Introduction
Thyroid hormones play a critical role in the
differentiation and proliferation of neurons in the brain and are
closely related to brain development (1). Hypothyroidism is a thyroid dysfunction
caused by insufficient synthesis, secretion or physiological
effects of thyroid hormones, which is characterized by an increase
in serum levels of thyroid-stimulating hormone (TSH) accompanied by
a reduction in the level of free thyroxine (FT4) and in some cases
a reduction in the level of free triiodothyronine (FT3) (2). Neonatal hypothyroidism may cause
growth and developmental disorders as well as mental retardation in
neonates (3,4). Neonatal hypothyroidism is a common
clinical condition and is one of the major causes of mental and
physical retardation in children (5). Neonatal screening in China in 2,000
indicated that the incidence of neonatal hypothyroidism was
approximately 1/3,624 in and that the condition severely endangers
the health of neonates and can cause irreversible damage following
the onset of clinical symptoms (6,7). The
pathogenesis and causes of neonatal hypothyroidism remain unclear;
however, it is reported that pregnant women's health and lifestyle
and the foetal environment are factors influencing development of
hypothyroidism (8). Thyroid
function tests are important methods for early detection and
intervention in neonatal hypothyroidism. The early detection of
neonatal hypothyroidism facilitates therapeutic intervention before
the onset of clinical symptoms and enables optimal physical and
intellectual development in neonates (9). The risk factors for neonatal
hypothyroidism vary and have been reported to include increased
gestational age at birth and birth weight. Neonates who had a
premature birth, low birth weight, post-term birth and fetal
macrosomia are at a higher risk of hypothyroidism compared with
neonates born at term (10-12).
In previous studies, neonatal hypothyroidism screening was mainly
performed by measuring thyroid-stimulating hormone (TSH) and free
thyroxine [(FT4); TSH+FT4] levels, which had low sensitivity and
specificity (13). Zhang et
al (14) showed that most
neonatal hyperthyrotropinemia is resolved with age, but some
infants will continue to have abnormal thyroid function, and should
be actively followed up to prevent hypothyroidism (14). Newborn disease screening is widely
performed and term neonates with congenital hypothyroidism can be
diagnosed in a timely manner. However, there are still no accurate
diagnostic criteria for abnormal thyroid function in premature
infants. Premature infants have clinical manifestations of
hypothyroidism, such as lethargy, anorexia, fatigue, constipation,
delayed jaundice, edema, dry skin and poor peripheral circulation.
Without timely intervention, hypothyroidism may have adverse
effects on neonates' growth, development, cognition and even cause
irreversible brain damage (15).
Based on this, in the present study, the risk
factors for neonatal hypothyroidism using gestational age at birth
in combination with TSH+FT4 levels were investigated to screen for
neonatal hypothyroidism and predict its occurrence.
Materials and methods
Study subjects
In total, 686 neonates with suspected hypothyroidism
(TSH >10 mIU/l) who were admitted to the The First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) between
January 2012 and January 2019 were included in this study for
retrospective analysis. From these, 70 with confirmed
hypothyroidism were randomly assigned to the patient group,
including 30 males and 40 females with an average age of 37.26±4.35
weeks. Hypothyroidism diagnosis was performed using the following
criteria provided by the Clinical Laboratory of The First
Affiliated Hospital of Chongqing Medical University: i) increased
serum TSH level (normal TSH level, 0.35-5.50 µIU/ml); ii) decreased
serum FT4 level (normal FT4 level, 0.89-1.76 ng/dl); and iii)
normal/decreased free triiodothyronine (FT3) level (normal FT3
level, 2.30-4.20 pg/ml). Neonates who did not meet the above
diagnostic criteria for hypothyroidism were excluded from the
patient group. In the patient group, the gestational age at birth
of 15 neonates was <37 weeks, 47 neonates was 37-42 weeks and 8
neonates was >42 weeks. The normal (control) group was comprised
of 70 neonates with normal thyroid function, including 36 males and
34 females with an average age of 39.58±4.23 weeks. These 70
neonates with normal thyroid function were recruited during the
same period as the patient group and were not screened from the 686
aforementioned neonates. The inclusion criteria for the control
group neonates were: i) born at The First Affiliated Hospital of
Chongqing Medical University; ii) live births; iii) had no
congenital malformation; iv) underwent their first examination at
the age of <3 days; v) parents provided written consent for
study participation; and vi) whose mothers underwent thyroid
function tests during pregnancy and had a normal range. The
exclusion criteria for the control group neonates were: i)
Levothyroxine® therapy prior to examination; ii) had
metabolic disorders; or iii) had incomplete examination data.
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) and signed informed consent was
obtained from the parents of all the neonates who were included in
the study.
Electrochemiluminescence (ECL) assays
and clinical information
Information on indicators (including sex,
gestational age at birth, Apgar score, birth weight, body length,
head circumference and heart rate) were collected from the neonates
in the patient and control groups. TSH and FT4 levels were first
measured in neonates 72 h after birth by the
electrochemiluminescence immunoassay using the DIX800
Chemiluminescence Analyzer (Beckman Coulter, Inc.) and FT3 and FT4
chemiluminescence immunoassay kits (access kit; cat. nos. 170611
and 171230) from Beckman Coulter, Inc. In this procedure, drops of
blood were collected from the neonatal heel and were tested by
filter paper. Venous blood samples (3 ml) were collected from the
elbow in tubes containing anticoagulant and centrifuged at 2,264 x
g for 30 min at 4˚C to extract serum.
Treatment regimen
Neonates with confirmed hypothyroidism were orally
administered 10-15 µg/kg/day thyroxine (Shandong Lubei
Pharmaceutical Co., Ltd.; SFDA approval no. H3702162). TSH and FT4
levels were re-examined at 2, 4 and 8 weeks of age and compared
between the control and patient groups. In addition, data were
gathered on maternal thyroid dysfunction, fetal macrosomia and
low-birth-weight neonates in the aforementioned groups. Diagnostic
criteria for maternal thyroid dysfunction were increased TSH;
decreased FT4; and normal or decreased FT3(16).
Statistical analyses
Data were analyzed using SPSS Statistics v.23.0 (IBM
Corp.). Measurement data are expressed as mean ± standard deviation
values using independent samples t-test. Numerical data are
presented as percentages and were analyzed using the χ2
test. One-way ANOVA was used to compare the mean among multiple
groups and the least significant difference (LSD) post hoc test was
used for pairwise comparison when the variance was homogeneous and
the Dunnett's T3 post hoc was used when the variance was
heterogeneous. The related risk factors for neonatal hypothyroidism
were subjected to univariate analysis and logistic regression
analysis was performed when univariate analysis demonstrated
significant differences. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of clinical and demographic
data between the patient and control groups
Sex, gestational age at birth, Apgar score, birth
weight, body length, head circumference and heart rate of the two
groups were collected and compared and the results showed that
there were no significant differences between the patient and
control groups in terms of sex, body length, head circumference and
delivery mode (P>0.05; Table I).
However, the patient group had a significantly lower gestational
age at birth, Apgar score, birth weight and heart rate compared
with the control group (P<0.05; Table I) indicating that the neonates with
hypothyroidism had poorer clinical indicators compared with the
control neonates.
 | Table IComparison of the clinical and
demographic data of neonates with hypothyroidism (patients) and
normal (control) neonates. |
Table I
Comparison of the clinical and
demographic data of neonates with hypothyroidism (patients) and
normal (control) neonates.
| Parameters | Patient group
(n=70) | Control group
(n=70) | Τ-test/χ2
test | P-value |
|---|
| Sex (male/female),
n | 30/40 | 36/34 | 1.032 | 0.310 |
| Gestational age
(weeks), mean ± SD | 37.26±4.35 | 39.58±4.23 | 3.199 | 0.002 |
| Apgar score (scores),
mean ± SD | 9.06±0.52 | 9.53±0.47 | 5.610 | <0.001 |
| Birth weight (g),
mean ± SD | 3.50±1.14 | 3.92±1.17 | 2.151 | 0.033 |
| Body length (cm),
mean ± SD | 48.49±5.23 | 50.19±5.44 | 1.885 | 0.062 |
| Head circumference
(cm), mean ± SD | 33.56±2.36 | 34.22±2.28 | 1.683 | 0.095 |
| Heart rate (bpm),
mean ± SD | 97.22±10.65 | 129.71±10.79 | 17.930 | <0.001 |
| Delivery modes
(eutocia/cesarean section), n | 48/22 | 52/18 | 0.560 | 0.454 |
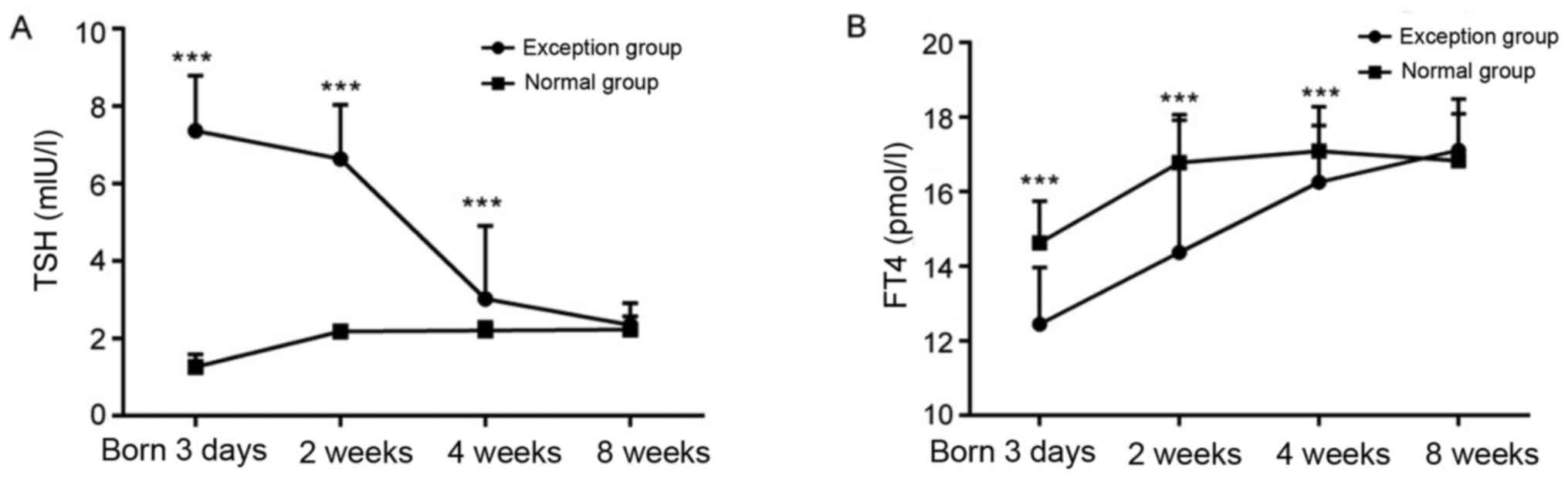
Neonates with hypothyroidism had
higher TSH levels and lower FT4 levels compared with controls
TSH and FT4 levels of the two groups were measured
by electrochemiluminescence immunoassay, and the results showed
that significantly higher TSH levels but lower FT4 levels at 3 days
of age were observed in the patient group compared with the control
group (P<0.05; Fig. 1A and
B). In addition, the TSH level
decreased, however FT4 levels increased during the 8 weeks of
treatment (Fig. 1). Following 8
weeks of treatment, there was no significant difference in the TSH
and FT4 levels between the patient and control groups (P>0.05;
Fig. 1). Thus, neonates may
gradually recover from hypothyroidism diagnosed at birth following
treatment.
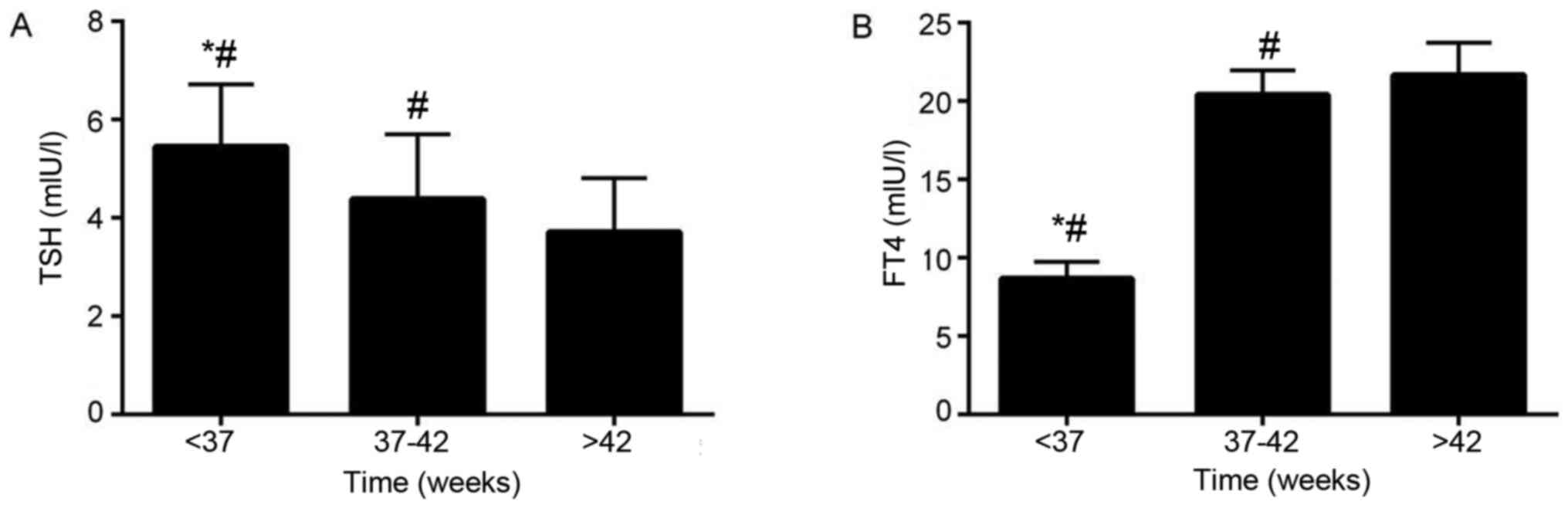
Effect of different gestational age at
birth on TSH and FT4 levels in neonates with hypothyroidism
Neonates with hypothyroidism were divided into
<37 weeks, 37-42 weeks, and >42 weeks according to
gestational age, and TSH and FT4 levels of neonates at different
gestational ages were compared. TSH and FT4 levels of newborns with
different gestational age were compared. The results showed that in
the patient group, the gestational age at birth of 15 neonates was
<37 weeks, 47 neonates was 37-42 weeks and 8 neonates was >
42 weeks. The TSH levels of neonates >42 weeks of gestational
age were significantly lower compared with neonates that were 37-42
weeks and <37 weeks of gestational age (FTSH=10.772,
PTSH <0.001; Fig.
2A). The FT4 levels of neonates with >42 weeks of
gestational age were significantly higher compared with neonates
that were 37-42 weeks and <37 weeks of gestational age
(FFT4=15.468 and PFT4 <0.001; Fig. 2B). The TSH levels of neonates with
37-42 weeks of gestational age were significantly lower compared
with neonates with <37 weeks of gestational age, while the FT4
levels of neonates with 37-42 weeks of gestational age were
significantly higher compared with neonates <37 weeks of
gestational age (Fig. 2). The
aforementioned results suggest that the younger the gestational age
at birth of neonates with hypothyroidism was, the more obvious the
degree of hypothyroidism was.
Combined use of TSH plus FT4 levels
and gestational age at birth had a higher predictive value compared
with TSH plus FT4 levels in screening for neonatal
hypothyroidism
Using a TSH level of >9 mIU/l, FT4 level of
<8.3 pmol/l and gestational age at birth of <37 weeks as
predictive values, the combined use of TSH+FT4 levels and
gestational age at birth to predict neonatal hypothyroidism
demonstrated a significantly higher positive predictive value,
sensitivity and accuracy compared with the use of only TSH+FT4
levels (P<0.001 vs. P=0.003 vs. P<0.001; Table II).
 | Table IIPredictive values for the combined use
of gestational age with TSH and FT4 values for screening neonatal
thyroid dysfunction. |
Table II
Predictive values for the combined use
of gestational age with TSH and FT4 values for screening neonatal
thyroid dysfunction.
| Screening method | Positive, %
(n/n) | Sensibility, %
(n/n) | Specificity, %
(n/n) | Accuracy, %
(n/n) | Negative predictive
value, % (n/n) | Positive predictive
value, % (n/n) |
|---|
| TSH+FT4+gestational
age | 11.66 (80/686) | 92.86 (65/70) | 97.56 (601/616) | 97.08 (666/686) | 99.17 (601/606) | 81.25 (65/80) |
| TSH+FT4 | 13.70 (94/686) | 74.29 (52/70) | 93.18 (574/616) | 91.25 (626/686) | 96.96 (574/592) | 55.32 (52/94) |
|
χ2 | 1.290 | 8.792 | 20.293 | 21.238 | 7.806 | 13.193 |
| P-value | 0.256 | 0.003 | <0.001 | <0.001 | 0.005 | <0.001 |
Univariate analyses of risk factors
for neonatal hypothyroidism
Univariate analyses was performed and revealed that
low gestational age at birth, maternal thyroid dysfunction, fetal
macrosomia and low birth weight were the underlying risk factors
for neonatal hypothyroidism (P<0.05; Table III).
 | Table IIIUnivariate analysis of the related
risk factors of neonatal hypothyroidism. |
Table III
Univariate analysis of the related
risk factors of neonatal hypothyroidism.
| Risk factors | Total number of
neonates, n | Patient group, (n=70)
n (%) | Normal group, (n=70)
n (%) | χ2 | P-value |
|---|
| Sex | | | | | |
|
Male | 69 | 30 (42.86) | 37 (52.86) | 1.403 | 0.236 |
|
Female | 71 | 40 (57.14) | 33 (47.14) | | |
| Gestational age,
weeks | | | | | |
|
<37 | 31 | 26 (34.1) | 5 (7.14) | 27.650 | <0.001 |
|
37-42 | 95 | 33 (47.14) | 62 (88.57) | | |
|
>42 | 14 | 11 (15.71) | 3 (4.29) | | |
| Maternal thyroid
function | | | | | |
|
Normal | 85 | 17 (24.29) | 68 (97.14) | 77.891 | <0.001 |
|
Abnormal | 55 | 53 (75.71) | 2 (2.86) | | |
| Fetal macrosomia
(>4,000 g) | | | | | |
|
Yes | 11 | 10 (14.29) | 3 (4.29) | 4.155 | 0.042 |
|
No | 129 | 60 (85.71) | 67 (95.71) | | |
| Low birth weight
(<2,500 g) | | | | | |
|
Yes | 18 | 16 (22.86) | 2 (2.86) | 12.495 | <0.001 |
|
No | 122 | 54 (77.14) | 68 (97.14) | | |
Low gestational age at birth, maternal
thyroid dysfunction and low birth weight are risk factors for
neonatal hypothyroidism
In logistics regression analysis, low gestational
age at birth, maternal thyroid dysfunction, fetal macrosomia and
low birth weight were included as dependent variables and neonatal
hypothyroidism as the independent variable. The analysis revealed
that low gestational age at birth, maternal thyroid dysfunction and
low birth weight significantly increased the risk of neonatal
hypothyroidism (R2=0.375, P<0.001; Table IV).
 | Table IVLogistic regression analyses of the
risk factors for neonatal hypothyroidism. |
Table IV
Logistic regression analyses of the
risk factors for neonatal hypothyroidism.
| | 95% CI |
|---|
| Variables | β-value | Wald value | OR | P-value | Upper limit | Lower limit |
|---|
| Gestational
age | 2.157 | 8.338 | 0.811 | <0.001 | 1.180 | 1.272 |
| Low birth
weight | 1.912 | 5.963 | 1.647 | <0.001 | 1.275 | 2.774 |
| Maternal thyroid
dysfunction | 1.267 | 4.892 | 1.618 | 0.002 | 1.074 | 3.121 |
Discussion
The present study demonstrated that the patient
group had a significantly lower gestational age at birth, lower
birth weight, slower heart rate, lower FT4 level and higher TSH
level compared with the control group. The patient group was
supplemented with thyroxine for 8 weeks, following which TSH and
FT4 levels significantly decreased and increased, respectively to
normal levels. A previous study reported similar findings wherein
the TSH and FT4 levels of neonates with thyroid dysfunction
normalized following 4 weeks of thyroxine therapy and reached
levels that were not significantly different compared with those of
healthy controls (17). This
indicated that treatment administered immediately following the
diagnosis of neonatal hypothyroidism has the ability to normalize
TSH and FT4 levels within a short period of time and effectively
prevent clinical symptoms. The present study also demonstrated that
gestational age at birth had a significant inverse association with
TSH level, but had a direct association with levels of FT4. The
reason for this may be because the thyroid gland developed further
with increased gestational age at birth and reached the normal
level. Neonates with at gestational age of <37 weeks were born
prematurely and their thyroid glands may have failed to develop
normally compared with neonates of a gestational age ≥37 weeks.
Premature neonates were more likely to develop hypothyroidism with
higher TSH and lower FT4 levels (18,19). A
relevant study indicated that the prevalence and positive screening
rate of hypothyroidism are higher in premature neonates, which
suggests that gestational age is closely related to hypothyroidism
(20). In the present study,
neonatal hypothyroidism was screened on the basis of TSH+FT4 levels
in neonates with a gestational age at birth of <37 weeks and the
results were compared with the screening results obtained using
only TSH+FT4 levels. This comparison revealed that the combined use
of TSH+FT4 levels and gestational age at birth was more effective
in predicting neonatal hypothyroidism with significantly improved
positive predictive value, sensitivity and accuracy. In the present
study, the additional use of fetal gestational age increased the
efficiency of early screening for thyroid function in neonates.
Analysis of the related risk factors for neonatal
hypothyroidism revealed low gestational age at birth, maternal
thyroid dysfunction and low birth weight to be risk factors. A
previous study looking at the effects of gestational age at birth
on neonatal hypothyroidism demonstrated that the fetal
hypothalamus-pituitary-adrenal (HPA) axis was located at the center
of the mechanisms controlling fetal growth and development by
stimulating the production of a large amount of thyroid hormones in
addition to performing the function of stabilizing their levels
(21). When FT4 levels increased,
the HPA axis performed negative feedback regulation to reduce TSH
secretion (22). However, when FT4
levels decreased, the HPA axis increased TSH secretion (23). In addition, gestational age at birth
was positively associated with HPA development and functions
(24). The gestational age at birth
was also demonstrated to be negatively associated with the
detection rate of hypothyroidism (25). A study investigating the effects of
maternal thyroid function on neonatal hypothyroidism demonstrated
that in pregnant women, TSH failed to pass through the placental
barrier while small amounts of TH3 and thyroxine successfully
passed to the fetus. In addition, thyrotropin receptor antibodies
passed through the placental barrier into the fetus, affecting the
levels of neonatal thyroid hormones thereby causing neonatal
thyroid dysfunction (26). Previous
findings have revealed that thyroid dysfunction in pregnant women
increases the rate of neonatal thyroid dysfunction (27). Maternal thyroid dysfunction may have
been responsible for neonatal hypothyroidism in the present study.
A study investigating the effects of birth weight on neonatal
hypothyroidism demonstrated that low-birth-weight neonates were
relatively underdeveloped; in addition, their HPA axis and thyroid
glands were not well developed leading to hypothyroidism. These
neonates were prone to non-thyroidal diseases and their thyroid
function was affected due to a variable degree of growth
retardation. In addition, they responded slowly to TSH changes and
thyroid gland reactions and had poor thyroid hormone regulation
(28). Due to limited iodine
reserves and thyroglobulin content, low-birth-weight neonates had a
negative iodine balance at early stages after birth and their
iodine intake capacity was extremely low (29). This failed to effectively support
the needs of neonatal growth and development increasing their risk
of hypothyroidism (29). The weight
of low-birth-weight neonates has also been reported to have more
propensity for neonatal thyroid dysfunction (30), which is consistent with the results
of the present study.
The present study had several limitations. Firstly,
there were 2 and 3 premature neonates with gestational age at birth
of <37 weeks in the control and patient groups, respectively.
Even though other congenital diseases were excluded premature
neonates have various problems that may interfere with their
thyroid function, therefore the study results may be biased to a
certain extent. As the present study was a preliminary study,
future studies with larger cohorts excluding premature neonates are
required to verify the findings of the present study. Secondly,
when calculating the predictive value and identifying risk factors,
the denominator was different. This may have been due to
non-matched control recruitment with a small sample size due to
inadequate birth weight and gestational age at birth. Future
studies with a higher number of cases are required to eliminate the
interference of the results of the aforementioned factors. Thirdly,
in the present study the overall heart rate was relatively low and
the average heart rate of the neonates was 97. Despite various
possible neonatal conditions, such as bradycardia being ruled out
in the present study severe hypothyroidism may be the cause of the
low heart rate detected in the present study. Future studies with
larger sample sizes are needed for the change in heart rate to be
further assessed. Future large scale studies are required to
analyze the predictive value of these indicators for neonates with
different degrees of hypothyroidism and premature neonates with
hypothyroidism. Furthermore, the study duration was short (8
weeks). Neonates with hypothyroidism were not followed up for
long-term thyroid function. Although neonates with hypothyroidism
were actively treated and thyroid function gradually returned to
normal, the long-term effects on children have not been analyzed.
This could be the focus of a future study. In the present study,
maternal thyroid dysfunction was not fully described i.e. whether
it was subclinical hypothyroidism, Hashimoto's thyroiditis,
hyperthyroidism or otherwise was not elaborated. Future studies
could investigate the effects of maternal thyroid dysfunction on
neonates.
In conclusion, in the present study TSH and FT4
levels increased and decreased, respectively in neonates with
hypothyroidism aged <3 days; these values normalized following
treatment with thyroxine. Gestational age at birth was negatively
associated with the TSH level, but was positively associated with
the FT4 level. The combined use of TSH+FT4 levels and gestational
age at birth is an improved predictor of neonatal hypothyroidism
and helps in early treatment and intervention. Low gestational age
at birth, maternal thyroid dysfunction and low birth weight
increased the risk of neonatal hypothyroidism; therefore, special
intervention should be administered to pregnant women with thyroid
dysfunction to reduce the incidence of neonatal hypothyroidism.
Based on the findings of the present study, pre-pregnancy screening
is strongly advocated especially for women planning for pregnancy
who have had thyroid dysfunction or have a family history of
thyroid disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL wrote the manuscript. JL, JC and QL designed the
study, collected the data and performed the statistical analyses.
All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University (Chonqing, China). The parents of the neonates provided
written consent for study participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarkar A, Sar P and Islam E: Hexavalent
chromium reduction by microbacterium oleivorans A1: A possible
mechanism of chromate-detoxification and -bioremediation. Recent
Pat Biotechnol. 9:116–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khordad E, Alipour F, Beheshti F, Hosseini
M, Rajabzadeh AA, Asiaei F and Seghatoleslam M: Vitamin C prevents
hypothyroidism associated neuronal damage in the hippocampus of
neonatal and juvenile rats: A stereological study. J Chem
Neuroanat. 93:48–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu WB, Chen HQ, Wang J, Lin F, Zhao H and
Zhang HH: Study on the cut-off value of TSH screening for neonatal
congenital hypothyroidism in Fujian Province. Chin J Child Health
Care. 11(13)2003.
|
|
4
|
Tian JR: Study on the cut-off value of TSH
screening for congenital hypothyroidism in neonates. China Health
Care Nutr. 29(124)2019.
|
|
5
|
Rahmani K, Yarahmadi S, Etemad K, Mehrabi
Y, Aghang N, Koosha A and Soori H: Intelligence quotient at the age
of six years of Iranian children with congenital hypothyroidism.
Indian Pediatrics. 55:121–124. 2018.PubMed/NCBI
|
|
6
|
Xu YH, Qin YF and Zhao ZY: Retrospective
study on neonatal screening for congenital hypothyroidism and
phenylketonuria in China in the past 22 years. Zhonghua Er Ke Za
Zhi. 47:18–22. 2009.PubMed/NCBI(In Chinese).
|
|
7
|
García M, Barrio R, García-Lavandeira M,
Garcia-Rendueles AR, Escudero A, Díaz-Rodríguez E, Del Blanco DG,
Fernández A, De Rijke YB, Vallespín E, et al: The syndrome of
central hypothyroidism and macroorchidism: IGSF1 controls TRHR and
FSHB expression by differential modulation of pituitary TGFβ and
Activin pathways. Sci Rep. 7(42937)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dayal D, Giri D and Senniappan S: A rare
association of central hypothyroidism and adrenal insufficiency in
a boy with Williams-Beuren syndrome. Ann Pediatr Endocrinol Metab.
22:65–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou W and Xi X: Screening of congenital
hypothyroidism and early intervention effect of levothyroxine.
China Pharm. 21:81–82. 2012.
|
|
10
|
Aubry G, Pontvianne M, Chesnais M,
Weingertner AS, Guerra F and Favre R: Prenatal diagnosis of fetal
Goitrous hypothyroidism in a Euthyroid mother: A management
challenge. J Ultrasound Med. 36:2387–2392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oguma M, Kobayashi M, Yamazaki M, Yokoyama
K, Morikawa S, Yamaguchi T, Yamagata T and Tajima T: Two siblings
with congenital central hypothyroidism caused by a novel mutation
in the IGSF1 gene. Clin Pediatr Endocrinol. 27:95–100.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Siffo S, Adrover E, Citterio CE, Miras MB,
Balbi VA, Chiesa A, Weill J, Sobrero G, González VG, Papendieck P,
et al: Molecular analysis of thyroglobulin mutations found in
patients with goiter and hypothyroidism. Mol Cell Endocrinol.
473:1–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thaker VV, Galler MF, Marshall AC,
Almodovar MC, Hsu HW, Addis CJ, Feldman HA, Brown RS and Levine BS:
Hypothyroidism in infants with congenital heart disease exposed to
excess iodine. J Endocr Soc. 1:1067–1078. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang CP: Correlation analysis of high
thyroid stimulating hormone outcome in newborns and congenital
hypothyroidism. Guangzhou Med J. 2:88–90. 2019.PubMed/NCBI
|
|
15
|
Tsagarakis S, Tzanela M and Dimopoulou I:
Diabetes insipidus, secondary hypoadrenalism and hypothyroidism
after traumatic brain injury: Clinical Implications. Pituitary.
8:251–254. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stagnaro-Green A, Abalovich M, Alexander
E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP,
Sullivan S, et al: Guidelines of the American Thyroid Association
for the diagnosis and management of thyroid disease during
pregnancy and postpartum. Thyroid. 21:1081–1125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fernandez Rodriguez B and Perez Diaz AJ:
Evaluation of a follow up protocol of infants born to mothers with
antithyroid antibodies during pregnancy. J Matern Fetal Neonatal
Med. 31:312–319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Asiaei F, Fazel A, Rajabzadeh AA, Hosseini
M, Beheshti F and Seghatoleslam M: Neuroprotective effects of
Nigella sativa extract upon the hippocampus in PTU-induced
hypothyroidism juvenile rats: A stereological study. Metab Brain
Dis. 32:1755–1765. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kobayashi M, Yagasaki H, Saito T, Nemoto
A, Naito A and Sugita K: Fetal goitrous hypothyroidism treated by
intra-amniotic levothyroxine administration: Case report and review
of the literature. J Pediatr Endocrinol Metab. 30:1001–1005.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McGrath N, Hawkes CP, McDonnell CM, Cody
D, O'Connell S, Mayne PD and Murphy NP: Incidence of congenital
hypothyroidism over 37 years in Ireland. Pediatrics.
142(e20181199)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Onal Z, Balkaya S, Ersen A, Mutlu N, Onal
H and Adal E: Possible effects of neonatal vitamin B12 status on
TSH-screening program: A cross-sectional study from Turkey. J
Pediatr Endocrinol Metab. 30:551–555. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Camargo Neto E, Schulte J, Pereira J,
Bravo H, Sampaio-Filho C and Giugliani R: Neonatal screening for
four lysosomal storage diseases with a digital microfluidics
platform: Initial results in Brazil. Genet Mol Biol. 41:414–416.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Valizadeh M, Nazeri P, Fazli F,
Mohammadian F, Kalantari S, Kamali K and Osali H: Application of
povidone-iodine at delivery significantly increases maternal
urinary iodine but not neonatal thyrotropin in an area with iodine
sufficiency. J Pediatr Endocrinol Metab. 30:967–972.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Braslavsky D, Mendez MV, Prieto L,
Keselman A, Enacan R, Gruneiro-Papendieck L, Jullien N, Savenau A,
Reynaud R, Brue T, et al: Pilot neonatal screening program for
central congenital hypothyroidism: Evidence of significant
detection. Horm Res Paediatr. 88:274–280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sarkar D and Singh SK: Neonatal
hypothyroidism affects testicular glucose homeostasis through
increased oxidative stress in prepubertal mice: Effects on GLUT3,
GLUT8 and Cx43. Andrology. 5:749–762. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lara NLM and Franca LR: Neonatal
hypothyroidism does not increase Sertoli cell proliferation in
iNOS(-/-) mice. Reproduction. 154:13–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Akangire G, Cuna A, Lachica C, Fischer R,
Raman S and Sampath V: Neonatal Graves' disease with maternal
hypothyroidism. AJP Rep. 7:e181–e184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen JG, Zhong YH, Cai XJ, Ye LX and Xie
CL: Effects of birth weight on thyroid stimulating hormone levels
and incidence of congenital hypothyroidism in neonates. Medical
Information. 30:52–53. 2017.
|
|
29
|
Leeuwen L, van Heijst AF, Vijfhuize S,
Beurskens LW, Weijman G, Tibboel D, van den Akker EL and
Ijsselstijn H: Nationwide evaluation of congenital hypothyroidism
screening during neonatal extracorporeal membrane oxygenation.
Neonatology. 111:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Novakovic TR, Dolicanin ZC and Djordjevic
NZ: Oxidative stress biomarkers in amniotic fluid of pregnant women
with hypothyroidism. J Matern Fetal Neonatal Med. 32:1105–1110.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qi HB and Zheng J: ACOG Guidelines for
management of fetal macrosomia: Essential introduction. J Chongqing
Med Univ. 42:925–928. 2017.(In Chinese).
|