 3 months. The thyroid function test
results are presented in Table I.
The patient was diagnosed with hyperthyroidism and treated with
methimazole (10 mg, 3 times daily). After 10 days of continuous
methimazole administration, the patient had aggravated symptoms and
developed paralysis of the limbs and labored breathing, with a
blood potassium level of 1.91 mmol/l (normal range, 3.5-5.3
mmol/l). Following symptomatic treatment, which included
intravenous and oral potassium supplementation, the patient
improved, and was subsequently treated with intermittent potassium
supplementation.
 | Table IThyroid function test results. |
Table I
Thyroid function test results.
| A, The People's
Hospital of Jizhou District, Tianjin |
|---|
| Laboratory test | Test result | Reference range |
|---|
| FT3, pmol/l | >30.8 | 3.5-6.5 |
| FT4, pmol/l | 87.24 | 10.44-24.38 |
| TSH µIU/ml | 0.009 | 0.35-5.29 |
| Anti-TPO, U/ml | 1,300.0 | ≤60 |
| Anti-TG, U/ml | 291.3 | ≤60 |
| TRAb, IU/l | 9.11 | 0-1.75 |
| B, Tianjin First
Center Hospital |
| Laboratory test | Test result | Reference range |
| FT3, pmol/l | 6.33 | 3.1-6.8 |
| FT4, pmol/l | 14.23 | 12.0-22.0 |
| TSH µIU/ml | 0.005 | 0.27-4.2 |
| Anti-TPO, U/ml | 345.5 | 0-34 |
| Anti-TG, U/ml | 287.9 | 0-115 |
| TRAb, IU/l | 5.25 | 0-1.75 |
However, the patient still had intermittent fatigue
and palpitations and was subsequently admitted to Tianjin First
Center Hospital (Tianjin, China). The patients thyroid was
diffusely enlarged on palpation and there was no exophthalmos. The
cardiac and respiratory examination findings were unremarkable. The
abdomen was soft and non-tender, without masses. The examination
results of the thyroid function test performed following admission
are also presented in Table I. The
erythrocyte sedimentation rate was normal, while the thyroid color
doppler ultrasound indicated that the thyroid gland double-lobe
gland was enlarged, and blood flow was normal. The patient was
diagnosed with Graves' disease and continued to be treated with
methimazole (10 mg, twice daily). Following treatment with
potassium supplementation, the fluctuation in the blood potassium
level was monitored at 3.17-4.67 mmol/l (normal range, 3.5-5.3
mmol/l); however, the symptoms, such as fatigue, palpitation and
hand tremors, appeared intermittently. By monitoring the finger
blood glucose level, it was demonstrated that the minimum finger
blood glucose level was 1.90 mmol/l, and the plasma blood glucose
level was 2.44 mmol/l. Therefore, a spontaneous hypoglycemia
episode was identified. The patient had no history of diabetes
mellitus or hypoglycemia and denied exposure to insulin or oral
antidiabetic drugs. The glycosylated hemoglobin level was 6.0%
(normal range, 4.0-6.0%). The patient had an oral glucose tolerance
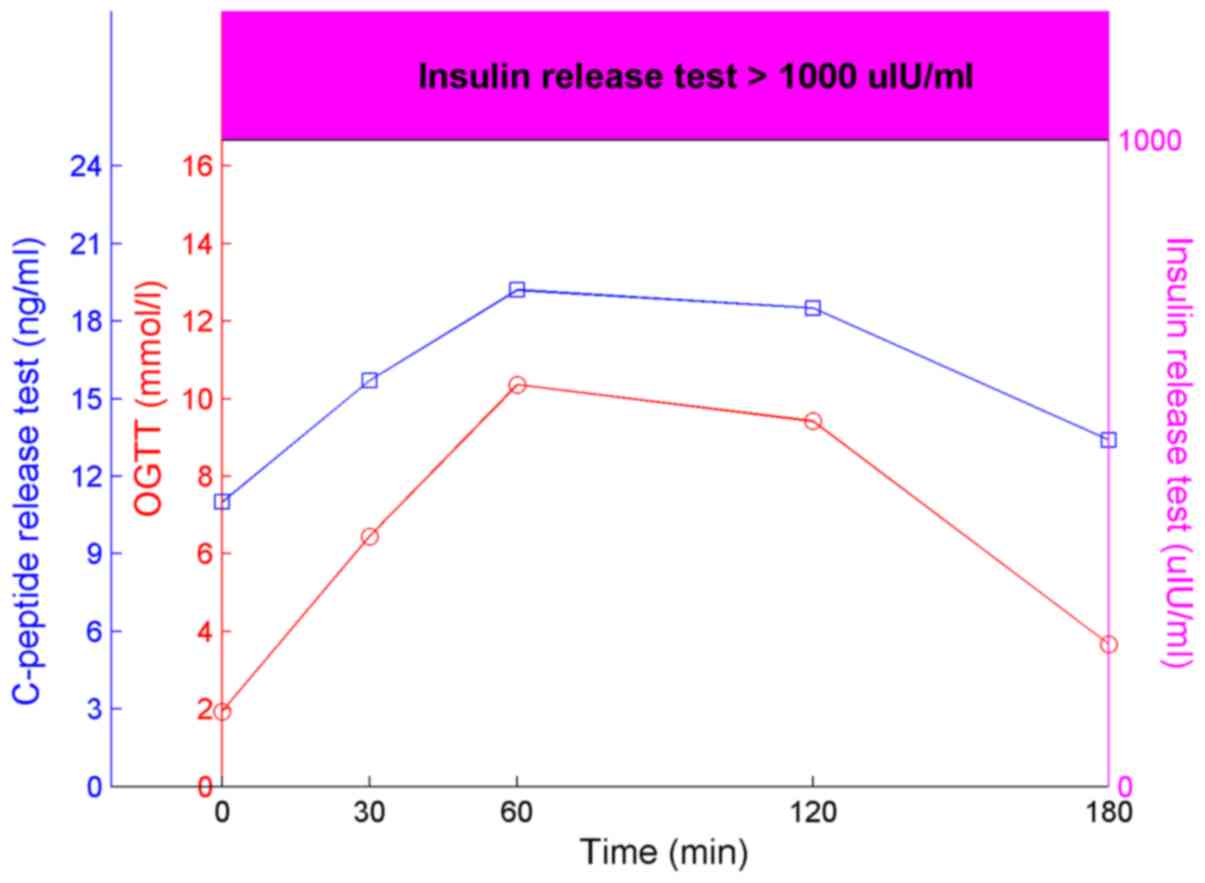
test (OGTT), insulin release test and C-peptide release test
(Fig. 1). The time intervals of
these tests were 0, 30, 60, 120 and 180 min. All results of the
insulin release test were >1,000 µIU/ml. IAA was positive, while
tests for glutamic acid decarboxylase antibody and islet cell
antibody were negative. There were no abnormalities in routine
blood, blood biochemistry, rheumatism immunity series, anti-nuclear
antibody spectrum and adrenal function tests. A pancreatic CT and
enhanced CT did not reveal any abnormalities. Using the Naranjo's
assessment scale (7), the causal
association had a score of 7 points (Table II). Considering hypoglycemia may be
secondary to methimazole-induced IAS, methimazole was discontinued.
The patient did not experience hypoglycemia again, once his eating
habits were changed, he had more meals a day but with a reduction
in the amount consumed. The patient was ingesting food higher in
protein and fiber to delay gastric emptying and a slowdown in the
insulin release rate. The examination before discharge indicated
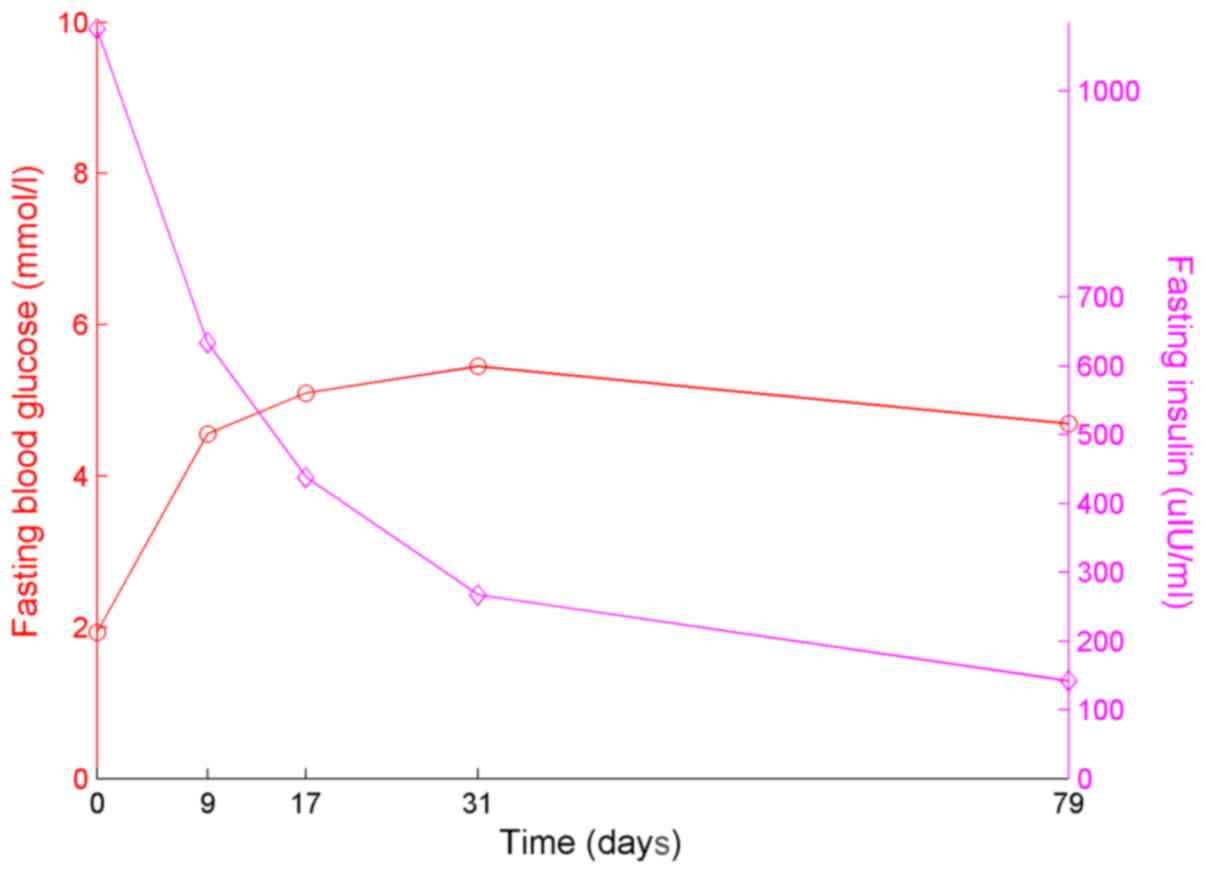
that the fasting serum insulin level (634.0 µIU/ml), was
significantly lower than before treatment. After 2 weeks, the
patient was treated with I-131 11 mCi. Outside of the hospital,
using blood glucose monitoring, a hypoglycemia episode did not
occur. Furthermore, the insulin level of the patient was lower, as
presented in Fig. 2. The fasting
blood glucose and insulin were recorded on days 0, 9, 17, 31 and
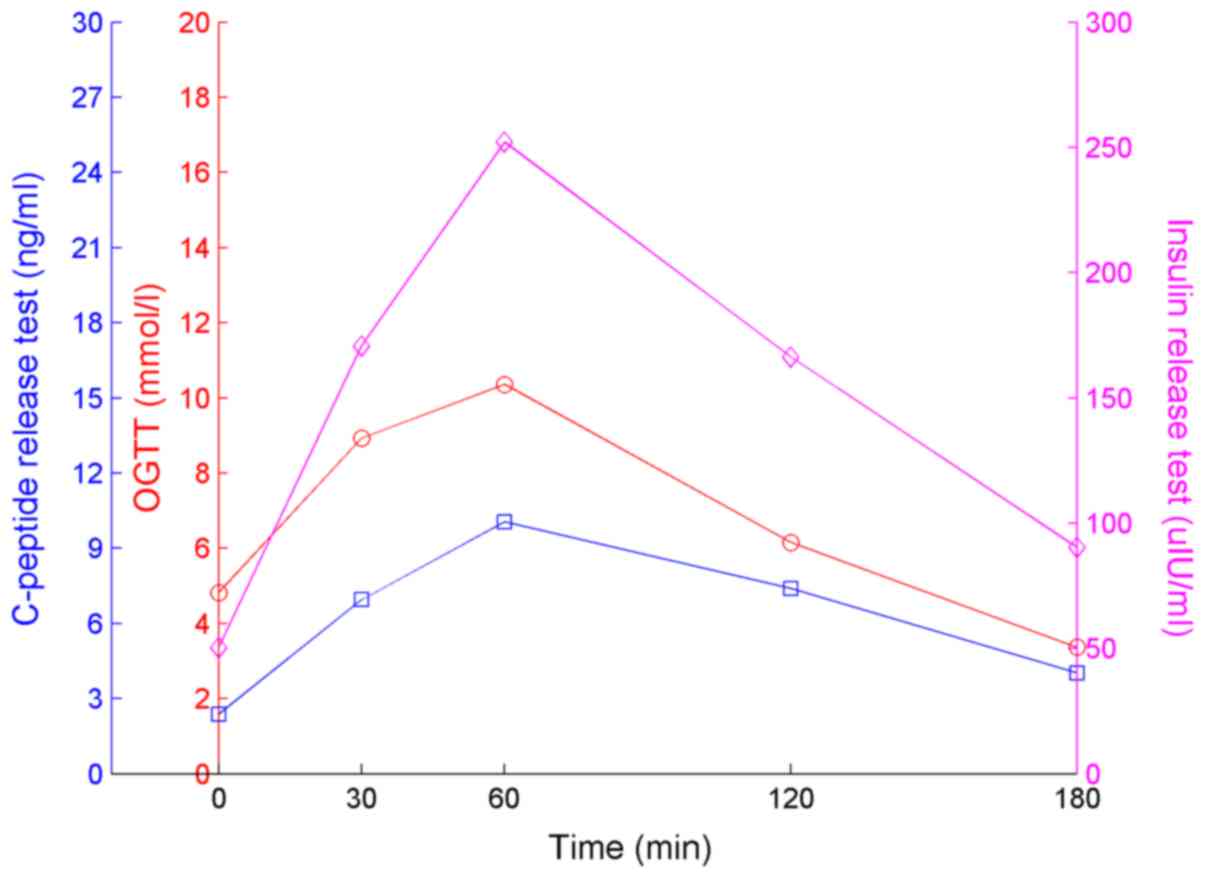
79. The results of the OGTT, insulin and C-peptide tests are
presented in Fig. 3 and were
obtained >2 months after discharge. The time intervals of these
tests were 0, 30, 60, 120 and 180 min. The insulin and C-peptide
levels had decreased markedly compared to those on admission, and
no hypoglycemia occurred.
 | Table IINaranjo's assessment scale. |
Table II
Naranjo's assessment scale.
| | Score | |
|---|
| Questions | Yes | No | Unknown | Answer | Score |
|---|
| 1. Are there previous
conclusive reports on this reaction? | +1 | 0 | 0 | Yes | 1 |
| 2. Did the adverse
event appear after the suspected drug was administered? | +2 | -1 | 0 | Yes | 2 |
| 3. Did the adverse
reaction improve when the drug was discontinued or a specific
antagonist was administered? | +1 | 0 | 0 | Yes | 1 |
| 4. Did the adverse
reaction reappear when the drug was readministered? | +2 | -1 | 0 | Unknown | 0 |
| 5. Are there
alternative causes (other than the drug) that could on their own
have caused the reaction? | -1 | +2 | 0 | No | 2 |
| 6. Did the reaction
reappear when a placebo was given? | -1 | +1 | 0 | Unknown | 0 |
| 7. Was the drug
detected in the blood (or other fluids) in concentrations known to
be toxic? | +1 | 0 | 0 | Unknown | 0 |
| 8. Was the reaction
more severe when the dose was increased, or less severe when the
dose was decreased? | +1 | 0 | 0 | Unknown | 0 |
| 9. Did the patient
have a similar reaction to the same or similar drugs in any
previous exposure? | +1 | 0 | 0 | No | 0 |
| 10. Was the adverse
event confirmed by any objective evidence? | +1 | 0 | 0 | Yes | 1 |
| Total score | | | | | 7 |
Discussion
The patient in the present case report presented
with hypokalemia and hypoglycemia following treatment for
hyperthyroidism with methimazole. Hypoglycemia may be easily missed
at diagnosis or misdiagnosed (5).
Following treatment with potassium supplementation, the patient
experienced symptoms, such as fatigue, palpitation and hand
tremors, which occurred intermittently. By monitoring finger blood
glucose levels, spontaneous hypoglycemia episodes were identified.
The patient had no history of diabetes mellitus and hypoglycemia,
or exposure to insulin or oral antidiabetic agents. Therefore,
hypoglycemia caused by oral hypoglycemic drugs or exogenous insulin
was excluded. The insulin and C-peptide release test revealed that
levels of insulin were elevated, and although the C-peptide level
was high, it was not synchronized with the insulin level. IAA was
indicated to be positive, and no pancreatic abnormalities were
identified following a pancreatic CT. The patient discontinued with
methimazole treatment. Using dietary management, hypoglycemia did
not return. Therefore, hypoglycemia was indicated to be caused by
IAS. IAS is a typical clinical symptom of recurrent, spontaneous
and severe hypoglycemia induced by non-exogenous insulin in the
blood and requires the identification of insulinoma. However,
pancreatic space-occupying lesions are often observed in the CT
images of patients with insulinoma, although some small insulinomas
are difficult to locate. The elevated serum insulin level in these
patients are synchronous with C-peptide, and IAA is negative. The
plasma C-peptide levels in patients with IAS have also been
indicated to be elevated, but it is much lower compared with the
insulin concentration in the blood, showing a state of separation
of C-peptide from insulin (8). IAA
has a high titer; therefore, detection of IAA, serum insulin and
C-peptide levels can assist with identification of this disorder
(9).
Using the Naranjo's assessment scale (7), the causal association had a score of 7
points (Table II), which supported
the possible causal relationship between the use of methimazole and
IAS. The pathogenesis of IAS is still unclear and it is currently
hypothesized to develop from the combined action of autoimmune
defects and IAA induced by the administration of specific drugs, on
the basis of susceptibility genes (4,6). Using
sulfur/sulfhydryl-containing drugs is an important treatment
method, and these include methimazole, captopril, D-penicillamine,
hydralazine, glutathione, methionine, mercaptans, imipenem,
penicillin G, α-lipoic acid and diltiazem (10). Any one of these can induce IAS, and
methimazole is the most common treatment option (11). It has been debated that
sulfur/sulfhydryl groups may be able to bind and reduce the
sulfhydryl bonds connecting insulin chains A and B, making
endogenous insulin more immunogenic (12). Studies have demonstrated that IAA in
patients with IAS is characterized by low affinity and high
capacity (4,13). IAA can also form an unstable complex
with insulin, reduce the concentration of serum-free insulin,
expand insulin storage capacity, and further stimulate islet B
cells to continue to secrete insulin. When the insulin-IAA complex
dissociates, a large amount of free insulin is released into the
blood to cause hyperinsulinemia, which in turn causes hypoglycemia
(14). The patient in the present
case report had a history of methimazole exposure, which supports
an etiological role for drugs with sulfhydryl groups in the
development of IAS.
From 2001 to September 2019, 64 cases of IAS induced
by methimazole were identified in the Chinese Biomedical Literature
Database (https://www.cnki.net/) and the patient
characteristics are presented in Table III. Among them, 16 patients were
males and 48 were females, 6 were >60-years-old and 58 were
<60-years-old. The reason for the increased amount of women
diagnosed may be that the incidence of hyperthyroidism in women is
significantly higher than in men, and the incidence of
hyperthyroidism in young people is higher than in people of old
age. Patients with hyperthyroidism may also have hypokalemia, and 2
out of 64 patients also had hypokalemia. The patient in the present
case report had Graves' disease with intermittent hypokalemia and
periodic paralysis, which is more common in young males.
Hypoglycemia occurs irregularly, and can manifest as hypoglycemia
at night or early in the morning and as reactive hypoglycemia
following the ingestion of food (5,6). The
degree of hypoglycemia in the cases was indicated to be different,
with 60 cases having blood glucose levels >2.8 mmol/l and 4
cases with levels >2.8 mmol/l. The hypoglycemia episode patient
described in the present case report was also irregular, and his
lowest fingertip blood glucose level was 1.90 mmol/l. A previous
study (10) have indicated that the
treatment of IAS first requires eliminating the inducement,
discontinuing the causative drugs, dietary management, delaying
gastric emptying by increasing the number of meals a day with a
reduction in the amount and ingestion of high protein and high
fiber food and slowing the release rate of insulin. α-Glucosidase
inhibitors have also been used to reduce or prevent hypoglycemic
episodes (4,13). In more severe or prolonged cases,
glucocorticoids, immunosuppressants and plasmapheresis may be
beneficial adjuvant therapies (15,16).
Of the 64 cases, 21 patients adjusted their diet, 38 patients were
treated with combined glucocorticoid therapy, 2 patients were
treated with combined α-glucosidase inhibitors and 3 patients were
treated with glucocorticosteroids and α-glucosidase inhibitors to
treat hypoglycemia. In the patient described in the present case
report, there was no recurrence of hypoglycemia following diet
management and the discontinuation of methimazole. When Graves'
disease is combined with IAS, treatment with I-131 is often
advocated (17). Of the 64
patients, 25 were treated with I-131, 19 were treated with
propylthiouracil (PTU), 4 were switched to I-131 therapy following
oral PTU treatment, 2 cases received surgical treatment and 14
cases were previously treated with methimazole only or it was not
explicitly stated. Patients with hyperthyroidism receiving
methimazole treatment may also develop IAS; however, not all the
patients had Graves' disease. The patient in the present case
report stopped methimazole treatment and was instead treated with
radioactive iodine for Graves' disease. In previous studies
(18,19) it has been hypothesized that HLA-DR4
is a genetic susceptibility gene for IAS and is associated with the
pathogenesis of IAS. In addition, studies have demonstrated that
84% of patients from Japan carry the HLA-DRB1*0406
allele (20), while European and
American populations primarily carry the HLA-DRB1*0403
allele (21,22). Unfortunately, the patient in the
current case report did not undergo genetic testing. Of the 64
patients previously reported, 3 were genetically tested (23), and all the patients were indicated
to carry the HLA-DRB1*0406 allele, which is consistent
with the reported results of previous Asian populations.
 | Table IIIIAS induced by methimazole from 2001
to September 2019 in China (n=64). |
Table III
IAS induced by methimazole from 2001
to September 2019 in China (n=64).
| Characteristic | Number of cases |
|---|
| Sex | |
|
Female | 48 |
|
Male | 16 |
| Age, years | |
|
<60 | 58 |
|
≤60 | 6 |
| Hypoglycemia
treatment | |
|
Diet
adjustment | 21 |
|
Diet
adjustment + glucocorticoids | 38 |
|
Diet
adjustment + α-glucosidase inhibitor | 2 |
|
Diet
adjustment + glucocorticoids +
α-glucosidase inhibitor | 3 |
| Treatment of
hyperthyroidism | |
|
I-131
therapy | 25 |
|
PTU | 19 |
|
First PTU
and then I-131 therapy | 4 |
|
Surgery | 2 |
|
Discontinued
methimazole without other expressions | 14 |
| Blood glucose | |
|
≥2.8 | 4 |
|
<2.8 | 60 |
| Hypokalemia | |
|
With | 2 |
|
Without | 62 |
| HLA typing | |
|
With | 3 |
|
Without | 61 |
The patient in the present case report exhibited
IAS. The patient was also previously diagnosed with Graves' disease
and suffered with intermittent hypokalemic periodic paralysis.
Hypoglycemia might be easily missed in diagnosis or misdiagnosed.
Monitoring fingertip blood glucose level is a convenient and
feasible method to determine this. The majority of patients with
IAS exhibit a good prognosis. However, it is a rare cause of
hypoglycemia in clinical practice, and it is susceptible to
misdiagnosis and missed diagnosis. This may lead to severe adverse
effects, such as severe hypoglycemia and even disturbance of
consciousness. For patients that also exhibit autoimmune diseases,
who have used or are using suspicious drugs and are developing
spontaneous hypoglycemia, insulin levels and IAA should be
monitored in time. Therefore, early diagnosis and appropriate
treatment should be conducted to reduce adverse consequences for
patients with IAS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RH and XJ designed the study, collected and analyzed
the clinical data, and wrote and revised the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was granted by The Tianjin First
Center Hospital (approval no. E2020011L).
Patient consent for publication
Informed consent was obtained from the patient,
including for the publication of this report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirata Y, Ishizu H and Ouchi N: Insulin
autoimmunity in a case of spontaneous hypoglycemia. J Japan
Diabetes Soc. 13:312–320. 1970.
|
|
2
|
Xiang D, Chen J, Xu M, Luo B, Wan C, Jin
J, Fan H and Fu L: Insulin autoimmune syndrome report of a case and
review of the literature. Chin J Endocrinol Metab. 64:94–97.
1985.(In Chinese).
|
|
3
|
Jendrzejewski J, Obołończyk Ł, Leczycka
ME, Utracka A, Ciura P, Makowski W and Sworczak K: A case report of
insulin autoimmune syndrome in a central european individual. Clin
Chem Lab Med. 56:e132–e134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Savas-Erdeve S, Yilmaz Agladioglu S, Onder
A, Peltek Kendirci HN, Bas VN, Sagsak E, Cetinkaya S and Aycan Z:
An uncommon cause of hypoglycemia: Insulin autoimmune syndrome.
Horm Res Paediatr. 82:278–282. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ismail AA: Testing for insulin antibodies
is mandatory in the differential diagnosis of hypoglycaemia in
nondiabetic subjects. Clin Endocrinol. 76:603–604. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Censi S, Mian C and Betterle C: Insulin
autoimmune syndrome: From diagnosis to clinical management. Ann
Transl Med. 6(335)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng C, Yang Y, Yang G, Ba J, Wang F, Dou
J, Gu W, Guo Q, Lv Z and Mu Y: Insulin autoimmune syndrome: A
report of 6 cases and literature review. Chin Gen Pract.
15:3313–3316. 2012.(In Chinese).
|
|
9
|
Okazaki-Sakai S, Yoshimoto S, Yagi K,
Wakasugi T, Takeda Y and Yamagishi M: Insulin autoimmune syndrome
caused by an adhesive skin patch containing loxoprofensodium.
Intern Med. 52:2447–2451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reis MZR, Fernandes VO, Fontenele EGP,
Sales APAM, Montenegro Junior RM and Quidute ARP: Insulin
autoimmune syndrome in an occidental woman: A case report and
literature review. Arch Endocrinol Metab. 62:566–570.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hunter A, Graham U and Lindsay JR: Insulin
autoimmune syndrome: A rare case of hypoglycaemia resolving with
immunosuppression. Ulster Med J. 87:34–36. 2018.PubMed/NCBI
|
|
12
|
Deguchi A, Okauchi Y, Suehara S and Mineo
I: Insulin autoimmune syndrome in a health supplement user: The
effectiveness of cornstarch therapy for treating hypoglycemia.
Intern Med. 52:369–372. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nasu T, Suzuki R, Okamoto Y, Miyata K, Uno
A, Nakao R, Kawashima A, Nakao T and Kondo M: Late postprandial
hypoglycemia due to bioactive insulin dissociation from
autoantibody leading to unconsciousness in a patient with insulin
autoimmune syndrome. Intern Med. 50:339–343. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y and Zhao T: Hypoglycemic coma due
to insulin autoimmune syndrome induced by methimazole: A rare case
report. Exp Ther Med. 8:1581–1584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saxon DR, McDermott MT and Michels AW:
Novel management of insulin autoimmune syndrome with rituximab and
continuous glucose monitoring. J Clin Endocrinol Metab.
101:1931–1934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chu JP, Zheng XW, Lu J, Zhong JY, Li JL,
Xu M and Lin F: Insulininduced autoimmune syndrome: Acase report.
Exp Ther Med. 12:3359–3362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu T: Analysis of 71 cases of insulin
autoimmune syndrome. Chin J Immunol. 32:1053–1055. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Uchigata Y, Eguchi Y, Takayama-Hasumi S,
Omon Y, Hirata Y, Kuwata S, Tokunaga K, Miyamoto M and Juji T:
Strong association of insulin autoimmune syndrome with HLA-DR4.
Lancet. 339:393–394. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsushita S, Takahashi K, Motoki M,
Komoriya K, Ikagawa S and Nishimura Y: Allele specificity of
structural requirement for peptides bound to
HLA-DRB1*0405 and-DRB1*0406 complexes:
Implication for the HLA-associated susceptibility to
Methimazole-induced insulin autoimmune syndrome. J Exp Med.
180:873–883. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Uchigata Y and Hirata Y: Insulin
autoimmune syndrome (IAS, Hirata disease). Ann Med Interne (Paris).
150:245–253. 1999.PubMed/NCBI
|
|
21
|
Censi S, Albergoni MP, Gallo N, Plebani M,
Boscaro M and Betterle C: Insulin autoimmune syndrome (Hirata's
Disease) in an Italian patient: A case report and review of the
literature. Clin Chem Lab Med. 56:889–895. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Uchigata Y, Hirata Y, Omori Y, Iwamoto Y
and Tokunaga K: Worldwide differences in the incidence of insulin
autoimmune syndrome (Hirata disease) with respect to the evolution
of HLA-DR4 alleles. Hum Immunol. 61:154–157.
2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen C, Wang W, Jin J, Ni W, Gu T, Bi Y
and Zhu D: Methimazole-induced insulin autoimmune syndrome in
Graves' disease: A case report and review on literature. J Clin
Pathol Res. 39:904–908. 2019.(In Chinese).
|