Introduction
Cardiovascular disease, cancer, diabetes and other
chronic non-communicable diseases are the most frequent causes of
death worldwide (1). Cardiovascular
diseases such as stroke, ischemic heart disease, atherosclerosis
and congestive heart failure are the major causes of death in
China, with the number of cardiovascular disease related deaths
expected to rise by 39 million between 2016 and 2030(2). Vascular smooth muscle cells (VSMCs)
are important components of blood vessels. Atherosclerosis,
hypertension and restenosis are associated with increased VSMC
proliferation, invasion and migration (3,4). VSMCs
maintain an organized, differentiated and contractile phenotype
under normal physiological conditions, and can be converted into a
proliferative, migratory and synthetic phenotype under various
stimuli, such as mechanical injury, activation of growth factors
(such as transforming growth factor-β and platelet-derived growth
factor), ligand-receptor signaling and increased hemodynamics,
eventually leading to an increase in proliferation, migration and
secretory activities of VSMCs (5).
MicroRNAs (miRNAs/miRs) are non-coding RNAs that
degrade mRNA or impede its translation to regulate the levels of
target genes (6,7). miRNAs play an important role in the
occurrence and development of cardiovascular diseases, such as
miR-665 which suppresses VSMC proliferation, invasion and migration
through targeting FGF9 and MEF2D (8), and it is hypothesized that miRNAs
could be used as novel targets in the treatment of cardiovascular
diseases (9,10). Studies have confirmed that miRNAs,
such as miR-541 promote VSMC proliferation and miR-146b-5p promote
VSMC proliferation and migration (11,12),
while miR-124 and miR-503 inhibit the proliferation of VSMCs
(13,14). A previous study demonstrated that
macrophage-derived miR-342-5p contributed to atherosclerosis by
inhibiting Akt1 expression (15).
Another study revealed that the levels of miR-342-5p in patients
with coronary heart disease were increased (16). Furthermore, Yan et al
(17) revealed that miR-342-5p
promoted endothelial cell migration and reduced angiogenesis by
targeting endoglin. Although the proliferation and differentiation
of VSMCs play a key role in the progression of cardiovascular
disease (18), the role of
miR-342-5p in VSMCs remains to be elucidated. Therefore, the
effects and mechanism of action of miR-342-5p on VSMC proliferation
and differentiation were explored in the current study.
Materials and methods
Cell culture
Mouse aortic vascular smooth muscle (MOVAS) cells
(American Type Culture Collection; 2x105 cells/well)
were cultured in 24-well plate with DMEM (Sigma-Aldrich; Merck
KGaA) containing 10% FBS (Sigma-Aldrich; Merck KGaA), 100 U/m
penicillin (Invitrogen; Thermo Fisher Scientific, Inc.) and 100 U/m
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C
in a humidified incubator with 5% CO2.
Cell transfection
Cells were divided into specific groups as follows
for transfection: i) Control group (cells with no transfected
material); mock group [50 nM miR-342-5p mimics negative control
(NC); GeneCopoeia, Inc.]; mimics group (50 nM miR-342-5p mimics;
GeneCopoeia, Inc.); NC group (50 nM miR-342-5p inhibitor NC;
GeneCopoeia, Inc.) and inhibitor group (50 nM miR-342-5p inhibitor;
GeneCopoeia, Inc.); ii) control + phosphatidylinositol 3-kinase
regulatory subunit α (PIK3R1)-3'-untranslated region (UTR) group
(50 ng/µl PIK3R1-3'-UTR expression plasmid), miR-342-5p +
PIK3R1-3'-UTR group (50 ng/µl PIK3R1-3'-UTR expression plasmid and
miR-342-5p mimics) and miR-342-5p + PIK3R1-3'-UTR mutant (mut)
group (miR-342-5p mimics and 50 ng/µl mut PIK3R1-3'-UTR); iii)
control group (cells with no transfected material), small
interfering (si)NC group (siPIK3R1 NC; Sigma-Aldrich; Merck KGaA)
and siPIK3R1 group (siPIK3R1; Sigma-Aldrich; Merck KGaA); iv) NC
group (miR-342-5p inhibitor NC), inhibitor group (miR-342-5p
inhibitor), NC + siPIK3R1 group (miR-342-5p inhibitor NC and 50 nM
siPIK3R1) and inhibitor + siPIK3R1 group (miR-342-5p inhibitor and
50 nM siPIK3R1); and v) mock group (miR-342-5p mimics NC), mimics
group (miR-342-5p mimics), mock + LY294002 (Cayman Chemical
Company) group (transfected with miR-342-5p mimics NC and treated
with 10 µmol/l LY294002) and mimics + LY294002 group (transfected
with miR-342-5p mimics and treated with 10 µmol/l LY294002).
Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to transfect the aforementioned
groups into the cells and incubated for 24 h at 37˚C in a
humidified incubator with 5% CO2. Sequences for miRNA
and siRNA used were as follows: miR-342-5p mimics,
5'-AGGGGUGCUAUCUGUGAUUGAG-3'; miR-342-5p mimics NC,
5'-UUUGUACUACACAAAAGUACUG-3'; miR-342-5p inhibitor NC,
5'-CAGUACUUUUGUGUAGUACAA-3'; siPIK3R1,
5'-GCAGAGGCACTCCTGATATATGATTT-3'; and siNC,
5'-AATTCACTCCAAGTCTCTTCC-3'.
Cell proliferation assay
Cell viability was measured using a Cell Counting
Kit (CCK)-8 cell proliferation assay kit (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocol. Following transfection and culturing of cells
(3x103 cells/well) for 0, 24, 48 and 72 h, CCK-8
solution was added into each well and cells were incubated in the
dark at 37˚C in a humidified incubator with 5% CO2 for 2
h. A microplate reader (Bio-Rad Laboratories, Inc.) was used to
determine the optical density of each well at a wavelength of 450
nm.
Wound healing assay
Cell migration was determined using a wound healing
assay. Cells were plated at a density of 4x105
cells/well in a six-well plate. A pipette tip was used to create a
straight wound in each well. Cells were washed twice with PBS
(Gibco; Thermo Fisher Scientific, Inc) to remove floating cells and
the remaining cells were cultured in DMEM at 37˚C in a humidified
incubator with 5% CO2 for 0 and 24 h. Subsequently, cell
migration was observed under an inverted phase contrast microscope
(magnification, x200; Olympus Corporation). The distance of cell
migration was measured by Image ProPlus v6.0 analysis software
(Media Cybernetics, Inc.). The relative migration distance = (0 h
migration distance -24 h migration distance)/0 h migration
distance.
Transwell assay
Transwell assay was performed to detect cell
invasion. Transwell chambers (8-µm pores; Corning, Inc.) were
placed in a 24-well plate. Cells (1x105) were
re-suspended in serum-free medium and plated into the upper chamber
which pre-coated with Matrigel® (BD Biosciences). DMEM
containing 10% FBS was added into the lower chamber and cells were
cultured at 37˚C in a humidified incubator with 5% CO2
for 24 h. The Transwell chambers were moved to another 24-well
plate, washed with PBS three times and then fixed in 4% methanol
solution for 30 min at room temperature and stained with 0.1%
crystal violet for 25 min at room temperature. Subsequently, the
transwell chambers were washed with PBS twice and non-migrating
cells in the upper chamber were removed using a cotton swab. The
chambers were then placed under an inverted fluorescence microscope
(Olympus Corporation) to observe cells and capture images
(magnification, x200).
Target gene prediction for
miR-342-5p
The target gene online prediction databases
TargetScan (http://www.targetscan.org/), miRDB (http://mirdb.org/) and miRBase (http://www.mirbase.org/) were used to predict
potential target genes and binding sites of miR-342-5p. Potential
target genes of miR-342-5p were further confirmed using
dual-luciferase reporter assays.
Dual luciferase reporter assay
The sequences of PIK3R1-3'-UTR was amplified from
cDNA derived from total RNA and PIK3R1-3'-UTR-mut was constructed
using a Site-Directed Mutagenesis kit (Stratagene; Agilent
Technologies, Inc.) according to the manufacturer's protocol. The
two target gene fragments were cloned into pmirGLO vectors (Promega
Corporation) and transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc), as aforementioned. Cells were harvested 24 h
after the transfection, and firefly and Renilla luciferase
activity was measured using a Dual-Luciferase® Reporter
Assay System (Promega Corporation). Firefly luciferase activity was
normalized to that of Renilla luciferase activity.
RT-qPCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Briefly, the
cells were mixed with Trizol reagent and subsequently transferred
to an Eppendrof™ tube and incubated on the bench at room
temperature. Then 200 µl chloroform was added, and mixed with the
cell/Trizol mixture and placed on ice for 5 min. The tubes were
centrifuged at 12,000 x g at 4˚C for 15 min, following which the
upper aqueous phase was transferred to a clean
EppendorfTM tube and 500 µl isopropanol was added, and
the tubes were incubated on ice for 10 min. Pre-cooled 75% ethanol
was subsequently added and the tubes were centrifuged for a second
time at 12,000 x g at 4˚C for 10 min. The supernatant was discarded
and the RNA pellet was air dried for 3 min, following which
pre-cooled 20 µl DEPC was added. A spectrophotometer (ND-1000;
NanoDrop Technologies; Thermo Fisher Scientific, Inc.) was used to
determine RNA purity and concentration at 260/280 nm. Total RNA (2
µg) was reverse transcribed into cDNA for mRNA detection using a
PrimeScript RT master mix kit (Takara Biotechnology Co., Ltd.) at
37˚C for 60 min, then 85̊C for 5 min and the samples were stored at
4˚C until further experimentation. qPCR was performed using SYBR
Premix Ex Taq (Takara Biotechnology Co., Ltd.) on the ABI7500
Real-Time PCR System (Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 5 sec; 40 cycles of 95˚C for 10 sec, 60˚C
for 30 sec; and a final extension step at 72˚C for 10 sec, with
storage of the samples at 4˚C. The relative expression of genes was
calculated using the 2-ΔΔCq method (19). U6, GAPDH and β-actin (GeneCopoeia)
served as internal controls. The following primer pairs were used
for the qPCR: miR-342-5p forward, 5'-CGGAGGGGTGCTATCTGTGA-3' and
reverse, 5'-AGTCGGCAATTGCACTGGAT-3'; U6 forward,
5'-CTCAGAGCGTGGTTCTCCGTCAC-3' and reverse,
5'-TATAAATCTTTACCCTGTTGGCAGT-3'; GAPDH forward,
5'-AGGTCGGTGTGAACGGATTTG-3' and reverse,
5'-TGTAGACCATGTAGTTGAGGTCA-3'; α-smooth muscle actin (α-SMA)
forward, 5'-CATCCGTAAAGACCTCTATGCCAAC-3' and
5'-ATGGAGCCACCGATCCACAA-3' and reverse,
5'-TCGGATACTTCAGCGTCAGGA-3'; vimentin forward,
5'-GAGAACTTTGCCGTTGAAGC-3' and reverse, 5'-GCTTCCTGTAGGTGGCAATC-3';
apelin (APLN) forward, 5'-TGCTCTGGCTCTCCTTGACT-3' and reverse,
5'-ATGGGTCCCTTATGGGAGAG-3'; zinc finger and BTB domain-containing
protein 39 (ZBTB39) forward, 5'-CGTGTTAACTAGGTCCCCTTTG-3' and
reverse, 5'-GTTCCTTTAATCCAGAAGGGCT-3'; PIK3R1 forward,
5'-AGCATTGGGACCTCACATTACACA-3' and reverse,
5'-ACTGGAAACACAGTCCATGCACATA-3'; myocyte-specific enhancer factor
2D (MEF2D) forward, 5'-CGCGAATTCACCATGGGGAGGAAAAAGATT-3' and
reverse, 5'-TGGCTCGAGTCACTTTAATGTCCAGGT-3'; and neurogenic locus
notch homolog protein 2 (NOTCH2) forward,
5'-CCCCTTGCCCTCTATGTACCA-3' and reverse,
5'-GGTAGGTGGGAAAGCCACACT-3'; β-actin forward,
5'-GCTGCGTGTGGCCCCTGAG-3' and reverse,
5'-ACGCAGGATGGCATGAGGGA-3'.
Western blot analysis
Cell lysates were extracted using RIPA buffer
(Beyotime Institute of Biotechnology) and the protein concentration
was determined with a Pierce bicinchoninic acid protein assay kit
(Thermo Fisher Scientific, Inc.). 10% SDS-PAGE was used to separate
the proteins (30 µg) according to the molecular weight of protein.
Samples were transferred to PVDF membranes (Bio-Rad Laboratories,
Inc.), which were subsequently blocked with 5% skimmed milk for 1
h. PVDF membranes were incubated with the following primary
antibodies: Anti-GAPDH (1:1,000; cat. no. ab8245); anti-Akt
(1:1,000; cat. no. ab8805); anti-α-SMA (1:200; cat. no. ab5694);
anti-vimentin (1:1,000, cat. no. ab92547); anti-phosphorylated
(p)-Akt (1:500; cat. no. ab38449); and anti-PIK3R1 (1:1,000; cat.
no. ab86714; all Abcam) overnight at 4˚C. Following primary
incubation, membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (H+L)
or goat anti-mouse IgG (H+L) secondary antibodies (1:1,000; cat.
nos. A0208 and A0216; Beyotime Institute of Biotechnology) for 1 h
at room temperature. Protein bands were visualized using the
BeyoECL Plus kit (Beyotime Institute of Biotechnology) and
quantified using ImageJ (version 5.0; National Institutes of
Health). The expression levels of GAPDH served as an internal
control.
Statistical analysis
Data are presented as the mean ± SD. Data were
analyzed using SPSS v21.0 software (IBM Corp.). Any significant
difference among different groups was analyzed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were performed in triplicate.
Results
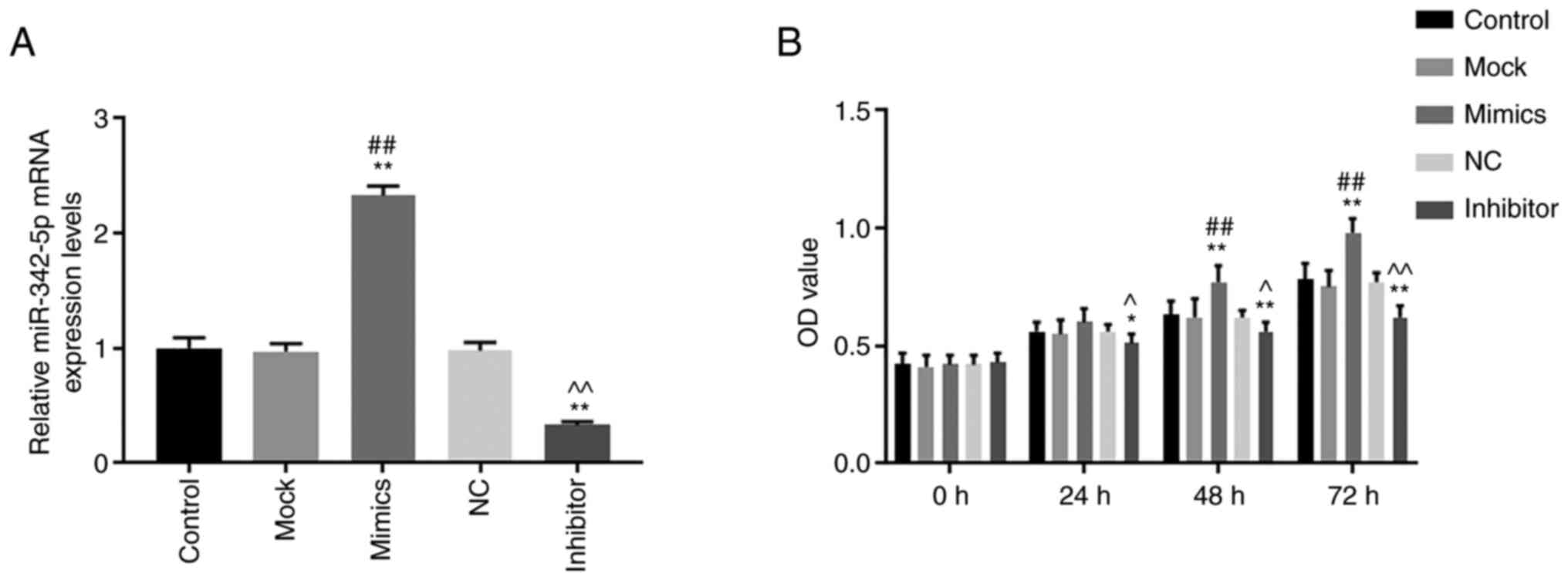
miR-342-5p mimics and inhibitors was
successfully expressed in MOVAS cells
The transfection efficiency of miR-342-5p was
determined using RT-qPCR. The levels of miR-342-5p mRNA were
significantly increased in the mimics group compared with that in
the control and mock groups (Fig.
1A), while mRNA levels of miR-342-5p were significantly
decreased in the inhibitor group compared with that in the control
and NC groups (Fig. 1A). The data
showed that MOVAS cells with high and low expression levels of
miR-342-5p were successfully constructed.
miR-342-5p promotes cell viability,
migration and invasion
The cell viability, migration and invasion of cells
were determined using CCK-8, wound healing and Transwell assays,
respectively. Cell viability was significantly higher in the mimics
group compared with that in the control and mock groups 48 and 72 h
after transfection (P<0.05; Fig.
1B), while cell viability in the inhibitor group was
significantly lower compared with control and NC groups at 24, 48
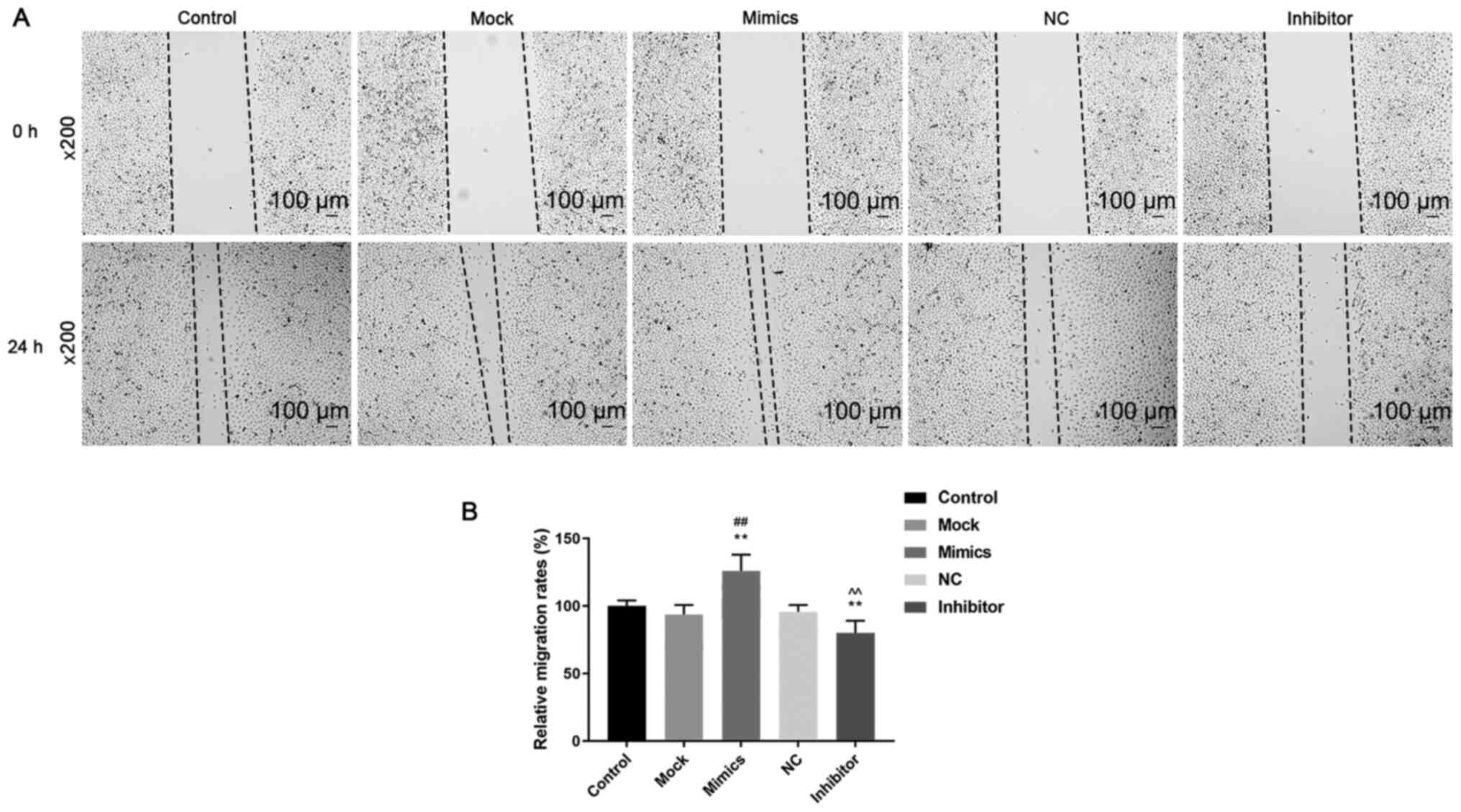
and 72 h after transfection (P<0.05; Fig. 1B). Cell migration (Fig. 2A and B) and invasion (Fig. 3A) was significantly increased in the
mimics group (P<0.05) compared with that in the control and mock
groups, whereas they were significantly decreased in the inhibitor
group (P<0.05) compared with that in the control and NC groups.
These results suggest that miR-342-5p increased cell viability and
promoted cell migration and invasion in MOVAS cells.
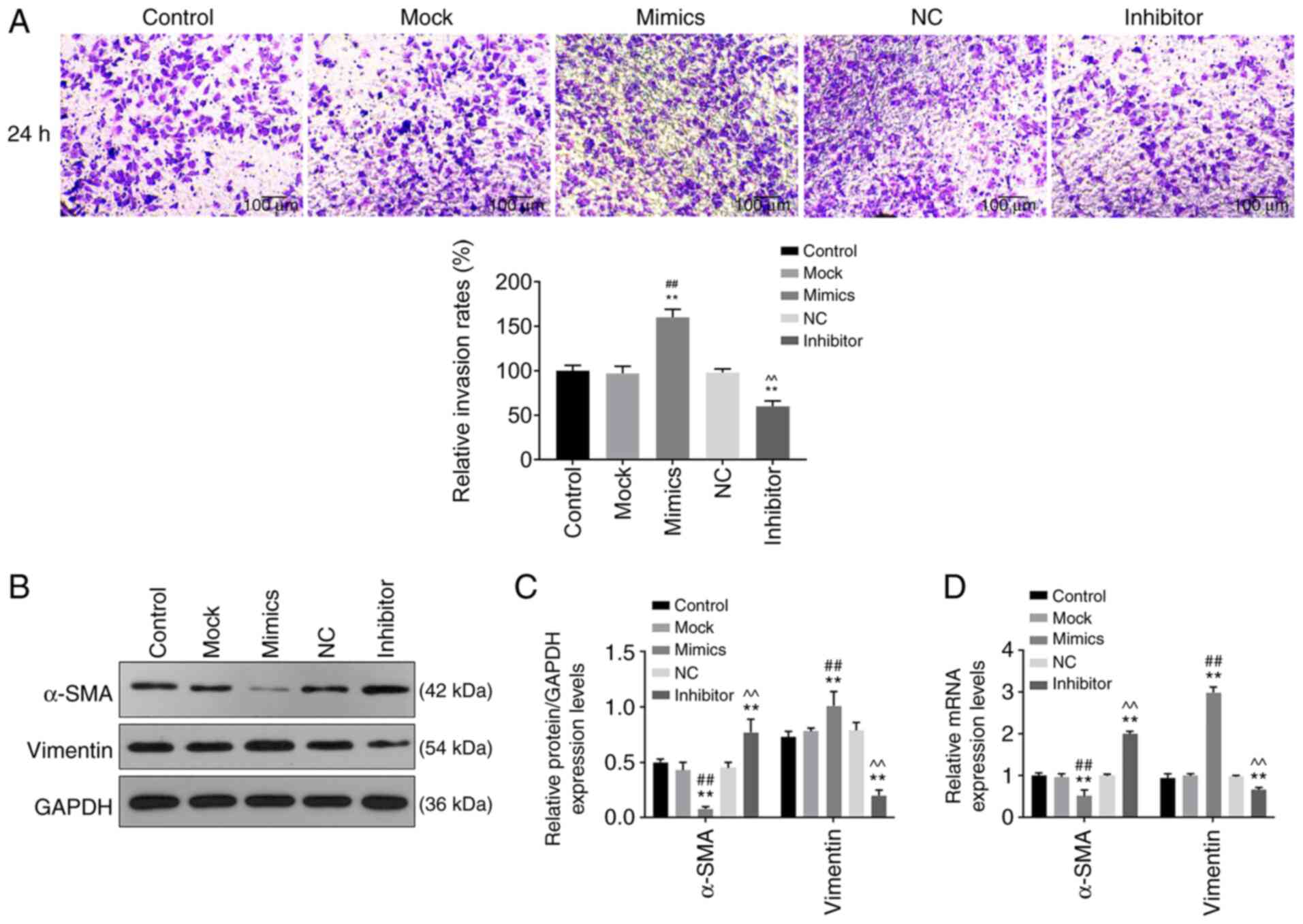 | Figure 3Role of miR-342-5p in cell invasion
and its effects on relative mRNA expression of vimentin and α-SMA.
MOVAS cells were transfected with control, mock, mimics, NC and
inhibitor, following which (A) cell invasion, and (B and C) protein
and (D) mRNA expression levels of target genes were determined
using Transwell assay, western blot analysis and reverse
transcription-quantitative PCR, respectively. (A) Representative
images of cell invasion in each experimental group and quantitative
analysis. Magnification, x200. α-SMA and vimentin protein
expression levels were determined using (B) western blotting and
(C) quantified and analyzed statistically. (D) The mRNA levels of
α-SMA and vimentin. **P<0.01 vs. control;
##P<0.01 vs. mock; ^^P<0.01 vs. NC.
miR-342-5p, microRNA-342-5p; α-SMA, α-smooth muscle actin; mock,
miR-342-5p mimic negative control; NC, miR-342-5p inhibitor
negative control; MOVAS, mouse aortic vascular smooth muscle. |
Protein and mRNA levels of a-SMA and
vimentin are regulated by miR-342-5p
The relative protein and mRNA levels of α-SMA and
vimentin were determined using western blot analysis and RT-qPCR,
respectively. Protein and mRNA expression levels of α-SMA in the
mimics group were downregulated compared with that in the control
and mock groups, while protein and mRNA expression levels of
vimentin were upregulated compared with that in the control and
mock groups (Fig. 3B-D). While the
protein and mRNA levels of α-SMA in the inhibitor group were
upregulated compared with that in the control and NC groups, and
the protein and mRNA levels of vimentin were downregulated compared
with that in the control and NC groups (Fig. 3B-D). These data revealed that
miR-342-5p mimics downregulated α-SMA expression and upregulated
vimentin expression, and the effects were reversed by the
miR-342-5p inhibitor.
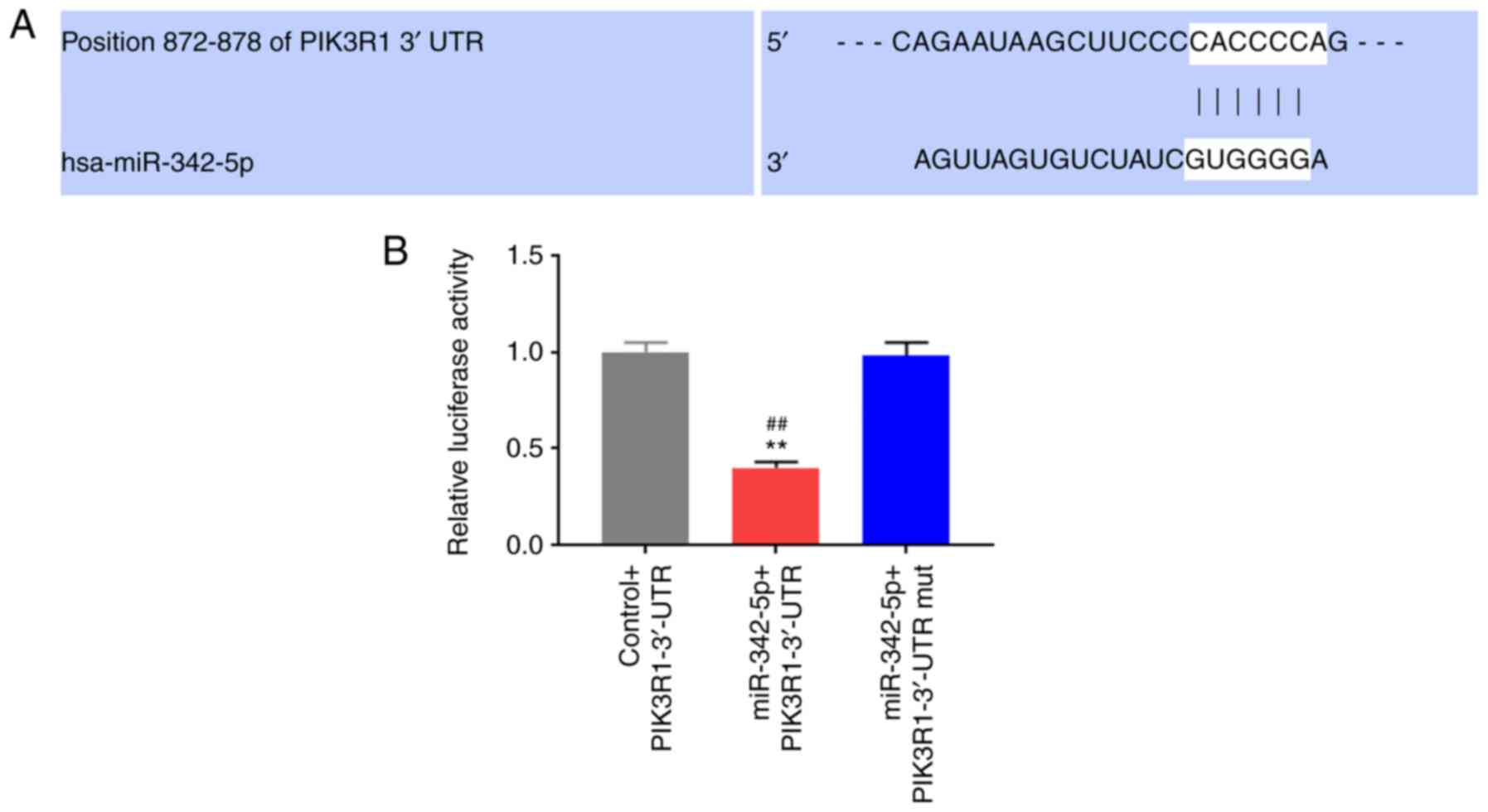
PIK3R1 is a target gene of
miR-342-5p
Online databases was applied to predict the target
gene of miR-342-5p (Fig. 4A-B) and
it has been reported that the APLN, ZBTB39, PIK3R1, MEF2D and
NOTCH2 genes may be associated with cardiovascular disease
(8,20-23),
therefore infer that APLN, ZBTB39, PIK3R1, MEF2D and NOTCH2 may
were potential target genes of miR-342-5p. The role of miR-342-5p
in the aforementioned target genes was examined using RT-qPCR. The
mRNA levels of APLN, ZBTB39, PIK3R1 and NOTCH2 in the mimics group
were significantly lower compared with that in the control and mock
groups, while MEF2D levels were significantly higher (P<0.05;
Fig. 4C), notably PIK3R1 was the
most significantly associated (P<0.01). The mRNA levels of APLN,
ZBTB39, PIK3R1 and NOTCH2 were significantly upregulated in the
inhibitor group compared with that in the control and NC groups
(Fig. 4D), and the upregulation in
expression was more significant for PIK3R1 (P<0.01). Based on
the significant results from the mRNA expression levels, to further
determine whether PIK3R1 was a target of miR-342-5p, target gene
online prediction databases were used to predict the binding sites
of PIK3R1 and miR-342-5p (Fig. 5A).
The binding sites were further verified using a dual luciferase
reporter assay. The results revealed that compared with the control
+ PIK3R1-3'-UTR and miR-342-5p + PIK3R1-3'-UTR mut groups, the
relative luciferase activity in the miR-342-5p + PIK3R1-3'-UTR
group was significantly decreased (P<0.05; Fig. 5B).
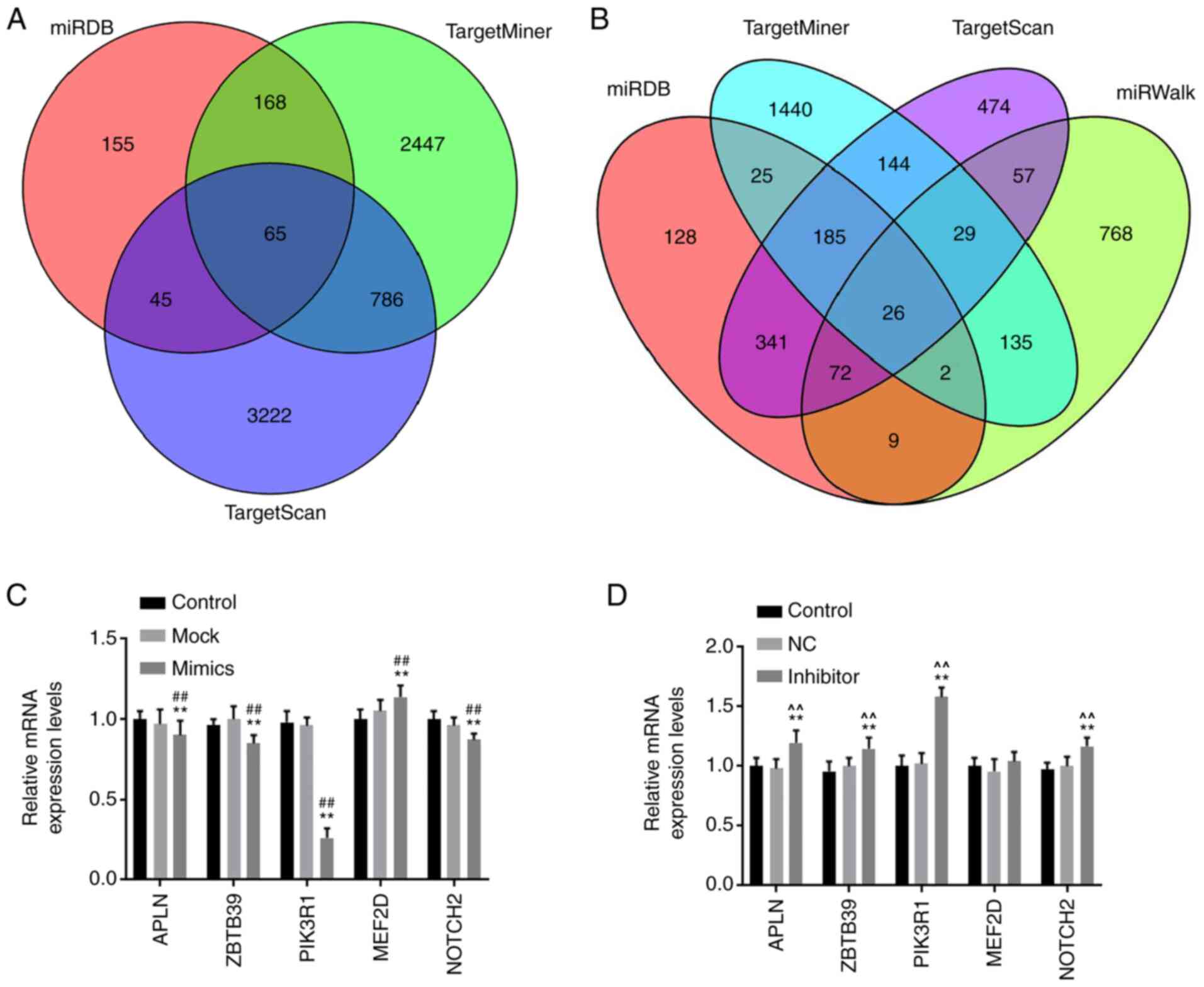 | Figure 4miR-342-5p target genes were
predicted using target gene prediction websites. (A) Venn diagrams
of miR-342-5p target genes, the target genes were predicted using
miRDB, TargetMiner and TargetScan. (B) Venn diagrams of miR-342-5p
target genes, the target genes were predicted using miRDB,
TargetMiner, TargetScan and miRWalk. MOVAS cells were transfected
with (C) control, mock or miR-342-5p mimic and (D) control, NC or
miR-342-5p inhibitor and the relative mRNA expression levels of
APLN, ZBTB39, PIK3R1, MEF2D and NOTCH2 were determined using
reverse transcription-quantitative PCR. **P<0.01 vs.
control; ##P<0.01 vs. mock; ^^P<0.01
vs. NC. miR, microRNA; mock, miR-342-5p mimic negative control; NC,
miR-342-5p inhibitor negative control; APLN, apelin; ZBTB39, zinc
finger and BTB domain-containing protein 39; PIK3R1,
phosphatidylinositol 3-kinase regulatory subunit α; MEF2D,
myocyte-specific enhancer factor 2D; NOTCH2, neurogenic locus notch
homolog protein 2. |
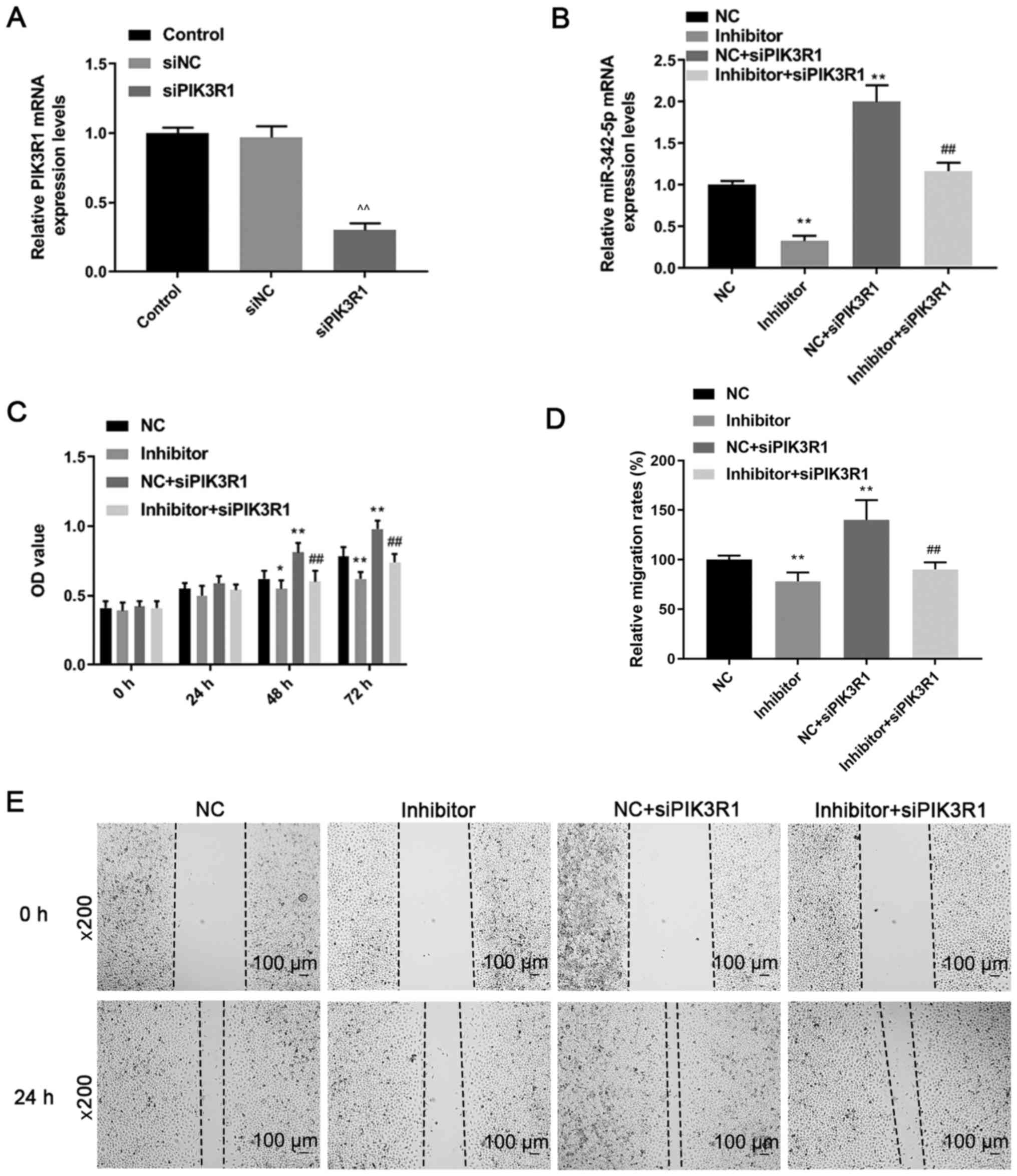
siPIK3R1 results in lower expression
of PIK3R1 in MOVAS cells
RT-qPCR was performed to determine the mRNA
expression levels of PIK3R1 following transfection with siPIK3R1.
PIK3R1 mRNA expression was downregulated in the siPIK3R1 group
compared with that in the control and siNC groups (P<0.05;
Fig. 6A). The results indicated
that MOVAS cells with low PIK3R1 expression were successfully
constructed.
siPIK3R1 reverses the effects of
miR-342-5p inhibitor on cell proliferation, migration and
invasion
Cells were co-transfected with miR-342-5p inhibitor
and siPIK3R1 and the RT-qPCR results (Fig. 6B) revealed that the miR-342-5p mRNA
expression levels in the inhibitor group were downregulated
compared with that in the NC group, while miR-342-5p levels were
upregulated in NC + siPIK3R1 compared with that in the NC group.
The mRNA expression levels of miR-342-5p in the inhibitor +
siPIK3R1 group was significantly lower compared with that in the NC
+ siPIK3R1 group (P<0.05; Fig.
6B).
Cell proliferation, migration and invasion were
determined by CCK-8, wound healing and Transwell assays,
respectively. Following transfection for 48 and 72 h, cell
proliferation was higher in the NC + siPIK3R1 group compared with
that in the NC group, while it was significantly lower in the
inhibitor group compared with that in the NC group (P<0.05;
Fig. 6C). The cell proliferation in
the inhibitor + siPIK3R1 group was significantly lower compared
with that in the NC + siPIK3R1 group (P<0.05; Fig. 6C). Compared with that in the NC
group, cell migration was decreased in the inhibitor group, but
increased in the NC + siPIK3R1 group (P<0.05; Fig. 6D and E). Cell migration in the inhibitor +
siPIK3R1 group was significantly lower compared with that in the NC
+ siPIK3R1 group, but was slightly higher compared with that in the
inhibitor group (P<0.05; Fig. 6D
and E). Cell invasion decreased in
the inhibitor group but increased in the NC + siPIK3R1 group
compared with that in the NC group (P<0.05; Fig. 7A and B). In addition, cell invasion was lower in
the inhibitor + siPIK3R1 group compared with that in the NC +
siPIK3R1 group (P<0.05; Fig. 7A
and B). The results of the current
study showed that PIK3R1 silencing increased miR-342-5p expression.
siPIK3R1 antagonized the effects of miR-342-5p inhibitor on cells,
increasing cell viability and promoting cell migration and
invasion.
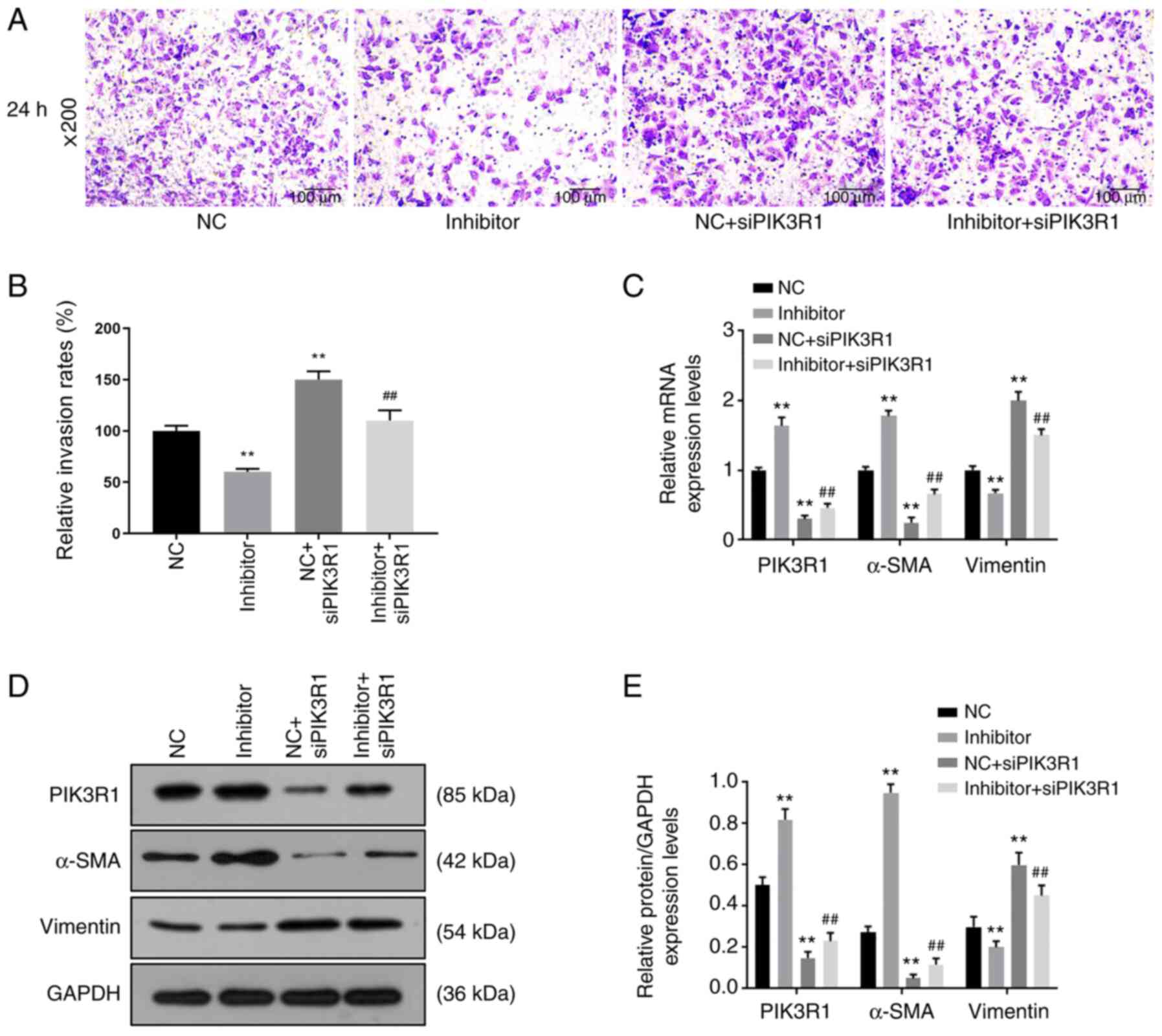 | Figure 7Roles of siPIK3R1, miR-342-5p
inhibitor and the combination of miR-342-5p inhibitor with siPIK3R1
in cell invasion and the relative expression of target genes. MOVAS
cells were transfected with NC, miR-342-5p inhibitor, NC + siPIK3R1
or miR-342-5p inhibitor + siPIK3R1, following which (A and B) cell
invasion, and (C) mRNA and (D and E) protein expression levels of
target genes were determined using Transwell assay, reverse
transcription-quantitative PCR and western blot analysis,
respectively. (A) Representative cell invasion images of the 5
experimental groups using an inverted fluorescence microscope at
x200 magnification. (B) Quantitative analysis of the rate of cell
invasion. The (C) mRNA and (D) protein expression levels of α-SMA,
PIK3R1 and vimentin. (E) The quantification of the protein levels
in 4 experimental groups. **P<0.01 vs. NC;
##P<0.01 vs. NC + siPIK3R1. miR-342-5p,
microRNA-342-5p; α-SMA, α-smooth muscle actin; PIK3R1,
phosphatidylinositol 3-kinase regulatory subunit α; siPIK3R1, small
interfering RNA targeting PI3K1; siNC, siPIK3R1 negative control;
NC, miR-342-5p inhibitor negative control. |
siPIK3R1 reverses the effects of
miR-342-5p inhibitor on the expression of a-SMA and vimentin
The mRNA and protein expression levels of PIK3R1,
α-SMA and vimentin were analyzed using RT-qPCR and western blot
analysis, respectively, following transfection with miR-342-5p
inhibitor and siPIK3R1. Compared with that in the NC group, the
mRNA and protein expression levels of PIK3R1 were upregulated in
the inhibitor group, but downregulated in the NC + siPIK3R1 and
inhibitor + siPIK3R1 groups (P<0.05; Fig. 7C-E). However, compared with that in
the NC + siPIK3R1 group, the mRNA and protein expression levels of
PIK3R1 were higher in the inhibitor + siPIK3R1 group, but lower
compared with that in the inhibitor group. The mRNA and protein
expression levels of α-SMA in the NC + siPIK3R1 and the inhibitor +
siPIK3R1 groups were downregulated compared with the NC group, but
were upregulated in the inhibitor group compared with that in the
NC group (P<0.05; Fig. 7C-E). By
contrast, the mRNA and protein expression levels of α-SMA in the
inhibitor + siPIK3R1 group were higher compared with that in the NC
+ siPIK3R1 group (P<0.05; Fig.
7C-E). The mRNA and protein expression levels of vimentin were
upregulated in NC + siPIK3R1 and inhibitor + siPIK3R1 groups, and
downregulated in the inhibitor group, when compared with that in
the NC group, while the mRNA and protein expression levels of
vimentin in the inhibitor + siPIK3R1 group were lower compared with
that in the NC + siPIK3R1 group (P<0.05; Fig. 7C-E). These results showed that
PIK3R1 silencing caused decreased PIK3R1 and α-SMA expression, and
increased vimentin expression. siPIK3R1 partially reversed the
effects of miR-342-5p inhibitor on the expressions of α-SMA, PIK3R1
and vimentin.
siPIK3R1 reverses the effects of
miR-342-5p inhibitor on p-Akt protein levels
The protein expression levels of p-Akt and Akt were
detected using western blot analysis following transfection with
miR-342-5p inhibitor and siPIK3R1. p-Akt protein levels were lower
in the inhibitor group compared with that in the NC group
(P<0.05; Fig. 8A and B), but was upregulated in the NC +
siPIK3R1 and inhibitor + siPIK3R1 groups compared with that in the
NC group (P<0.01; Fig. 8A and
B). The p-Akt protein levels in
inhibitor + siPIK3R1 group was lower compared with that in the NC +
siPIK3R1 group. These results revealed that p-Akt protein levels
were downregulated after inhibiting miR-342-5p expression, and that
p-Akt expression was upregulated and the Akt signaling pathway was
activated following PIK3R1 silencing. In addition, siPIK3R1
partially reversed the inhibitory effects of miR-342-5p inhibitor
on p-Akt protein expression levels. The ratio of p-Akt/Akt was
reduced in inhibitor group, while increased in NC + siPIK3R1 group
compared with NC group; and the ratio of p-Akt/Akt was lower in
inhibitor + siPIK3R1 group than that in NC + siPIK3R1 group
(P<0.01; Fig. 8C).
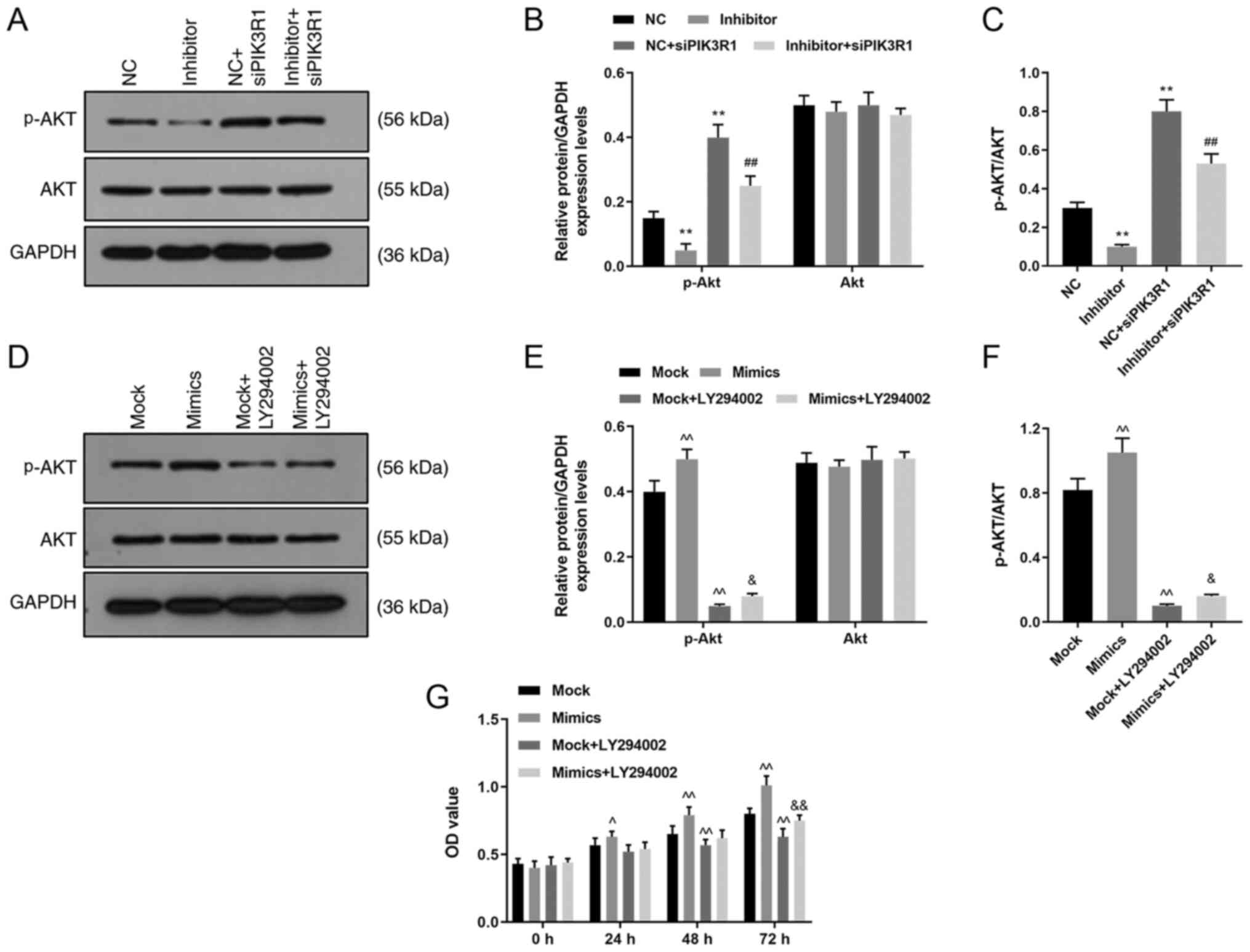 | Figure 8Role of siPIK3R1 and LY294002 in the
Akt signaling pathway. MOVAS cells were transfected with NC,
miR-342-5p inhibitor, NC + siPIK3R1 or miR-342-5p inhibitor +
siPIK3R1 and the p-Akt and Akt protein expression levels were
determined using (A) western blot analysis and (B) quantified, and
analyzed statistically. (C) The ratio of p-AKT/AKT was quantified
in each experiment group. MOVAS cells were transfected with mock,
mimics, mock + LY294002 or mimics + LY294002 and the p-Akt and Akt
protein levels were analyzed using (D) western blot analysis and
(E) quantified, and analyzed statistically. (F) The ratio of
p-AKT/AKT was quantified in each experiment group. (G) Cell
Counting Kit-8 was used to determine cell proliferation following
transfection. **P<0.01 vs. NC; ##P<0.01
vs. NC + siPIK3R1; ^P<0.05, ^^P<0.01
vs. mock; and &P<0.05,
&&P<0.01 vs. mock + LY294002. p,
phosphorylated; NC, miR-342-5p inhibitor negative control; si,
small interfering; PIK3R1, phosphatidylinositol 3-kinase regulatory
subunit α; mock, miR-342-5p mimic negative control; OD, optical
density; NC, miR-342-5p inhibitor negative control. |
miR-342-5p activates the Akt signaling
pathway and reverses the effect of LY294002
The cells were transfected with miR-342-5p mimics or
NC, and treated with PI3K inhibitor (LY294002) to confirm the role
of miR-342-5p in the Akt signaling pathway and the p-Akt and Akt
protein expression levels were subsequently determined using
western blot analysis. The data revealed that p-Akt protein levels
were higher in the mimics group compared with that in the mock
group (P<0.05; Fig. 8D and
E), but were downregulated in mock
+ LY294002 and mimics + LY294002 groups compared with that in the
mock group (P<0.05; Fig. 8D and
E). Moreover, the p-Akt protein
level was upregulated in the mimics + LY294002 group compared with
that in the mock + LY294002 group (P<0.05; Fig. 8D and E). However, there were no significant
differences in the total Akt protein expression levels among the
four groups (P<0.05; Fig. 8D and
E). The ratio of p-Akt/Akt was
increased in the mimic group, while reduced in the mock + LY294002
group compared with the mock group; and the ratio of p-Akt/Akt was
higher in the mimic + LY294002 group than that in the mock +
LY294002 group (P<0.05; Fig.
8F). Thus, miR-342-5p upregulated p-Akt levels and activated
the Akt pathway and miR-342-5p could activate the AKT pathway,
which was inhibited by LY294002.
To investigate the role of the Akt pathway in cells,
cell proliferation was examined using a CCK-8 assay. Following 24
and 48 h of transfection, cell proliferation increased in the
miR-342-5p mimics group, but decreased in the mock + LY294002 group
compared with that in the mock group after 48 h of transfection
(P<0.05; Fig. 8E). In addition,
cell proliferation was also increased in the mimics + LY294002
group after 72 h compared with that in the mock + LY294002 group
(P<0.05; Fig. 8E). Inhibition of
the Akt signaling pathway decreased cell proliferation, and this
decrease could be reversed by miR-342-5p transfection.
Discussion
The proliferation and migration of VSMCs can trigger
vascular lesions, leading to abnormal functioning of the heart or
cause diseases such as hypertension and diabetes (24). A recent study suggested that some
miRNAs (such as miR-665 and miR-143/-145) may play important roles
in the development of cardiovascular diseases (8,25). In
the present study, miR-342-5p was shown to improve cell migration
and invasion, promote cell proliferation and the phenotypic
transformation of VSMCs (as shown by the altered α-SMA and vimentin
expression profiles). PIK3R1 was found to be a target gene for
miR-342-5p, and miR-342-5p could regulate the proliferation and
differentiation of VSMCs via activation of the Akt signaling
pathway through inhibition of PIK3R1.
In previous studies, Yan et al (17) found that miR-342-5p was involved in
mediating angiogenesis in human umbilical cord tissue samples;
Ahmadi et al (16) found
that the mRNA expression level of miR-342-5p in peripheral blood
mononuclear cells in patients with coronary heart disease was
increased and Ge et al (26)
concluded that serum miR-342-5p levels might be a novel biomarker
for pertussis. To further investigate the role of miR-342-5p in
MOVAS cells, in vitro transfection was performed to increase
or suppress the expression of miR-342-5p in the cells for
subsequent experiments. miR-342-5p mimics significantly upregulated
miR-342-5p levels in MOVAS cells compared with that in the control
group, whereas miR-342-5p inhibitor downregulated miR-342-5p
levels. Abnormally increased proliferation of VSMCs plays a key
role in the in the development of atherosclerosis and in restenosis
in some diseases (27-29),
and miR-92 regulates vascular smooth muscle cell function by
targeting KLF4 during vascular restenosis and injury (29). In VSMC injury, VSMCs undergo
phenotype switching by transforming from a differentiated and
contractile phenotype to a proliferative, migratory and synthetic
state. (30) This can lead to
increased proliferation, migration and secretory abilities and
contributes to the development of cardiovascular diseases (31). In the present study, high expression
of miR-342-5p in MOVAS cells increased cell viability, migration
and invasion, which, could be reversed by inhibiting the expression
of miR-342-5p. These results indicated that miR-342-5p might
promote the proliferation and phenotypic transformation of
VSMCs.
Increases in VSMCs proliferation causes cell
migration, expression of chemokines and regulation of extracellular
substrates (32,33). VSMCs are considered to have two
phenotypes: Contractile (differentiated) phenotype and synthetic
(undifferentiated or dedifferentiated) phenotype (34). Contractile VSMCs belongs to the
mature type, which has poor proliferation and migration ability,
and elevated protein expression levels of contractile markers, such
as α-SMA, calponin H1 and smooth muscle protein 22-α (35). However, the expression levels of
these contractile markers were reversed in the synthetic VSMC
phenotype, which also present with a simultaneous increase in the
protein expression of synthetic phenotype markers, such as vimentin
and osteopontin (36,37). Thus, phenotypic transformation of
VSMCs is characterized by expressional changes of these markers.
Yan et al (17) demonstrated
that overexpression of miR-342-5p upregulated mesenchymal phenotype
markers, such as atlastin 1, vimentin, Twist-related protein 1,
β-catenin and α-SMA. The results from the present study showed that
overexpression of miR-342-5p increased vimentin and reduced α-SMA
mRNA and protein expression levels, and regulate VSMC phenotypic
conversion; however, low expression levels of miR-342-5p decreased
vimentin and increased α-SMA expression. This indicates that
overexpression of miR-342-5p inhibited the expression of
contractile markers but promoted the expression of synthetic
phenotype markers, thus possibly inducing the phenotypic
transformation of VSMCs from a contractile phenotype to a synthetic
phenotype. Conversely, low expression of miR-342-5p could inhibit
phenotypic transformation of VSMCs.
In the current study, it was found that PIK3R1 was a
target gene for miR-342-5p, and silencing PIK3R1 could reverse the
effect of miR-342-5p inhibitors on the VSMCs. Akt is a threonine
protein kinase and its signaling pathway is involved in the
regulation of a variety of biological processes, including
proliferation, growth, metabolism, angiogenesis and metastasis
(38,39). Several studies have confirmed that
the PI3K/Akt signaling pathway plays an important role in the
regulation of VSMC phenotypic transformation (40-42).
The results from the present study revealed that the Akt pathway
was activated by silencing PIK3R1. However, inhibition of
miR-342-5p mRNA expression could suppress the Akt pathway, which
was partially reversed by siPIK3R1. The results revealed that
reduction of PIK3R1 could activate the Akt signaling pathway. The
cell proliferation of VSMCs and protein expression levels of p-Akt
were significantly inhibited following treatment with LY294002,
indicating that the Akt pathway was inhibited by LY294002. Under
the effects of high miR-342-5p expression, cell proliferation
increased and the Akt pathway was partially activated.
The present study demonstrated that miR-342-5p could
enhance cell proliferation, and promote cell migration, invasion
and phenotypic transformation of MOVAS cells, which may be
associated with the activation of the Akt pathway induced by PIK3R1
inhibition. Other studies have also confirmed the role of
miR-342-5p in atherosclerosis or angiogenesis using animal studies,
in vitro functional experiments and in human tissue samples
(15-17).
However, whether miR-342-5p can be used as a marker in vascular
diseases remains to be further elucidated.
In conclusion, miR-342-5p could improve cell
proliferation, promote cell migration and invasion, increase
vimentin and decrease α-SMA expression. PIK3R1 was a target gene of
miR-342-5p, and decreased PIK3R1 expression could improve cell
proliferation, promote cell migration and invasion, and activate
the Akt signaling pathway. Thus, miR-342-5p may promote the
proliferation and differentiation of VSMCs via regulating the Akt
signaling pathway through targeting PIK3R1. The results from the
present study may provide a novel insight into the treatment of
cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SB made substantial contributions to the conception
and design of the study, and drafted and revised the manuscript for
important intellectual content. QP, WL, CZ and ZL acquired the
data, and performed analysis and interpretation. All authors have
read and approved the final manuscript and agree to be accountable
for the accuracy and integrity of the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lisy K, Campbell JM, Tufanaru C, Moola S
and Lockwood C: The prevalence of disability among people with
cancer, cardiovascular disease, chronic respiratory disease and/or
diabetes: A systematic review. Int J Evid Based Healthc.
16:154–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stevens W, Peneva D, Li JZ, Liu LZ, Liu G,
Gao R and Lakdawalla DN: Estimating the future burden of
cardiovascular disease and the value of lipid and blood pressure
control therapies in China. BMC Health Serv Res.
16(175)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang CH, Ciou JS, Chen ST, Kok VC, Chung
Y, Tsai JJ, Kurubanjerdjit N, Huang CF and Ng KL: Identify
potential drugs for cardiovascular diseases caused by
stress-induced genes in vascular smooth muscle cells. PeerJ.
4(e2478)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tykocki NR, Boerman EM and Jackson WF:
Smooth muscle ion channels and regulation of vascular tone in
resistance arteries and arterioles. Compr Physiol. 7:485–581.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang W, Chen S, Zhang Z, Wang C and Liu
C: FAM3B mediates high glucose-induced vascular smooth muscle cell
proliferation and migration via inhibition of miR-322-5p. Sci Rep.
7(2298)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nazari-Jahantigh M, Egea V, Schober A and
Weber C: MicroRNA-specific regulatory mechanisms in
atherosclerosis. J Mol Cell Cardiol. 89:35–41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Andreou I, Sun X, Stone PH, Edelman ER and
Feinberg MW: miRNAs in atherosclerotic plaque initiation,
progression, and rupture. Trends Mol Med. 21:307–318.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li K, Pan J, Wang J, Liu F and Wang L:
MiR-665 regulates VSMCs proliferation via targeting FGF9 and MEF2D
and modulating activities of Wnt/β-catenin signaling. Am J Transl
Res. 9:4402–4414. 2017.PubMed/NCBI
|
|
9
|
Nguyen MA, Karunakaran D and Rayner KJ:
Unlocking the door to new therapies in cardiovascular disease:
MicroRNAs hold the key. Curr Cardiol Rep. 16(539)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu R, Liu X, Zhu Y and He Z: MiRNAs:
Potential diagnostic and therapeutic targets for cerebral
ischaemia. Neurol Res. 38:86–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jia YY, Zhao JY, Li BL, Gao K, Song Y, Liu
MY, Yang XJ, Xue Y, Wen AD and Shi L: miR-592/WSB1/HIF-1α axis
inhibits glycolytic metabolism to decrease hepatocellular carcinoma
growth. Oncotarget. 7:35257–35269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Jiang M, Xu Z, Huang H, Gong P,
Zhu H and Ruan C: miR-146b-5p promotes VSMC proliferation and
migration. Int J Clin Exp Pathol. 8:12901–12907. 2015.PubMed/NCBI
|
|
13
|
Choe N, Kwon DH, Shin S, Kim YS, Kim YK,
Kim J, Ahn Y, Eom GH and Kook H: The microRNA miR-124 inhibits
vascular smooth muscle cell proliferation by targeting S100
calcium-binding protein A4 (S100A4). FEBS Lett. 591:1041–1052.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bi R, Ding F, He Y, Jiang L, Jiang Z, Mei
J and Liu H: miR-503 inhibits platelet-derived growth
factor-induced human aortic vascular smooth muscle cell
proliferation and migration through targeting the insulin receptor.
Biomed Pharmacother. 84:1711–1716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M,
Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C and
Schober A: The microRNA-342-5p fosters inflammatory macrophage
activation through an Akt1- and microRNA-155-dependent pathway
during atherosclerosis. Circulation. 127:1609–1619. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ahmadi R, Heidarian E, Fadaei R, Moradi N,
Malek M and Fallah S: miR-342-5p expression levels in coronary
artery disease patients and its association with inflammatory
cytokines. Clin Lab. 64:603–609. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yan XC, Cao J, Liang L, Wang L, Gao F,
Yang ZY, Duan JL, Chang TF, Deng SM, Liu Y, et al: miR-342-5p is a
notch downstream molecule and regulates multiple angiogenic
pathways including notch, vascular endothelial growth factor and
transforming growth factor β signaling. J Am Heart Assoc.
5(e003042)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rivard A and Andres V: Vascular smooth
muscle cell proliferation in the pathogenesis of atherosclerotic
cardiovascular diseases. Histol Histopathol. 15:557–571.
2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong JC, Zhang ZZ, Wang W, McKinnie SMK,
Vederas JC and Oudit GY: Targeting the apelin pathway as a novel
therapeutic approach for cardiovascular diseases. Biochim Biophys
Acta Mol Basis Dis. 1863:1942–1950. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu G, Luo Z, Zhou Y, Zhang L, Wu Y, Ding L
and Shi Y: Uncovering the pharmacological mechanism of Carthamus
tinctorius L. On cardiovascular disease by a systems pharmacology
approach. Biomed Pharmacother. 117(109094)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cai W, Zhang J and Yang J, Fan Z, Liu X,
Gao W, Zeng P, Xiong M, Ma C and Yang J: MicroRNA-24 attenuates
vascular remodeling in diabetic rats through PI3K/Akt signaling
pathway. Nutr Metab Cardiovasc Dis 621-632, 2019.
|
|
23
|
Meester JAN, Verstraeten A, Alaerts M,
Schepers D, Van Laer L and Loeys BL: Overlapping but distinct roles
for NOTCH receptors in human cardiovascular disease. Clin Genet.
95:85–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Reddy MA, Das S, Zhuo C, Jin W, Wang M,
Lanting L and Natarajan R: Regulation of vascular smooth muscle
cell dysfunction under diabetic conditions by miR-504. Arterioscler
Thromb Vasc Biol. 36:864–873. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao W, Zhao SP and Zhao YH:
MicroRNA-143/-145 in cardiovascular diseases. Biomed Res Int.
2015(531740)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ge Y, Zhao K, Qi Y, Min X, Shi Z, Qi X,
Shan Y, Cui L, Zhou M, Wang Y, et al: Serum microRNA expression
profile as a biomarker for the diagnosis of pertussis. Mol Biol
Rep. 40:1325–1332. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Psaltis PJ and Simari RD: Vascular wall
progenitor cells in health and disease. Circ Res. 116:1392–1412.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Deng S, Zhang Y, Wang Y, Lu X and Jiang Q:
MicroRNA-92 regulates vascular smooth muscle cell function by
targeting KLF4 during vascular restenosis and injury. Int J Clin
Exp Pathol. 12:4253–4262. 2019.PubMed/NCBI
|
|
30
|
Kingsley K, Huff JL, Rust WL, Carroll K,
Martinez AM, Fitchmun M and Plopper GE: ERK1/2 mediates PDGF-BB
stimulated vascular smooth muscle cell proliferation and migration
on laminin-5. Biochem Biophys Res Commun. 293:1000–1006.
2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chiong M, Cartes-Saavedra B,
Norambuena-Soto I, Mondaca-Ruff D, Morales PE, García-Miguel M and
Mellado R: Mitochondrial metabolism and the control of vascular
smooth muscle cell proliferation. Front Cell Dev Biol.
2(72)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Park ES, Kang SI, Yoo KD, Lee MY, Yoo HS,
Hong JT, Shin HS, Kim B and Yun YP: Camptothecin inhibits
platelet-derived growth factor-BB-induced proliferation of rat
aortic vascular smooth muscle cells through inhibition of PI3K/Akt
signaling pathway. Exp Cell Res. 319:982–991. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim S, Zhan Y, Izumi Y, Yasumoto H, Yano M
and Iwao H: In vivo activation of rat aortic platelet-derived
growth factor and epidermal growth factor receptors by angiotensin
II and hypertension. Arterioscler Thromb Vasc Biol. 20:2539–2545.
2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pei C, Qin S, Wang M and Zhang S:
Regulatory mechanism of human vascular smooth muscle cell
phenotypic transformation induced by NELIN. Mol Med Rep.
12:7310–7316. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang G, Jacquet L, Karamariti E and Xu Q:
Origin and differentiation of vascular smooth muscle cells. J
Physiol. 593:3013–3030. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhong W, Li B, Yang P, Chen R, Wang C,
Wang Z, Shao C, Yuan W and Yan J: CD137-CD137L interaction
modulates neointima formation and the phenotype transformation of
vascular smooth muscle cells via NFATc1 signaling. Mol Cell
Biochem. 439:65–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ersahin T, Tuncbag N and Cetin-Atalay R:
The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu T, Zhu H, Li D, Lang Y, Cao L, Liu Y,
Wu W and Chen D: Luteolin inhibits angiotensin II-stimulated VSMC
proliferation and migration through downregulation of Akt
phosphorylation. Evid Based Complement Alternat Med.
2015(931782)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brown DJ, Rzucidlo EM, Merenick BL, Wagner
RJ, Martin KA and Powell RJ: Endothelial cell activation of the
smooth muscle cell phosphoinositide 3-kinase/Akt pathway promotes
differentiation. J Vasc Surg. 41:509–516. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ponnusamy A, Sinha S, Hyde GD, Borland SJ,
Taylor RF, Pond E, Eyre HJ, Inkson CA, Gilmore A, Ashton N, et al:
FTI-277 inhibits smooth muscle cell calcification by up-regulating
PI3K/Akt signaling and inhibiting apoptosis. PLoS One.
13(e0196232)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fang H, Yang S, Luo Y, Zhang C, Rao Y, Liu
R, Feng Y and Yu J: Notoginsenoside R1 inhibits vascular smooth
muscle cell proliferation, migration and neointimal hyperplasia
through PI3K/Akt signaling. Sci Rep. 8(7595)2018.PubMed/NCBI View Article : Google Scholar
|