Introduction
Diabetes is the most commonly diagnosed metabolic
disorder in clinical practice; it is estimated that >280 million
people have diabetes worldwide (1,2). The
long-term development and progression of diabetes can affect
multiple organs, leading to the occurrence of diabetic
complications (3). Moreover,
diabetic retinopathy (DR) is a common complication of diabetes and
is a major cause of vision-loss globally (4,5). DR is
caused by high-glucose mediated damage of the blood vessels in the
light-sensitive tissue of retina (4,5).
However, despite the development of various therapeutic approaches,
such as intravitreal injections of steroids, anti-vascular
endothelial growth factor agents and laser photocoagulation,
blindness will inevitably occur in ~10% of patients with DR
(6). Therefore, investigation into
novel therapeutic approaches remains of great importance.
Along with hyperglycemic conditions, the progression
of DR also involves in multiple genetic factors (7,8). For
example, the polymorphism of Aldose Reductase gen has been found to
be a risk factor of DR with type-2 diabetes (9). The genetic variant in miR-449b is
significantly associated with Caucasian patients with type-1
diabetes (10). Thus, the
identification and functional characterization of genetic factors
involved in the molecular pathogenesis of DR may provide novel
insights into the treatment of DR (11). TIMP metallopeptidase inhibitor 3
(Timp3) is a critical component in glucose homeostasis, and
intravitreal injection of Timp3 has protective effects in a
retinopathy mouse model (12,13).
Furthermore, microRNA (miRNA/miR)-365 can target Timp3 to promote
the development of DR (14). In a
previous study, preliminary bioinformatics analysis results
revealed that miR-365 could also target ribosomal protein SA
pseudogene 52 (RPSAP52), which is an oncogenic long non-coding
(lnc)RNA in pituitary tumors (15).
Therefore, the aim of the present study was to investigate the
interactions between miR-365, RPSAP52 and Timp3 in DR.
Patients and methods
Research subjects
This study was approved by the Ethics Committee of
The Fourth People's Hospital of Shenyang (Shenyang, China).
Research subjects included 60 patients with DR (age, 47-68 years),
60 patients with diabetes (age, 47-68 years) and 60 healthy
controls (age, 47-68 years). All these participants were enrolled
at the aforementioned hospital between March 2017 and March 2019.
All patients were diagnosed for the first time and no other
clinical disorders were diagnosed. No therapies were performed
within 3 months before admission. The basic information of the
three groups of participants is presented in Table I. According to National Institute on
Alcohol Abuse and Alcoholism, heavy alcohol drinking defined as
average intake of >7 drinks per week for women and >14 drinks
per week for men (16). No
significant differences in basic information, except body mass
index were identified among the three groups. All participants
signed informed consent.
 | Table IBasic information of the three groups
of participants. |
Table I
Basic information of the three groups
of participants.
| | Diabetic retinopathy
(n=60) | Diabetes (n=60) | Control (n=60) |
|---|
| Sex | | | |
|
Male | 40 (66.7%) | 40 (66.7%) | 40 (66.7%) |
|
Female | 20 (33.3%) | 20 (33.3%) | 20 (33.3%) |
| Age, years | 56.3±7.1 | 56.4±7.0 | 56.4±7.1 |
| Mean BMI | 26.6±2.8a | 25.5±2.8a | 21.6±2.1 |
| Habits | | | |
|
Current
smoker | 30 (50%) | 30 (50%) | 30 (30%) |
|
Heavy
alcohol drinking | 36 (60%) | 35 (58.3%) | 35 (58.3%) |
Plasma and retinal pigment epithelial
(RPE) cells
Blood (5 ml) was extracted under fasting conditions
from all participants before the initiation of therapies. Blood
samples were centrifuged at 1,200 x g in EDTA tubes at 4˚C for 10
min to prepare plasma samples. The human RPE cell line ARPE-19
(American Type Culture Collection; cat. no. CRL-2302) was used.
Cells were cultivated under conditions of 37˚C, 5% CO2
with DMEM/F12 Medium (Thermo Fisher Scientific, Inc.) containing
10% FBS (Sigma-Aldrich; Merck KGaA).
Cell transfections
Expression vectors of RPSAP52 (accession no.
NR_026825.2) and Timp3 (accession no. NM_000362.5) were established
using pcDNA3.1 vector (Sigma-Aldrich; Merck KGaA) between
EcoRI and EcoRV cleavage sites as the backbone.
Negative control (NC) miRNA (5'-GUGUGGUUCGGAAAUUCCACAG-3') and
miR-365 mimic (5'-UAAUGCCCCUAAAAAUCCUUAU-3') were also purchased
from Sigma-Aldrich (Merck KGaA). Cells were cultivated at 75-85%
confluence and manually counted using a hemocytometer. Then,
106 cells were transfected with 50 nM miRNA or 10 nM
vector via transient transfections using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). In
total, two control groups were included: i) Control (C),
untransfected cells; and ii) NC, empty pcDNA3.1 or miRNA NC
transfection. Transfections were performed at 37˚C for 6 h. Cells
were collected at 24 h post-transfection to perform the following
experiments.
Luciferase reporter assay
The potential binding site of miR-365 on RPSAP52 was
predicted by IntaRNA 2.0 (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp).
The RPSAP52 vector was constructed by inserting the full-length
cDNA of RPSAP52 (accession no. NR_026825.2) into pmirGLO vector
(Promega Corporation). RPSAP52 vector was co-transfected into
106 cells with miRNA NC or miR-365 mimic via
aforementioned methods. A dual-luciferase reporter gene assay
system (Promega Corporation) was used to measure luciferase
activity in cells collected at 24 h post-transfection.
Renilla luciferase activity was used to normalize the
relative luciferase activity.
RNA preparations and reverse
transcription-quantitative PCR (RT-qPCR)
RNAiso Plus (Takara Biotechnology Co., Ltd.) was
used to extract total RNA from both in vitro cultivated
cells and in vivo plasma from patients. Cells were
cultivated in DMEM/F12 medium containing 10, 20 and 30 mM D-glucose
(Shanghai Aladdin Bio-Chem Technology Co., Ltd.) at 37˚C for 24 h
before use. LookOut® DNA Erase (Sigma-Aldrich; Merck
KGaA) was used to digest all RNA samples to remove genomic DNA.
Total RNAs were reverse transcribed into cDNAs using the SSRT IV
system (Thermo Fisher Scientific, Inc.) under the following
conditions: 25˚C for 10 min, 55˚C for 30 min and 85˚C for 10 min.
The qPCR reaction mixtures were prepared using SYBR Green PCR kit
(Takara Biotechnology Co., Ltd.). GAPDH was used as endogenous
control for the expression levels of RPSAP52 and Timp3. PureLink
miRNA Isolation kit (Thermo Fisher Scientific, Inc.) was used to
extract miRNAs from the samples. All-in-One™ miRNA RT-qPCR reagent
kit (GeneCopoeia, Inc.) was used to measure the expression of
miR-365 with U6 as endogenous control. Primer sequences were:
RPSAP52 forward, 5'-GGACCTGGGAGAAGCTTCTG-3' and reverse,
5'-CCAGGAGTGAAGTGGCCAGC-3'; Timp3 forward,
5'-CCAGGACGCCTTCTGCAACT-3' and reverse, 5'-CATCTTGGTGAAGCCTCGGT-3';
GAPDH forward, 5'-CATCACTGCCACCCAGAAGACTG-3' and reverse,
5'-ATGCCAGTGAGCTTCCCGTTCAG-3'; and miR-365 forward,
5'-TAATGCCCCTAAAAATCCTT-3' and reverse,
5'-GCGAGCACAGAATTAATACGAC-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'. PCR conditions were as follows:
Initial denaturation at 95˚C for 10 min; 40 cycles at 95˚C for 10
sec and 56˚C for 50 sec; final annealing at 72˚C for 10 min. The
2-ΔΔCq method (17) was used to calculate the fold changes
of gene expression levels across samples. All PCR reactions were
repeated three times.
Western blot analysis
Transfected cells were harvested at 24 h
post-transfection and were washed twice with PBS. Cells were mixed
with RIPA solution (Beyotime Institute of Biotechnology) to prepare
cell lysates. A bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) was used to measure the protein
concentration, followed by protein denaturation in boiling water at
100˚C for 10 min. Then, 25 µg protein was separated by 12% SDS-PAGE
and was transferred to PVDF membranes. After being blocked with 5%
skim milk for 1 h at 4˚C, rabbit primary antibodies of Timp3
(1:500; cat. no. ab39185; Abcam) and GAPDH (1:1,000; cat. no.
ab8425 Abcam) were incubated with the membranes at 4˚C for 12 h.
Then, horseradish peroxidase-conjugated goat anti-rabbit (IgG)
secondary antibody (1:1,000; cat. no. ab97051; Abcam) was further
incubated with the membranes at 25˚C for 2 h. An ECL assay (EMD
Millipore) was performed to produce signals, which were normalized
using ImageJ v1.48 software (National Institutes of Health).
Flow cytometry to analyze cell
apoptosis
Transfected cells were harvested at 24 h
post-transfection and were seeded onto a 6-well plate
(105 cells per well), followed by cell culturing in
DMEM/F12 medium containing 30 mM D-glucose under 5% CO2
at 37˚C for 48 h. Cells cultured in DMEM/F12 medium without
D-glucose under 5% CO2 at 37˚C (normal condition) were
used as the control. Subsequently, cells were stained with 10 µl
FITC-Annexin V and 10 µl propidium iodide (0.5 mg/ml) at 4˚C in the
dark (BD Biosciences), followed by detection of apoptotic cells
using flow cytometry. The apoptotic rate was calculated using the
percentage of early and late apoptotic cells. Flowjo7.6.1 software
(BD Biosciences) was used to analyze flow cytometry data with the
BD Accuri C6 flow cytometer (BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent replicates obtained in each experiment. SPSS
V17.0 (IBM Corp.) was used to perform data analysis. Differences
between two groups were analyzed by unpaired t-test. Differences
among multiple groups were assessed by one-way ANOVA and post hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
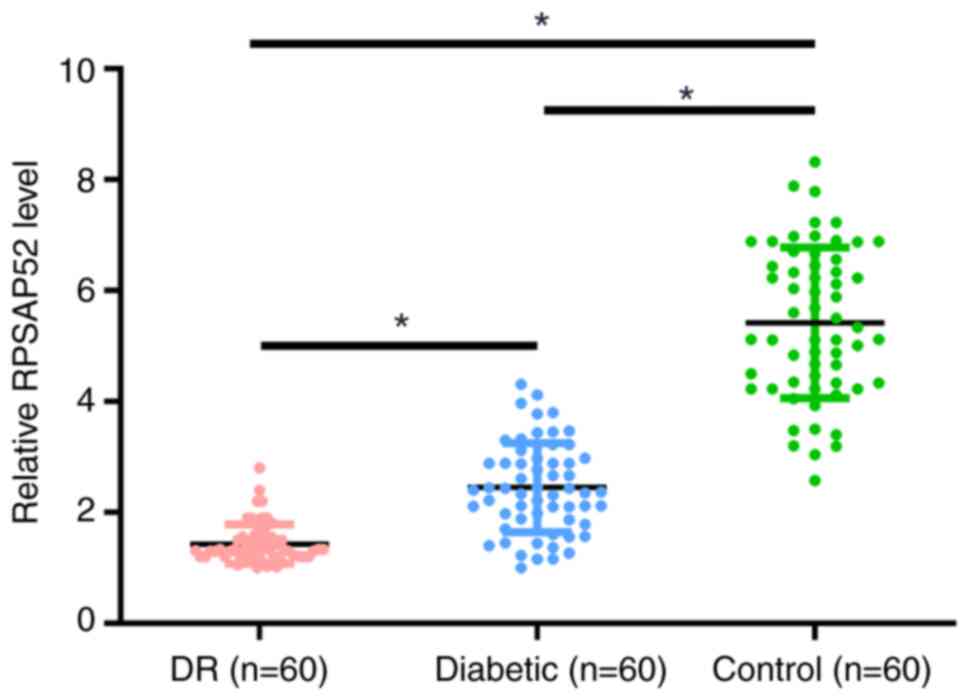
Differential expression of RPSAP52 in
the three groups of participants
The differential expression of RPSAP52 in the three
groups of participants was assessed by measuring the expression of
RPSAP52 in plasma of patients with DR (n=60), patients with
diabetes (n=60) and healthy controls (n=60). It was demonstrated
that the plasma expression levels of RPSAP52 were significantly
lower in the DR group compared with the other two groups (3.3- and
2.0-fold, respectively; P<0.05; Fig.
1). Moreover, the diabetic group had lower plasma expression
levels of RPSAP52 compared with the control group (1.7-fold;
P<0.05).
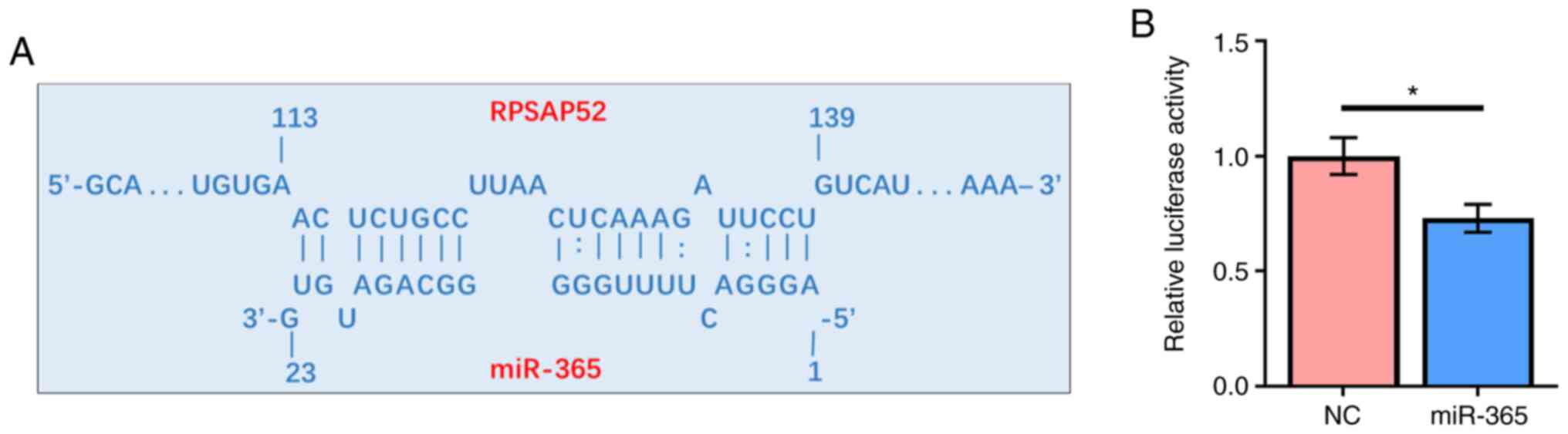
RPSAP52 may directly interact with
miR-365
Prediction of the interaction between RPSAP52 and
miR-365 was performed by IntaRNA 2.0. It was identified that
RPSAP52 and miR-365 could form base pairing with each other
(Fig. 2A). The interaction between
them was assessed by dual luciferase reporter assay. Compared with
ARPE-19 cells transfected with RPSAP52 vector and miRNA NC, cells
co-transfected with RPSAP52 vector and miR-365 mimic had a
significantly lower relative luciferase activity (Fig. 2B; P<0.05).
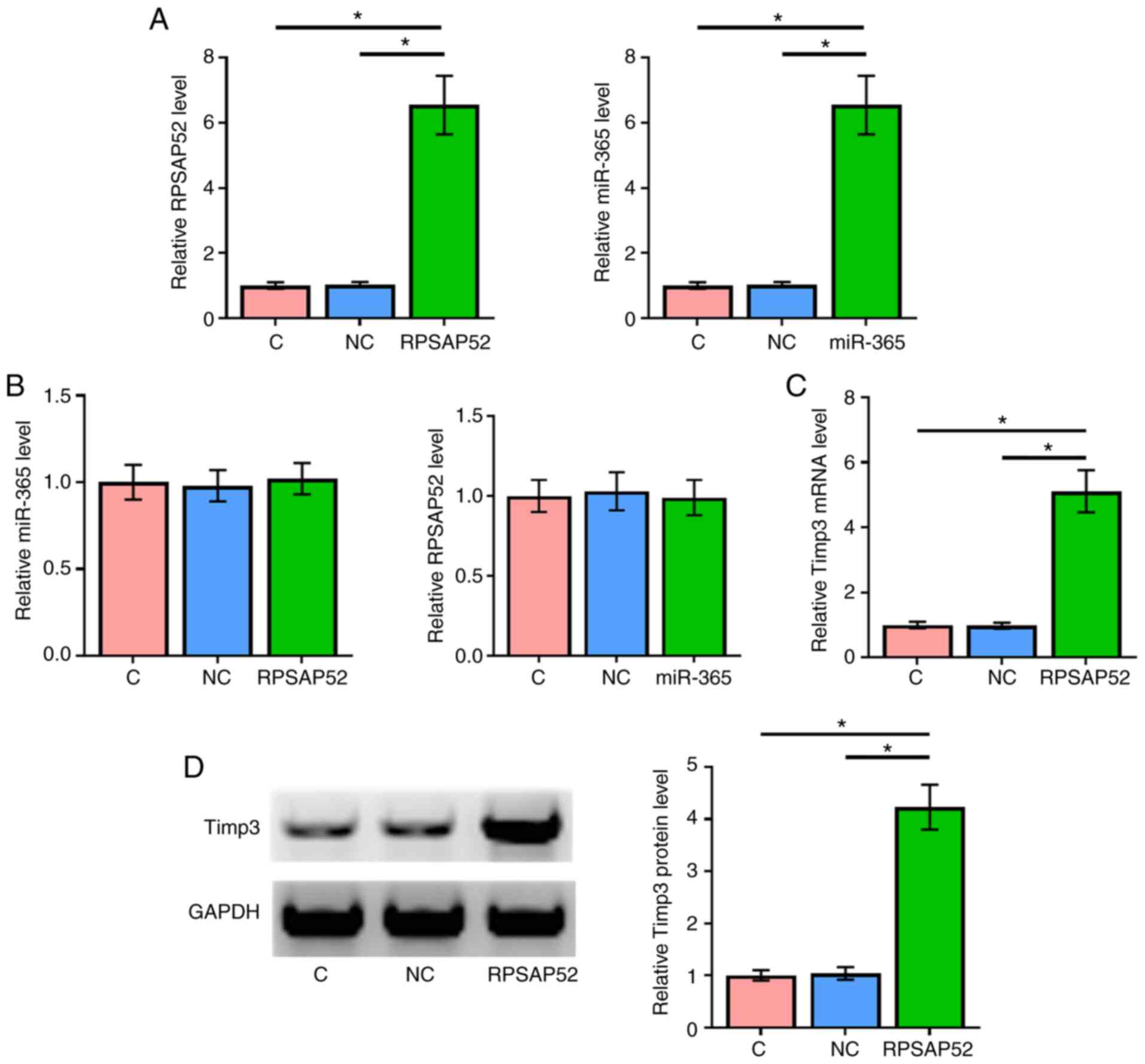
RPSAP52 and miR-365 did not affect the
expression of each other, but RPSAP52 upregulated Timp3
To further evaluate the interactions between RPSAP52
and miR-365, ARPE-19 cells were transfected with RPSAP52 vector and
miR-365 mimic. Overexpression of RPSAP52 and miR-365 were
demonstrated by RT-qPCR (Fig. 3A;
P<0.05). Compared with the NC and C groups, overexpression of
RPSAP52 and miR-365 did not affect the expression of one another
(Fig. 3B).
The effects of overexpressing RPSAP52 on the
expression of Timp3 at mRNA (Fig.
3C) and protein (Fig. 3D)
levels were assessed by RT-qPCR and western blotting, respectively.
Compared with C and NC groups, overexpression of RPSAP52 led to the
significant upregulation of Timp3 (P<0.05).
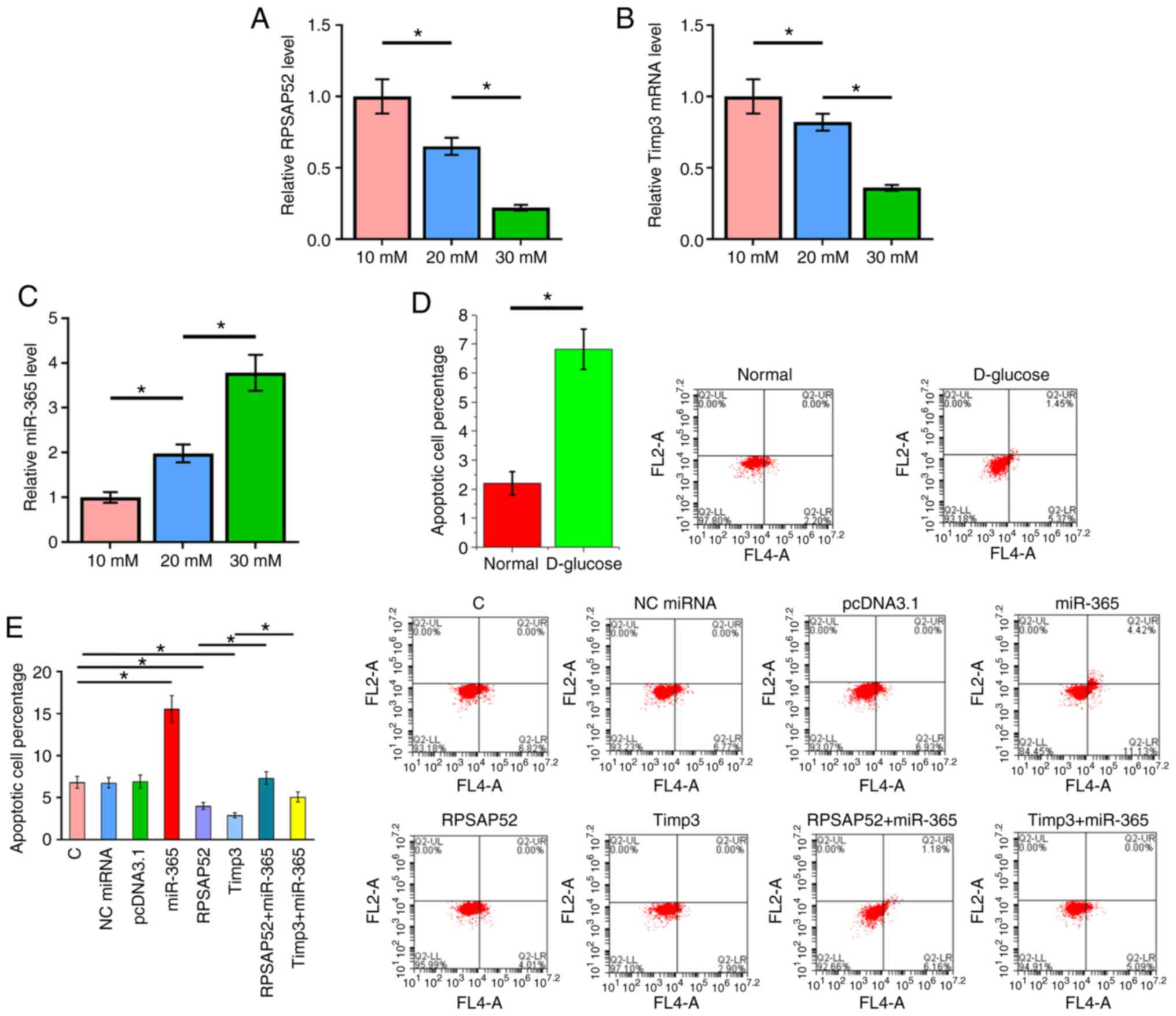
RPSAP52 regulates the miR-365/Timp3
axis to suppress D-glucose-induced ARPE-19 cell apoptosis
The RT-qPCR results indicated that D-glucose
treatment led to downregulation of RPSAP52 (Fig. 4A; P<0.05) and Timp3 (Fig. 4B; P<0.05), but upregulated
miR-365 (Fig. 4C; P<0.05).
Cells apoptosis under 30 mM D-glucose treatment
after the overexpression of RPSAP52, miR-365 and Timp3 was detected
by cell apoptosis assay. The results identified that the apoptotic
rate under normal conditions was 2.2±0.4%, while the apoptotic rate
of the C group under 30 mM D-glucose treatment was 6.82±0.7%, which
was significantly different (Fig.
4D; P<0.05). Furthermore, overexpression of Timp3 in ARPE-19
cells was evaluated by RT-qPCR (Fig.
S1). Compared with the C group, overexpression of RPSAP52 and
Timp3 led to a decreased apoptotic rate of ARPE-19 cells induced by
high D-glucose treatment. Moreover, overexpression of miR-365 had
an opposite role and reduced the effects of RPSAP52 and Timp3
overexpression (Fig. 4E;
P<0.05).
Discussion
The present study investigated the roles of RPSAP52
in DR. The results suggested that RPSAP52 was downregulated in DR
and may regulate the miR-365/Timp3 axis to participate in the
regulation of RPE cell apoptosis induced by high D-glucose
conditions.
RPSAP52 is a newly identified lncRNA with known
functions in pituitary tumors (15). In pituitary tumors, RPSAP52 is
overexpressed and can sponge multiple miRNAs, such as miR-15a,
miR-15b and miR-16, to regulate the expression of high mobility
group A proteins, thus promoting the proliferation of cancer cells
(15). It has been reported that
RPSAP52 is abundant in the cytoplasm and interacts with the RNA
binding protein insulin-like growth factor 2 mRNA-binding protein
2, and can also play in a role in sarcomas (18). The development and progression of
diabetes and its complications can affect the expression of
multiple lncRNAs (19); however, to
the best of our knowledge, the role of RPSAP52 in diabetes or its
complications has not previously reported. The present study
identified the downregulation of RPSAP52 in patients with DR and
patients with diabetes. Furthermore, high D-glucose treatment led
to downregulation of RPSAP52 in RPE. Plasma levels of RPSAP52 were
also significantly lower in patients with DR compared with healthy
controls. Therefore, the downregulation of RPSAP52 may be induced
by the hyperglycemic condition of patients with diabetes. Moreover,
with the development of diabetic complications, such as DR, plasma
levels of RPSAP52 can be further downregulated.
RPE cells participate in multiple functions of the
eyes, such as light absorption, epithelial transport, spatial
buffering of ions and the visual cycle (20). DR induces the apoptosis of RPE cells
to reduce the functions of RPE (21). Moreover, the present results
indicated a reduced apoptotic rate of RPE cells induced by high
D-glucose treatment after overexpression of RPSAP52. Therefore,
RPSAP52 may play a protective role in DR.
Previous studies have reported that miR-365 is
dysregulated in several human malignancies, such as ovarian cancer
(22) and lung cancer (23), and can serve a role in the
regulation of the cell cycle and apoptosis (24). Furthermore, high expression of
miR-365 is found in the aqueous humor of patients with cataracts
(25). It has also been reported
that miR-365 can target Timp3 to promote DR and increase oxidative
stress (14). Timp3, which is a
multifunctional protein, can inhibit matrix metalloproteinases
(26). Moreover, it has been shown
that a mutation of Timp3 can cause Sorsby funfus dystrophy
(27). Timp3 also serves a critical
role in glucose homeostasis (12,13),
and it has been reported that intravitreal injection of Timp3 can
improve retinopathy in a mouse model (12,13).
The present results suggested that RPSAP52 could directly interact
with miR-365 and overexpression of RPSAP52 caused downregulation of
Timp3, which indicated that RPSAP52 may target Timp3 via miR-365.
To the best of our knowledge, the present study was the first to
identify the involvement of the interaction between Timp3 and
miR-365 in RPE cell apoptosis. Moreover, the results suggested that
miR-365 may not target RPSAP52. Instead, RPSAP52 could sponge
miR-365 to upregulate Timp3, resulting in inhibited RPE cell
apoptosis.
It has been previously reported that high D-glucose
level induces cell death (28). The
present study also confirmed that high D-glucose treatment can
induce ARPE-19 cell apoptosis. In addition, the present study did
not analyze the correlations between expression levels of RPSA52,
Timp3 or miR-365 and the percentage of apoptotic cells. Thus, these
will be the focus of future studies.
In conclusion, the present results indicated that
RPSAP52 was downregulated in DR and may regulate the miR-365/Timp3
axis to regulate RPE cell apoptosis.
Supplementary Material
Figure S1. Expression vector of Timp3
up-regulated Timp3 mRNA level in ARPE‑19 cells. ARPE‑19 cells were
transfected with expression vector of Timp3. The mRNA level of
Timp3 was assessed by RT‑qPCR at 24 h post‑transfection.
*P<0.05. C, control; NC, negative control; RT‑qPCR, reverse
transcription‑quantitative PCR.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The data in this work are available from the
corresponding author on reasonable request.
Authors' contributions
TN and DL designed the experiments. TN and YA
performed the experiments. TN and TL analyzed and interpreted the
data. TN wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Fourth People's Hospital of Shenyang. Informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dagenais GR, Gerstein HC, Zhang X, McQueen
M, Lear S, Lopez-Jaramillo P, Mohan V, Mony P, Gupta R, Kutty VR,
et al: Variations in diabetes prevalence in low-, middle-, and
high-income countries: Results from the prospective urban and rural
epidemiological study. Diabetes Care. 39:780–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA.
317:2515–2523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oei L, Rivadeneira F, Zillikens MC and Oei
EHG: Diabetes, diabetic complications, and fracture risk. Curr
Osteoporos Rep. 13:106–115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee R, Wong TY and Sabanayagam C:
Epidemiology of diabetic retinopathy, diabetic macular edema and
related vision loss. Eye Vis (Lond). 2(17)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nentwich MM and Ulbig MW: Diabetic
retinopathy-ocular complications of diabetes mellitus. World J
Diabetes. 6:489–499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Duh EJ, Sun JK and Stitt AW: Diabetic
retinopathy: Current understanding, mechanisms, and treatment
strategies. JCI Insight. 2(e93751)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hampton BM, Schwartz SG, Brantley MA Jr
and Flynn HW Jr: Update on genetics and diabetic retinopathy. Clin
Ophthalmol. 9:2175–2193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mishra B, Swaroop A and Kandpal RP:
Genetic components in diabetic retinopathy. Indian J Ophthalmol.
64:55–61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wihandani DM, Suastika K, Bagiada INA and
Malik SG: Polymorphisms of aldose reductase (ALR2) regulatory gene
are risk factors for diabetic retinopathy in type-2 diabetes
mellitus patients in Bali, Indonesia. Open Ophthalmol J.
12:281–288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu E, Kaidonis G, McComish BJ, Gillies
MC, Abhary S, Essex RW, Chang JH, Pal B, Daniell M, Lake S, et al:
MicroRNA-related genetic variants are associated with diabetic
retinopathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci.
60:3937–3942. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Babapoor-Farrokhran S, Jee K, Puchner B,
Hassan SJ, Xin X, Rodrigues M, Kashiwabuchi F, Ma T, Hu K,
Deshpande M, et al: Angiopoietin-like 4 is a potent angiogenic
factor and a novel therapeutic target for patients with
proliferative diabetic retinopathy. Proc Natl Acad Sci USA.
112:E3030–E3039. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hewing NJ, Weskamp G, Vermaat J, Farage E,
Glomski K, Swendeman S, Chan RV, Chiang MF, Khokha R, Anand-Apte B
and Blobel CP: Intravitreal injection of TIMP3 or the EGFR
inhibitor erlotinib offers protection from oxygen-induced
retinopathy in mice. Invest Ophthalmol Vis Sci. 54:864–870.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Federici M, Hribal ML, Menghini R, Kanno
H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo
V, et al: Timp3 deficiency in insulin receptor-haploinsufficient
mice promotes diabetes and vascular inflammation via increased
TNF-alpha. J Clin Invest. 115:3494–3505. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Wang J, Zhang J, Chen X, Yang Y, Wang F,
Li W, Awuti M, Sun Y, Lian C, Li Z, et al: miR-365 promotes
diabetic retinopathy through inhibiting Timp3 and increasing
oxidative stress. Exp Eye Res. 168:89–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
D'Angelo D, Mussnich P, Sepe R, Raia M,
Del Vecchio L, Cappabianca P, Pellecchia S, Petrosino S, Saggio S,
Solari D, et al: RPSAP52 lncRNA is overexpressed in pituitary
tumors and promotes cell proliferation by acting as miRNA sponge
for HMGA proteins. J Mol Med (Berl). 97:1019–1032. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
National Institute on Alcohol Abuse and
Alcoholism. Helping patients who drink too much: A clinician's
guide: Updated 2005 edition (no. 7). US Department of Health and
Human Services, national institutes of health, national institute
on alcohol abuse and alcoholism, 2007. urihttps://my.ireta.org/sites/ireta.org/files/NIAAA_Clinicians_Guide_Helping_Patients.pdfsimplehttps://my.ireta.org/sites/ireta.org/files/NIAAA_Clinicians_Guide_Helping_Patients.pdf.
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oliveira-Mateos C, Sánchez-Castillo A,
Soler M, Obiols-Guardia A, Piñeyro D, Boque-Sastre R,
Calleja-Cervantes ME, de Moura MC, Martínez-Cardús A, Rubio T, et
al: The transcribed pseudogene RPSAP52 enhances the oncofetal
HMGA2-IGF2BP2-RAS axis through LIN28B-dependent and independent
let-7 inhibition. Nat Commun. 10(3979)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leung A and Natarajan R: Long noncoding
RNAs in diabetes and diabetic complications. Antioxid Redox Signal.
29:1064–1073. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xia T and Rizzolo LJ: Effects of diabetic
retinopathy on the barrier functions of the retinal pigment
epithelium. Vision Res. 139:72–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Xu C, Wang Y and Zhang X:
MicroRNA-365 inhibits ovarian cancer progression by targeting
Wnt5a. Am J Canc Res. 7:1096–1106. 2017.PubMed/NCBI
|
|
23
|
Qi J, Rice SJ, Salzberg AC, Runkle EA,
Liao J, Zander DS and Mu D: MiR-365 regulates lung cancer and
developmental gene thyroid transcription factor 1. Cell Cycle.
11:177–186. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y,
Du X and Han W: MicroRNA-365, down-regulated in colon cancer,
inhibits cell cycle progression and promotes apoptosis of colon
cancer cells by probably targeting cyclin D1 and Bcl-2.
Carcinogenesis. 33:220–225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dunmire JJ, Lagouros E, Bouhenni RA, Jones
M and Edward DP: MicroRNA in aqueous humor from patients with
cataract. Exp Eye Res. 108:68–71. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang J, Chen M, Hughes D, Chumanevich AA,
Altilia S, Kaza V, Lim CU, Kiaris H, Mythreye K, Pena MM, et al:
CDK8 selectively promotes the growth of colon cancer metastases in
the liver by regulating gene expression of TIMP3 and matrix
metalloproteinases. Cancer Res. 78:6594–6606. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Weber BH, Vogt G, Pruett RC, Stöhr H and
Felbor U: Mutations in the tissue inhibitor of metalloproteinases-3
(TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet.
8:352–356. 1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Y, Xu F, Xiao H and Han F: Long
noncoding RNA BDNF-AS inversely regulated BDNF and modulated
high-glucose induced apoptosis in human retinal pigment epithelial
cells. J Cell Biochem. 119:817–823. 2018.PubMed/NCBI View Article : Google Scholar
|