Introduction
Respiratory syncytial virus (RSV) is one of the most
common causative pathogens of infant respiratory tract infection
worldwide (1,2), and RSV pneumonia is a leading cause of
hospitalization and mortality among neonates (3,4). RSV
is a segmented, negative-sense, single-stranded RNA virus belonging
to the family Paramyxoviridae (genus, Pneumovirus). The RSV
genome encodes 11 proteins, among which the transmembrane proteins
G and F are the primary determinants of pathogenicity (5,6).
Protective antibodies and cellular immunity are induced against
these two proteins, promoting T helper
(Th)1/Th2 imbalance and the release of a
series of cytokines, such as interferon (INF)-γ, interleukin
(IL)-4, IL-5 and IL-13, which ultimately results in
immunopathological injury of the lower respiratory tract (7-10).
RSV can also stimulate the production of asthma-associated factors,
enhance allergen sensitization and induce Th1 and
Th2 reactions, which may result in the development of
asthma (11,12). RSV is primarily transmitted via
air-borne droplets, but also via indirect contact with contaminated
respiratory secretions from children with RSV. RSV-associated
pathological changes include congestion and edema of the nasal and
pharyngeal mucosa, necrosis and exfoliation of the bronchial
mucosa, degeneration of alveolar epithelial cells, and necrosis and
atrophy of the alveoli (7). Infants
and young children are susceptible to RSV, though neonates are
susceptible to more severe infections (3,13). The
neonatal airway is not fully developed, with a narrow internal
diameter, poor elastic support and increased mucus secretion
following inflammation, making it more easily blocked than that of
older children (7,14,15).
In addition, neonatal immune function, and especially that of the
local airway, is also underdeveloped with lower levels of secretory
IgA, which serve an anti-infectious role (16). Maternally transmitted antibodies can
effectively protect neonates from RSV infection but the degree of
protection is directly associated with the RSV antibody titer of
the mother (17). Moreover, immune
complexes formed with RSV and maternally transmitted antibodies
deposit in the lungs causing airway inflammation and
hyperresponsiveness (18), which
may increase susceptible to RSV infection in the neonatal
period.
Compared with older children, the neonatal symptoms
of RSV pneumonia are more serious and often atypical, with
prominent manifestations including a cough, choking on milk,
spitting and tachypnea (19).
Currently, there are no specific clinical treatments for RSV
infection, and symptomatic supportive treatments are still the
primary therapeutic methods for neonates with RSV pneumonia. These
include oxygen inhalation, atomization and keeping the respiratory
tract unobstructed. Moreover, the American Academy of Pediatrics
and the National Institute for Health and Care Excellence
guidelines agree on supportive management only, which consists of
respiratory support and hydration (20-22).
Ribavirin, which is a commonly used antiviral drug, selectively
inhibits RSV, though its efficacy is controversial (23,24). A
previous study used ribavirin and a placebo to conduct a
prospective, double-blind controlled trial on 83 infants with RSV
pneumonia (25). The clinical
indicators of the treatment group, including hospitalization time,
oxygen inhalation and mechanical ventilation time, were not
significantly different compared with those of the control group
(25). Therefore, ribavirin is not
currently recommended for routine clinical use. Palivizumab is the
only prophylactic treatment available for RSV, but is not used to
treat acute infection (7,26). In 1957, Alick Isaacs discovered IFN,
which was confirmed to exhibit broad-spectrum antiviral effects
(27). Following viral infection
in vivo, the levels of IFN tend to increase (28). IFN binds to specific receptors and
activates antiviral protein genes, which results in the generation
of antiviral proteins that inhibit viral replication and prevent
the spread of inflammation (28).
IFN can also promote the phagocytic and antigen-presenting
functions of alveolar macrophages, and increase the secretion of
inflammatory cytokines in the alveoli, enhancing the immune
response and promoting viral inhibition and clearance (29). Previous studies have confirmed the
role of IFN in RSV pneumonia (30,31),
and some practitioners in China have used IFN to treat RSV
pneumonia, though efficacy and safety data for its use remains
limited. In order to provide a clinical basis for the use of IFN in
infants with RSV pneumonia, the efficacy and safety of IFN were
retrospectively analyzed in the present study.
Materials and methods
The present study is a retrospective analysis
approved by the Ethics Committee of Children's Hospital of
Chongqing Medical University. Neonates with RSV pneumonia were
divided into two groups according to the use of IFN therapy, and
all other routine treatments remained the same during
hospitalization. Finally, the general clinicopathological
characteristics, clinical signs and symptoms, auxiliary examination
results, as well as the efficacy and adverse effects of IFN
treatment were collected and analyzed.
Inclusion criteria
The neonates were hospitalized in the Neonatal
Diagnosis and Treatment Center of the Children's Hospital of
Chongqing Medical University (Chongqing, China) between February
2011 and March 2012, and were diagnosed with RSV pneumonia. The
diagnostic criteria for neonatal RSV pneumonia were as follows: i)
Clinical symptoms and signs, such as cough, rhinorrhea, tachypnea,
spitting, wheezing and dry or moist rales; ii) chest X-ray
manifestations, such as small patch shadows in both lungs,
coarsening of the lung texture, irregular linear shadow and
emphysema; iii) routine blood test results with normal or slightly
reduced white blood cell counts, and a relatively elevated
proportion of lymphocytes; and iv) on the day of admission, a
disposable sterile sputum suction tube was used to absorb part of
the laryngeal secretion for examination after tracheal intubation.
The direct immunofluorescence method was used to detect RSV-Ag in
the laryngeal secretion samples (32), and an RSV-Ag-positive sample was
considered to be an affirmative diagnosis of RSV infection. Normal
full-term neonates with complete medical history data were 37 to 42
weeks (260 to 293 days) of age, weighed >2.5 kg but ≤4.0 kg, and
the age at admission was <28 days.
Exclusion criteria
Neonates with congenital heart disease, congenital
immunodeficiencies, bronchial and pulmonary dysplasia and
incomplete medical data were excluded. Premature neonates were also
excluded. A number of studies have reported that these are risk
factors for severe RSV pneumonia which may affect the accuracy of
the current study (13,33,34).
Grouping method
Neonates eligible for enrollment were divided into
the treatment and the control groups according to the use of IFN.
In the treatment group, IFN-α1b (1 µg/kg; Beijing Tri-Prime Gene
Pharmaceutical Co., Ltd.) was intramuscularly injected once a day
for 3 days. The neonates in the control group were not administered
IFN. During hospitalization, both groups received warmth
preservation, sputum aspiration, atomization with 10% saline,
hydration, oxygen support, antibiotics for bacterial co-infections
(confirmed by sputum bacterial culture; Table IV) and other symptomatic supportive
therapies (backslapping and posture changing).
 | Table IVThe comparison of bacterial spectrum
between the treatment and control group. |
Table IV
The comparison of bacterial spectrum
between the treatment and control group.
| Bacterial
spectrum | Total, n=191 | Treatment group,
n=81 | Control group,
n=110 |
χ2 | P-value |
|---|
| Escherichia
coli (%) | 41 (21.5) | 19 (23.4) | 22 (20.0) | 0.331 | 0.565 |
| Klebsiella
pneumoniae (%) | 39 (20.4) | 18 (22.2) | 21 (19.1) | 0.281 | 0.596 |
| Staphylococcus
aureus (%) | 33 (17.2) | 12 (14.8) | 21 (19.1) | 0.597 | 0.440 |
| Acinetobacter
baumannii (%) | 25 (13.1) | 9 (11.1) | 16 (14.5) | 0.484 | 0.487 |
| Pseudomonas
aeruginosa (%) | 19 (9.9) | 9 (11.1) | 10 (9.1) | 0.213 | 0.645 |
Patients
All neonates were examined and treated at the
Children's Hospital of Chongqing Medical University (Chongqing,
China). A total of 2,381 neonates were diagnosed with neonatal
pneumonia at the Neonatal Diagnosis and Treatment Center between
February 2011 and March 2012, among which 1,196 cases received
sputum examination, and a total of 428 cases (35.8%) were
RSV-Ag-positive. According to the inclusion and exclusion criteria,
286 neonates with RSV pneumonia were included and 126 (69 male and
57 female neonates) received IFN-α1b treatment. By contrast, 160
neonates in the control group (87 male and 73 female patients) did
not receive IFN treatment. Aside from IFN, all patients in both
groups received the same routine treatments.
RSV-Ag examination and sputum
bacterial culture
Laryngeal secretion specimens were tested using the
D3 Ultra DFA Respiratory Virus Screening & ID Kits (Diagnostic
Hybrids, Inc.) in the department of clinical laboratory of the
Children's Hospital of Chongqing Medical University, and the
specimens were prepared in strict accordance with the
manufacturer's instructions. Sputum was oscillated and mixed with a
oscillating mixer (NHWY-200F, Aipu Food Industry Co., Ltd.) for
10-15 sec and centrifuged (400-600 x g; Heraeus Labofuge 400R;
Thermo Fisher Scientific, Inc.) for 5-10 min at room temperature.
The supernatant was discarded and the precipitate was washed three
times with 5% PBS and centrifuged (400-600 x g) for 5-10 min at
room temperature. The supernatant and mucus layer were absorbed,
and the washing and centrifugation steps repeated until all mucus
was completely absorbed. Subsequently, 0.5-1 ml 5% PBS was added to
the precipitate and a suspension was formed by repeated blowing and
suction; the cell suspension was then added to an eight-well plate
(25 µl per well). The specimens were completely air-dried, fixed
with 100% acetone for 5-10 min at 20-25˚C, and then air-dried once
more. Finally, the specimens were stained with the DFA reagent,
which contains fluorescently-labeled monoclonal antibodies against
RSV antigen and is part of the aforementioned kit, for 15 min at
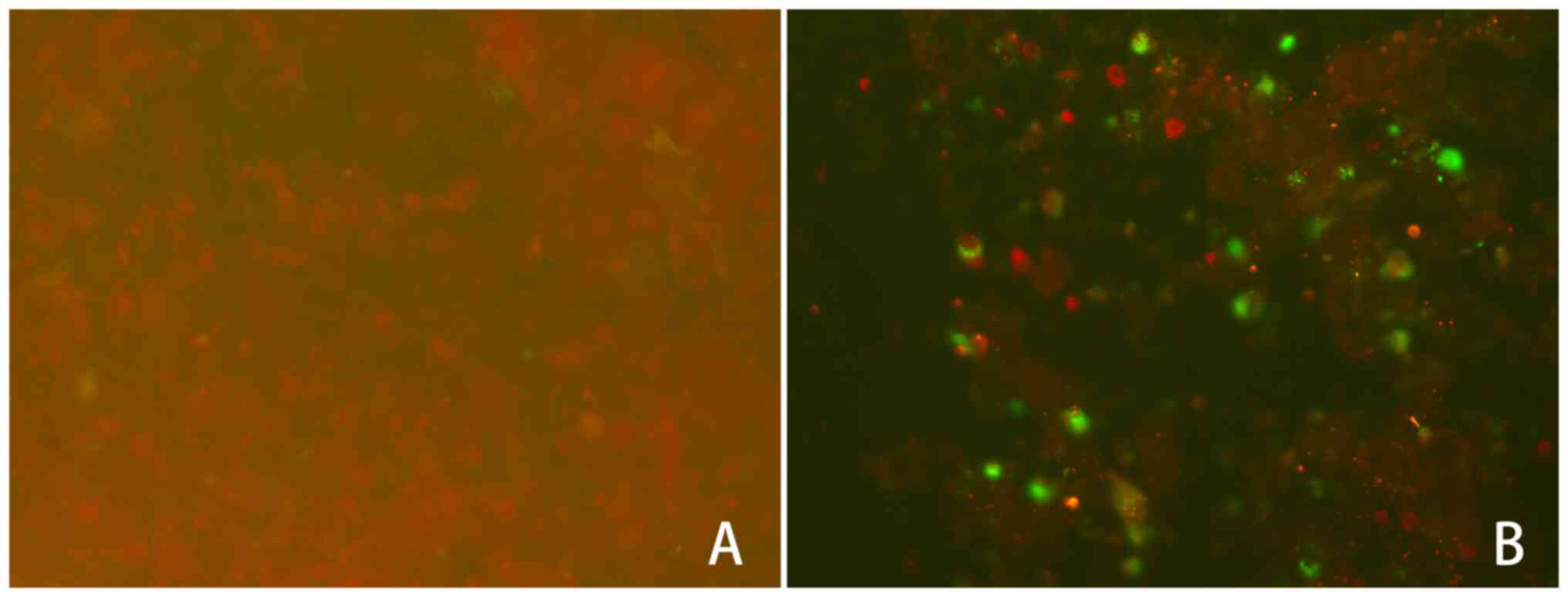
37˚C. The results were observed by fluorescence microscopy
(magnification, x200; Nikon TE2000-S, Nikon Corporation). The
nucleus and/or cytoplasm of RSV-Ag-positive cells were
distinguished by green fluorescence, while the cells without an
antigen-antibody reaction were stained red (Fig. 1). For scoring, specimens with >10
cells were determined to be positive (+), 20-0 cells were
considered as positive (++) and >50 cells were considered to be
positive (+++), with the cells being counted manually. The
specimens were also cultured on chocolate agar plates and (Autobio
Diagnostics Co., Ltd.) and blood agar plates (Autobio Diagnostics
Co., Ltd.) at 37˚C. After inoculation, the specimens were placed in
an incubator containing 3-10% CO2 and cultured for 18-24
h at 37±2˚C. The results were analyzed using a bacterial
identification instrument (VITEK® 2 COMPACT;
bioMérieux).
Data collection
Clinicopathological information including sex, age,
gestational age, duration from onset to admission, birth weight,
feeding methods and admission time were collected. Clinical
symptoms and signs including fever, rhinorrhea, cough, tachypnea,
perilabial cyanosis, choking on milk, spitting, three concave sign,
wheezing, dry and moist rales were also assessed. Auxiliary
examination results were collected, including those from RSV-Ag
testing, sputum bacterial cultures and chest X-rays. Adverse
effects after IFN-α1b administration were also recorded, including
fever, chills, tachycardia, rash, infection at the injection site,
scleredema and hemorrhage.
Statistical analysis
Statistical analysis was performed using SPSS
(v23.0; IBM Corp.). The measurement data are presented as the mean
± standard deviation, and unpaired t-tests were used to compare the
means between samples. The counting data are presented as
percentages, and the χ2 test was used to compare the
percentages between samples. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of clinicopathological
characteristics
There was no significant difference in the sex
distribution between the two groups (χ2 =0.004;
P=0.948). There were 51 (40.5%) breastfed neonates in the treatment
group and 70 (43.8%) in the control group, and there was no
significant difference in these results (χ2=0.310;
P=0.578). There were also no significant differences in age,
gestational age, weight and duration from onset to admission
between the treatment and the control groups (Table I).
 | Table IThe comparison of general information
between the treatment and control group. |
Table I
The comparison of general information
between the treatment and control group.
| General
information | Treatment group
(n=126) | Control group
(n=160) |
χ2/(t) | P-value |
|---|
| Age (day) | 15.7±5.2 | 16.8±4.9 | 1.821 | 0.070 |
| Gestational age
(day) | 275.3±7.0 | 274.8±7.7 | 0.607 | 0.544 |
| Birth weight
(g) | 3,258.8±404.1 | 3,291.4±374.4 | 0.705 | 0.481 |
| Duration from onset
to admission (day) | 3.7±2.1 | 3.9±2.3 | 0.652 | 0.515 |
| Breast feeding
rate, % | 40.5 (51/126) | 43.8 (70/160) | 0.310 | 0.578 |
| Sex | | | 0.004 | 0.948 |
|
Male | 69 | 87 | | |
|
Female | 57 | 73 | | |
Comparison of signs and symptoms
From 286 total cases, there were 75 instances of
fever, including 27 in the treatment group and 48 in the control
group. Incidences of the primary respiratory symptoms in both
groups [fever, cough, rhinorrhea, tachypnea, perilabial cyanosis,
choking on milk, spitting, wheezing, three concave sign (the upper
sternal fossa, the supraclavicular fossa, and the intercostal space
appearing obviously depressed on inhalation), and dry and moist
rales] are presented in Table II.
There were no significant differences in the aforementioned
clinical symptoms between the two groups.
 | Table IIThe comparison of symptoms and signs
between treatment and control group prior to treatment. |
Table II
The comparison of symptoms and signs
between treatment and control group prior to treatment.
| Clinical data | Treatment group
(%) | Control group
(%) |
χ2 | P-value |
|---|
| Fever | 27 (21.4) | 48 (30.0) | 2.677 | 0.102 |
| Cough | 120 (95.2) | 146 (91.3) | 1.724 | 0.189 |
| Rhinorrhea | 54 (42.9) | 77 (48.1) | 0.788 | 0.375 |
| Tachypnea | 119 (94.4) | 141 (88.1) | 3.406 | 0.065 |
| Perilabial
cyanosis | 84 (66.7) | 110 (68.8) | 0.140 | 0.708 |
| Choking on
milk | 72 (57.1) | 108 (67.5) | 3.242 | 0.072 |
| Spitting | 43 (34.1) | 58 (36.3) | 0.139 | 0.709 |
| Wheezing | 22 (17.5) | 35 (21.9) | 0.861 | 0.353 |
| Three concave
sign | 24 (19.0) | 39 (24.4) | 1.165 | 0.280 |
| Moist rales | 81 (64.3) | 86 (53.8) | 3.220 | 0.073 |
| Dry rales | 11 (8.7) | 17 (10.6) | 0.287 | 0.592 |
Comparison of auxiliary examination
results
In total, 286 neonates were RSV-Ag-positive. Among
them, 102 (35.7%) cases were positive (+), 138 (48.3%) were
positive (++), and 46 (16%) cases were positive (+++). There were
no significant differences in the degree of RSV-Ag positivity
between the treatment and the control groups (Table II). Of the 286 cases, 191 (66.8%)
were positive for bacterial co-infection (sputum bacterial
culture), including those with Escherichia coli (21.5%,
41/191), Klebsiella pneumoniae (20.4%, 39/191),
Staphylococcus aureus (17.2%, 33/191), Acinetobacter
baumannii (13.1%, 25/191) and Pseudomonas aeruginosa
(9.9%, 19/191). However, there were no significant differences in
sputum bacterial culture-positive rate and bacterial spectrum
between the two groups (P>0.05; Tables III and IV). There were 269 cases (94.1%) with
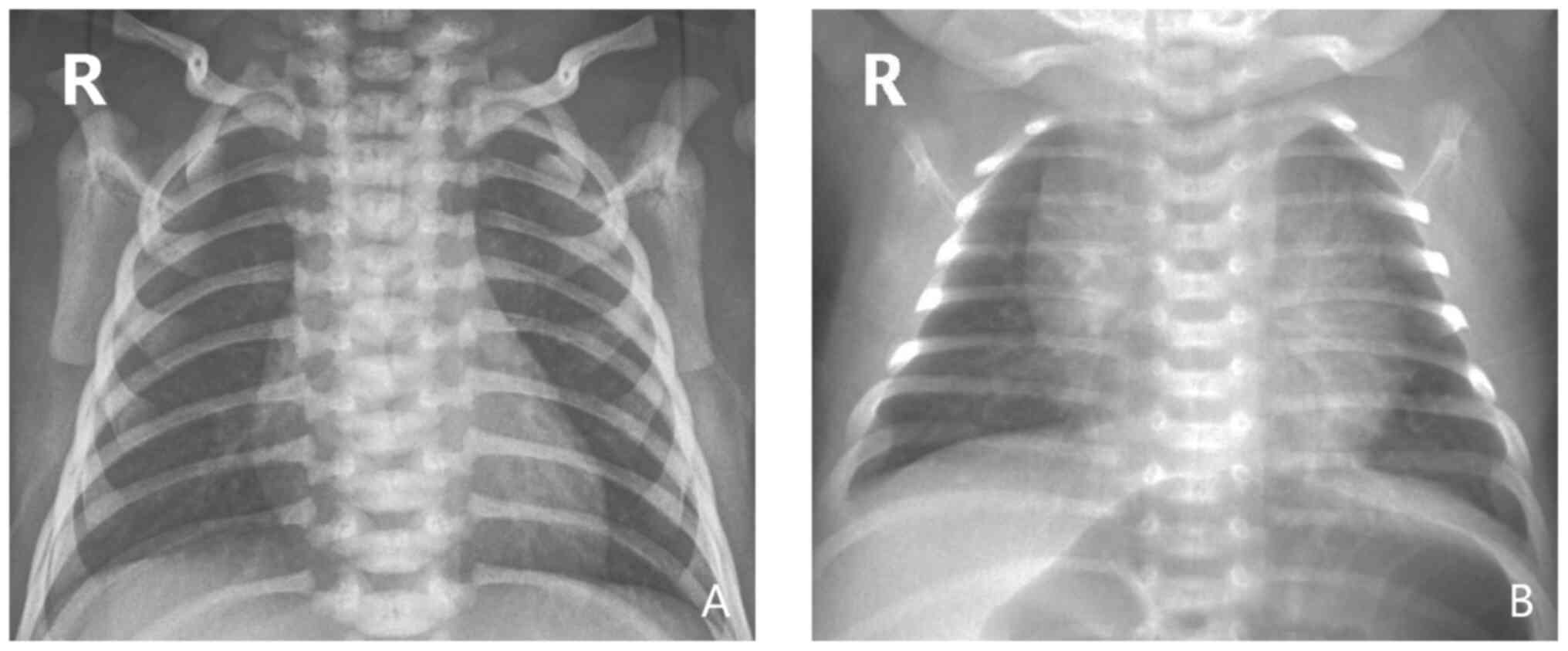
abnormal chest X-ray findings, including blurred pulmonary texture,
hyperinflation and visible flocculent shadow in the middle and
inner side of the lung (Fig. 2).
However, there was no significant difference in the positive rate
of chest X-ray findings between the two groups (P>0.05; Table III).
 | Table IIIThe comparison of auxiliary
examination between the treatment and control group. |
Table III
The comparison of auxiliary
examination between the treatment and control group.
| Auxiliary
examination | Total (n=286)
(%) | Treatment group
(n=126) (%) | Control group
(n=160) (%) |
χ2 | P-value |
|---|
| RSV-Ag | | | | | |
|
+ | 102 (35.7) | 40 (31.7) | 62 (38.8) | 1.507 | 0.220 |
|
++ | 138 (48.3) | 64 (50.8) | 74 (46.3) | 0.583 | 0.445 |
|
+++ | 46 (16.0) | 22 (17.5) | 24 (15.0) | 0.316 | 0.574 |
| Sputum bacterial
culture (+) | 191 (66.8) | 81 (64.3) | 110 (68.8) | 0.633 | 0.426 |
| Chest X-ray
exhibiting pneumonia or increased lung texture | 269 (94.1) | 115 (91.3) | 154 (96.3) | 3.127 | 0.077 |
Comparison of therapeutic effects
The relief time from primary symptoms (such as
cough, tachypnea, choking on milk, perilabial cyanosis and moist
rales) in the treatment group was significantly lower compared with
the control group (P<0.05). The oxygen inhalation time of the
treatment group was also decreased, compared with that of the
control group, and the difference was statistically significant
(P<0.05). Compared with the control group, the mean number of
hospitalization days was marginally decreased in the treatment
group, but there was no significant difference between the two
groups (P=0.132; Table V). All
patients in both groups were cured and discharged, and no
mortalities occurred.
 | Table VThe comparison of therapeutic effect
between the treatment and control group. |
Table V
The comparison of therapeutic effect
between the treatment and control group.
| | Treatment
group | Control group | |
|---|
| Clinical data | n | Time (day) | n | Time (day) | t value | P-value |
| Cough | 120 | 4.4±1.8 | 146 | 7.8±3.7 | 9.763 | <0.001 |
| Tachypnea | 119 | 5.2±2.1 | 141 | 6.2±3.1 | 2.953 | 0.003 |
| Choking on
milk | 72 | 3.9±2.1 | 108 | 5.3±3.4 | 3.463 | 0.001 |
| Perilabial
cyanosis | 84 | 5.1±2.3 | 110 | 6.3±3.9 | 2.579 | 0.011 |
| Moist rales | 81 | 6.5±2.1 | 86 | 7.5±3.3 | 2.386 | 0.018 |
| Oxygen inhalation
time | 126 | 5.6±1.7 | 160 | 6.1±1.8 | 2.110 | 0.036 |
| Hospitalization
time | 126 | 9.6±2.7 | 160 | 10.3±4.7 | 1.510 | 0.132 |
Adverse effects of IFN
administration
Adverse effects such as shivering, tachycardia,
rash, infection at the injection site, scleredema and hemorrhage
were not observed in the treatment group following IFN-α1b
administration. However, two cases experienced fever 2-6 h after
treatment. The temperatures of the neonates were all <38˚C, and
after physical cooling (a warm water sponge bath for ~5 min),
decreased to within a normal range. No special drug treatment was
required and there were no recurrences of fever.
Discussion
RSV is one of the most common pathogens associated
with infant respiratory tract infection. In the present study, the
RSV-Ag-positive rate was 35.8%, suggesting that RSV is one of the
primary causative agents of neonatal pneumonia. Infantile RSV
pneumonia is largely characterized by symptoms such as high fever,
marked dyspnea and wheezing; however, the symptoms and signs of
neonatal RSV pneumonia lack specificity (35). In the current study, the key
clinical manifestations of RSV pneumonia in neonates were cough,
tachypnea, choking on milk, perilabial cyanosis and moist rales,
accounting for 93.0, 90.9, 62.9, 67.8 and 58.4% of the total cases,
respectively. The majority of the patients exhibited a normal
temperature or low fever, and wheezing was not prominent.
Therefore, it is difficult to distinguish RSV from non-RSV
pneumonia according to clinical manifestations only. In the present
study, the RSV-positive rate according to chest X-ray examination
was 94.1%, and the key manifestations were blurred lung texture,
hyperinflation and flocculation in the middle and inner lung. There
were no significant differences in chest X-ray findings between
neonates with RSV pneumonia and non-RSV infection, and the
specificity was poor (36).
Therefore, in the epidemic season, neonates with cough, tachypnea,
choking on milk (amongst other clinical manifestations) should be
more vigilantly assessed, and a more definite diagnosis of RSV
should aim to be achieved.
There are currently a number of methods that are
used to diagnose RSV infection, such as virus isolation, electron
microscopy and the detection of viral antigen, antibodies and
nucleic acids (37-40).
Antigen detection is reportedly more sensitive (41), especially immunolabeling technology
(including direct or indirect immunofluorescence, immunoenzymatic
and ELISA assays), which can be used to quickly and effectively
detect viral antigens. In the current study, RSV was qualitatively
detected in laryngeal secretion samples using fluorescein-labeled
specific monoclonal antibodies. The method has been confirmed by
three evaluation centers of the World Health Organization, with a
sensitivity and specificity of 95 and 86%, respectively (42). Moreover, the method is rapid, simple
and convenient for broad clinical application. In the present
study, fluorescence (i.e. RSV infection) was categorized into three
degrees (+, ++ and +++) according to the number of virus particles.
To reduce bias, the proportions of neonates at each level were
compared between the two groups, and no significant differences
were observed.
A third of all cases of community acquired pneumonia
are co-infections with viruses and bacteria; as such, RSV pneumonia
is also often associated with bacterial infection (43). In the current study, 66.8% of
neonatal RSV pneumonia was associated with bacterial infection, and
the primary causative agents included Escherichia coli,
Streptococcus pneumoniae and Staphylococcus aureus,
accounting for 21.5, 20.4 and 17.2% of the sputum culture-positive
cases, respectively. Similar studies have reported that
gram-negative bacteria are the most common cause of co-infection in
RSV pneumonia, followed by gram-positive organisms, and the
bacterial spectrum was similar to that observed in the present
study (44). However, a previous
study reported that group B hemolytic streptococci are the
predominant pathogens in neonates aged 0 to 21 days, and that
Streptococcus pneumoniae is the predominant pathogen in
neonates aged >3 weeks (45).
Due to the high rate of bacterial co-infection in neonates with RSV
pneumonia, the use of antibiotic treatment is receiving increased
attention. Once the results of sputum culture are clear (before
those of drug sensitivity testing are clear), appropriate
antibiotics for common bacteria can be empirically selected. These
are predominantly against gram-negative bacteria, though the
treatment of gram-positive organisms is also frequently required. A
reduction in interfering factors (such as bacterial infection)
allows for a more accurate evaluation of the efficacy and safety of
IFN treatment, which is more effective in the absence of bacterial
co-infection. However, since ~2/3 of hospitalized neonates also
present with bacterial infections, such cases were not excluded
from the present study. To limit experimental bias, the bacterial
spectrum and the proportion of neonates with bacterial infections
were compared, and no significant differences were observed between
the two groups. Moreover, studies have demonstrated that type I
IFNs (IFN-α and -β) also serve an important role in antibacterial
immunity (46,47). In the present study, 66.8% (191/286)
of the neonates were infected with both bacteria and RSV, thus
whether exogenous IFN has therapeutic effects in both types of
infection requires further investigation. In addition, a variety of
natural compounds have indicated antimicrobial potential in the
treatment of pneumonia, both in clinical and research settings
(48,49). Therefore, the combined use of IFN
with other therapeutic strategies may hold considerable potential
for the treatment of RSV-associated pneumonia.
Numerous studies have reported that the level of
RSV-Igor is increased in children with RSV pneumonia, while the
levels of IFN-α, IFN-γ and IL-2 are decreased or undetectable
(50-52).
It is speculated that the inhibition of cellular immune function in
the early stages of RSV infection may be associated with the
reduced efficacy of IFN in vivo (53,54).
Therefore, the administration of exogenous IFN is particularly
important. A further study has indicated that the use of IFN can
alleviate clinical symptoms and reduce the duration of infantile
RSV infection, and no associated complications were observed
(47). In the present study, the
remission time of primary RSV symptoms was significantly lower in
the IFN treatment group compared with the control group
(P<0.05), and the oxygen inhalation time was also significantly
reduced (P<0.05). These results suggest that the use of IFN-α1b
in the treatment of neonatal RSV pneumonia may promote the relief
of symptoms, which is in line with the findings of a similar study
(55).
No significant differences in hospitalization time
were revealed between the two groups in the present study, which
may be associated with the need for a more adequate observation
time (and the detection of adverse effects of IFN) during
hospitalization. However, it was also observed that the
hospitalization times of neonates with simple RSV infection
(without bacterial co-infection) were shorter than those for
neonates with co-infections (RSV and bacteria). This may be
associated with the bacteriological imbalance after antibiotic use,
and may be the primary reason for the prolonged hospitalization
time. Antibiotic-associated diarrhea is a recognized adverse
reaction to antibiotics such as amoxicillin, which is more likely
to occur in neonates (24).
Additionally, only 2 neonates experienced low fever during the IFN
treatment period. After physical cooling, the temperatures of these
individuals returned to normal without any other adverse effects,
suggesting that the short-term use of IFN in neonates is safe.
In conclusion, IFN effectively alleviates the signs
and symptoms of neonatal RSV pneumonia with few short-term adverse
effects. However, these findings were not all observed in the same
time period, and the number of cases was limited, thus, the results
do not completely represent the clinical conditions of RSV
pneumonia in neonates. Therefore, it is necessary to design further
multi-center prospective studies to accurately analyze the
therapeutic, as well as the long-term adverse effects of IFN for
the treatment of neonatal RSV pneumonia.
The format and content of the manuscript have been
checked in accordance with STROBE guideline (56).
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 81701709) and
Chongqing Science and Technology Commission (grant no.
cstc2016shmszx130026).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
LH, QL and HZ designed the study. LH wrote the main
manuscript text. LH and LY analyzed all data and prepared all
figures. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current retrospective review study was approved
by the Ethics Committee of Children's Hospital of Chongqing Medical
University. The requirement for consent was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ralston SL, Lieberthal AS, Meissner HC,
Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa
NF, Mendonca EA, et al: Clinical practice guideline: The diagnosis,
management, and prevention of bronchiolitis. Pediatrics.
134:e1474–e1502. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bennett MV, Mclaurin K, Ambrose C and Lee
HC: Population-based trends and underlying risk factors for infant
respiratory syncytial virus and bronchiolitis hospitalizations.
PLoS One. 13(e0205399)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oren E, Frere J and Yom-Tov E and Yom-Tov
E: Respiratory syncytial virus tracking using internet search
engine data. BMC Public Health. 18(445)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stein RT, Bont LJ, Zar H, Polack FP, Park
C, Claxton A, Borok G, Butylkova Y and Wegzyn C: Respiratory
syncytial virus hospitalization and mortality: Systematic review
and meta-analysis: Incidence of RSV hospitalization and mortality.
Pediatr Pulmonol. 52:556–569. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goodwin E, Gilman MSA, Wrapp D, Chen M,
Ngwuta JO, Moin SM, Bai P, Sivasubramanian A, Connor RI, Wright PF,
et al: Infants infected with respiratory syncytial virus generate
potent neutralizing antibodies that lack somatic hypermutation.
Immunity. 48:339:–349.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taleb SA, Al Thani AA, Al Ansari K and
Yassine HM: Human respiratory syncytial virus: Pathogenesis, immune
responses, and current vaccine approaches. Eur J Clin Microbiol
Infect Dis. 37:1817–1827. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Griffiths C, Drews SJ and Marchant DJ:
Respiratory syncytial virus: Infection, detection, and new options
for prevention and treatment. Clin Microbiol Rev. 30:277–319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meissner HC: Viral bronchiolitis in
children. N Engl J Med. 374:1793–1794. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu X, Zhou X, Hu Y, Liu C and Wang J:
Neutralization of nerve growth factor (NGF) inhibits the Th2
response and protects against the respiratory syncytial virus (RSV)
infection. Immunol Res. 65:721–728. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hijano DR, Siefker DT, Bishwas S, Jaligama
S, Vu LD, Tillman H, Finkelstein D, Saravia J, You D and Cormier
SA: Type I interferon potentiates IgA immunity to respiratory
syncytial virus infection during infancy. Sci Rep.
8(11034)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
James KM, Gebretsadik T, Escobar GJ, Wu P,
Carroll KN, Li SX, Walsh EM, Mitchel EF, Sloan C and Hartert TV:
Risk of childhood asthma following infant bronchiolitis during the
respiratory syncytial virus season. J Allergy Clin Immun.
132:227–229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang HL and Lü FF: Research advance of
association between viral bronchiolitis and asthma in children.
Chin J Pract Pediatr. 32:895–900. 2017.
|
|
13
|
Hall CB, Weinberg GA, Blumkin AK, Edwards
KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR,
Williams JV, et al: Respiratory syncytial virus-associated
hospitalizations among children less than 24 months of age.
Pediatrics. 132:e341–e348. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hislop AA: Airway and blood vessel
interaction during lung development. J Anat. 201:325–334.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ostadabbas S, Bulach C, Ku DN, Anderson LJ
and Ghovanloo M: A passive quantitative measurement of airway
resistance using depth data. Conf Proc IEEE Eng Med Biol Soc.
2014:5743–5747. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stensballe LG, Kofoed PE, Nante EJ, Sambo
M, Jensen IP and Aaby P: Duration of secretory IgM and IgA
antibodies to respiratory syncytial virus in a community study in
Guinea-Bissau. Acta Paediatr. 89:421–426. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chu HY, Steinhoff MC, Magaret A, Zaman K,
Roy E, Longdon G, Formica MA, Walsh EE and Englund JA: Respiratory
syncytial virus transplacental antibody transfer and kinetics in
mother-infant pairs in Bangladesh. J Infect Dis. 210:1582–1589.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson JE, Gonzales RA, Olson SJ, Wright
PF and Graham BS: The histopathology of fatal untreated human
respiratory syncytial virus infection. Mod Pathol. 20:108–119.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wollmeister E, Alvarez AE, Bastos JCS,
Marson FAL, Ribeiro JD, Baracat ECE, Arns CW and Riccetto AGL:
Respiratory syncytial virus in Brazilian infants-Ten years, two
cohorts. J Clin Virol. 98:33–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gadomski AM and Scribani MB:
Bronchodilators for bronchiolitis. Cochrane Database Syst Rev.
2014(CD001266)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mazur NI, Martinón-Torres F, Baraldi E,
Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H,
Papadopoulos NG, et al: Lower respiratory tract infection caused by
respiratory syncytial virus: Current management and new
therapeutics. Lancet Respir Med. 3:888–900. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Institute for Health and Care
Excellence: Bronchiolitis in children: diagnosis and
management|Guidance and guidelines NICE. Available from: urihttps://www.
nice.org.uk/guidance/NG9simplehttps://www.
nice.org.uk/guidance/NG9, 2015.
|
|
23
|
Israel S, Rusch S, DeVincenzo J, Boyers A,
Fok-Seang J, Huntjens D, Lounis N, Mariёn K, Stevens M and René
Verloes R: Effect of oral JNJ-53718678 (JNJ-678) on disease
severity in healthy adult volunteers experimentally inoculated with
live respiratory syncytial virus (RSV): A placebo-controlled
challenge study. Open Forum Infect Dis. 3 (Suppl 1)(S650)2016.
|
|
24
|
Xing Y and Proesmans M: New therapies for
acute RSV infections: Where are we? Eur J Pediatr. 178:131–138.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guerguerian AM, Gauthier M, Lebel MH,
Farrell CA and Lacroix J: Ribavirin in ventilated respiratory
syncytial virus bronchiolitis. A randomized, placebo-controlled
trial. Am J Respir Crit Care Med. 160:829–834. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Manzoni P, Paes B, Lanctôt KL, Dall'Agnola
A, Mitchell I, Calabrese S, Maule M, Girardi E, Harimoto T and Li
A: Outcomes of infants receiving palivizumab prophylaxis for
respiratory syncytial virus in Canada and Italy: An international,
prospective cohort study. Pediatr Infect Dis J. 36:2–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Isaacs A and Baron S: Antiviral action of
interferon in embryonic cells. Lancet. 2:946–947. 1960.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Welliver RC Sr: The immune response to
respiratory syncytial virus infection: Friend or foe? Clin Rev
Allergy Immunol. 34:163–173. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reassessment of the indications for
ribavirin therapy in respiratory syncytial virus infections.
American academy of pediatrics committee on infectious diseases.
Pediatrics. 97:137–140. 1996.
|
|
30
|
Bem RA, Domachowske JB and Rosenberg HF:
Animal models of human respiratory syncytial virus disease. Am J
Physiol Lung Cell Mol Physiol. 301:L148–L156. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Van-Schaik SM, Obot N, Enhorning G, Hintz
K, Gross K, Hancock GE, Stack AM and Welliver RC: Role of
interferon gamma in the pathogenesis of primary respiratory
syncytial virus infection in BALB/c mice. J Med Virol. 62:257–266.
2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wen S, Yu M, Zheng G, Lv F, Chen X, Lin L,
Li C and Zhang H: Changes in the etiology of viral lower
respiratory tract infections in hospitalized children in Wenzhou,
China: 2008-2017. J Med Virol. 92:982–987. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Glezen WP, Greenberg SB, Atmar RL, Piedra
PA and Couch RB: Impact of respiratory virus infections on persons
with chronic underlying conditions. JAMA. 283:499–505.
2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mori M, Morio T, Ito S, Morimoto A, Ota S,
Mizuta K, Lwata T, Hara T and Saji T: Risks and prevention of
severe RS virus infection among children with immunodeficiency and
Down's syndrome. J Infect Chemother. 20:455–459. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rostad CA: Respiratory syncytial virus:
Spectrum of clinical manifestations and complications in children.
Pediatr Ann. 48:e349–e353. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rahmati MB, Ahmadi M, Malekmohamadi
Hasanpur S, Zare SH and Jafari M: The significance of chest
ultrasound and chest X-ray in the diagnosis of children clinically
suspected of pneumonia. J Med Life. 8:50–53. 2015.PubMed/NCBI
|
|
37
|
Tai CC, Tsai CH, Huang YH, Lee CL, Chen HP
and Chan YJ: Detection of respiratory viruses in adults with
respiratory tract infection using a multiplex PCR assay at a
tertiary center. J Microbiol Immunol Infect, Aug 12, 2020 (Online
ahead of print).
|
|
38
|
Allen AJ, Gonzalez-Ciscar A, Lendrem C,
Suklan J, Allen K, Bell A, Baxter F, Crulley S, Fairlie L, Hardy D,
et al: Diagnostic and economic evaluation of a point-of-care test
for respiratory syncytial virus. ERJ Open Res. 6:00018–2020.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Alidjinou EK, Lefebvre N, Dewilde A, Mäki
M, Hober D and Engelmann I: Evaluation of the reverse transcription
strand invasion based amplification (RT-SIBA) RSV assay, a rapid
molecular assay for the detection of respiratory syncytial virus.
Diagn Microbiol Infect Dis. 95:55–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Percze K, Szakács Z, Scholz É, András J,
Szeitner Z, Kieboom CH, Ferwerda G, Jonge MI, Gyurcsányi RE and
Mészáros T: Aptamers for respiratory syncytial virus detection. Sci
Rep. 7(42794)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Leonardi GP, Wilson AM, Dauz M and Zuretti
AR: Evaluation of respiratory syncytial virus (RSV) direct antigen
detection assays for use in point-of-care testing. J Virol Methods.
213:131–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
McDonald JC and Quennec P: Utility of a
respiratory virus panel containing a monoclonal antibody pool for
screening of respiratory specimens in nonpeak respiratory syncytial
virus season. J Clin Microbiol. 10:2809–2811. 1993.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cilla G, Sarasua A, Montes M, Arostegui N,
Vicente D, Pérez-Yarza E and Pérez-Trallero E: Risk factors for
hospitalization due to respiratory syncytial virus infection among
infants in the Basque Country, Spain. Epidemiol Infect.
134:506–513. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shah BA, Singh G, Naik MA and Dhobi GN:
Bacteriological and clinical profile of community acquired
pneumonia in hospitalized patients. Lung India. 27:54–57.
2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
McIntosh K: Community acquired pneumonia
in children. New Engl J Med. 346:429–437. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gottschalk RA, Dorrington MG, Dutta B,
Krauss KS, Martins AJ, Uderhardt S, Chan WP, Tsang JS,
Torabi-Parizi P, Fraser ID and Germain RN: IFN-mediated negative
feedback supports bacteria class-specific macrophage inflammatory
responses. Elife. 8(e46836)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu SY, Sanchez DJ and Cheng G: New
developments in the induction and antiviral effectors of type I
interferon. Curr Opin Immunol. 23:57–64. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rath S and Padhy RN: Antibacterial
efficacy of five medicinal plants against multidrug-resistant
enteropathogenic bacteria infecting under-5 hospitalized children.
J Integr Med. 13:45–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Blonk B and Cock IE: Interactive
antimicrobial and toxicity profiles of Pittosporum angustifolium
Lodd. extracts with conventional antimicrobials. J Integr Med.
17:261–272. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gut W, Pancer K, Abramczuk E, Cześcik A,
Dunal-Szczepaniak M, Lipka B and Litwińska B: RSV respiratory
infection in children under 5 y.o.-dynamics of the immune response
Th1/Th2 and IgE. Przegl Epidemiol. 67:17–22, 105-109.
2013.PubMed/NCBI(In English, Polish).
|
|
51
|
Cormier SA, Shrestha B, Saravia J, Lee GI,
Shen L, DeVincenzo JP, Kim YI and You D: Limited type I interferons
and plasmacytoid dendritic cells during neonatal respiratory
syncytial virus infection permit immunopathogenesis upon
reinfection. J Virol. 88:9350–9360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sedeyn K, Schepens B and Saelens X:
Respiratory syncytial virus nonstructural proteins 1 and 2:
Exceptional disrupters of innate immune responses. PLoS Pathog.
15(e1007984)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hijano DR, Vu LD, Kauvar LM, Tripp RA,
Polack FP and Cormier SA: Role of type I interferon (IFN) in the
respiratory syncytial virus (RSV) immune response and disease
severity. Front Immunol. 10(566)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Laura MS and Steven MV: Function and
modulation of type I interferons during respiratory syncytial virus
infection. Vaccines (Basel). 8(177)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Greenberg SB: Viral pneumonia. Infect Dis
Clin North Am. 5:603–621. 1991.PubMed/NCBI
|
|
56
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative. The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Lancet. 370:1453–1457. 2007.PubMed/NCBI View Article : Google Scholar
|