Introduction
Inflammatory processes are generally divided into
four stages: Initiation, inflammation, resolution and tissue repair
(1,2). Macrophages mainly serve important
roles throughout the whole process of inflammation and have
different effects in different stages (2,3).
Consequently, macrophages have been divided into two types
according to different and opposite functional states
(pro-inflammatory and anti-inflammatory); these two types of
macrophages are characterized by different phenotypes (1). A previous study suggested that
macrophages differentiated into the M1 or M2 type are involved in
the restoration of homeostasis and tissue repair by switching gene
expression (3).
Macrophages are able to change their phenotype in
response to numerous different stimuli and this process is termed
polarization (4). In the 1990s,
M2-type macrophages were first recognized as anti-inflammatory and
tissue-repairing macrophages (5).
This phenotype is different from that of typical inflammatory
macrophages (M1) (5,6). M1-type macrophages are produced after
monocytes are stimulated with lipopolysaccharide (LPS) or
interferon-γ (IFN-γ); M2-type macrophages are produced after
monocytes are stimulated with interleukin (IL)-10 or IL-4 (1,7). In
most wound repairs, M1-type macrophages are important in initiating
the inflammatory response to produce exudation; in the later
inflammatory stage, most of M1-type macrophages switch to the M2
phenotype to promote inflammation resolution and tissue repair
(8). However, a previous study
demonstrated that in spinal cord injury (SCI), the microenvironment
of damaged spinal cord is consistently filled with M1-type
macrophages that do not switch to the M2 phenotype (9). Ultimately, the consistent effect of
M1-type macrophages results in irreversible spinal cord tissue
destruction (9). Thus, it is
important to promote M1 to M2 switching in macrophages and maintain
the presence of M2-type macrophages to promote tissue repair in
SCI.
Currently, regulating macrophage polarization using
various agents has been considered a novel potential strategy for a
number of diseases. For example, blocking CD64 with neutralizing
antibodies induces M2 polarization of macrophages to decrease M1
cells and increase M2 cells in acute inflammatory diseases, such as
ovarian cyst (10). In addition,
some strategies involve cell-cell interactions. Cheng et al
(11) demonstrated that conditioned
medium from neural stem cells (NSCs) promoted M2 polarization of
macrophages by suppressing the expression of inducible nitric oxide
synthase (iNOS). Matsubara et al and Kano et al
(12,13) also reported that conditioned stem
cell medium induced spinal cord tissue recovery that was associated
with promoting M2 polarization of macrophages. Thus, it is
considered that there is a specific interaction between NSCs and
macrophages, but the mechanism is still unknown.
The present study explored the effect of NSCs on
macrophages and demonstrated that NSCs induce M2 polarization and
suppress M1-polarization of macrophages, which is beneficial for
tissue repair and neural regeneration through the secretion of
IL-4. Knockdown of IL-4 in NSCs by specific short hairpin (sh)RNA
abolished the promotion of M2 polarization. NSCs suppressed
activation of the NF-κB/p65 pathway in an IL-4-dependent manner.
Hence, the results of the present study demonstrated the novel
effect of NSCs on macrophages and the underlying mechanism, which
may provide useful information for its potential translational
application in NSCs transplantation.
Materials and methods
NSC isolation and culturing
All procedures performed on experimental animals
were approved by the Animal Care and Use Committee of Sun Yat-sen
University (Guangdong, China).
Rats
All animal protocols were approved by China Council
on Animal care and Sun Yet-San University Committee and have
therefore been performed in accordance with the ethical standards
laid down in the 1964 Declaration of Helsinki and its later
amendments. Adult male Wistar rats (200-250 g; 6 weeks of age) were
purchased from animal facility of Sun Yat-sen University and housed
in stainless steel cages. Rats were fed under controlled
environment at 25±3˚C, humidity of 40-65% and 12 h light/dark
cycle, and free access to food and drink water. Normal food (Animal
Facility of Sun Yat-sen University) was provided.
Rats were anesthetized with 1% pentobarbital sodium
(40-45 mg/kg) to cause minimum pain and sacrificed by
CO2 asphyxiation. To be sacrificed, animals were placed
into euthanasia cases, then 100% CO2 were injected into
cases at the filling rate of 30% per min. Death was confirmed by
cardio-respiratory arrest and loss of reflexes. The duration of
animal experiments was <30 min.
NSCs were isolated from the fetal brains of
embryonic day 14 rats, which were extracted from pregnant
Sprague-Dawley (SD) rats and identified as previously described
(14). Briefly, the fetal brain
tissue was mechanically dissected and dissociated in Hanks Balanced
Salt Solution and the cell suspension was centrifuged at 4˚C, 111 x
g for 5 min. The supernatant was discarded and the cell pellet was
diluted to a single-cell suspension. NSCs (1x105) were
plated on a T25 culture flask (Corning Inc.) containing Dulbecco's
modified Eagle's medium (DMEM)/F-12 nutrient mixture, 2% B27, 1%
penicillin/streptomycin, 1% l-glutamine (all purchased from Gibco;
Thermo Fisher Scientific, Inc.), 20 ng/ml fibroblast growth
factor-2 (FGF-2) and 20 ng/ml epidermal growth factor (EGF; both
purchased from PeproTech, Inc.). NSCs were cultured at 37˚C in 5%
CO2 and were passaged weekly by digestion with Accutase
(EMD Millipore) in the medium described above. All NSCs (passage
2-3) used in this study were between passages 2 and 4.
To induce neural differentiation, cells were plated
at a density of 2x105 cells/well in 6- or 12-well
tissue-culture plates and allowed to adhere for 24 h at 37˚C, at
which time cells were switched to neural differentiated medium
consisting of basic medium supplemented with 2% B27, 1%
penicillin/streptomycin and 1% l-glutamine. The medium was changed
every 2-3 days (14,15).
Monocytes were isolated from the femurs of adult SD
rats as previously described (2).
In co-culture system, cells were differentiated into the
macrophages when culturing in DMEM supplemented with 20 ng/ml
macrophage colony-stimulating factor (M-CSF) (PeproTech, Inc.) at
37˚C in 5% CO2 for 7 days (12).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells according to the
manufacturer's protocol (16) and 2
µg of total DNA-free RNA was used to synthesize cDNA using the
ReverTra Ace® qPCR RT kit (Toyobo Life Science). The
reactions were set up in 96-well plates using 1 µl cDNA with
Thunderbird SYBR qPCR Mix (Toyobo Life Science), to which
gene-specific forward and reverse PCR primers were added. RT-qPCR
was performed under the following conditions: 95˚C for 10 min,
followed by 40 cycles of 95˚C for 10 sec and 55˚C for 34 sec. These
analyses were performed to detect the expression of CD58, iNOS,
CD206, CD163, IL-4, IL-10, IL-13, tumor necrosis factor (TNF)-α,
IFN-γ and β-actin was used as an internal control. Primer sequences
were as demonstrated in Table
I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| CD85 | F:
TCACGGCTGAGATTCGACAG |
| | R:
GTTTTGTGACGGACTGAGGTTAT |
| Inducible nitric
oxide synthase | F:
GAGGAGAGAGATCCGGTTCACA |
| | R:
CCGCATTAGCACAGAAGCAA |
| CD206 | F:
GACAGATATGAACAAGCATTCC |
| | R:
TGAACATCTGAGAGTCCTGTC |
| CD163 | F:
CGTCACCTTGGAAACAGAGACAG |
| | R:
AGATCTCCACACGTCCAGAACAG |
| IL-4 | F:
ACCTTGCTGTCACCCTGTTCT |
| | R:
AGCTCGTTCTCCGTGGTGT |
| IL-10 | F:
CAGACCCACATGCTCCGAGA |
| | R:
CAAGGCTTGGCAACCCAAGTA |
| IL-13 | F:
GCTCTCGCTTCGCTTGGTGGTC |
| | R:
CATCCGAGGCCTTTTGGTTACAG |
| Tumor necrosis
factor-α Interferon-γ | F:
TCAGTTCCATGGCCCAGAC |
| | R:
GTTGTCTTTGAGATCCATGCCATT |
| | F:
TCGCACCTGATCACTAACTTCTTC |
| | R:
CGACTCCTTTTCCGCTTCC |
| β-actin | F:
CCTCTTTGCATGTCTCACTC |
| | R:
AATGTCACGCACGATTTCC |
Western blotting analysis
Cells were lysed in RIPA buffer (25 mM Tris-HCl pH
7.5, 150 mM NaCl, 1% Na-deoxycholale, 0.5 M EDTA, 1% Nonidet P40,
0.1% SDS, Beijing Solarbio Science & Technology Co., Ltd; cat.
no. R0010), total protein was extracted, and the protein
concentration was determined with a bicinchoninic acid assay.
SDS-PAGE gels (10 and 12%) were loaded with 20 µg/lane of total
protein. The separated proteins were transferred by electro
blotting to PVDF membranes. The membranes were blocked with 5%
non-fat dry milk in TBST at room temperature for 2 h (50 mM Tris;
pH 7.6; 150 mM NaCl; and 0.1% Tween-20) and incubated with the
primary antibody overnight at 4˚C in 5% non-fat dry milk in TBST.
Immunology-labeling was detected using ECL reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The primary antibodies used were
as follows: Anti-p65 (1:1,000, Room temperature, 45 min, Cell
Signaling Technology, Inc; cat. no. 8242), anti-phospho-p65
(1:1,000; Room temperature, 45 min; Cell Signaling Technology Inc;
cat. no. 93H1), anti-IL-4 antibody (1:500; Room temperature, 45
min, Proteintech Group, Inc; cat. no. 66142), anti-β-actin
(1:10,000; Room temperature, 45 min, Sigma-Aldrich; Merck KGaA;
cat. no. A5316) and anti-GAPDH (1:10,000; Room temperature, 45 min,
Sigma-Aldrich; Merck KGaA; cat. no. G9545). The secondary antibody
(1:1,000; Cell Signaling Technology, Inc.) were incubated for 1 h
at room temperature.
Transfection
Recombinant lentiviruses for IL-4 silencing (shIL-4)
and control lentivirus (scramble) were obtained from Shanghai
Genechem Co., Ltd. For transfection, cells in the log phase were
plated at a concentration of 1x105 cells/well in 6-well
plates and transfected with the scramble and shIL-4 (MOI: 20) in
DMEM/F12 with 10% FBS. Polybrene (Beijing Solarbio Science &
Technology Co., Ltd; cat. no. H8761) was added as an enhancing
reagent to improve transduction efficiency at a concentration of 10
mg/ml. After 8 h, the medium was changed with fresh complete
medium. Cells were harvested for the co-culture system 24 h after
transfection.
Immunofluorescence confocal
microscopy
Macrophages (5x103/well) were transferred
to flat-bottom 96-well plates at the indicated density. After being
treated and incubated, the cells were fixed with 4%
paraformaldehyde in 15 min at room temperature, permeabilized with
0.5% Triton X-100 in PBS for 10 min at room temperature, blocked
with PBS containing 1% BSA in 15 min at room temperature, and then
incubated with an antibody against p65 (1:50; Cell Signaling
Technology Inc.) overnight at the 4˚C. After being washed with PBS,
the cells were incubated with an Alexa Fluor-488-conjugated
anti-rabbit secondary antibody (1:1,000; Invitrogen; Thermo Fisher
Scientific, Inc; cat. no. A11029.) for 1 h at room temperature
before being imaged with a confocal microscope using a LCPlanFl
objective (magnification x200; Olympus Corporation).
Enzyme linked immunosorbent assay for
the quantitation of IL-4, IL-10 and IL-13
The ELISA of IL-4 (Abcam; cat. no. ab215089), IL-10
(Abcam; cat. no. ab185986) and IL-13 (Abcam; cat. no. ab100553)
were performed using ELISA kits. The protocol was as follows:
Prepare all reagents, working standards, and the culture medium.
Remove unused microplate strips from the plate frame, return them
to the foil pouch containing the desiccant pack, and reseal. Wash
each well three times with PBS (300 µl/well) using a squirt bottle,
multi-channel pipette, manifold dispenser or autowasher. Complete
removal of liquid at each step is essential to good performance.
Remove any remaining Wash Buffer by aspirating or decanting. Invert
the plate and blot it against clean paper towels. Add 100 µl of
each serially diluted protein standard or culture medium per well
including a zero standard. Ensure reagent addition is uninterrupted
and completed within 15 min. Cover/seal the plate and incubate for
2 h at room temperature. Add 100 µl of Detection Antibody in
working concentration to each well. Cover/seal the plate and
incubate for 1 h at room temperature. Add 200 µl of Substrate
Solution to each well. Incubate for 20 min at room temperature.
Protect from light. Add 50 µl of Stop Solution to each well. If
color change does not appear uniform, gently tap the plate to
ensure thorough mixing. Determine the optical density of each well
within 20 min, using a microplate reader set to 450 nm.
Flow cytometric analysis
Single-cell suspensions were prepared and stained
with fluorochrome-conjugated antibodies (Invitrogen; Thermo Fisher
Scientific, Inc, cat. no. A35029) at the room temperature for 1 h.
Data were collected on a BD LSRII flow cytometer (BD Biosciences)
and analyzed with FlowJo software V2.0 (Tree Star, Inc.). Data were
acquired as the fraction of labeled cells within a live-cell gate
set for 50,000 events. For flow cytometric sorting, cells were
stained with specific antibodies (APC Mouse Anti-Human HLA-DR;
1:200; BD Biosciences; cat. no. 559868; PE Mouse Anti-Human CD163;
1:200; BD Biosciences; cat. no. 556018; BV421 Mouse Anti-Human
CD206, 1:200, BD Biosciences; cat. no. 564062) and isolated on a BD
FACSAria cell sorter (BD Biosciences) (17).
Statistical analysis
Data were represented as the mean ± SD of three
independent repeats. Shapiro-Wilk test was used to analyze the
normality of data and one-way ANOVA with Levene's test was used to
test the homogeneity of variance, followed by the Bonferroni post
hoc test for multiple comparisons. The analyses were performed
using SPSS (version 16.0; SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
NSCs induce M2-polarization and
suppress M1-polarization of macrophages
To determine the interactions between NSCs and
macrophages, an in vitro co-culture system was established
to investigate the effect of NSCs on macrophages. The frequencies
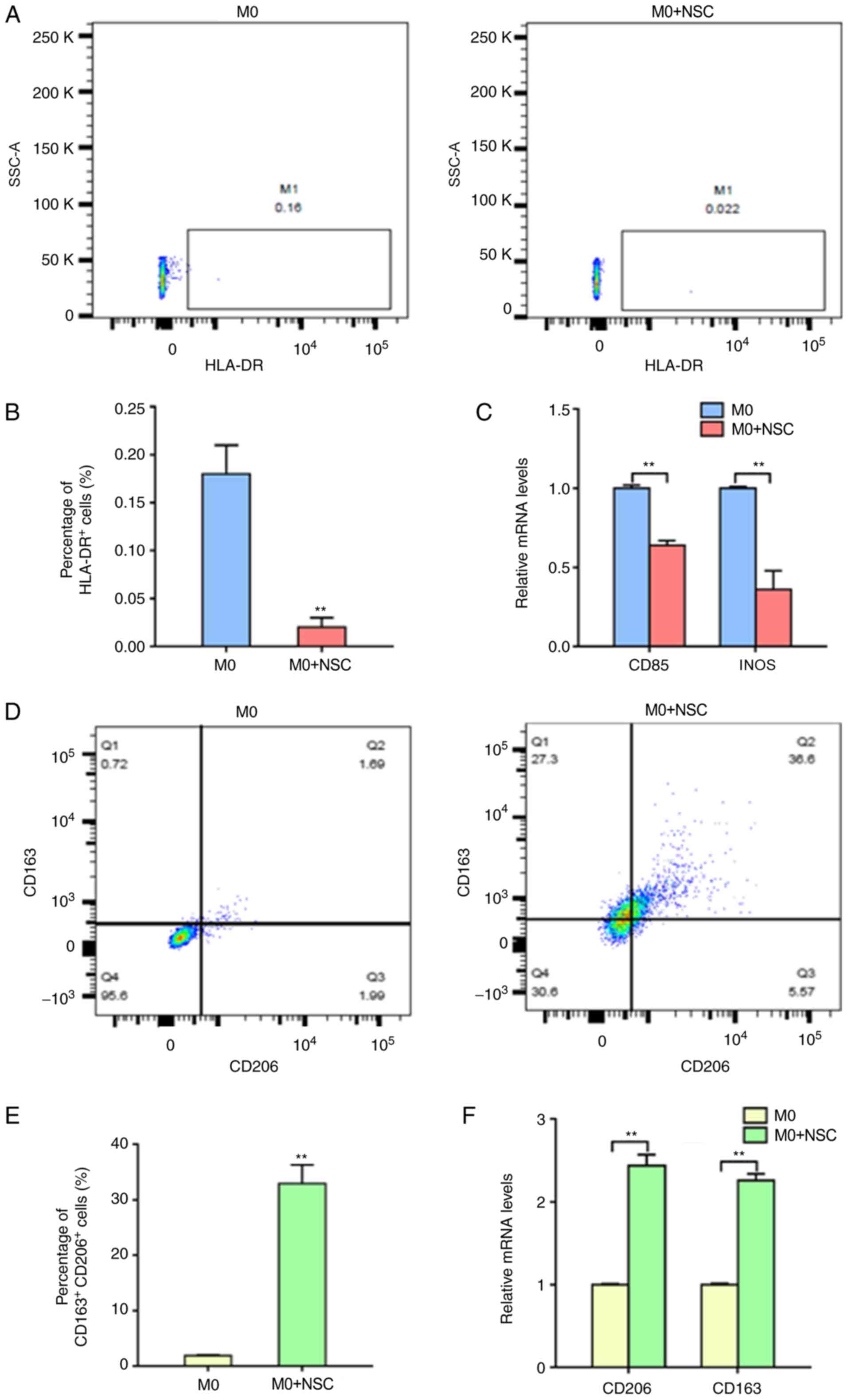
of macrophages by flow cytometry was measured. The results
demonstrated that the M1-type macrophages (HLA-DR+) were
significantly reduced from 0.18±0.03 to 0.02±0.01% after co-culture
with NSCs (Fig. 1A and B). The RT-qPCR results demonstrated that
the mRNA expression levels of the M1-type macrophage markers (CD85
and iNOS) were significantly decreased in co-cultured cells
compared with M0-macrophages alone (Fig. 1C). In contrast, flow cytometric
analysis demonstrated that the M2-type macrophages
(CD206+ and CD163+) were significantly
increased from 1.87±0.17 to 32.93±3.43% after co-culture with NSCs
(Fig. 1D and E). Additionally, the RT-qPCR results
demonstrated that the mRNA expression levels of the M2-type
macrophage markers (CD206 and CD163) were significantly increased
in co-cultured cells compared with M0-type macrophages cultured
alone (Fig. 1F). These results
suggested that NSCs induce M2 polarization and suppress M1
polarization in macrophages.
NSCs induce M2 polarization of
macrophages in an IL-4-dependent manner
Previous studies have demonstrated that stimulation
with different cytokines affects macrophage polarization (M1 or M2)
(4,18,19).
IL-4 or IL-13 stimulation induces M2 polarization; TNF-α or IFN-γ
stimulation induces M1 polarization (1). To confirm whether these cytokines were
involved in the interactions between NSCs and macrophages, NSCs
were cultured in neural differentiation medium. The RT-qPCR and
ELISA results demonstrated that the mRNA and protein expression
levels of IL-4, but not IL-13 or IL-10 were significantly increased
in cells with the increase in time (Fig. 2A and B). In contrast, no significant differences
were observed in the mRNA or protein expression levels of TNF-α and
IFN-γ during neural differentiation (Fig. 2C and D). To further confirm whether the
secretion of IL-4 was crucial for NSC-induced M2 polarization, a
target-specific lentivirus was used to knock down the expression
levels of IL-4 in NSCs (NSCshIL-4), then the
NSCshIL-4 were co-cultured with macrophages. The RT-qPCR
results demonstrated that the mRNA expression levels of the M1-type
macrophage marker (CD85 and iNOS) were significantly increased in
macrophages co-cultured with NSCsshIL-4 compared with
those of macrophages cultured alone (Fig. 2E). By contrast, the mRNA expression
levels of the M2-type macrophages marker (CD206 and CD163) were
significantly decreased in macrophages co-cultured with
NSCshIL-4 compared with those of macrophages cultured
alone (Fig. 2F). These results
suggested that NSCs induce M2 polarization of macrophages in an
IL-4-dependent manner.
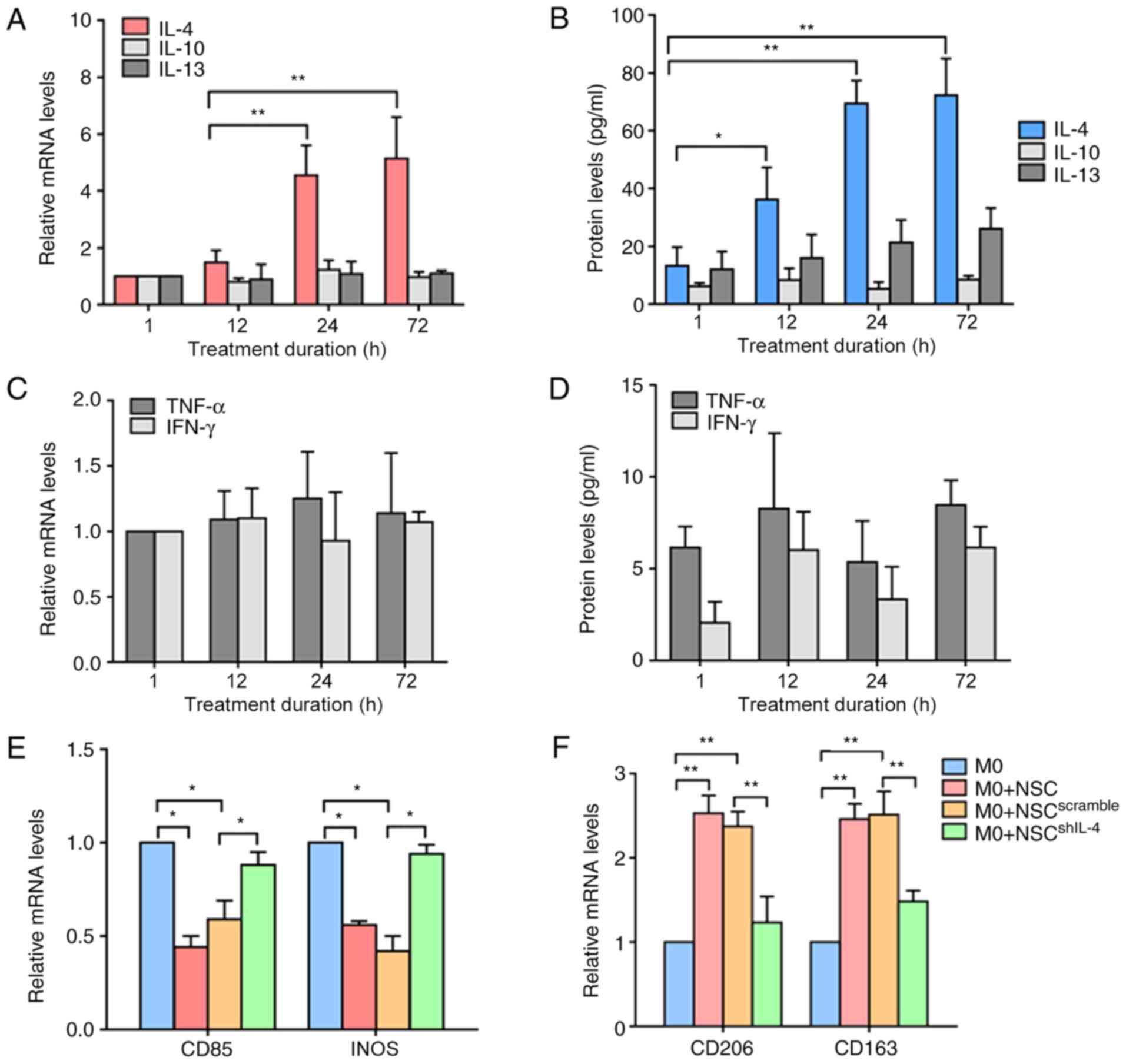 | Figure 2NSCs induce M2 polarization of
macrophages by upregulating IL-4. (A) RT-qPCR analyses of IL-4,
IL-10 and IL-13 expression in NSCs during neural differentiation.
(B) ELISA analyses of IL-4, IL-10 and IL-13 concentrations in the
culture medium of NSCs during neural differentiation. (C) RT-qPCR
analyses of TNF-α and IFN-γ expression in NSCs during neural
differentiation. (D) ELISA analyses of TNF-α and IFN-γ
concentrations in the culture medium of NSCs during neural
differentiation. (E and F) RT-qPCR analyses of CD85, inducible
nitric oxide synthase, CD206 and CD163 expression in macrophages
co-cultured with NSCs. *P<0.05,
**P<0.01. NSCs, neural stem cells; IL, interleukin;
RT-qPCR, reverse transcription-quantitative PCR; TNF, tumor
necrosis factor; IFN, interferon; INOS, inducible nitric oxide
synthase; M0, non-polarized macrophage. |
NSCs suppress the nuclear factor
(NF)-κB/p65 pathway to promote M2-polarization of macrophages in an
IL-4-dependent manner
Activation of the NF-κB/p65 pathway by inflammatory
cytokines such as TNF-α in macrophages induce the M1 polarization
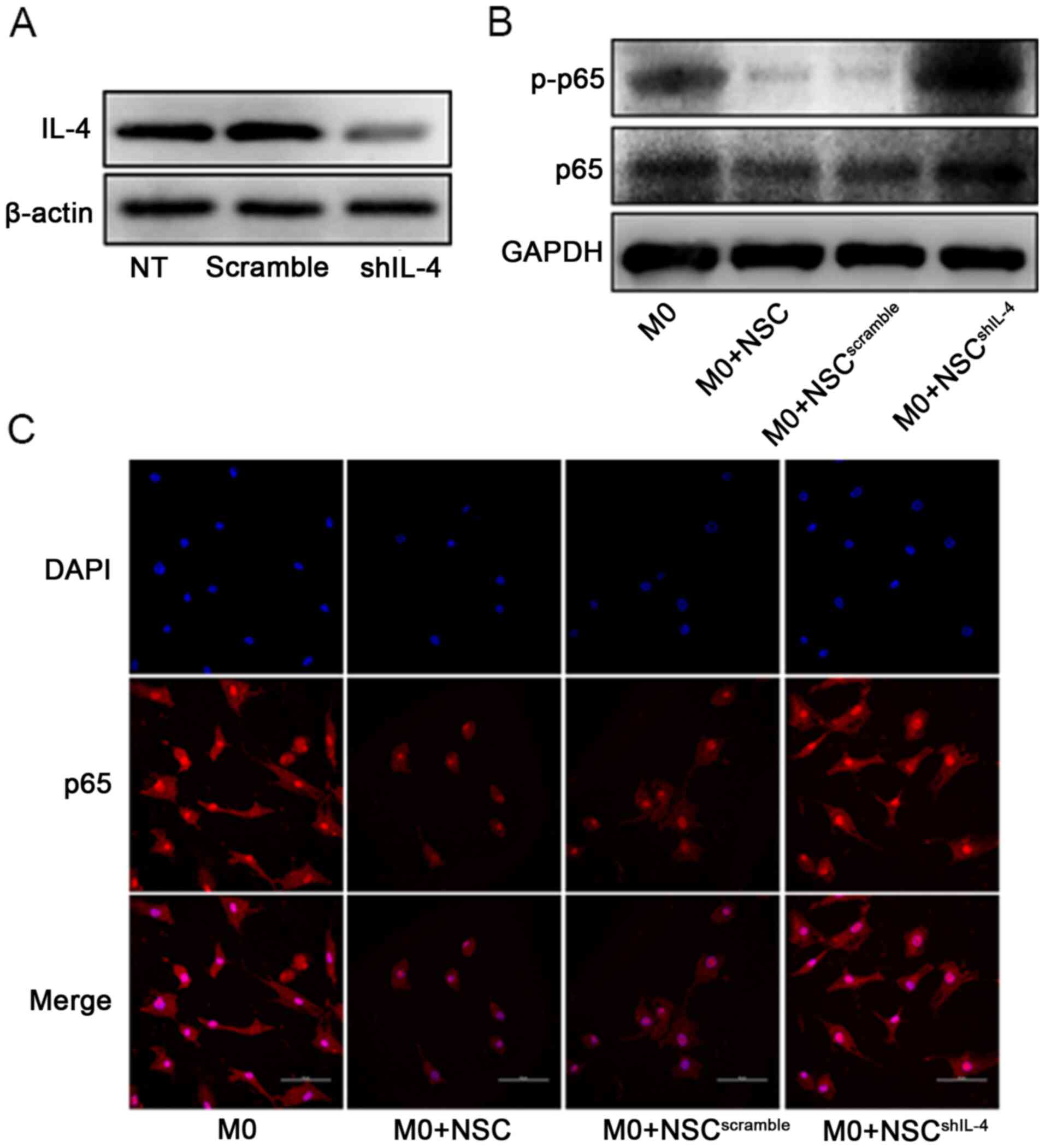
of macrophages (20,21). To determine whether IL-4 was crucial
for NSC-mediated inhibition of NF-ΚB/p65 pathway activation, a
target-specific lentivirus was used to knock down the expression
levels of IL-4 in NSCs (Fig. 3A),
then the NSCshIL-4 were co-cultured with macrophages.
The results demonstrated that silencing IL-4 expression
significantly diminished the inhibitory effect of NSCs on p65
translocation (from cytoplasm to nucleus) and p65 phosphorylation
in macrophages (Fig. 3B and
C). These results suggested that
NSCs suppress the NF-κB/p65 pathway to promote M2 polarization of
macrophages in an IL-4-dependent manner.
Discussion
The present study established an in vitro
co-cultured system to investigate the interactions between NSCs and
macrophages and the effect of NSCs on the polarization of
macrophages. The results demonstrated that M1-type macrophages
markers, such as CD85 and iNOS, were significantly decreased in
macrophages that were co-cultured with NSCs compared with those of
macrophages cultured alone. In addition, the number of
HLA-DR-positive cells was decreased in this co-cultured system. By
contrast, M2-type macrophage markers, such as CD206 and CD163, were
significantly increased in macrophages that were co-cultured with
NSCs compared with those of macrophages cultured alone. In
addition, CD206 and CD163 double-positive cells were decreased in
this co-cultured system. Additionally, IL-4 mRNA and protein
expression levels were significantly increased during neural
differentiation. Knockdown of IL-4 expression in NSCs diminished
the M2 polarization-promoting effect on macrophages and the
NF-κB/p65 pathway was the primary pathway involved in this
process.
Macrophages are important in a number of processes,
such as inflammation, tumorigenesis and tissue repair (22). M1/M2 polarization is a specific
characteristic of macrophages that divides macrophages into M1-type
macrophages and M2-type macrophages (23). M1-type macrophages are termed
inflammatory macrophages because this type of cell is related to
inflammatory production; M2-type macrophages are termed healing
macrophages because they have an anti-inflammatory effect and have
been reported to promote tissue repair (1).
As previously mentioned, a number of diseases are
caused by the imbalances in M1/M2 macrophage phenotypes (24,25).
For example, M1-type macrophages attack tumors and prevent tumor
growth (26). In contrast,
infiltration of M2-type macrophages promotes tumor deterioration
(27). In chronic inflammatory
processes, inflammatory cytokine production by M1-type macrophages
leads to cancer or dysregulation and promote tissue destruction,
followed by the M2-type macrophage response, promoting tissue
repair in these situations (28).
In SCI, macrophages also have an important role in the pathological
mechanism. Previous studies reported that both types of
macrophages, which have opposing neurotoxic and neuroprotective
functions, are recruited (8,29,30).
It has been reported that consistent infiltration of M1-type
macrophages is strongly related to numerous pathological processes
of SCI, such as neuronal death and demyelination (31,32).
On the other hand, studies have reported that the M2-type
macrophages switches from the M1-type macrophages. M2-type
macrophages act as anti-inflammatory cells, expressing IL-4 and
IL-10 and generating TGF-β, while downregulating the expression of
numerous proinflammatory cytokines, such as IL-1, IL-6, IL-18,
TNF-α and IFNγ (8). These mediators
of M2-type macrophages suppress inflammatory responses and promote
tissue remodeling and repair (33,34).
Thus, increasing the population of M2-type macrophages and
maintaining the survival of this type of cell in the
microenvironment of damaged tissue may represent a promising
strategy for tissue repair after SCI.
A number of clinical trials have reported the
efficacy of the stem cell transplantation as a potential strategy
to repair damaged tissue and recover locomotor functional after SCI
(15,35,36).
These studies demonstrated that the transplanted cells
differentiate into neurons, which mainly rebuilt neural circuits to
promote tissue repair and locomotor function recovery (37). In addition, the current study
indicated that transplanted cells have been considered to modulate
the polarization of macrophages to improve the microenvironment.
However, the mechanism by which transplanted NSCs affect
macrophages in the inflammatory microenvironment is still unclear.
A previous study reported that the medium from NSCs has
anti-inflammatory effects in vitro and in vivo,
suppressing the expression of inflammatory cytokines in macrophages
and injured spinal cord tissues (11). Matsubara et al (12) reported that conditioned medium from
stem cells induced spinal cord tissue recovery by promoting M2
polarization of macrophages. To the best of our knowledge, the
current study, provided the first evidence of the interaction
between NSCs and macrophages. The results of the present study
demonstrated that NSCs promoted M2 polarization in macrophages in
an in vitro co-cultured system. Nakajima et al
(38) demonstrated similar results
that MSC transplantation promoted M2 polarization of macrophages
and prevented M1 polarization of macrophages through the secretion
of IL-4 and IL-13.
The underlying mechanism of NSC-induced M2
polarization of macrophages remains unclear. M1-type macrophages
are produced after monocytes are stimulated with LPS or IFN-γ, and
M2-type macrophages are produced after monocytes are stimulated
with IL-10 or IL4(7). Based on
these findings, this study investigated whether IL-4, IL-10 and
IL-13 are involved in the interactions between NSCs and
macrophages. The results of the present study demonstrated that the
production of IL-4, but not IL-10 or IL-13, was increased in NSCs
during neural differentiation. Additionally, knockdown of IL-4
expression in NSCs diminished the promotion of M2 polarization in
the co-culture system. These findings suggested that NSCs induce M2
polarization of macrophages in an IL-4-dependent manner, which has
not been reported previously to the best of our knowledge.
M1-type macrophages have strong cytotoxicity and
anti-proliferative activities. They have been reported to promote
neuron death, prevent neuronal differentiation and tissue
destruction after SCI (39-41).
Activation of the NF-κB/p65 pathway by inflammatory cytokines such
as TNF-α and IL-1 in macrophages is important for M1 polarization
(42,43). The present study investigated the
effect of NSCs on the NF-κB/p65 pathway in macrophages; the results
demonstrated that NSCs suppress the activation of NF-κB/p65
signaling by suppressing the phosphorylation and translocation of
p65 in an IL-4-dependent manner. Taken together, the results of the
present study suggested that NSCs induce M2 polarization and
suppress the activation of NF-κB/p65 signaling to prevent M1
polarization in an IL-4-dependent manner. In future studies, the
effect and mechanism of M2-type macrophage protection of neurons
and promotion of neuronal differentiation in SCI will be
investigated.
There are some limitations of the current study;
in vivo experiments are better to indicate the effects of
IL-4 on improved tissue repair in SCI. Additionally, the current
study should discuss more information regarding the mechanisms of
IL-4 induced macrophage polarization. Also, studies on the negative
effects of M1 macrophage on the neuronal differentiation are
required in the future.
In conclusion, the results of the present study
suggested that NSCs secrete the cytokine IL-4 to induce M2
polarization of macrophages and suppress activation of NF-κB/p65
signaling to prevent M1 polarization of macrophages during neural
differentiation. The results of the present study may provide
useful information to overcome the challenges of tissue repair in
SCI and propose a potential therapeutic strategy for improving
locomotor function recovery.
Acknowledgement
The authors would like to thank Dr Yue Hu (The First
Affiliated Hospital of Sun Yat-sen University) for their assistance
with the experiments.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81971151) and the Guangdong
Natural Science Foundation (grant no. 2019A1515010241;
2020A1515010265).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ and XL designed the study. ZJ, XJ and XL
conducted the study. YL, JS and CC collected the data. ZJ, XJ, JS,
CC, and XL analyzed the data. ZJ, XJ, YL, and XL interpreted the
data. ZJ, XJ and XL drafted the manuscript. ZJ and XJ revised the
manuscript. All authors take responsibility for the integrity of
the data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Care and Use
Committee of Sun Yat-sen University (approval no. 4557, Guangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Funes SC, Rios M, Escobar-Vera J and
Kalergis AM: Implications of macrophage polarization in
autoimmunity. Immunology. 154:186–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Varga T, Mounier R, Horvath A, Cuvellier
S, Dumont F, Poliska S, Ardjoune H, Juban G, Nagy L and Chazaud B:
Highly dynamic transcriptional signature of distinct macrophage
subsets during sterile inflammation, resolution, and tissue repair.
J Immunol. 196:4771–4782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fahlman C, Jacobsen FW, Veiby OP, McNiece
IK, Blomhoff HK and Jacobsen SE: Tumor necrosis factor-alpha
(TNF-alpha) potently enhances in vitro macrophage production from
primitive murine hematopoietic progenitor cells in combination with
stem cell factor and interleukin-7: Novel stimulatory role of p55
TNF receptors. Blood. 84:1528–1533. 1994.PubMed/NCBI
|
|
7
|
He Y, Gao Y, Zhang Q, Zhou G, Cao F and
Yao S: IL-4 switches microglia/macrophage M1/M2 polarization and
alleviates neurological damage by modulating the JAK1/STAT6 pathway
following ICH. Neuroscience. 437:161–171. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kong X and Gao J: Macrophage polarization:
A key event in the secondary phase of acute spinal cord injury. J
Cell Mol Med. 21:941–954. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kigerl KA, Gensel JC, Ankeny DP, Alexander
JK, Donnelly DJ and Popovich PG: Identification of two distinct
macrophage subsets with divergent effects causing either
neurotoxicity or regeneration in the injured mouse spinal cord. J
Neurosci. 29:13435–13444. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Akinrinmade OA, Chetty S, Daramola AK,
Islam MU, Thepen T and Barth S: CD64: An attractive
immunotherapeutic target for M1-type macrophage mediated chronic
inflammatory diseases. Biomedicines. 5(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng Z, Bosco DB, Sun L, Chen X, Xu Y,
Tai W, Didier R, Li J, Fan J, He X and Ren Y: Neural stem
cell-conditioned medium suppresses inflammation and promotes spinal
cord injury recovery. Cell Transplant. 26:469–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsubara K, Matsushita Y, Sakai K, Kano
F, Kondo M, Noda M, Hashimoto N, Imagama S, Ishiguro N, Suzumura A,
et al: Secreted ectodomain of sialic acid-binding Ig-like lectin-9
and monocyte chemoattractant protein-1 promote recovery after rat
spinal cord injury by altering macrophage polarity. J Neurosci.
35:2452–2464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kano F, Matsubara K, Ueda M, Hibi H and
Yamamoto A: Secreted ectodomain of sialic acid-binding ig-like
lectin-9 and monocyte chemoattractant protein-1 synergistically
regenerate transected rat peripheral nerves by altering macrophage
polarity. Stem Cells. 35:641–653. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen N, Cen JS, Wang J, Qin G, Long L,
Wang L, Wei F, Xiang Q, Deng DY and Wan Y: Targeted inhibition of
leucine-rich repeat and immunoglobulin domain-containing protein 1
in transplanted neural stem cells promotes neuronal differentiation
and functional recovery in rats subjected to spinal cord injury.
Crit Care Med. 44:e146–e157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Peng Z, Long L, Tuo Y, Wang L, Zhao
X, Le W and Wan Y: Wnt4-Modified NSC transplantation promotes
functional recovery after spinal cord injury. FASEB J. 34:82–94.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang Q, Shi M, Shen Y, Cao Y, Zuo S, Zuo
C, Zhang H, Gabrilovich DI, Yu Y and Zhou J: COX-1-Derived
thromboxane A2 plays an essential role in early B-cell development
via regulation of JAK/STAT5 signaling in mouse. Blood.
124:1610–1621. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Orecchioni M, Ghosheh Y, Pramod AB and Ley
K: Macrophage polarization: Different gene signatures in
M1(LPS+) vs. classically and M2(LPS-) vs.
alternatively activated macrophages (vol 10, 1084, 2019). Front
Immunol. 24(1084)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miller JE, Ahn SH, Marks RM, Monsanto SP,
Fazleabas AT, Koti M and Tayade C: IL-17A Modulates peritoneal
macrophage recruitment and m2 polarization in endometriosis. Front
Immunol. 14:1082020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gu X, Zhang Y, Li D, Cai H, Cai L and Xu
Q: N6-Methyladenosine demethylase FTO promotes M1 and M2 macrophage
activation. Cell Signal. 69(109553)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Zhang J, Jiang X, Yang L, Zhang
Q, Wang B, Cui L and Wang X: Hydroxytyrosol inhibits LPS-induced
neuroinflammatory responses via suppression of TLR-4-mediated
NF-kappaB P65 activation and ERK signaling pathway. Neuroscience.
426:189–200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bagheri M, Mostafavinia A, Abdollahifar
MA, Amini A, Ghoreishi SK, Chien S, Hamblin MR, Bayat S and Bayat
M: Combined effects of metformin and photobiomodulation improve the
proliferation phase of wound healing in type 2 diabetic rats.
Biomed Pharmacother. 123(109776)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Milich LM, Ryan CB and Lee JK: The origin,
fate, and contribution of macrophages to spinal cord injury
pathology. Acta Neuropathol. 137:785–797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu YC, Zou XB, Chai YF and Yao YM:
Macrophage polarization in inflammatory diseases. Int J Biol Sci.
10:520–529. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Labonte AC, Tosello-Trampont AC and Hahn
YS: The role of macrophage polarization in infectious and
inflammatory diseases. Mol Cells. 37:275–285. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai YH, Yin KM, Su T, Ji F and Zhang S:
CTHRC1 in ovarian cancer promotes M2-like polarization of
tumor-associated macrophages via regulation of the STAT6 signaling
pathway. Onco Targets Ther. 13:5743–5753. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Dvorak HF: Tumors: Wounds that do not
heal-redux. Cancer Immunol Res. 3:1–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hauk TG, Muller A, Lee J, Schwendener R
and Fischer D: Neuroprotective and axon growth promoting effects of
intraocular inflammation do not depend on oncomodulin or the
presence of large numbers of activated macrophages. Exp Neurol.
209:469–482. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li SY, Nie K, Zhang QX, Guo ML, Qiu YH, Li
Y, Gao Y and Wang L: Macrophage migration inhibitory factor
mediates neuroprotective effects by regulating inflammation,
apoptosis and autophagy in Parkinson's disease. Neuroscience.
416:50–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ghasemlou N, Lopez-Vales R, Lachance C,
Thuraisingam T, Gaestel M, Radzioch D and David S:
Mitogen-Activated protein kinase-activated protein kinase 2 (MK2)
contributes to secondary damage after spinal cord injury. J
Neurosci. 30:13750–13759. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ghasemlou N, Bouhy D, Yang J, López-Vales
R, Haber M, Thuraisingam T, He G, Radzioch D, Ding A and David S:
Beneficial effects of secretory leukocyte protease inhibitor after
spinal cord injury. Brain. 133:126–138. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Marchetti V, Yanes O, Aguilar E, Wang M,
Friedlander D, Moreno S, Storm K, Zhan M, Naccache S, Nemerow G, et
al: Differential macrophage polarization promotes tissue remodeling
and repair in a model of ischemic retinopathy. Sci Rep.
76(1038)2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ahuja CS, Nori S, Tetreault L, Wilson J,
Kwon B, Harrop J, Choi D and Fehlings MG: Traumatic spinal cord
injury-repair and regeneration. Neurosurgery. 80:S9–S22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Saberi H, Firouzi M, Habibi Z, Moshayedi
P, Aghayan HR, Arjmand B, Hosseini K, Razavi HE and Yekaninejad MS:
Safety of intramedullary schwann cell transplantation for
postrehabilitation spinal cord injuries: 2-Year follow-up of 33
cases. J Neurosurg Spine. 15:515–525. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Assinck P, Duncan GJ, Hilton BJ, Plemel JR
and Tetzlaff W: Cell transplantation therapy for spinal cord
injury. Nat Neurosci. 20:637–647. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nakajima H, Uchida K, Guerrero AR,
Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT,
Johnson WE and Baba H: Transplantation of mesenchymal stem cells
promotes an alternative pathway of macrophage activation and
functional recovery after spinal cord injury. J Neurotrauma.
29:1614–1625. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kaushal V, Koeberle PD, Wang Y and
Schlichter LC: The Ca2+-activated K+ channel
KCNN4/KCa3.1 contributes to microglia activation and nitric
oxide-dependent neurodegeneration. J Neurosci. 27:234–244.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bao F, Bailey CS, Gurr KR, Bailey SI,
Rosas-Arellano MP, Dekaban GA and Weaver LC: Increased oxidative
activity in human blood neutrophils and monocytes after spinal cord
injury. Exp Neurol. 215:308–316. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rathore KI, Kerr BJ, Redensek A,
Lopez-Vales R, Jeong SY, Ponka P and David S: Ceruloplasmin
protects injured spinal cord from iron-mediated oxidative damage. J
Neurosci. 28:12736–12747. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kroner A, Greenhalgh AD, Zarruk JG, Passos
Dos Santos R, Gaestel M and David S: TNF and increased
intracellular iron alter macrophage polarization to a detrimental
M1 phenotype in the injured spinal cord. Neuron. 83:1098–1116.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Brykczynska U, Geigges M, Wiedemann SJ,
Dror E, Boni-Schnetzler M, Hess C, Donath MY and Paro R: Distinct
transcriptional responses across tissue-resident macrophages to
short-term and long-term metabolic challenge. Cell Rep.
30:1627–1643. 2020.PubMed/NCBI View Article : Google Scholar
|