Introduction
Massive subretinal hemorrhage (SRH) is a devastating
complication that can occur in patients with both wet and dry
age-related macular degeneration (AMD). Due to the toxic effect of
the accumulated iron compounds on the photoreceptors and retinal
pigment epithelium (RPE), the therapeutic solution must be found
very quickly, otherwise, the visual prognosis is reserved (1-3).
Subretinal injections with recombinant tissue plasminogen activator
(rTPA) have been used for some time and the results are highly
variable from case to case.
Alteplase is a thrombolytic agent, a glycoprotein,
produced by recombinant deoxyribonucleic acid (DNA) synthesis in
cell culture (4). It is approved by
Food and Drug Administration for intravenous administration in
acute ischemic stroke, pulmonary embolism, acute myocardial
infarction, and occluded catheters (5,6). From
its inactive form, it becomes active after fibrin coupling, which
eventually results in the transformation of plasminogen into
plasmin.
Without treatment, in a massive SRH, in the most
common cases, the final visual acuity of the patients is only light
perception. The subretinal use of rTPA led to the improvement of
these results (7,8), despite possible retinal toxicity
observed in cats and rabbits (9,10).
Moreover, using immunostaining with antibodies against
brain-specific home box/POU domain protein 3a (Brn3a), in a mouse
model of glaucoma, a degeneration of retinal ganglion cells was
observed, determined by up-regulating of the tissue plasminogen
activation (11). Brn3a is a
transcription factor expressed in the central and peripheral
sensory nervous system such as trigeminal ganglion or retinal
ganglion cells (RGCs) in rodents and probably in humans. In the
report described herein, it was shown that the increase in IOP
caused the increase of the proteolytically active tPA, which led to
a reduction of Brn3a and RGCs in mice.
However, there is no consensus regarding the
indications for this procedure, the dose of the substance, not even
the surgical technique. We describe three cases of massive SRH
determined by AMD, from our experience, in which we used the
subretinal injection of alteplase.
Case reports
The study was performed accordingly to the
Institutional Guidelines of the Ponderas Academic Hospital,
Bucharest, Romania. Approval was obtained from the Institutional
Review Board and the Ethics Committee of the Ponderas Academic
Hospital. All procedures conformed to the tenets of the World
Medical Association Declaration of Helsinki. All patients gave
their written informed consent.
A series of three cases of SRH are described. In all
cases, a 25-gauge pars plana vitrectomy (PPV) with hyaloid removal
was performed. In eyes with vitreous hemorrhage, all blood and
membranes were removed from the retinal surface. For the subretinal
injection, a 1 ml insulin syringe and a 38-gauge cannula were used.
Actilyse® 50 mg (Boehringer Ingelheim International
GMBH, Germany) was used. After the powder and solvent were properly
mixed according to the instructions, 0.6-0.7 ml of solution was
drawn into the syringe. On average 0.3 ml of the substance was
injected through the lower part of the macula, until a bullous
retinal detachment was obtained. Depending on the size of the clot,
more or less of the substance was injected or a second injection
was made in another part of the macula. In some cases, a quick
dislocation of the clot through the injected site was observed.
After total fluid-air exchange, 1.25 mg/0.05 ml bevacizumab
(Avastin®, Roche, Switzerland) injection was
intravitreally injected in all patients (12). Face-down position was recommended in
all cases for the next 24 h. Patients were evaluated on the first
day, 1 week and 1 month after surgery.
Case 1
A 74-year old male with neovascular AMD in both
eyes, with anti-vascular endothelial growth factor (VEGF) therapy
in the right one, presented due to a sudden decrease of visual
acuity and central scotoma for 2-3 days in his right eye. Due to
cardiovascular risk, the patient was treated with anti-aggregating
agents and oral anticoagulants for several years. Best-corrected
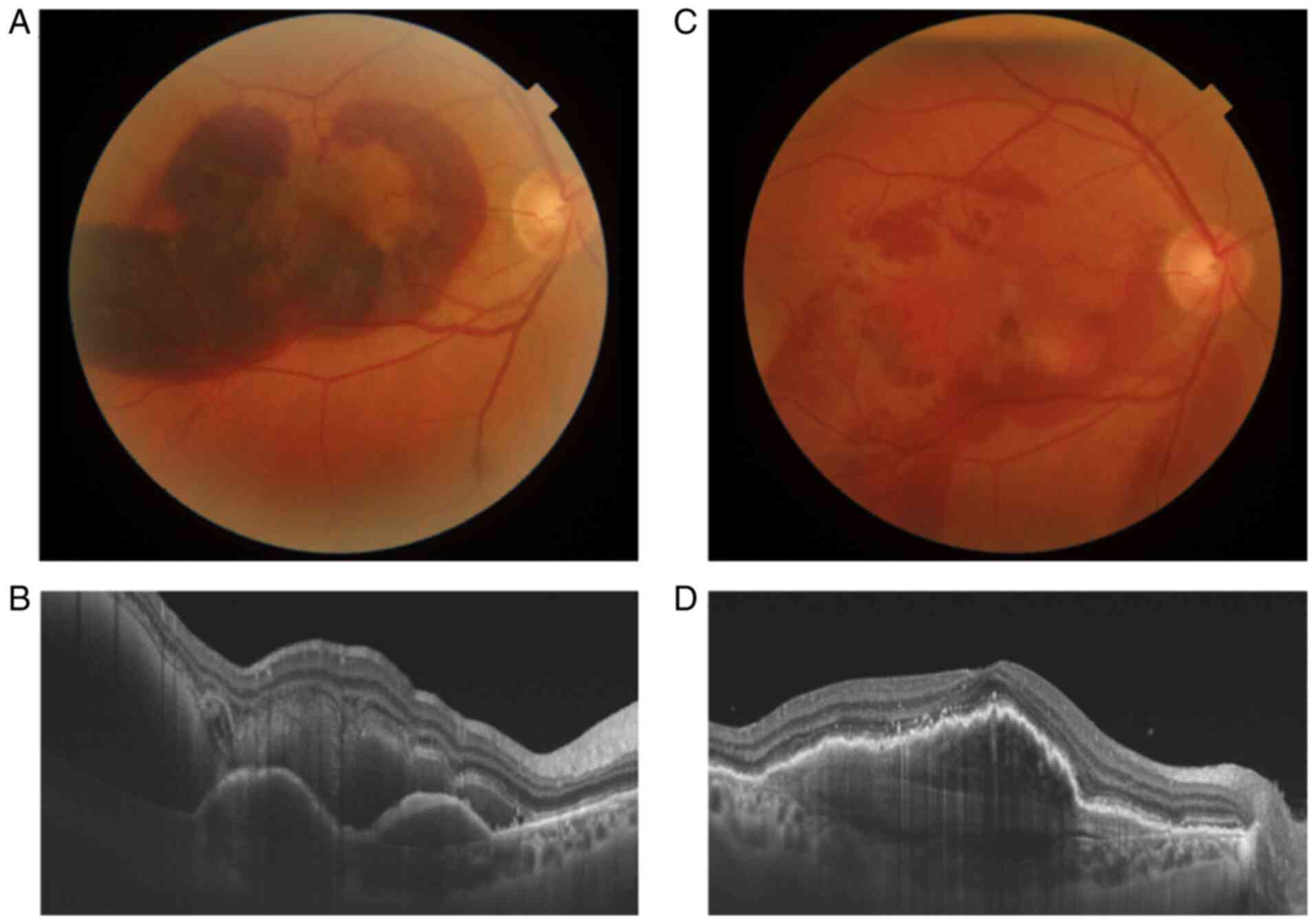
visual acuity (BCVA) at the presentation was hand motion. At the
fundoscopic examination, a massive SRH was observed affecting the
entire macular surface. Optical coherence tomography (OCT) revealed
a significant retinal thickening. The patient was submitted to PPV,
with subretinal rTPA injection and intravitreal bevacizumab.
Fluid-gas exchange was performed at the end of the surgery. Due to
gas and diffuse vitreous hemorrhage, weakly transparent, BCVA was
hand motion the first 2 weeks postoperatively. One month after the
surgery BCVA was 0.8 (decimal fraction), with a significant
improvement of the fundoscopic and spectral domain optical
coherence tomography (SD-OCT) examination (Fig. 1). Clinical examination revealed the
presence of the blood clot inferior, below the peripheral retina.
The patient followed regular checkups, with intravitreal injections
of bevacizumab as needed.
Case 2
A 60-year old male patient was referred with acute
vision loss for 2 days in the right eye. Without ophthalmological
history, being in treatment with oral anticoagulants for 3 years.
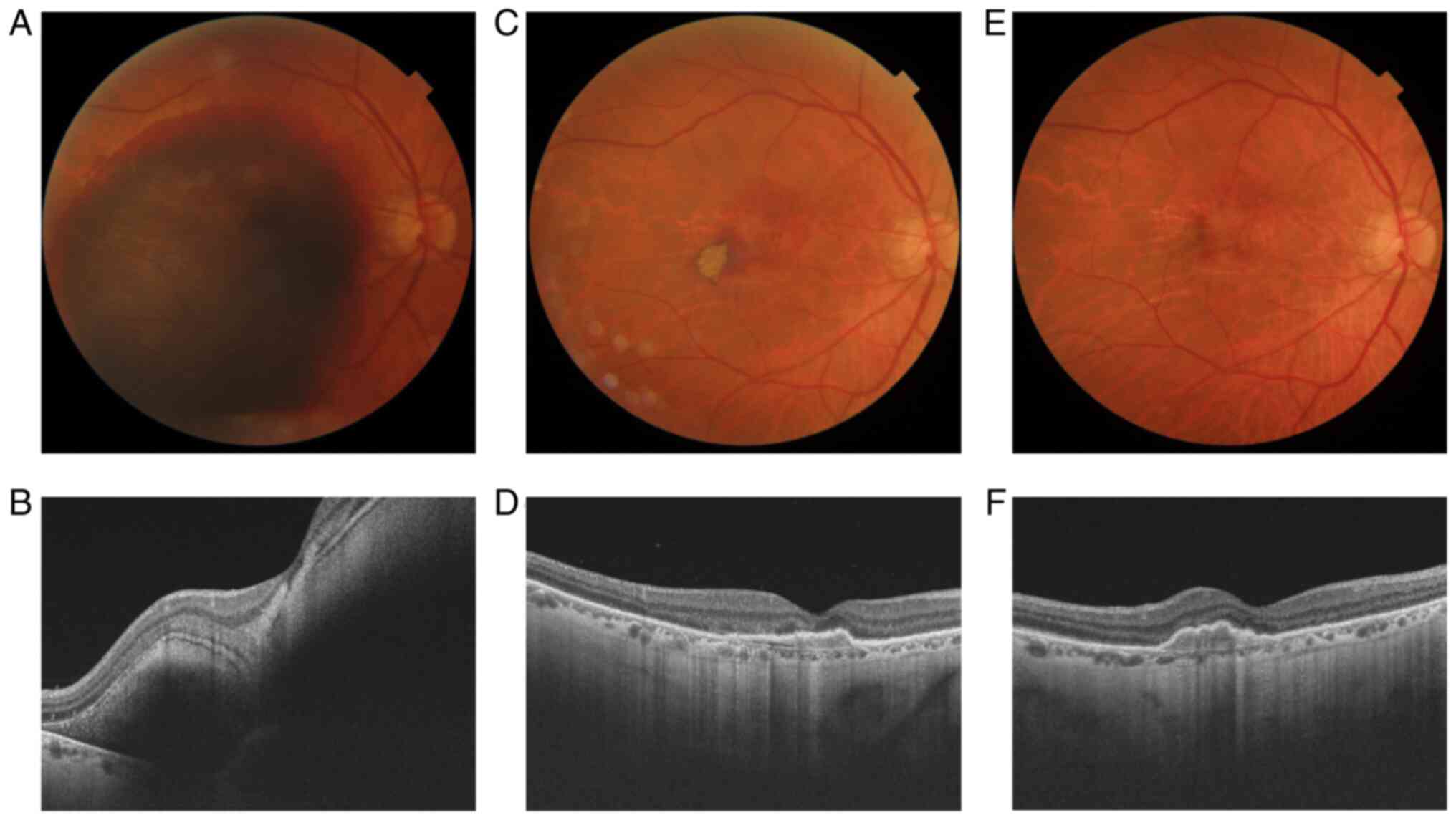
BCVA at presentation was hand motion. The fundoscopic examination
revealed an important SRH in the macular area, with a marked
increase in retinal thickness on SD-OCT. PPV and subretinal rTPA
injection were performed on the same day. Intraoperatively, a
slight movement of the clot was already observed. Seven days after
the surgery the BCVA was 0.05. The fundoscopic aspect improved
significantly, a few small areas of blood below the retina and
under the RPE persisted. On SD-OCT the retinal thickness is close
to normal, foveolar depression is also observed. In one month no
postoperative complications appeared, BCVA was 0.2, fundoscopic and
SD-OCT appearance were preserved or even small improvements were
observed (Fig. 2).
Case 3
A 70-year old woman with neovascular AMD, treated
with anti-VEGF agents, was referred to our clinic for a severe SRH.
The last injection with bevacizumab was 10 months before this
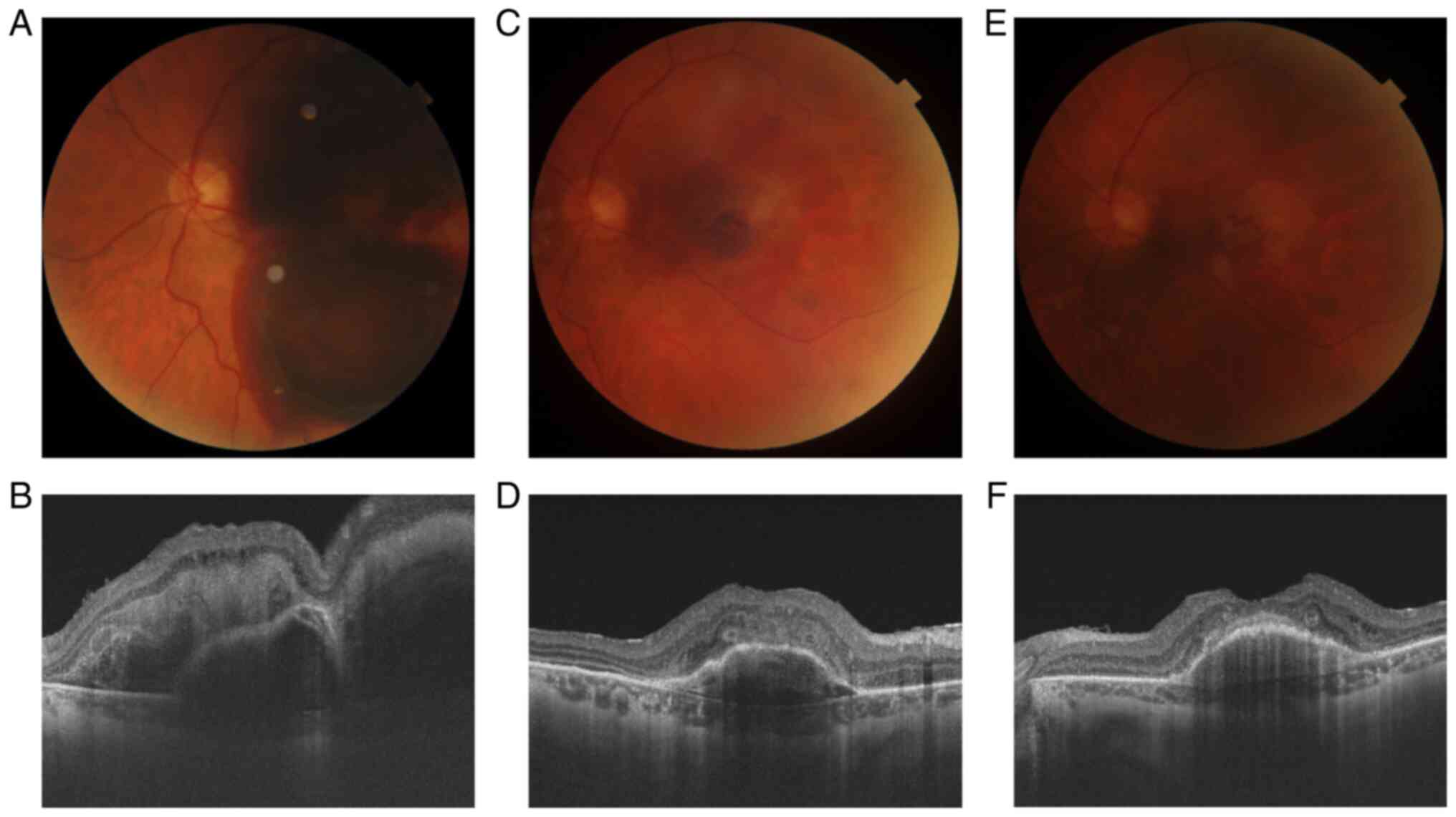
event. Visual acuity decreased to hand motion for about 4 days. The
clinical aspect of the retina was similar to the previous cases, a
massive submacular hemorrhage. On SD-OCT, a subretinal and under
RPE hemorrhages were observed. The same surgical steps were
followed in this case. BCVA was counting fingers after the first
week and 0.16 after one month, the patient was very happy that he
returned to visual acuity as before the event. At the fundoscopic
examination, the remains of hemorrhage were visualized the first
week postoperatively. The SRH disappeared almost completely over a
month, an area of RPE atrophy and pigmentary changes remained at
the macula level. The SD-OCT showed an RPE detachment, with
possible RPE rolls and a significant distortion of the outer layers
of the retina after 1 week. The aspect has improved significantly
over a month (Fig. 3). The patient
followed regular check-ups, with intravitreal injections of
bevacizumab or aflibercept as needed.
Discussion
AMD is one of the most common causes of SRH in
adults. Therefore the definitive visual acuity of each patient,
regardless of the therapeutic solution chosen, also depends on the
degree of macular damage before bleeding. In our practice, we have
9 cases of SRH for which subretinal injections with rTPA were
performed. Eight of them were caused by AMD and a single one by a
choroidal neovascular membrane in a high myopic eye. In most cases,
the baseline visual acuity was hand motion or light perception and
at least counting fingers after the surgery. Case number 1
presented above is the one in which the best postoperative visual
acuity was obtained. The postoperative clinical and OCT appearance
was spectacular in all cases.
The prognostic factors of postoperative visual
acuity was observed to be visual acuity before hemorrhage occurred
and the time elapsed since the onset of the SRH. The most frequent
contraindication for rTPA injection was late presentation. Late
presentation means poor visual potential due to neurosensory
retinal distress (13). All cases
in which a visual acuity greater than counting fingers was
obtained, presented in the first 5 days of onset. The maximum
duration of the SRH for which we used this technique was 3 weeks,
and postoperative visual acuity was counting fingers at 1 m. We did
not have any intraoperative complications due to alteplase or other
factors. Regarding postoperative complications only one case of
rhegmatogenous retinal detachment, caused by a peripheral retinal
break, was observed.
Regarding the surgical technique used, we considered
that it is one of the most optimal in terms of the applications
during the surgery and after it. Probably one of the most widely
used methods is for the surgeon to hold the syringe and an
assistant to push the plunger. In other words, this technique
requires a good collaboration between team members and a good
training of the entire team of surgeons and assistant. Another
method to inject the alteplase is using the viscous fluid control
unit. This is a semiautomated technique that requires an insulin
syringe of 1-ml adapted to the viscous fluid control unit and a
41-gauge cannula. This technique probably offers more control and
stability than the assisted one. In our opinion what makes our
technique more practical and easy to use is that all operative
steps can be performed by a single surgeon (14). No additional time is needed for clot
lysis or to prepare special injection devices (15). There are no studies that compare the
functional results of these surgical techniques, so at the moment
the choice is strictly related to the comfort and possibilities of
the surgeon. Based on our experience the fluid-air exchange is
sufficient for the mobilization of the clot, the injection of an
expandable gasis not necessary. The decision to inject an anti-VEGF
drug at the end of the operation seems to be quite important,
considering the pathophysiological mechanism of the SRH.
In massive hemorrhages, a larger amount of alteplase
was needed for clot dislocation and dissolution. However, even in
these patients, no dose-dependent toxic effect was observed. Also,
no special attitude was taken towards patients treated with
anticoagulant or anti-aggregating agents. All the patients followed
the treatment recommended by the cardiologist. Moreover, the
injection with anti-VEGF agent at the end of the surgery had
exactly this purpose, to prevent subsequent bleeding, which did not
occur in any of the cases.
Studying the maximum duration of the SRH for which
surgery is worthwhile is one of our future goals. Another purpose
is a long-term follow-up of patients in whom the subretinal
alteplase was injected and their comparison with those in whom a
less invasive therapeutic solution was decided.
SRH is a common complication of the AMD which can
lead to irreversible loss of visual acuity. Due to intravitreal
injections with anti-VEGF agents, AMD in most patients can be kept
under control. Thus, surgery has become an indication only for
complicated cases of SRH. Subretinal injection with rTPA is a
viable solution in patients with massive SRH who are addressed on
time. In our practice, this technique has become the first
therapeutic approach that we adopt in such cases.
Acknowledgements
Not applicable.
Funding
No funding was received. This research did not
receive any specific grant from funding agencies in the public,
commercial, or not-for-profit sectors.
Availability of data and materials
The first author and the corresponding author have
full access to all the data and materials in the study, and the
data are available from the corresponding author on reasonable
request.
Authors' contributions
RO, FB and RB participated in conceptualization,
design and writing of the original draft. MB, MZ and UO were
involved in the analysis and visualization of the results, the
acquisition of the data and literature research. DSB, RO and FB did
a critical interpretation of the data for the study and edited the
manuscript. All authors revised the manuscript for important
intellectual content, read and approved the final version of the
manuscript to be published.
Ethics approval and consent to
participate
The study was retrospectively approved by the
Institutional Review Board and the Ethics Committee of Ponderas
Academic Hospital (Bucharest, Romania). All procedures conformed to
the tenets of the World Medical Association Declaration of
Helsinki.
Patient consent for publication
Written informed consent was obtained from all the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JH, Lee MY and Lee WK: Incidence and
risk factors of massive subretinal hemorrhage in retinal
angiomatous proliferation. PLoS One. 12(e0186272)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maranduca MA, Branisteanu D, Serban DN,
Branisteanu DC, Stoleriu G, Manolache N and Serban IL: Synthesis
and physiological implications of melanic pigments. Oncol Lett.
17:4183–4187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Munteanu M, Rosca C and Stanca H:
Sub-inner limiting membrane hemorrhage in a patient with Terson
syndrome. Int Ophthalmol. 39:461–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Michel P, Lindsay P, Martins S, Pandian
JD, Caso V, Kim JS, Bryer A, Anderson C, Feigin V, Sandercock P,
et al: Alteplase (recombinant tissue Plasminogen Activator,
rt-PA) for the Treatment of Acute Ischemic Stroke. Application for
inclusion of a new individual medicine in the WHO Model List of
Essential Medicines (EML) For the 2019 WHO Expert Committee on the
Selection and Use of Essential Medicines and Canadian Stroke
Network. Alteplase for AIS: WHO EML 2019 application. urihttps://www.who.int/selection_medicines/committees/expert/22/applications/s12.5.2_alteplase.pdf?ua=1simplehttps://www.who.int/selection_medicines/committees/expert/22/applications/s12.5.2_alteplase.pdf?ua=1.
|
|
5
|
Stanca HT, Petrović Z and Munteanu M:
Transluminal Nd:YAG laser embolysis - a reasonable method to
reperfuse occluded branch retinal arteries. Vojnosanit Pregl.
71:1072–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harvison PJ: Alteplase. In: xPharm: The
Comprehensive Pharmacology Reference. Elsevier Inc., Amsterdam,
pp1-5, 2007.
|
|
7
|
Fine HF, Iranmanesh R, Del Priore LV,
Barile GR, Chang LK, Chang S and Schiff WM: Surgical outcomes after
massive subretinal hemorrhage secondary to age-related macular
degeneration. Retina. 30:1588–1594. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh RP, Patel C and Sears JE: Management
of subretinal macular haemorrhage by direct administration of
tissue plasminogen activator. Br J Ophthalmol. 90:429–431.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Johnson MW, Olsen KR, Hernandez E, Irvine
WD and Johnson RN: Retinal toxicity of recombinant tissue
plasminogen activator in the rabbit. Arch Ophthalmol. 108:259–263.
1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hrach CJ, Johnson MW, Hassan AS, Lei B,
Sieving PA and Elner VM: Retinal toxicity of commercial
intravitreal tissue plasminogen activator solution in cat eyes.
Arch Ophthalmol. 118:659–663. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chintala SK: Tissue and urokinase
plasminogen activators instigate the degeneration of retinal
ganglion cells in a mouse model of glaucoma. Exp Eye Res.
143:17–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stanca HT, Stanca S, Tabacaru B, Boruga M
and Balta F: Bevacizumab in Wet AMD treatment: A tribute to the
thirteen years of experience from the beginning of the anti-VEGF
era in Romania. Exp Ther Med. 18:4993–5000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moraru AD, Costin D, Moraru RL, Costuleanu
M and Brănișteanu DC: Current diagnosis and management strategies
in pachychoroid spectrum of diseases (Review). Exp Ther Med.
20:3528–3535. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Haupert CL, McCuen BW, Jaffe GJ, Steuer
ER, Cox TA, Toth CA, Fekrat S and Postel EA: Pars plana vitrectomy,
subretinal injection of tissue plasminogen activator, and fluid-gas
exchange for displacement of thick submacular hemorrhage in
age-related macular degeneration. Am J Ophthalmol. 131:208–215.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Novelli FJD, Preti RC, Monteiro MLR,
Nóbrega MJ and Takahashi WY: A new method of subretinal injection
of tissue plasminogen activator and air in patients with submacular
hemorrhage. Retina. 37:1607–1611. 2017.PubMed/NCBI View Article : Google Scholar
|